Abstract
Aims/Introduction
Low bodyweight and hypoalbuminemia are independently associated with osteoporosis. In this study, the relationship among the Geriatric Nutritional Risk Index (GNRI), bone mineral density (BMD) and osteoporosis in type 2 diabetes mellitus patients was explored, and the GNRI predictive value was evaluated.
Materials and Methods
We enrolled 225 men and 192 women with type 2 diabetes mellitus. Their general condition, and laboratory and BMD data were collected. Spearman’s partial correlation analysis adjusting for age, body mass index and albumin was used for exploring the association among the GNRI, BMD and bone metabolism markers. Statistical analyses, including multivariate regression analysis and receiver operating characteristic curve analysis, were also applied in this study.
Results
On Spearman’s partial correlation analysis, GNRI was positively associated with BMD and albumin‐corrected calcium (r = 0.145–0.561, P < 0.01). For the multivariate regression analysis, we observed that the GNRI was dramatically related to high total lumbar, total hip, femur neck BMD and osteoporosis (odds ratio 0.857 for men and 0.927 for women, all P < 0.05). The area under the receiver operating characteristic curve of the GNRI (0.876 for men and 0.704 for women, all P < 0.01) was the largest compared with that of albumin and body mass index in osteoporosis prediction.
Conclusions
In this study, it was shown that the GNRI was positively correlated with BMD, and inversely correlated with osteoporosis in type 2 diabetes mellitus patients. In addition, compared with body mass index, albumin and age, the GNRI was a more powerful indicator for osteoporosis.
Keywords: Bone mineral density, Geriatric nutritional risk index, Osteoporosis
The Geriatric Nutritional Risk Index is positively correlated with bone mineral density and inversely correlated with osteoporosis in type 2 diabetes mellitus patients. In addition, compared with body mass index and albumin, the Geriatric Nutritional Risk Index was a more powerful indicator for osteoporosis.

Introduction
In recent years, osteoporosis, especially for low‐energy fractures, has become prevalent among the aging population, resulting in disability, poor quality of life and even death of patients1. In general, for patients with type 2 diabetes mellitus, the risk of osteoporosis is much higher than that in the normal population 2, 3. In addition, the risk still varies greatly from one case to another, even after modulating for age, duration of diabetes and body mass index (BMI)4, 5. The risk factors of osteoporosis in the normal population have been shown in previous studies6; however, because of the particularity of metabolic disease, the risk factors of osteoporosis in people with type 2 diabetes mellitus might be quite different. Therefore, it is crucial to discuss them more specifically.
Previous studies have shown that low bodyweight and hypoalbuminemia are separately related to lumbar spine osteoporosis for people with diabetes 7, 8. Recently, the Geriatric Nutritional Risk Index (GNRI) has been applied as a simple and available tool to evaluate the results based on serum albumin and the ratio between the actual and ideal bodyweight 9. For the past several years, a variety of studies have shown and validated the predictive property of the GNRI for patients with pyogenic liver abscess10, sepsis institutionalized11, hemodialysis12 and pneumonia13. However, the GNRI has rarely been used to assess the nutrient condition of type 2 diabetes mellitus patients. Furthermore, the value of the GNRI in predicting osteoporosis in type 2 diabetes mellitus patients has not been explored yet.
Therefore, the present study aimed to determine the relationship between the GNRI and osteoporosis in type 2 diabetes mellitus patients. The predictive properties of the GNRI, albumin, BMI and age for osteoporosis in patients with type 2 diabetes mellitus were ulteriorly assessed.
Methods
Study population
In the present cross‐sectional study, the participants were 447 Chinese older adults with type 2 diabetes mellitus (age >50 years, including 255 men and 192 postmenopausal women). The participants visited the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University for evaluating and treating type 2 diabetes mellitus, and were enrolled between January 2017 to December 2017. For the participants, the exclusion criteria were as follows: (i) malignant tumor and severe heart, liver or kidney diseases; (ii) pituitary, thyroid, parathyroid, adrenal and gonadal diseases; (iii) long‐term bedridden; (iv) individuals concomitantly taking drugs affecting the bone metabolism, such as calcium, vitamin D and bisphosphonates; and (v) patients without available information. This study obtained approval from the ethics committee of the Second Affiliated Hospital of Wenzhou Medical University (No. LCKY2017‐21), and obtained the written informed consent of all participants following the Declaration of Helsinki.
Clinical assessment and health history
Bodyweight and height were measured with light clothing and without shoes. The body mass index (BMI; kg/m2), defined as the bodyweight (kg) per square of height (m2), was calculated for each patient. Blood pressure (mmHg) was measured by a mercury sphygmomanometer in the supine position after resting for 5 min. The duration of diabetes was counted by years, starting from the time point the patients was diagnosed with type 2 diabetes based on the medical record, up to carrying out the blood tests and measuring bone mineral density (BMD) values. Smoking and drinking history were considered as never or ever.
Biochemical indexes
Serum samples were collected at 06.00 hours after overnight fasting (at least 8 h). The glucose metabolism indexes, including fasting blood glucose (FBG) and glycosylated hemoglobin, were measured according to standard methodology. In addition, the levels of lipids in serum, including total cholesterol (TC), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C) and low‐density lipoprotein cholesterol (LDL‐C) were analyzed. Furthermore, the bone metabolic indicators, which contained parathyroid hormone, procollagen of type I N‐propeptide, β‐isomerized C‐terminal telopeptides and 25‐hydroxy‐vitamin were also detected. Additionally, other biochemical markers, such as blood creatinine, uric and calcium, were recorded.
BMD index
Dual‐energy X‐ray absorptiometry (Hologic‐Discovery, Bedford, MA, USA) was used to measure the BMD value of each patient at three positions: total lumbar, femur neck and total hip. The standard of osteoporosis in the World Health Organization criterion was evaluated by the BMD, the value of which was >2.5 standard deviations below the T‐score14.
GNRI
The GNRI was calculated based on the parameters, including height (H; cm), actual bodyweight (kg), ideal bodyweight (kg) and the level of serum albumin (g/L), and the calculation formula is shown as follows9:
The WL0 in the above equation represented the ideal weight, which can be further calculated by the following equations:
Statistical analysis
The statistical analyses were processed dividedly for men and women. In this study, mean ± standard deviation was applied to measure continuous variables, and the proportion was applied to measure categorical variables. The difference of a quantitative variable between two groups was investigated using the independent two‐sample t‐test or the Mann–Whitney U‐test, and Pearson’s 2‐test was used to test for differences in the distribution of a categorical variable. Spearman’s partial correlation analysis adjusting for age, BMI and albumin was applied to determine the relationships between GNRI, BMD and bone metabolism markers. Multivariate linear regression analysis was carried out to show the relationship among BMD at the lumbar spine, hip and femoral neck, and other parameters. The hazard ratios of risk factors for osteoporosis in people with type 2 diabetes mellitus was further assessed by sex subanalysis. Furthermore, the receiver operating characteristic curve was applied to estimate the predictive property of the GNRI for osteoporosis, and the areas under the receiver operating characteristic curve (AUC) were also calculated.
Results
Analysis of baseline characteristics
The baseline characteristics of all participants are represented in Table 1. In this study, 447 patients had type 2 diabetes, with the average age ranging from 66.10 ± 9.47 years, and BMI ranging from 24.41 ± 3.66 kg/m2. Figure S1 shows the distribution of BMD in type 2 diabetes mellitus patients. The average BMD at the total lumbar, femur neck and total hip of men were higher than those in women (1.073 vs 0.925, 0.827 vs 0.738, 0.896 vs 0.809, respectively, and all P < 0.01). Compared with women, the levels of FBG, TC, TG, HDL‐C, LDL‐C, PINH and the incidence of osteoporosis were markedly lower in men. In addition, a history of smoking or drinking was drastically higher among men than that among women.
Table 1.
Patients characteristics, stratified by sex
| Total patients (n = 447) | Men patients (n = 255) | Women patients (n = 192) | P | |
|---|---|---|---|---|
| Age (years) | 66.10 ± 9.47 | 64.95 ± 9.79 | 67.63 ± 8.82 | 0.003 |
| Diabetes duration (years) | 8.02 ± 6.82 | 7.64 ± 6.90 | 8.52 ± 6.70 | 0.177 |
| Systolic blood pressure (mmHg) | 139.44 ± 22.27 | 136.92 ± 21.53 | 142.79 ± 22.84 | 0.006 |
| Diastolic blood pressure (mmHg) | 77.42 ± 12.56 | 78.26 ± 12.60 | 76.30 ± 12.45 | 0.103 |
| BMI (kg/m2) | 24.41 ± 3.66 | 23.97 ± 3.25 | 25.00 ± 4.07 | 0.003 |
| Smoking (current or ever) | 28.6% | 49.4% | 1.0% | 0.000 |
| Drinking (current or ever) | 21.7% | 36.9% | 1.6% | 0.000 |
| Laboratory findings | ||||
| FBG (mmol/L) | 8.85 ± 3.18 | 8.53 ± 2.72 | 9.27 ± 3.66 | 0.030 |
| HbA1c (mmol/L) | 9.85 ± 2.36 | 10.14 ± 2.55 | 9.46 ± 2.02 | 0.003 |
| TC (mmol/L) | 4.47 ± 1.22 | 4.29 ± 1.09 | 4.69 ± 1.35 | 0.001 |
| TG (mmol/L) | 1.82 ± 1.57 | 1.65 ± 1.10 | 2.05 ± 2.01 | 0.009 |
| HDL‐c (mmol/L) | 1.00 ± 0.26 | 0.96 ± 0.25 | 1.06 ± 0.26 | 0.000 |
| LDL‐c (mmol/L) | 2.58 ± 0.93 | 2.51 ± 0.88 | 2.67 ± 0.99 | 0.087 |
| Albumin (g/L) | 37.65 ± 4.67 | 38.26 ± 4.17 | 36.83 ± 4.95 | 0.001 |
| Creatinine (µmol/L) | 71.98 ± 31.75 | 78.64 ± 30.00 | 63.15 ± 31.92 | 0.000 |
| Uric (μmol/L) | 314.19 ± 98.01 | 327.74 ± 101.06 | 296.15 ± 90.96 | 0.001 |
| PTH (pg/mL) | 43.60 ± 22.18 | 43.08 ± 21.04 | 44.43 ± 24.10 | 0.739 |
| PINP (ng/mL) | 14.95 ± 22.08 | 13.14 ± 19.76 | 17.36 ± 24.67 | 0.045 |
| β‐CTX (ng/mL) | 0.38 ± 0.24 | 0.35 ± 0.25 | 0.41 ± 0.23 | 0.072 |
| 25(OH)D (ng/mL) | 58.42 ± 22.71 | 60.54 ± 20.93 | 55.71 ± 24.66 | 0.155 |
| Calcium (mmol/L) | 2.19 ± 0.11 | 2.19 ± 0.11 | 2.19 ± 0.11 | 0.812 |
| GNRI | 101.72 ± 9.65 | 101.36 ± 9.30 | 102.22 ± 10.09 | 0.355 |
| BMD | ||||
| Total lumbar (g/cm2) | 1.009 ± 0.312 | 1.073 ± 0.363 | 0.925 ± 0.201 | 0.000 |
| Femur neck (g/cm2) | 0.788 ± 0.148 | 0.827 ± 0.135 | 0.738 ± 0.149 | 0.000 |
| Total hip (g/cm2) | 0.858 ± 0.162 | 0.896 ± 0.148 | 0.809 ± 0.166 | 0.000 |
| Osteoporosis | 15.1% | 9.8% | 21.9% | 0.001 |
Values are mean ± standard deviation or number (%). P < 0.05 was deemed significant (comparison between men and women). 25(OH)D, 25‐hydroxy‐vitamin; β‐CTX, β‐isomerized C‐terminal telopeptides; BMD, bone mineral density; BMI, body mass index; FBG, fasting blood glucose; GNRI, Geriatric Nutritional Risk Index; HbA1c, glycosylated hemoglobin; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol; PINP, procollagen of type I N‐propeptide; PTH, parathyroid hormone; TC, total cholesterol; TG, triglyceride.
Spearman’s partial correlations among GNRI, BMD and bone metabolism markers
The GNRI was found to be positively and significantly associated with BMD at all bone sites (Figure 1). After adjusting for age, BMI and albumin, Spearman’s partial correlation analysis showed that GNRI was still related to BMD procollagen of type I N‐propeptide and albumin‐corrected calcium (Table 2). The Pearson’s correlation coefficients (r) were 0.341, 0.410, 0.355, 0.278 and 0.145 at total lumbar, total hip, femur neck procollagen of type I N‐propeptide and albumin‐corrected calcium, respectively, in men, and 0.561, 0.495, 0.538, 0.307 and 0.209 in women, respectively. The GNRI has a negative association with parathyroid hormone (r = −0.321 in men and r = −0.315 in women, P < 0.05).
Figure 1.
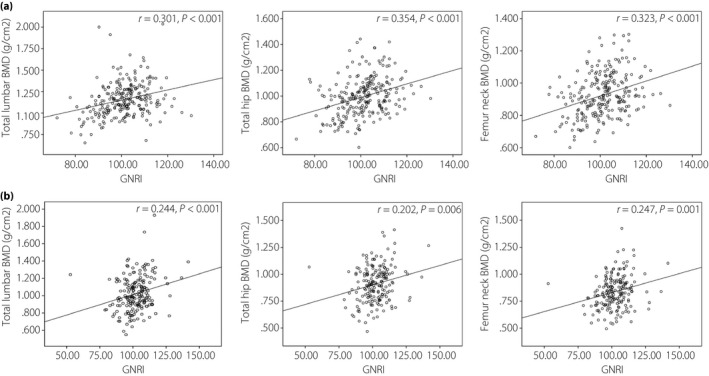
Scatter diagrams showing the correlation between the Geriatric Nutritional Risk Index (GNRI) and bone mineral density (BMD).
Table 2.
Correlation analysis between the Geriatric Nutritional Risk Index, bone mineral density and bone metabolism markers adjusted for body mass index, albumin and age
| Variables | Men | Women | ||
|---|---|---|---|---|
| r | P | r | P | |
| Total lumbar BMD | 0.341 | 0.000 | 0.561 | 0.001 |
| Total hip BMD | 0.410 | 0.000 | 0.495 | 0.002 |
| Femur neck BMD | 0.355 | 0.000 | 0.538 | 0.001 |
| PTH | −0.321 | 0.011 | −0.315 | 0.048 |
| PINP | 0.278 | 0.005 | 0.307 | 0.050 |
| β‐CTX | 0.031 | 0.825 | −0.130 | 0.436 |
| 25(OH)D | 0.049 | 0.725 | −0.062 | 0.713 |
| Albumin‐corrected Calcium | 0.145 | 0.025 | 0.209 | 0.018 |
Linear regression analyses for BMD
Tables 3 and 4 show multivariate linear regression analysis for BMD between BMI and age. Sex, age and BMI have a great influence on the incidence of osteoporosis. Therefore, we carried out a subgroup analysis of sex, age and BMI. After adjusting for age, diabetic duration, systolic blood pressure, diastolic blood pressure, smoking, drinking, TC, TG, HDL‐C, LDL‐C, creatinine, uric, glycosylated hemoglobin and FBG, the GNRI was independently correlated with BMD of total lumbar, hip and femoral neck in the group with BMI <24 (β values were 0.314, 0.357 and 0.431 in men, and 0.123, 0.252 and 0.398 in women). In women with BMI >24, the GNRI was independently associated with the BMD of the total lumbar, hip and femoral neck (β values were 0.302, 0.295 and 0.364), but no independent correlation was shown for men. In men aged <65 years men, the GNRI was independently associated with the BMD of the total hip and femoral neck (β values were 0.277 and 0.392, respectively). For women aged <65 years, the GNRI was independently associated with the BMD of the total lumbar, hip and femoral neck (β values were 0.227, 0.290 and 0.369, respectively). In men aged >65 years, the GNRI was independently associated with the BMD of the total lumbar, hip and femoral neck (β values were 0.280, 0.335 and 0.277, respectively). In women aged >65 years, the GNRI was independently associated with the BMD of the total lumbar (β values were 0.296).
Table 3.
Geriatric Nutritional Risk Index was independent association with bone mineral density based on the cross‐categorization of body mass index and sex
| 18.5 ≤ BMI < 24 | BMI ≥24 | |||
|---|---|---|---|---|
| β | P | β | P | |
| Men | ||||
| Total lumbar | 0.314 | 0.013 | 0.083 | 0.536 |
| Total hip | 0.357 | 0.004 | 0.072 | 0.590 |
| Femur neck | 0.431 | <0.001 | 0.05 | 0.711 |
| Women | ||||
| Total lumbar | 0.123 | 0.282 | 0.302 | 0.042 |
| Total hip | 0.252 | 0.049 | 0.295 | 0.047 |
| Femur neck | 0.398 | 0.005 | 0.364 | 0.037 |
Table 4.
Geriatric Nutritional Risk Index was independently associated with bone mineral density based on the cross‐categorization of age and sex
| Age <65 years | Age ≥65 years | |||
|---|---|---|---|---|
| Β | P | β | P | |
| Men | ||||
| Total lumbar | 0.194 | 0.112 | 0.280 | 0.044 |
| Total hip | 0.277 | 0.021 | 0.335 | 0.017 |
| Femur neck | 0.392 | 0.001 | 0.277 | 0.045 |
| Women | ||||
| Total lumbar | 0.227 | 0.043 | 0.296 | 0.048 |
| Total hip | 0.290 | 0.028 | 0.219 | 0.136 |
| Femur neck | 0.369 | 0.006 | 0.125 | 0.424 |
Logistic regression analyses for osteoporosis in participants
The relationship between the GNRI and osteoporosis was analyzed by logistic regression method, and the results are shown in Table 5. Although adjusting for age, diabetes duration, TC, TG, HDL‐C, LDL‐C, creatinine, uric, glycosylated hemoglobin and FBG reduced the odds ratio, the relationship between GNRI and osteoporosis were still significant (odds ratio 0.860 in men and 0.927 in women, P < 0.05).
Table 5.
Logistic regression analysis for osteoporosis
| Variables | Men | Women | ||||
|---|---|---|---|---|---|---|
| SE | Odds ratio (95% CI) | P | SE | Odds ratio (95% CI) | P | |
| Age | 0.035 | 0.994 (0.928, 1.063) | 0.853 | 0.026 | 1.058 (1.004, 1.114) | 0.033 |
| Diabetes duration | 0.049 | 1.005 (0.913, 1.106) | 0.922 | 0.036 | 1.011 (0.943, 1.085) | 0.754 |
| TC | 0.012 | 1.691 (0.142, 2.009) | 0.246 | 0.965 | 0.633 (0.096, 4.197) | 0.636 |
| TG | 1.148 | 0.126 (0.007, 2.293) | 0.162 | 0.343 | 1.076 (0.550, 2.107) | 0.830 |
| HDL‐c | 3.486 | 0.029 (0.000, 26.819) | 0.309 | 1.474 | 4.889 (0.272, 8.739) | 0.282 |
| LDL‐c | 2.404 | 0.168 (0.002, 18.669) | 0.458 | 1.080 | 1.354 (0.163, 11.236) | 0.779 |
| Creatinine | 0.015 | 0.995 (0.966, 1.024) | 0.715 | 0.008 | 0.998 (0.982, 1.015) | 0.848 |
| Uric | 0.005 | 1.002 (0.993, 1.011) | 0.714 | 0.003 | 1.003 (0.997, 1.010) | 0.294 |
| HbA1c | 0.137 | 1.071 (0.819, 1.400) | 0.616 | 0.119 | 0.957 (0.758, 1.208) | 0.711 |
| FPG | 0.138 | 0.909 (0.693, 1.191) | 0.489 | 0.054 | 1.032 (0.929, 1.147) | 0.560 |
| GNRI | 0.048 | 0.860 (0.783, 0.944) | 0.002 | 0.031 | 0.927 (.872, 0.985) | 0.014 |
FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin; HDL‐c, High‐density lipoprotein cholesterol; LDL‐c, Low‐density lipoprotein cholesterol; SE, standard error; TC, total cholesterol; TG, triglyceride.
Predictive property of the GNRI for osteoporosis
In Figure 2, the predictabilities of the GNRI, albumin, BMI and age for osteoporosis were uncovered by the receiver operating characteristic curves. Compared with albumin, BMI and age, the AUC of the GNRI was the highest and statistically significant (0.876 for men and 0.704 for women). The optimal cut‐off of the GNRI for predicting osteoporosis was 98.2, with sensitivity of 72.1% and specificity 95.8% in men. The optimal cut‐off of GNRI for predicting osteoporosis was 99.5, with sensitivity of 69.8% and specificity 69.0% in women.
Figure 2.
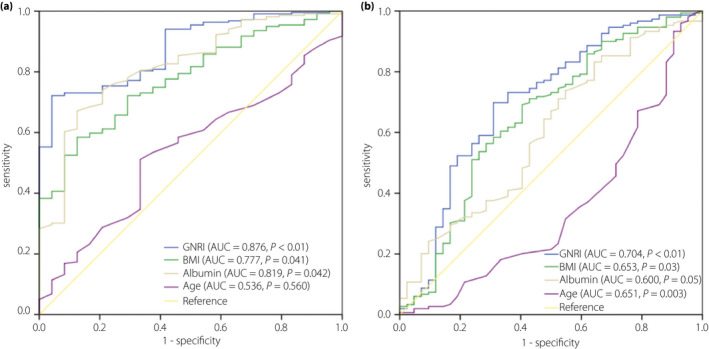
(a) Receiver operating characteristic analysis of the Geriatric Nutritional Risk Index (GNRI), body mass index (BMI), albumin and age for osteoporosis among men. (b) Receiver operating characteristic analysis of GNRI, BMI, albumin and age for osteoporosis among women. AUC, area under the curve.
Discussion
In the present study, it was reported that GNRI was initially used for estimating the risk of malnutrition‐related complications in the aging population15, and has been applied as a reliable indicator for a variety of diseases16, 17, 18. According to previous studies, the GNRI was adopted in this work, and the results showed that the GNRI was positively related to the BMD of the total lumbar, femur neck and total hip, and inversely related to osteoporosis in type 2 diabetes mellitus patients. In addition, compared with albumin and BMI, the predictability of the GNRI for osteoporosis was more powerful. Because of the simplicity of the GNRI to assess nutriture merely based on blood albumin, bodyweight and height, the GNRI is a convenient method to estimate nutriture and osteoporosis in type 2 diabetes mellitus patients. In addition, nutritional supplementation might be an effective method to reduce the incidence of osteoporosis.
The nutriture of the patients with type 2 diabetes mellitus was negatively impacted, despite the relevant ingestion19. Diabetes accelerates the loss of muscle strength, quality and serum albumin 20, which draws attention to the protein and energy balance in nutrition. It was shown in our work that a low GNRI was a significant risk factor for reduced BMD and osteoporosis in type 2 diabetes mellitus patients. To our knowledge, this is the first time that the direct association among GNRI and BMD, osteoporosis in type 2 diabetes mellitus patients has been shown. Therefore, GNRI might be a convenient and reliable indicator for the BMD status in patients with type 2 diabetes mellitus. The GNRI of the participants was 101.72 ± 9.65, which showed that the nutritional status was not severe. After grouping the patients into three different groups according to the GNRI, the GNRI at a low status was related to a low level of BMD and serum calcium. Accordingly, it has been partially proven that malnutrition plays a role in the low level of BMD in patients with a low GNRI.
We also observed a sexual dimorphism between GNRI and osteoporosis. No correlation between GNRI and BMD was found in the group with BMI >24 kg/m2. A discordant association between BMI and sexual hormones in men and women might partially explain our finding. Testosterone and sex hormone‐binding globulin levels decreased in men with increased BMI, whereas a favorable association was shown between BMI and serum estrogen concentrations in postmenopausal women21, 22. Karim et al. suggested that these associations can be explained by the increased fat mass with increased peripheral aromatization after menopause. Another putative mechanism is increased plasma levels of leptin. A recent meta‐analysis showed that high levels of leptin were positively associated with BMD in postmenopausal women.
As serum albumin and bodyweight were applied to develop the GNRI, it reflected the nutriture and enabled comprehensive assessment of the above variables. As a consequence, the GNRI value – the integration of both serum albumin and BMI – was a complementary indicator improving the diagnostic accuracy and reducing the limitations. As shown in Figure 2a,b, the AUC of GNRI was the largest compared with that of BMI and serum albumin, which was also statistically significant.
The relationship between nutrition and bone mass in chronic obstructive pulmonary disease23, chronic kidney disease24, non‐alcoholic fatty liver disease25 and cardiovascular disease26 has been well developed. As a consequence, it has been proven that GNRI can be applied as a convenient and reliable indicator for the BMD conditions of patients with the above diseases. In addition, it has been shown that there is a dramatically inverse relationship between GNRI and BMD in rheumatoid arthritis patients27. As the association is caused by protein that originates from animals and plants 28, it is crucial to guarantee the intake of enough protein from the diet.
There are several plausible mechanisms that might explain why the GNRI may be associated with osteoporosis. First, previous studies have shown that the intestinal absorption of calcium is upregulated by the high intake of protein29, 30. Consistent with this, a positive relationship between the GNRI and calcium was represented in both men and women (r = 0.400 for men and 0.423 for women). The role of calcium supplementation in the treatment of osteoporosis has been extensively studied31. Second, supplementing protein in the diet can effectively increase muscle strength and body coordination. Furthermore, bones and joints can be protected by the enhanced muscularity32, 33. Third, several studies have shown that the high intake of protein might contribute to the increased serum IGF‐I level34. Yakar et al showed that low levels of insulin‐like growth factor‐1 in mice contributed to reduced bone strength35. Furthermore, another study proved that low insulin‐like growth factor‐1 levels played a role in increasing the risk of fracture for postmenopausal women, independent of BMD36. Therefore, high calcium, increased muscularity and high insulin‐like growth factor‐1 might provide evidence about the mechanisms of the significant relationship between the GNRI and osteoporosis. In the present study, based on the logistic regression analyses, the odds ratio for osteoporosis was 0.857 in men and 0.927 in women.
However, there were some limitations in the present work. First, the causality of the GNRI and BMD was difficult to evaluate in this cross‐sectional study. As the present study was based on the former data, several key parameters could not be further analyzed, affecting the selection of controls. Second, all the serum samples were collected only once from the participants, and BMD at each position was also collected once, leading to the deviations in the GNRI and BMD. Third, some relevant parameters that affected the results might have been neglected in the present study, such as the menopausal status, estrogen level, dietary habits, physical activity and previous fractures.
In conclusion, a low GNRI was significantly associated with osteoporosis and low levels of BMD in type 2 diabetes mellitus patients. Compared with serum albumin and BMI, the predictive ability of GNRI for osteoporosis was superior. In summary, GNRI can be applied to evaluate the appropriate nutritional intervention.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Distribution of bone mineral density (BMD).
Acknowledgments
The authors thank the staff at the Department of Endocrinology and Metabolism, the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, and all the patients who participated in the study. This work was supported by grants from the Project of Zhejiang Provincial Department of Health (2016KYB194), General Research Project of Zhejiang Provincial Education Department (Y201534290), and Wenzhou Science and Technology Bureau (Y20120163). The funders played no role in the design of this study; the collection, analysis and interpretation of data; or preparation of the manuscript.
J Diabetes Investig 2020; 11: 956–963
References
- 1. Yuan L‐Q, Lin X, Xiong D, et al Epidemiology and management of osteoporosis in the People’s Republic of China: current perspectives. Clin Interv Aging 2015; 10: 1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janghorbani M, Van Dam RM, Willett WC, et al Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495–505. [DOI] [PubMed] [Google Scholar]
- 3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta‐analysis. Osteoporos Int 2007; 18: 427–444. [DOI] [PubMed] [Google Scholar]
- 4. Schacter GI, Leslie WD. DXA‐based measurements in diabetes: can they predict fracture risk? Calcif Tissue Int 2017; 100: 150–164. [DOI] [PubMed] [Google Scholar]
- 5. Moayeri A, Mohamadpour M, Mousavi S, et al Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta‐analysis. Ther Clin Risk Manag 2017; 13: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holm JP, Hyldstrup L, Jensen JB. Time trends in osteoporosis risk factor profiles: a comparative analysis of risk factors, comorbidities, and medications over twelve years. Endocrine 2016; 54: 241–255. [DOI] [PubMed] [Google Scholar]
- 7. Afshinnia F, Chacko S, Zahedi T. Association of lower serum cholesterol levels with higher risk of osteoporosis in type 2 diabetes. Endocr Pract 2007; 13: 620–628. [DOI] [PubMed] [Google Scholar]
- 8. Schacter GI, Leslie WD. Diabetes and bone disease. Endocrinol Metab Clin North Am 2017; 46: 63–85. [DOI] [PubMed] [Google Scholar]
- 9. Bouillanne O, Morineau G, Dupont C, et al Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 10. Xu J, Zhou X, Zheng C. The geriatric nutritional risk index independently predicts adverse outcomes in patients with pyogenic liver abscess. BMC Geriatr 2019; 19: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin YT, Liu CJ, Chen TJ, et al Pyogenic liver abscess as the initial manifestation of underlying hepatocellular carcinoma. Am J Med 2011; 124: 1158–1164. [DOI] [PubMed] [Google Scholar]
- 12. Komatsu M, Okazaki M, Tsuchiya K, et al Geriatric nutritional risk index is a simple predictor of mortality in chronic hemodialysis patients. Blood Purif 2015; 39: 281–287. [DOI] [PubMed] [Google Scholar]
- 13. Mitani Y, Oki Y, Fujimoto Y, et al Relationship between functional independence measure and geriatric nutritional risk index in pneumonia patients in long‐term nursing care facilities. Geriatr Gerontol Int 2017; 17: 1617–1622. [DOI] [PubMed] [Google Scholar]
- 14. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368–381. [DOI] [PubMed] [Google Scholar]
- 15. Rizzoli R. Nutrition: its role in bone health. Best Pract Res Clin Endocrinol Metab 2008; 22: 813–829. [DOI] [PubMed] [Google Scholar]
- 16. Kang SH, Cho KH, Park JW, et al Geriatric nutritional risk index as a prognostic factor in peritoneal dialysis patients. Perit Dial Int 2013; 33: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szeto C‐C, Kwan BC‐H, Chow K‐M, et al Geriatric nutritional risk index as a screening tool for malnutrition in patients on chronic peritoneal dialysis. J Ren Nutr 2010; 20: 29–37. [DOI] [PubMed] [Google Scholar]
- 18. Wada H, Dohi T, Miyauchi K, et al Prognostic impact of the geriatric nutritional risk index on long‐term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol 2017; 119: 1740–1745. [DOI] [PubMed] [Google Scholar]
- 19. Donnelly A. Nutritional requirements in malnutrition and diabetes mellitus. Nur Stand 2018; 33: 69–76. [DOI] [PubMed] [Google Scholar]
- 20. Xu J, Pan X, Liang H, et al Association between skeletal muscle mass to visceral fat area ratio and arterial stiffness in Chinese patients with type 2 diabetes mellitus. BMC Cardiovasc Disord 2018; 18: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karim R, Mack WJ, Hodis HN, et al Influence of age and obesity on serum estradiol, estrone, and sex hormone binding globulin concentrations following oral estrogen administration in postmenopausal women. J Clin Endocrinol Metab 2009; 94: 4136–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osuna JA, Gomez‐Perez R, Arata‐Bellabarba G, et al Relationship between BMI, total testosterone, sex hormone‐binding‐globulin, leptin, insulin and insulin resistance in obese men. Arch Androl 2006; 52: 355–361. [DOI] [PubMed] [Google Scholar]
- 23. Corsonello A, Scarlata S, Pedone C, et al Treating COPD in older and oldest old patients. Curr Pharm Des 2015; 21: 1672–1689. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka S, Ito M. [Bone and nutrition. Nutrition care of renal osteodystrophy]. Clin Calcium 2015; 25: 1057–1062. [PubMed] [Google Scholar]
- 25. Adams LA, Anstee QM, Tilg H, et al Non‐alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017; 66: 1138–1153. [DOI] [PubMed] [Google Scholar]
- 26. O'Keefe JH, Bergman N, Carrera‐Bastos P, et al Nutritional strategies for skeletal and cardiovascular health: hard bones, soft arteries, rather than vice versa. Open Heart 2016; 3: e000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tokumoto H, Tominaga H, Arishima Y, et al Association between bone mineral density of femoral neck and geriatric nutritional risk index in rheumatoid arthritis patients treated with biological disease‐modifying anti‐rheumatic drugs. Nutrients 2018; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wengreen HJ, Munger RG, West NA, et al Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res 2004; 19: 537–545. [DOI] [PubMed] [Google Scholar]
- 29. Civitelli R, Villareal DT, Agnusdei D, et al Dietary L‐lysine and calcium metabolism in humans. Nutrition 1992; 8: 400–405. [PubMed] [Google Scholar]
- 30. Kerstetter JE, O'Brien KO, Caseria DM, et al The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 2005; 90: 26–31. [DOI] [PubMed] [Google Scholar]
- 31. Uenishi K. [Bone and Nutrition. Calcium intake and bone health]. Clin Calcium 2015; 25: 959–966. [PubMed] [Google Scholar]
- 32. Schurch MA, Rizzoli R, Slosman D, et al Protein supplements increase serum insulin‐like growth factor‐I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double‐blind, placebo‐controlled trial. Ann Int Med 1998; 128: 801–809. [DOI] [PubMed] [Google Scholar]
- 33. Martin Jimenez JA, Consuegra Moya B, Martin Jimenez MT. [Nutritional factors in preventing osteoporosis]. Nutr Hosp 2015; 32(Suppl 1): 49–55. [DOI] [PubMed] [Google Scholar]
- 34. Chevalley T, Rizzoli R, Manen D, et al Arginine increases insulin‐like growth factor‐I production and collagen synthesis in osteoblast‐like cells. Bone 1998; 23: 103–109. [DOI] [PubMed] [Google Scholar]
- 35. Yakar S, Canalis E, Sun H, et al Serum IGF‐1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res 2009; 24: 1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salminen H, Saaf M, Ringertz H, et al The role of IGF‐I and IGFBP‐1 status and secondary hyperparathyroidism in relation to osteoporosis in elderly Swedish women. Osteoporos Int 2008; 19: 201–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Distribution of bone mineral density (BMD).


