Abstract
Individuals with a monophasic glucose response curve (GRC) during a 75‐g oral glucose tolerance test have a higher risk for type 2 diabetes than those with a biphasic GRC. However, no studies have addressed the association between GRC type and insulin clearance. Thus, we studied 49 healthy non‐obese Japanese men. We divided study participants into the monophasic or biphasic group based on the shape of their GRC. We evaluated tissue‐specific insulin sensitivity and insulin clearance using a two‐step hyperinsulinemic‐euglycemic clamp. The monophasic group had more visceral fat, lower insulin clearance and lower muscle insulin sensitivity than the biphasic group, whereas liver and adipose tissue insulin sensitivity, and insulin secretion were comparable. In conclusion, healthy non‐obese men with a monophasic GRC have lower insulin clearance and muscle insulin sensitivity.
Keywords: Insulin clearance, Insulin resistance, Shape of glucose response curve
A monophasic glucose response curve during 75‐g oral glucose tolerance test in apparently healthy non‐obese men is associated with reduced insulin clearance, lower muscle insulin sensitivity and more visceral fat accumulation compared with a biphasic curve.

Introduction
To prevent type 2 diabetes, an optimal screening method for identifying individuals at high risk should be developed. Glucose levels during a 75‐g oral glucose tolerance test (OGTT) are used to diagnose diabetes. Recently, independent of glucose levels, the shape of the glucose response curve (GRC) during an OGTT has been recognized as a risk factor for type 2 diabetes development1, 2. When the shape of the GRC is classified as monophasic or biphasic based on glucose concentrations during OGTT, individuals with a monophasic curve have a higher risk for type 2 diabetes than those with a biphasic curve1, 2.
However, the pathophysiological features of each type are not fully understood. Some reports have suggested that a monophasic curve is associated with impaired insulin secretion and decreased insulin sensitivity compared with a biphasic curve3, 4. However, the metabolic features of both types of curves in apparently healthy non‐obese individuals have not been elucidated yet.
Some data support the hypothesis that decreased insulin clearance is primarily observed in healthy individuals and might elicit insulin resistance, suggesting that reduced insulin clearance could be a upstream risk factor for the onset of type 2 diabetes5. Our group showed impaired insulin clearance even in apparently healthy non‐obese men, and this is associated with modestly lower insulin sensitivity in muscle6. However, no studies have addressed the association between GRC type and insulin clearance.
Thus, the present study investigated the association between the shape of the GRC during an OGTT and tissue‐specific insulin sensitivity, insulin clearance and insulin secretion in healthy, non‐obese Japanese men.
Methods
Study participants
The shape of the GRC was evaluated in participants of the Sportology Center Core Study7. To assess the role of the shape of the GRC in apparently healthy non‐obese men, we chose those with a body mass index of 21.0 to <25.0 kg/m2 and no risk factors for cardiovascular disease. We defined cardiometabolic risk factors assessed in the present study as hyperglycemia, dyslipidemia and hypertension7. The ethics committee of Juntendo University approved this study, and this study was carried out in accordance with the principles outlined in the Declaration of Helsinki.
Study design
We carried out the OGTT and a two‐step hyperinsulinemic‐euglycemic clamp with a glucose tracer. Each step lasted 180 min, with a constant insulin infusion rate of 10 mU/m2/min at the first step, and 20 mU/m2/min at the second step. Intrahepatic lipid and intramyocellular lipid levels were measured with 1H‐magnetic resonance spectroscopy8. The percentage of body fat and fat‐free mass were measured using the bioimpedance method (InBody 720; Biospace, Tokyo, Japan). Furthermore, the abdominal visceral fat area and subcutaneous fat area were also estimated by magnetic resonance imaging. These methods have been previously reported in detail7.
Calculations
GRC type was defined based on glucose concentrations during the 2‐h OGTT. A monophasic GRC was defined as a gradual increase in glucose concentration until a peak was reached and followed by a subsequent >4.5 mg/dL decrease in glucose concentration. A biphasic GRC was defined as having a >4.5 mg/dL rise in glucose concentrations after the decline in glucose concentrations3, 4.
Muscle, liver and adipose tissue insulin sensitivity and metabolic clearance rate of insulin (MCRI) were evaluated using a two‐step hyperinsulinemic‐euglycemic clamp6, 7, 9.
Statistical analysis
Data are shown as the mean ± standard deviation. Data that did not have a normal distribution were log‐transformed as required. We compared the data using an unpaired Student’s t‐test or χ2‐test. All statistical tests were two‐sided with a significance level of 5%. We used SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA) for the statistical analyses.
Results
Based on the GRC during the 75‐g OGTT, we divided all participants into either the monophasic (n = 31) or biphasic group (n = 18). Glucose and insulin levels were higher in the monophasic group (Table 1; Figure 1). The monophasic group had a significantly higher area under the curve of glucose during the 75‐g OGTT than the biphasic group, whereas the area under the curve of insulin and the insulinogenic index were not significantly different between the groups. Although percentage body fat, subcutaneous fat area, intrahepatic lipid and intramyocellular lipid were comparable between the two groups, the monophasic group had significantly higher visceral fat area. MCRI was lower in the monophasic group, which contributed to increased insulin levels during the clamp. Muscle insulin sensitivity was significantly lower in the monophasic group. The rate of disappearance, mainly reflecting muscle glucose uptake, was comparable between the groups. These data suggest that the rate of disappearance was maintained by increased steady‐state serum insulin due to lower insulin clearance in the monophasic group. Adipose tissue and hepatic insulin sensitivity were comparable between both groups.
Table 1.
Clinical characteristics of the biphasic and monophasic groups
| Overall (n = 49) | Biphasic group (n = 18) | Monophasic group (n = 31) | P | |
|---|---|---|---|---|
| Age (years) | 40.2 ± 5.3 | 39.9 ± 5.5 | 40.3 ± 5.2 | 0.811 |
| BMI (kg/m2) | 23.1 ± 1.0 | 22.9 ± 1.2 | 23.2 ± 0.9 | 0.331 |
| Family history of type 2 diabetes (%) | 14 (28.6%) | 4 (22.2%) | 10 (32.3%) | 0.453 |
| Body fat (%) | 20.1 ± 5.0 | 18.8 ± 4.0 | 20.9 ± 5.4 | 0.159 |
| Fasting plasma glucose (mg/dL) | 93.2 ± 6.8 | 93.6 ± 6.5 | 93.0 ± 7.1 | 0.778 |
| Fasting serum insulin (μU/mL) | 4.9 ± 2.1 | 4.36 ± 1.83 | 5.20 ± 2.14 | 0.169 |
| AUC‐glucose during OGTT (mg·min/dL·103) | 15.6 ± 2.3 | 14.5 ± 1.7 | 16.2 ± 2.4 | 0.008 |
| AUC‐insulin during OGTT (μU·min/mL·103) | 5.2 ± 2.8 | 4.4 ± 2.4 | 5.7 ± 3.0 | 0.144 |
| Insulinogenic index | 0.95 ± 0.68 | 1.10 ± 0.68 | 0.86 ± 0.68 | 0.236 |
| Free fatty acid (μEq/L) | 335 ± 105 | 322.4 ± 110.5 | 342.6 ± 103.4 | 0.523 |
| HbA1c (%) | 4.9 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.2 | 0.701 |
| High‐molecular‐weight adiponectin (ng/mL) | 1.82 ± 1.21 | 2.01 ± 1.34 | 1.71 ± 1.14 | 0.402 |
| Intramyocellular lipid in TA (S‐fat/Cre) | 3.2 ± 1.9 | 2.7 ± 1.8 | 3.5 ± 1.9 | 0.158 |
| Intramyocellular lipid in SOL (S‐fat/Cre) | 12.8 ± 6.8 | 11.7 ± 6.7 | 13.5 ± 6.9 | 0.370 |
| Intrahepatic lipid (%) | 1.9 ± 3.2 | 1.8 ± 3.3 | 2.0 ± 3.3 | 0.878 |
| Abdominal visceral fat area (cm2) | 75.3 ± 28.0 | 62.6 ± 24.2 | 82.7 ± 27.8 | 0.014 |
| Abdominal subcutaneous fat area (cm2) | 106 ± 40 | 94.2 ± 38.0 | 113.6 ± 39.9 | 0.102 |
| VO2peak (mL/kg per min) | 36.0 ± 7.0 | 37.3 ± 8.3 | 35.2 ± 6.1 | 0.320 |
| MCRI during the second step | 610.9 ± 83.3 | 641.7 ± 71.9 | 593.0 ± 85.4 | 0.048 |
| SSSI during the second step (μU/mL) | 36.4 ± 5.2 | 33.8 ± 4.2 | 37.9 ± 5.2 | 0.006 |
| %Reduction in EGP/SSSI during the first step (%/μU·mL−1) | 3.7 ± 1.0 | 3.9 ± 0.9 | 3.7 ± 0.9 | 0.424 |
| Rd during the second step (mg/kg FFM·min−1) | 8.6 ± 2.0 | 9.1 ± 2.3 | 8.3 ± 1.8 | 0.189 |
| Rd/SSSI during the second step (mg/kg FFM·min−1 ·μU−1·mL) | 0.24 ± 0.08 | 0.27 ± 0.09 | 0.22 ± 0.06 | 0.020 |
| %FFA suppression/insulin during the first step (%/μU·mL−1) | 4.54 ± 1.35 | 4.57 ± 1.26 | 4.52 ± 1.42 | 0.904 |
Data are the mean ± standard deviation.
AUC, area under the curve; BMI, body mass index; Cre, creatine signal; EGP, endogenous glucose production; FFM, fat‐free mass; HbA1c, glycated hemoglobin; MCRI, metabolic clearance rate of insulin; OGTT, oral glucose tolerance test; Rd, rate of disappearance; S‐fat, methylene signal intensity; SOL, soleus muscle; SSSI, steady‐state serum insulin; TA, tibialis anterior muscle.
Bold values indicate P values with significant differences between the two groups.
Figure 1.
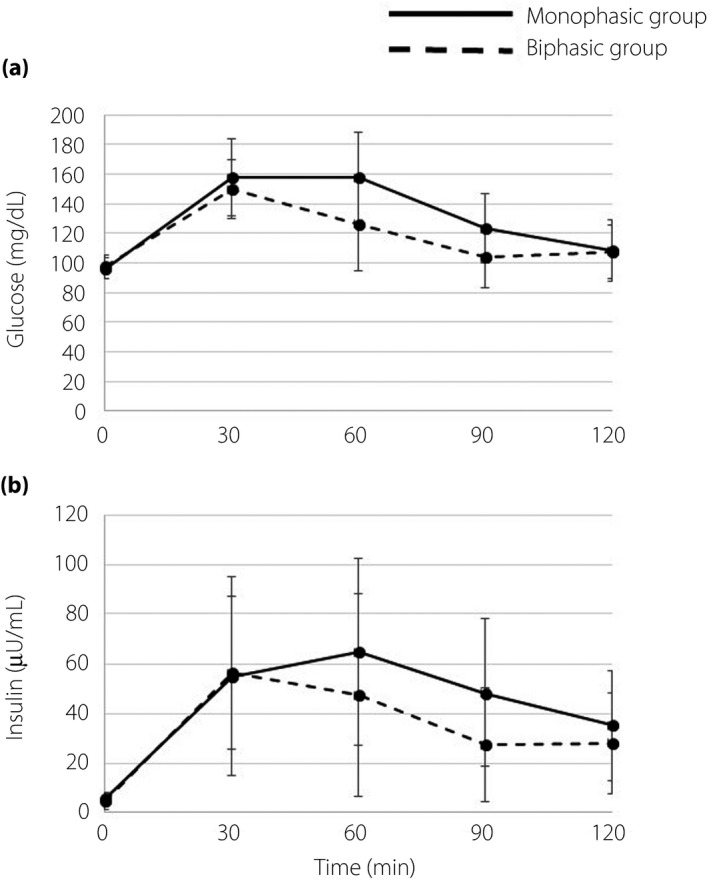
(a) Plasma glucose and (b) insulin levels during oral glucose tolerance tests in individuals in the monophasic group (solid lines) and biphasic group (dashed lines). Data are reported as the mean ± standard deviation.
Discussion
Recent data suggest that decreased insulin clearance might elicit insulin resistance and thus increase the risk of type 2 diabetes5, 6, 10, 11. Individuals with a monophasic curve are at higher risk for future type 2 diabetes1, 2, but the association between GRC type and insulin clearance has not been addressed. The present study showed that healthy non‐obese men with a monophasic GRC during OGTT have more visceral fat, lower insulin clearance and lower muscle insulin sensitivity.
We showed that decreased insulin clearance is observed even in apparently healthy men, which seems to be a compensatory mechanism for maintaining glucose uptake with modest muscle insulin resistance6. Similarly, in the present study, modest insulin resistance in participants with a monophasic curve was compensated by decreased MCRI. Thus, low MCRI could be viewed as early metabolic change compensating for modest insulin resistance. In contrast, several animal models showed that impaired insulin clearance and resulting hyperinsulinemia are not compensatory mechanisms against insulin resistance, but rather could be upstream factors that induce the development of insulin resistance and adiposity10, 11. Thus, individuals with a monophasic curve have lower insulin clearance and relative muscle insulin resistance.
In contrast, individuals with a biphasic curve have high insulin sensitivity and insulin clearance. Accordingly, in individuals with a biphasic curve, the later rise in glucose levels could be due to enhanced insulin clearance, which contributes to preventing hypoglycemia through high muscle insulin sensitivity. A previous study showed that aerobic exercise for 1 year enhances muscle insulin sensitivity12; however, the glucose infusion rate during a glucose clamp did not increase as anticipated, because aerobic exercise increased MCRI by 87% and reduced steady‐state serum insulin, suggesting that enhanced insulin clearance could compensate for higher insulin sensitivity.
The mechanisms underlying reduced insulin clearance in the monophasic group remain unclear. In the Hispanic cohort, insulin clearance has been shown to be highly hereditary, and chromosomal loci associated with insulin clearance have been identified13. Thus, reduced insulin clearance might be genetically determined, at least partially. Modest visceral fat accumulation might also contribute to reduced insulin clearance, but it is also possible that resulting hyperinsulinemia as a result of reduced insulin clearance might simultaneously induce visceral fat accumulation and insulin resistance5.
In conclusion, a monophasic curve during 75‐g OGTT is associated with reduced insulin clearance, lower muscle insulin sensitivity and more visceral fat accumulation compared with a biphasic curve.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Mutsuko Yoshikawa, Miyuki Iwagami, Naoko Daimaru, Eriko Magoshi and Emi Miyazawa for their excellent technical assistance. We also thank Hikari Taka and Tsutomu Fujimura (Juntendo University) for carrying out liquid chromatography–mass spectrometry analysis. Funding was obtained from a High Technology Research Center Grant, Strategic Research Foundation at Private Universities and KAKENHI (23680069, 26282197, 17K19929) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from the Japan Diabetes Foundation, Suzuken Memorial Foundation, Mitsukoshi Welfare Foundation and Diabetes Masters Conference.
J Diabetes Investig 2020; 11: 874–877
References
- 1. Abdul‐Ghani MA, Lyssenko V, Tuomi T, et al The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev 2010; 26: 280–286. [DOI] [PubMed] [Google Scholar]
- 2. Manco M, Nolfe G, Pataky Z, et al Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR‐RISC cohort. Metabolism 2017; 70: 42–50. [DOI] [PubMed] [Google Scholar]
- 3. Tschritter O, Fritsche A, Shirkavand F, et al Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003; 26: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 4. Kim JY, Michaliszyn SF, Nasr A, et al The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care 2016; 39: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergman RN, Piccinini F, Kabir M, et al Hypothesis: role of reduced hepatic insulin clearance in the pathogenesis of type 2 diabetes. Diabetes 2019; 68: 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaga H, Tamura Y, Takeno K, et al Correlates of insulin clearance in apparently healthy non‐obese Japanese men. Sci Rep 2017; 7: 1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takeno K, Tamura Y, Kawaguchi M, et al Relation between insulin sensitivity and metabolic abnormalities in japanese men with BMI of 23–25 kg/m2 . J Clin Endocrinol Metab 2016; 101: 3676–3684. [DOI] [PubMed] [Google Scholar]
- 8. Tamura Y, Tanaka Y, Sato F, et al Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005; 90: 3191–3196. [DOI] [PubMed] [Google Scholar]
- 9. Sugimoto D, Tamura Y, Takeno K, et al Clinical features of nonobese, apparently healthy, Japanese men with reduced adipose tissue insulin sensitivity. J Clin Endocrinol Metab 2019; 104: 2325–2333. [DOI] [PubMed] [Google Scholar]
- 10. Poy MN, Yang Y, Rezaei K, et al CEACAM1 regulates insulin clearance in liver. Nat Genet 2002; 30: 270–276. [DOI] [PubMed] [Google Scholar]
- 11. Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda). 2019; 34: 198–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oshida Y, Yamanouchi K, Hayamizu S, et al Long‐term mild jogging increases insulin action despite no influence on body mass index or VO2 max. J Appl Physiol (1985); 1989; 66: 2206–2210. [DOI] [PubMed] [Google Scholar]
- 13. Guo X, Cui J, Jones MR, et al Insulin clearance: confirmation as a highly heritable trait, and genome‐wide linkage analysis. Diabetologia 2012; 55: 2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]


