Abstract
Allogeneic hematopoietic stem cell transplantation (allo-SCT) offers cure for a variety of conditions, in particular, but not limited to, hematologic malignancies. However, it can be associated with life-threatening complications, including graft-versus-host disease (GVHD) and infections, which are factors limiting its widespread use. Technical advances in the field of microbiome research have allowed for a better understanding of the microbial flora of the human intestine, as well as dissection of their interactions with the host immune system in allo-SCT and posttransplant complications. There is growing evidence that the commensal microbiome is frequently dysregulated following allo-SCT and that this dysbiosis can predispose to adverse clinical outcomes, especially including acute intestinal GVHD and reduced overall survival. In this review, we discuss the interactions between the microbiome and the components of the immune system that play a major role in the pathways leading to the inflammatory state of acute intestinal GVHD. We also discuss the microbiome-centered strategies that have been devised or are actively being investigated to improve the outcomes of allo-SCT patients in regard to acute intestinal GVHD.
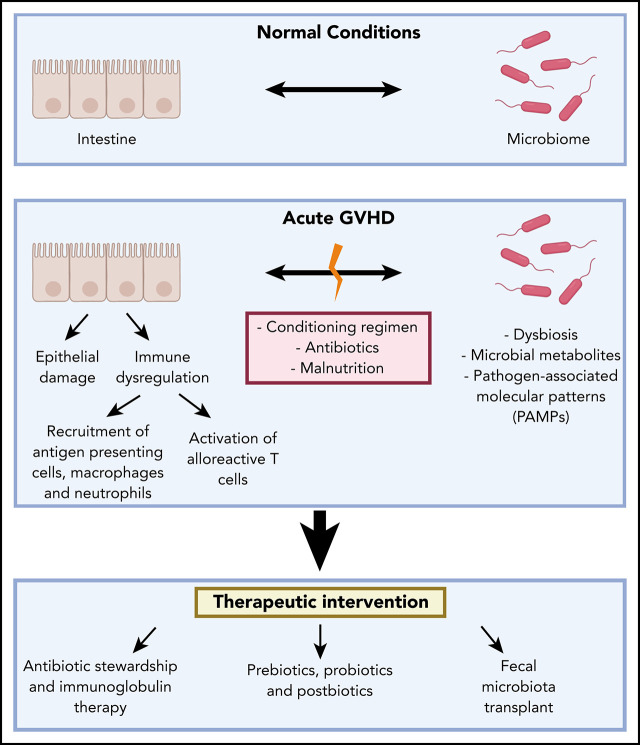
Visual Abstract
Introduction
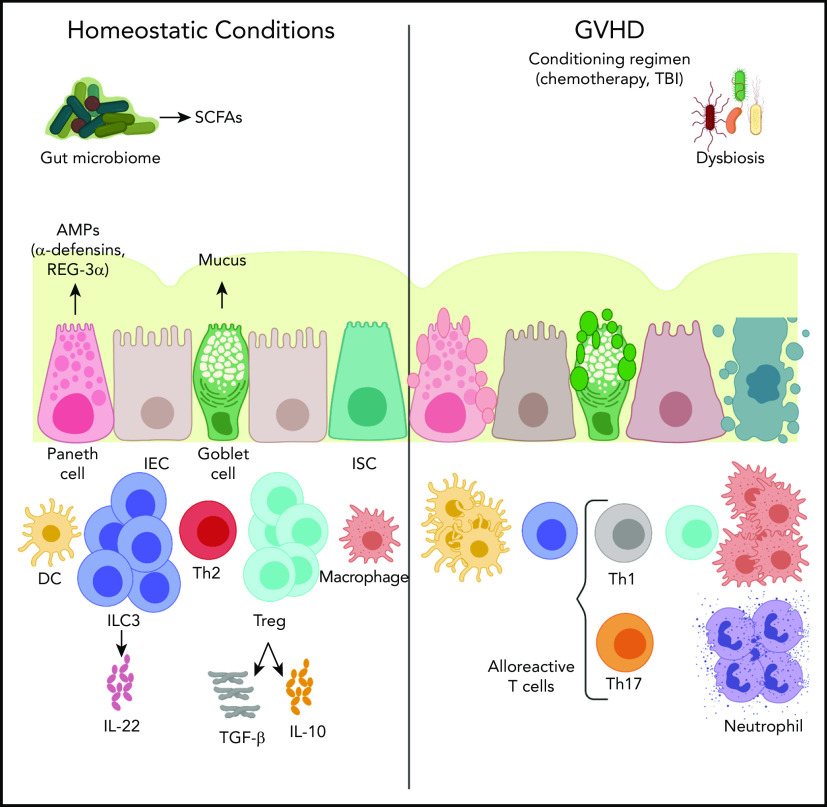
Acute graft-versus-host disease (GVHD) remains a major limitation to the wide application of allogeneic stem cell transplantation (allo-SCT). Resulting primarily from alloreactive T cells attacking healthy tissues, GVHD remains a major source of morbidity and mortality.1 Recent work has identified the microbiome as a key modulator of GVHD, particularly acute intestinal GVHD. In this review, we summarize the state of the field regarding the microbiome, intestinal immunity, and GVHD (Figure 1).
Figure 1.
The cross talk of the microbiome and the intestinal immune system in acute GVHD. Under physiologic conditions, the intestinal epithelial cell (IEC) surface maintains an intact barrier that prevents bacterial translocation into host tissue. In addition, Paneth cells produce antimicrobial proteins (AMPs), such as α-defensins and regenerating islet-derived 3α (REG-3α), that further protect against pathogenic organisms and provide trophic signals to intestinal stem cells (ISC). Goblet cells secrete mucus that solidifies the barrier separating the microbiota and host tissues. In allo-SCT, a conditioning regimen often consisting of chemotherapy, with or without total body irradiation (TBI), interrupts the integrity of the intestinal barrier bacterial translocation into host tissue. This leads to neutrophil infiltration into the small intestine producing reactive oxygen species that further contribute to the barrier damage. Bacterial translocation also leads to monocyte activation that mediates T helper 17 cell (Th17) differentiation, which, in turn, leads to macrophage and neutrophil accumulation into the inflammatory sites, as well as dendritic cell (DC) activation that leads to alloreactive cytotoxic T-cell homing. Microbiota-derived short-chain fatty acids (SCFAs) serve as an energy source for IECs and play a protective role in GVHD by inducing regulatory T cells (Treg) that secrete the anti-inflammatory cytokines transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), as well as group 3 innate lymphoid cells (ILC3) that secrete interleukin-22 (IL-22) and, again, mediate anti-inflammatory effects. Th1, T helper 1 cell; Th2, T helper 2 cell.
The microbiome is a complex and dynamic community of diverse organisms that can include bacteria, viruses, fungi, archaea, and other eukaryotes that include ∼1013 to 1014 microbial organisms across the human body.2-4 The lower intestinal tract is the site of heaviest colonization by microbes and is typically dominated by bacteria.5 The metabolic activities of commensal microbes and their host interactions can protect against pathogens and contribute to normal immune function.6 Disruption of this normal homeostasis, or dysbiosis, has the potential to perturb the balance between immune tolerance and activation and has been associated with a variety of disease states.7-12
The cross talk between the microbiome and acute intestinal GVHD, in particular, has long been investigated.13-15 Studies in the 1970s demonstrated that mice maintained in microbiome-devoid isolators13 or treated with antibiotics effective against gut microbes14 develop a very mild form of acute intestinal GVHD. More recent studies have implicated the microbiome, especially the loss of microbial diversity, in the development of acute intestinal GVHD.16,17 Next-generation sequencing technologies have broadened our understanding of the complex interplay between the microbial repertoire and the pathophysiology of acute intestinal GVHD, paving the road for translation of novel strategies targeting the microbiome in clinical practice.18,19
Alteration of the microbiome by aspects of SCT and role in GVHD
Effect of conditioning regimen
The interplay between the microbiome and the host immune system starts at the level of the first host defense mechanism, the mucosal intestinal barrier. The intestinal epithelium includes many cell types that comprise intestinal epithelial cells (IECs), including intestinal stem cells (ISCs), goblet cells, and Paneth cells, which assume different functions. The IECs provide the physical and biochemical barriers that separate the intestinal lining from luminal components and are primarily responsible for nutrient absorption, whereas goblet cells secrete mucus that further solidifies the intestinal barrier.20 Thus, a primary function of the intestinal barrier is to limit penetration of microbes and their products into host tissues. In allo-SCT, total body irradiation and chemotherapy cause intestinal mucosal damage that may lead to bacterial translocation and subsequent infection and bacteremia.21 In fact, intestinal radiosensitivity is significantly linked to the gut microbiota. Germ-free stem cell transplantation (SCT) mouse models treated with γ-irradiation have fewer apoptotic endothelial cells and reduced infiltration by lymphocytes in their small intestinal villi compared with animals that have acquired a microbiota.22
Paneth cells, an epithelial cell subset predominantly residing in the base of small intestinal crypts,23 have been particularly well studied in the setting of GVHD. Paneth cells are best known for secretory granules rich in microbicidal peptides.24 Upon sensing bacteria or bacterial products, Paneth cells release antimicrobial proteins, such as α-defensins, which play a role in suppressing potentially pathogenic organisms while relatively preserving commensals.25 Paneth cells also provide an epithelial niche for leucine-rich repeat-containing G-protein–coupled receptor 5–positive ISCs that maintain the epithelium. They exert trophic effects on ISCs through epidermal growth factor, transforming growth factor-α, WNT3, and NOTCH signaling pathways.26 Paneth cells are exquisitely targeted during GVHD, resulting in a dramatic reduction in the expression of α-defensins in the small intestines and associated alterations in the normal microbial environment, with expansions of potential pathogens (eg, Escherichia coli) that normally constitute only a small proportion of the intestinal microbiota.27 Although these changes can occur in the absence of irradiation, suggesting an alloreactive T-cell–dependent mechanism, Paneth cell loss occurs earlier and is more prolonged in mice receiving irradiation-based conditioning, suggesting that conditioning enhances Paneth cell damage directly and indirectly, contributing to subsequent GVHD.27 In a separate study, the enumeration of duodenal Paneth cells was demonstrated to correlate with GVHD outcomes.28 Regenerating islet-derived 3-α is another antimicrobial protein secreted by Paneth cells that has been identified as a plasma biomarker indicative of acute GVHD of the lower gastrointestinal (GI) tract.29
Disruption of gut microbiome diversity
Intestinal inflammation due to GVHD can lead to a major shift in the composition of the intestinal microbiota, which has been linked, in turn, to adverse outcomes after allo-SCT.30 Using mouse models, 1 study showed that experimentally induced GVHD led to a large shift within the phylum Firmicutes, with an increase in Lactobacillales and a decrease in Clostridiales.31 Interestingly, elimination of Lactobacillales from the flora of mice before SCT aggravated GVHD, whereas reintroduction of the predominant species of Lactobacillus resulted in significant protection against GVHD.31 Similar microbiome changes were observed in patients who developed GVHD following allo-SCT.31 The loss in diversity with expansion of Lactobacillus spp. has been observed in other studies,32 supporting the notion that maintaining a diverse population of commensal organisms may improve outcomes after allo-SCT.32-34 The diversity of the intestinal microbiota at the time of engraftment can serve as an independent predictor of mortality in allo-SCT, with worse outcomes associated with decreased intestinal diversity. Increased microbial diversity from low to intermediate to high increased 3-year overall survival rates in recipients of allo-SCT from 36% to 60% to 67%, respectively.33 A recently published article examined the microbial composition of fecal samples from 1362 patients undergoing allo-SCT at 4 centers; it revealed patterns of dysbiosis characterized by loss of diversity and domination of single taxa.35 Greater diversity in intestinal microbiota was associated with a lower mortality.35 Altered microbial diversity during the process of allo-SCT facilitates the intestinal luminal expansion of pathogenic organisms that have been associated with bacteremia post allo-SCT, including viridans streptococci, vancomycin-resistant Enterococcus (VRE), and facultative anaerobic gram-negative bacteria.36 Enterococcal domination of the gut flora was found to be associated with a ninefold increase in the risk of VRE bacteremia, and proteobacterial domination led to a fivefold increase in the risk of gram-negative bacteremia.37
Although the bacterial microbiome has been studied most extensively with respect to GVHD, the virome and mycobiome also likely play important roles and warrant further exploration. Emerging data suggest that enteric viruses are regulated and, in turn, regulate other microbial constituents of the intestine, such as bacteria, fungi, and parasites, through a series of processes called “transkingdom interactions.”38 Similarly, the role of fungi cannot be underestimated. Fungal wall components, such as a-mannan recognition by C-type lectin receptor and mediation of T helper 17 (Th17) cell responses in GVHD, have been linked to the pathogenesis of GVHD.39 Different pathogen recognition receptors (PRRs), such as dectin-1, dectin-2, mannose receptor, and Toll-like receptor-2 (TLR2), are known to be implicated in inducing these responses,40 but many questions regarding fungal compositions and infections in SCT patients and correlation with outcomes require further investigation and large-scale profiling.
Impact of antibiotics
The exact mechanisms underlying alterations in microbial flora leading to acute intestinal GVHD are still incompletely understood, although certain clinical parameters in the peritransplant setting can explain some of these changes. These include the low oral food intake of SCT patients, the oral medications they are receiving, the disruption of the intestinal epithelium integrity as will be detailed later, changes in organ function and subsequent effects on metabolism, and changes in gut absorption and transit time.20 Antibiotics can also have a profound impact on the intestinal microbiome. Prophylactic and therapeutic antibiotics are commonly used in the SCT process, and alterations in the microbial flora vary depending on the antibiotic regimen and their corresponding spectrum of microbial coverage. Agents with relatively narrow coverage have been associated with decreased acute GVHD severity.41,42 For instance, compared with ciprofloxacin and metronidazole, prophylaxis with rifaximin better preserves the microbial flora in patients undergoing allo-SCT.42 An ongoing phase 2 clinical trial is currently exploring different antibiotic regimens for prevention or treatment of infections in neutropenic patients while avoiding major shifts in the gut bacterial flora (NCT02641236).
Role of nutrition
The impaired nutritional status that patients experience after allo-SCT due to associated nausea, vomiting, anorexia, and mucositis is associated with an adverse prognosis.43,44 Poor nutrition can be linked to reduced microbial diversity and, during allo-SCT, may mediate an increased risk for acute GVHD. Total parenteral nutrition (TPN) is commonly used in allo-SCT patients, including patients with acute intestinal GVHD, to improve their nutritional status.45 It is still debatable whether oral nutrition is preferred over TPN in the setting of GI GVHD.46 General guidelines recommend the use of a graded GVHD diet, which gradually transitions patients from a liquid diet to solid foods once the volume of diarrhea has decreased to <500 mL/d.45 The exact effect of TPN on the microbiome has not been well established. Some studies suggested that oral nutrition might be more beneficial than TPN in preserving the digestive tract and barrier function and decreasing the incidence of severe acute GVHD.47,48 In another study, receiving TPN for >10 days was associated with loss of Blautia spp. and increased GVHD mortality.32 The NEPHA trial is a randomized multicenter clinical trial that compares the effects of enteral nutrition vs parenteral nutrition on early toxicity after allo-SCT, including mortality and early immunological and infectious toxicities.49
A commonly recommended diet for SCT patients with acute intestinal GVHD includes limited amounts of fats, fiber, lactose, acidic foods, and other potential irritants.45 However, there are limited data to support this recommendation, and given our understanding of the role of microbiome in healthy patients and extrapolating from research done in patients with inflammatory bowel disease, patients undergoing SCT may benefit from the earlier dietary addition of fibers, vegetables, and fruits to prevent GVHD and to assist in mucosal healing after GI GVHD. Immunomodulating diets, including those rich in nutrients, such as omega-3 polyunsaturated long-chain fatty acids, antioxidants, and specific amino acids (eg, arginine), have been shown to improve immune system cell function and reduce inflammation through regulation of T-cell responses.50 In a recent study, lactose was shown to provide a substrate for Enteroccocus growth that was associated with a greater incidence of GVHD and mortality. A lactose-free diet limits Enteroccocus growth, reduces the severity of GVHD, and improves survival in murine allo-SCT recipients with GVHD.51
Mechanisms of dysbiosis-mediated GVHD
PRRs
Epithelial damage caused by conditioning regimens prior to allo-SCT can lead to pathogen-associated molecular patterns (PAMPs) crossing the intestinal barrier and activating the innate immune system.52 In turn, the innate immune system activates alloreactive donor T cells, which are the principal mediators in the development of GVHD. The host innate immune cells detect PAMPs through certain PRRs that exist in distinct forms, depending on the cell type and the cellular compartment. TLRs are a type of PRRs recognizing various PAMPs, including bacterial lipoproteins recognized by TLR2, lipopolysaccharide recognized by TLR4, flagellin recognized by TLR5, immune complexes containing DNA recognized by TLR9, and RNA recognized by TLR3, TLR7, and TLR13.53-55
NOD-like receptors (NLRs) are another type of PRRs that are primarily found in the intracellular compartment, as opposed to TLRs that can be intracellular and extracellular,56 and function through recognizing intracellular PAMPs and danger-associated molecular patterns.57 Examples of NLRs include NOD1, NOD2, and NALPs.58 Although NOD1 is ubiquitously expressed, NOD2 expression is restricted to intestinal Paneth cells and innate immune cells.56 Signaling through NLRs is essential for the function of the inflammasome, a multiprotein complex that mediates inflammatory responses. NLRs are involved in upregulating a subset of inflammatory cytokines, including interleukin-1α (IL-1α) and IL-18.58 A recent article showed that, on the other hand, host NOD-like receptor family pyrin domain-containing 6, which is known to regulate innate immune responses and GI homeostasis, plays a pathogenic role in GI GVHD, independently of the composition of the gut microbiome.59
Other types of PRRs include sialic acid–binding Ig-like lectins, which are expressed by monocytes, neutrophils, natural killer cells, eosinophils, and basophils and are characterized by their inhibitory effects, attenuating danger-associated molecular pattern–mediated inflammation and, thus, serving an important regulatory function.60
Microbial metabolites
The microbiome can also influence the integrity of protective IECs through metabolites produced as byproducts of fermentation of dietary components and host-derived glycans. Butyrate is 1 intestinal microbiota-derived short-chain fatty acid (SCFA) that serves as an important energy source for IECs61; it can be diminished following allo-SCT.62 Local administration of exogenous butyrate was shown to restore histone acetylation and improve IEC junctional integrity, limit apoptosis, and mitigate GVHD.63 Moreover, rationally altering the composition of the intestinal microbiome toward high-butyrate producers was also shown to mitigate GVHD.63 In another study, the administration of high butyrate–producing Clostridia organisms results in increased intestinal regulatory T cells (Tregs), which play an important role in regulating gut inflammatory responses through several mechanisms, including the release of anti-inflammatory cytokines, such as IL-10.64,65 Tregs are protective against GVHD, likely through suppression of alloreactive T cells that mediate GVHD.66 Furthermore, SCFAs can induce IL-22 responses in innate lymphoid cells (ILCs), which can exert anti-inflammatory and regenerative effects on IECs.67
Indole is another important metabolite derived from tryptophan by tryptophanase-expressing commensal bacteria. Indole can regulate biofilm formation and mucosal barrier function, and it can also modulate the expression of pro- and anti-inflammatory genes by IECs.34 Indole-3-aldehyde activates the aryl hydrocarbon receptor on ILCs, mediates colonization resistance, and induces IL-22 secretion,68,69 which contributes to epithelial regeneration and improves GVHD-related mortality in mice models.70 A study showed that early exposure to antibiotics was associated with lower levels of 3-indoxyl sulfate in the urine of allo-SCT recipients, which was further associated with a higher transplant-related mortality.71 Similarly, stool specimens from patients with active GVHD were shown to have reduced 3-indoxyl sulfate levels and a prominent shift of the microbial flora from commensal bacteria toward Enterococci.34 Together, these findings indicate that the microbial metabolome can significantly impact on the pathogenesis of GVHD, although more research in this field needs to be conducted.
Innate immune system and T-cell differentiation
Different arms of the immune system modulate intestinal inflammation during the process of acute GVHD. The exposure to gut microbial flora and bacterial translocation during acute intestinal GVHD leads to neutrophil infiltration in the small intestine, which contributes to tissue damage partially through the production of reactive oxygen species; this, in turn, contributes to the pathogenesis of GVHD.72 Antigen-producing cells also play a critical role in mediating GVHD, primarily through directing T-cell activation and differentiation.73 Dendritic cells from mesenteric lymph nodes mediate homing of T cells to the intestines and focal intestinal damage.74,75 Monocytes also play a role in GVHD through promoting Th17 cell differentiation that, in turn, can recruit macrophages and neutrophils to inflammatory sites.76 In addition to these cell populations, ILCs are a group of immune cells that are of lymphoid lineage and assume innate immune roles.77 Group 3 ILCs play a particularly important role in maintaining the intestinal barrier function and were found to be reduced in GVHD.78 These tissue-resident cells produce Th17 family cytokines, such as IL-22.79 Group 3 ILCs express major histocompatibility complex (MHC) class II, promote tolerance to commensal bacteria by presenting commensal bacterial antigens to T cells without associated costimulation and, hence, limiting intestinal inflammation and T-cell hyperactivation.80 Recently, a study demonstrated that the microbiota influences MHC class II expression on IECs, which, in turn, present antigens, activate CD4+ T cells, and mediate lethal GI GVHD. MHC class II expression was found to depend on the IL-12/interferon-γ axis, representing a potential therapeutic strategy.81
Adaptive immune system
Activation of the adaptive immune system is an early event during development of acute GVHD. Th1 cell–mediated tissue damage can then sustain the inflammatory cycle of GVHD. The exposure of intestinal tissues to bacterial products is also associated with recruitment of IL-10 and transforming growth factor-β–secreting Tregs, as well as IL-22– and IL-17–secreting Th17 cells.82,83 In germ-free animal models, there is a shift to a predominantly Th2 cell response and a reduction in Th1 and Th17 cells, which could be reversed by floral colonization of the gut. For example, segmented filamentous bacteria, species belonging to commensal clostridia-related bacteria, and Lactobacillus johnsonii stimulate Th17 cells in the small intestine.83-85 Th17 cells are an important player in intestinal GVHD, as was revealed by the analysis of organ tissues at the time of GVHD diagnosis showing Th17 cell differentiation and STAT3 signaling in cases of severe GVHD.86,87 Moreover, the cytokine IL-6 that acts upstream of Th17 cell differentiation has also been identified as a potential target to prevent GVHD in murine models.88 The anti–IL-6 monoclonal antibody tocilizumab showed promising results in GVHD prophylaxis in a phase 1/2 study. Interestingly, effector CD8+ T cells also undergo Th17 cell–like differentiation under the key direction of IL-6, contributing to the pathogenesis of GVHD.89,90
B cells are also influenced by alterations in the microbiota and may play an important role in acute GVHD. The intestinal mucosa is essential for B-cell development, where extracellular signals from commensal organisms lead to a diverse repertoire of gut immunoglobulins.91 The intestinal flora continuously stimulates gut-associated lymphoid tissues, such as Peyer’s patches and lymphoid follicles where germinal center formation is induced, leading to immunoglobulin A production, particularly through NOD1-mediated signaling.92
Potential of microbiota as therapeutic intervention
In light of the strong association of the microbiota with allo-SCT outcomes and the pathogenesis of acute intestinal GVHD, there is a growing body of research developing strategies to target the microbiome as part of clinical practice. Interventions that target the microbiome in the setting of allo-SCT can be categorized into antibiotics, probiotics, prebiotics, and postbiotics. Fecal microbiota transplantation (FMT) has also been investigated as a strategy to directly transfer bacteria, bacterial products, and metabolites.
Antibiotic and immunoglobulin therapy
Antibiotic interventional strategies to minimize microbial alterations in the allo-SCT setting include the use of narrow-spectrum antibiotics, such as rifaximin, and minimizing the duration of antibiotic use. This is especially important in the preservation of commensal bacteria that exert anti-inflammatory effects post-SCT, such as Blautia spp.,32 as well as potentially preventing the growth of mucus-degrading bacteria, such as Akkermansia muciniphila.93
Oral immunoglobulin administration has been investigated as a strategy to reduce pathogenic bacteria, such as E coli, while increasing beneficial bacteria, such as Lactobacillus reuteri, to improve GVHD outcomes. In a haploidentical SCT murine model, immunoglobulin yolk antibodies from hens immunized with heat-inactivated E coli, Clostridium perfringens, and Salmonella typhimurium were administered starting 2 days before allo-SCT through day 28 after allo-SCT; treatment resulted in a decrease in acute GVHD scores and improved survival.94
Immunoglobulin therapies are promising alternatives to traditional broad-spectrum antibiotics that could help to reduce collateral damage to the microbiome owing to their targeted effects, although their role in reducing dysbiosis remains to be investigated.
Prebiotics, probiotics, and postbiotics
Prebiotics are indigestible carbohydrates that are preferentially metabolized by commensal gut bacteria producing nutrients used by IECs.95,96 The beneficial impact of fiber prebiotics on intestinal immunity has been demonstrated in a variety of studies. The indigestible oligo- and polysaccharides are made of chains of sugars that are fermented by commensal intestinal bacteria, resulting in the production of SCFA serving as a nutrient and energy source.97 As discussed above, SCFAs, including butyrate, acetate, and propionate, mediate anti-inflammatory cytokine production, preserve intestinal barrier integrity, and play an important role in regulating intestinal immune function.97-99 Other prebiotics include the polysaccharide inulin (derived from vegetables such as the Jerusalem artichoke), its fructo-oligosaccharide derivative (known as inulin-type fructans), as well as some oligosaccharides (eg, xylo-oligosaccharides and galacto-oligosaccharides). These have been shown in randomized clinical trials to be associated with increased Bifidobacterium growth and SCFA concentrations.100-102
Probiotics are selected beneficial live microorganisms that are introduced to the intestinal tract orally. Administration of Lactobacillus spp. has been shown to improve GVHD in mice models.31,103 Based in part on these results, a randomized trial evaluating supplementation with Lactobacillus rhamnosus GG was performed in allo-SCT patients; however, probiotic administration did not significantly modify gut microbiome diversity or impact on the incidence or severity of GVHD.104 Notably, possible negative effects from probiotic use have also been reported, such as Lactobacillus acidophilus sepsis in an immunocompromised patient with mantle cell lymphoma undergoing autologous SCT after excessive consumption of L acidophilus–enriched yogurt.105 Other probiotic bacteria have also been used, such as Bifidobacterium spp., which have been associated with resistance to infection by E coli in mice106 and improved response to immunotherapy with checkpoint inhibitors.107 The advent of genetic engineering technologies could allow genetic engineering of probiotic bacteria to secrete compounds with beneficial effects, such as antimicrobial molecules that help to kill pathogenic organisms while preserving commensal bacteria. Indeed, a recent study showed that the commensal bacteria Blautia producta BPSCSK secrete a lantibiotic that reduces intestinal colonization by VRE.108
Postbiotics are metabolite-based therapies that aim to identify the molecules depleted in a certain disease and supplement the diet with the molecule itself or a precursor molecule that the microbial organisms convert into the bioactive molecule.109 SCFAs, indole, and indole derivatives are previously discussed metabolites that may be beneficial as postbiotics, especially with encouraging recent reports showing that increasing the level of indole or its derivatives in the gut following SCT remarkably reduced the risk of GVHD in murine models without compromising the graft-versus-leukemia effect of alloreactive T cells.110
FMT
Going beyond introducing selected beneficial organisms, FMT as the ultimate probiotic therapy has experienced increasing interest, especially with its successful use in refractory Clostridium difficile infection. This disease has become the classical example of a dysbiosis-associated syndrome.111 In a pilot study, 13 patients who underwent allo-SCT received third-party FMT capsules at a median of 27 days after allo-SCT. FMT was found to be feasible and well tolerated, with 1 treatment-related adverse event: abdominal pain. Analysis of stool composition and urine 3-indoxyl sulfate concentration indicated improvement in intestinal microbiome diversity after FMT that was associated with expansion of stool-donor taxa.112 The optimal systematic approach to the administration of FMT remains unclear. One of the most extensive experiences with FMT in the allo-SCT setting evaluated transplantation of a patient’s own microbiome (auto-FMT) that was collected and preserved pre-SCT (NCT02269150).113 Although clinical results have yet to be reported, the investigators found that, in this study of 25 patients, those randomized to receive auto-FMT exhibited effective restoration of microbial diversity to pre–allo-SCT levels.113
Notably, however, concerns over possible FMT-related sepsis in immunocompromised hosts through bacterial translocation or norovirus infection have been reported in the setting of FMT for inflammatory bowel disease and in patients with C difficile infection.114,115 Another case report described post-FMT irritable bowel syndrome–like symptoms, such as bloating and constipation, that were thought to be due to methane-predominant bacterial overgrowth contracted from the FMT donor.116 A recent report describes 2 cases of bacteremia with extended-spectrum β-lactamase–producing E coli after FMT performed in 2 independent clinical trials, leading to the death of 1 of the 2 patients.117 Both cases were linked to the same stool donor, by means of genomic sequencing, who was qualified for fecal material donation before routine tests for extended-spectrum β-lactamase–producing organisms were included in the donor-screening protocol.117 Long-term follow-up and larger studies to evaluate outcomes of allo-SCT patients undergoing FMT and strategies to improve donor screening to prevent adverse events are needed to elucidate the safety of this approach.
Conclusions and future perspectives
The role of the microbiota in SCT has long been investigated, but the past decade has witnessed great advances in our understanding of the intricacies of the cross talk between the microbiome and the immune system in SCT, in general, and in acute intestinal GVHD, in particular. There seem to be major microbial shifts that commonly occur during the course of allo-SCT and specifically around the period of antibiotic use. Microbial diversity appears to play a crucial and independent role in predicting survival in allo-SCT and the incidence and severity of acute GVHD. Although more research is warranted, beneficial associations of Lactobacillales, Clostridiales, the Eubacterium limosum group and genus Blautia, Bacteroidetes species, and Clostridium clusters IV and XIVa have been observed in multiple studies. In contrast, enterococcal spp., proteobacterial spp., and C difficile have been associated with worsened GVHD outcomes. The role of the microbiota in immune-mediated tumor control remains to be fully established. In a retrospective single-center study of patients who underwent allo-SCT, the composition of the intestinal microbiota was analyzed, and an association between the abundance of E limosum and a decreased risk for relapse/progression of disease post-SCT was identified.118 However, the overall diversity of the intestinal microbiota was not found to be associated with relapse and progression of disease.118 It is likely that new high-throughput technologies will transform the field, because they are already showing us the limit of our knowledge. A recent study recapitulated thousands of species-level genome bins, 77% of which have never been described before, using large-scale metagenomics, cautioning us that we have a long way to go before deciphering all the intricacies of the microbiome and its role in health and diseases.119 The National Institutes of Health Human Microbiome Project is 1 of the first large-scale initiatives to characterize the human microbiome and its dynamic changes in relation to health-related outcomes.120 Integrating the microbiome and multi-omics studies is the subject of intense development, including a recent analysis using high-throughput metabolomics to characterize the major changes in the metabolic profile of SCT recipients showing significant variations in host- and microbiota-derived metabolites potentially affecting allogeneic immune cell reactivity.121
Novel strategies that have the potential to improve outcomes of SCT and reduce GVHD have already entered the translational phase and are being investigated in the clinic. These research efforts will likely determine the potential roles for FMT, prebiotics, probiotics, antibiotics, and other microbiome-centered therapies in SCT and GVHD. Advances in molecular biology, next-generation sequencing, and genetic engineering will likely revolutionize the therapeutic applications of the microbiome by developing highly specialized prebiotics that could precisely shift the microbiomes toward predetermined organisms. Engineering probiotics could increase safety by preventing off-target effects and allow for therapeutic elimination of introduced bacteria should probiotic-related septicemia develop. Further advances could come with development of new powerful diagnostic strategies to detect and quantify dysbiosis to allow early intervention. Finally, improving our understanding of patient-specific ecosystems could allow the design of biomarkers of disease, as well as “precision” therapies. The ultimate goal would be to profile a patient’s microbiome and identify features that could predict incidence and severity of GVHD, potentially allowing for an early microbiome-targeted intervention. Future research efforts will further dissect the intricacies of the interactions between the players of the immune system in acute GVHD and the microbiome and better elucidate the mechanisms underlying how the microbiome can modulate the immune system to improve patients’ health and outcomes.
Acknowledgment
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant HL124112.
Authorship
Contribution: H.R. and R.R.J. wrote the manuscript.
Conflict-of-interest disclosure: R.R.J. is on the board of directors or an advisory committee for Seres Therapeutics, Inc. and Kaleido Biosciences, Inc.; has consulted for Ziopharm Oncology, Merck, MicrobiomeDX, and Karius; and holds patents with or receives royalties from Seres Therapeutics, Inc. H.R. declares no competing financial interests.
Correspondence: Robert R. Jenq, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: rrjenq@mdanderson.org.
REFERENCES
- 1.Maeda Y. Pathogenesis of graft-versus-host disease: innate immunity amplifying acute alloimmune responses. Int J Hematol. 2013;98(3):293-299. [DOI] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staffas A, Burgos da Silva M, van den Brink MRM. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease [published correction appears in Blood. 2017;129(15):2204]. Blood. 2017;129(8):927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dicksved J, Halfvarson J, Rosenquist M, et al. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J. 2008;2(7):716-727. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez A, Stombaugh J, Lozupone C, Turnbaugh PJ, Gordon JI, Knight R. The mind-body-microbial continuum. Dialogues Clin Neurosci. 2011;13(1):55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupton JR. Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr. 2004;134(2):479-482. [DOI] [PubMed] [Google Scholar]
- 12.Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(4):795-806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res. 1971;45(3):577-588. [PubMed] [Google Scholar]
- 14.van Bekkum DW, Roodenburg J, Heidt PJ, van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974;52(2):401-404. [DOI] [PubMed] [Google Scholar]
- 15.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308(6):302-307. [DOI] [PubMed] [Google Scholar]
- 16.Whangbo J, Ritz J, Bhatt A. Antibiotic-mediated modification of the intestinal microbiome in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(2):183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18(5):283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salipante SJ, Sengupta DJ, Rosenthal C, et al. Rapid 16S rRNA next-generation sequencing of polymicrobial clinical samples for diagnosis of complex bacterial infections. PLoS One. 2013;8(5):e65226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan XC, Huttenhower C. Meta’omic analytic techniques for studying the intestinal microbiome. Gastroenterology. 2014;146(6):1437-1448.e1. [DOI] [PubMed] [Google Scholar]
- 20.Peled JU, Hanash AM, Jenq RR. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood. 2016;128(20):2395-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3-20, NaN-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci USA. 2005;102(37):13254-13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141-153. [DOI] [PubMed] [Google Scholar]
- 24.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1(2):113-118. [DOI] [PubMed] [Google Scholar]
- 25.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356-368. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood. 2012;120(1):223-231. [DOI] [PubMed] [Google Scholar]
- 28.Levine JE, Huber E, Hammer STG, et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood. 2013;122(8):1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara JL, Harris AC, Greenson JK, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118(25):6702-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taur Y, Jenq RR, Ubeda C, van den Brink M, Pamer EG. Role of intestinal microbiota in transplantation outcomes. Best Pract Res Clin Haematol. 2015;28(2-3):155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(8):1373-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(5):640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peled JU, Gomes ALC, Devlin SM, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020;382(9):822-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamboj M, Chung D, Seo SK, et al. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant. 2010;16(11):1576-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeiffer JK, Virgin HW. Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science. 2016;351(6270):aad5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Velden WJ, Plantinga TS, Feuth T, Donnelly JP, Netea MG, Blijlevens NM. The incidence of acute graft-versus-host disease increases with Candida colonization depending the dectin-1 gene status. Clin Immunol. 2010;136(2):302-306. [DOI] [PubMed] [Google Scholar]
- 40.van der Velden WJFM, Netea M, de Haan A, Huls G, Donnelly P, Blijlevens NN. Role of the mycobiome in human acute graft-versus-host disease Biol Blood Marrow Transplant. 2019;19(2):329-332. [DOI] [PubMed] [Google Scholar]
- 41.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber D, Oefner PJ, Dettmer K, et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(8):1087-1092. [DOI] [PubMed] [Google Scholar]
- 43.Papadopoulou A, Lloyd DR, Williams MD, Darbyshire PJ, Booth IW. Gastrointestinal and nutritional sequelae of bone marrow transplantation. Arch Dis Child. 1996;75(3):208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulte C, Reinhardt W, Beelen D, Mann K, Schaefer U. Low T3-syndrome and nutritional status as prognostic factors in patients undergoing bone marrow transplantation. Bone Marrow Transplant. 1998;22(12):1171-1178. [DOI] [PubMed] [Google Scholar]
- 45.van der Meij BS, de Graaf P, Wierdsma NJ, et al. Nutritional support in patients with GVHD of the digestive tract: state of the art. Bone Marrow Transplant. 2013;48(4):474-482. [DOI] [PubMed] [Google Scholar]
- 46.van der Meij BS, Wierdsma NJ, Janssen JJWM, Deutz NEP, Visser OJ. If the gut works, use it! But does the gut work in gastrointestinal GvHD? Bone Marrow Transplant. 2017;52(3):466-469. [DOI] [PubMed] [Google Scholar]
- 47.Seguy D, Berthon C, Micol JB, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation. 2006;82(6):835-839. [DOI] [PubMed] [Google Scholar]
- 48.Svahn BM, Remberger M, Heijbel M, et al. Case-control comparison of at-home and hospital care for allogeneic hematopoietic stem-cell transplantation: the role of oral nutrition. Transplantation. 2008;85(7):1000-1007. [DOI] [PubMed] [Google Scholar]
- 49.Lemal R, Cabrespine A, Pereira B, et al. Could enteral nutrition improve the outcome of patients with haematological malignancies undergoing allogeneic haematopoietic stem cell transplantation? A study protocol for a randomized controlled trial (the NEPHA study). Trials. 2015;16(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lye AD, Hayslip JW. Immunonutrition: does it have a role in improving recovery in patients receiving a stem cell transplant? Nutr Cancer. 2012;64(4):503-507. [DOI] [PubMed] [Google Scholar]
- 51.Stein-Thoeringer CK, Nichols KB, Lazrak A, et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science. 2019;366(6469):1143-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754-2759. [PubMed] [Google Scholar]
- 53.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5(10):975-979. [DOI] [PubMed] [Google Scholar]
- 54.Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev. 2012;250(1):216-229. [DOI] [PubMed] [Google Scholar]
- 55.Hidmark A, von Saint Paul A, Dalpke AH. Cutting edge: TLR13 is a receptor for bacterial RNA. J Immunol. 2012;189(6):2717-2721. [DOI] [PubMed] [Google Scholar]
- 56.Shaw MH, Reimer T, Kim YG, Nuñez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20(4):377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lechtenberg BC, Mace PD, Riedl SJ. Structural mechanisms in NLR inflammasome signaling. Curr Opin Struct Biol. 2014;29:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inohara N, Ogura Y, Chen FF, Muto A, Nuñez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276(4):2551-2554. [DOI] [PubMed] [Google Scholar]
- 59.Toubai T, Fujiwara H, Rossi C, et al. Host NLRP6 exacerbates graft-versus-host disease independent of gut microbial composition. Nat Microbiol. 2019;4(5):800-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30(1):357-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17(12):1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romick-Rosendale L, Haslam D, Lane A, et al. Short chain fatty acids are reduced after hematopoietic stem cell transplant in humans and are associated with modifications of the gut microbiome. Biol Blood Marrow Transplant. 2018;24(3):S87-S88. [DOI] [PubMed] [Google Scholar]
- 63.Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease [published correction appears in Nat Immunol. 2016;17(10):1235]. Nat Immunol. 2016;17(5):505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232-236. [DOI] [PubMed] [Google Scholar]
- 65.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells [published correction appears in Nature;506(7487):254]. Nature. 2013;504(7480):446-450. [DOI] [PubMed] [Google Scholar]
- 66.Edinger M, Powrie F, Chakraverty R. Regulatory mechanisms in graft-versus-host responses. Biol Blood Marrow Transplant. 2009;15(suppl 1):2-6. [DOI] [PubMed] [Google Scholar]
- 67.McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142(1):24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372-385. [DOI] [PubMed] [Google Scholar]
- 70.Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528(7583):560-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weber D, Jenq RR, Peled JU, et al. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(5):845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwab L, Goroncy L, Palaniyandi S, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014;20(6):648-654. [DOI] [PubMed] [Google Scholar]
- 73.MacDonald KP, Shlomchik WD, Reddy P. Biology of graft-versus-host responses: recent insights. Biol Blood Marrow Transplant. 2013;19(suppl 1):S10-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koyama M, Cheong M, Markey KA, et al. Donor colonic CD103+ dendritic cells determine the severity of acute graft-versus-host disease. J Exp Med. 2015;212(8):1303-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim TD, Terwey TH, Zakrzewski JL, et al. Organ-derived dendritic cells have differential effects on alloreactive T cells. Blood. 2008;111(5):2929-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinhardt K, Foell D, Vogl T, et al. Monocyte-induced development of Th17 cells and the release of S100 proteins are involved in the pathogenesis of graft-versus-host disease. J Immunol. 2014;193(7):3355-3365. [DOI] [PubMed] [Google Scholar]
- 77.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145-149. [DOI] [PubMed] [Google Scholar]
- 78.Hanash AM, Dudakov JA, Hua G, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765-774. [DOI] [PubMed] [Google Scholar]
- 80.Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498(7452):113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koyama M, Mukhopadhyay P, Schuster IS, et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity. 2019;51(5):885-898.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Round JL, Lee SM, Li J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luzza F, Parrello T, Monteleone G, et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165(9):5332-5337. [DOI] [PubMed] [Google Scholar]
- 85.Ivanov II, Frutos RL, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ratajczak P, Janin A, Peffault de Latour R, et al. Th17/Treg ratio in human graft-versus-host disease. Blood. 2010;116(7):1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Betts BC, Sagatys EM, Veerapathran A, et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J Leukoc Biol. 2015;97(4):807-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tawara I, Koyama M, Liu C, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17(1):77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gartlan KH, Markey KA, Varelias A, et al. Tc17 cells are a proinflammatory, plastic lineage of pathogenic CD8+ T cells that induce GVHD without antileukemic effects. Blood. 2015;126(13):1609-1620. [DOI] [PubMed] [Google Scholar]
- 90.Tajima M, Wakita D, Noguchi D, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205(5):1019-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wesemann DR, Portuguese AJ, Meyers RM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501(7465):112-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146(6):1477-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouazzaoui A, Huber E, Dan A, et al. Reduction of aGVHD using chicken antibodies directed against intestinal pathogens in a murine model. Blood. 2017;129(8):1052-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberfroid MB. Introducing inulin-type fructans. Br J Nutr. 2005;93(suppl 1):S13-S25. [DOI] [PubMed] [Google Scholar]
- 96.Andermann TM, Rezvani A, Bhatt AS. Microbiota manipulation with prebiotics and probiotics in patients undergoing stem cell transplantation. Curr Hematol Malig Rep. 2016;11(1):19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61(1):37-41. [DOI] [PubMed] [Google Scholar]
- 99.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lecerf JM, Dépeint F, Clerc E, et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr. 2012;108(10):1847-1858. [DOI] [PubMed] [Google Scholar]
- 101.Childs CE, Röytiö H, Alhoniemi E, et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr. 2014;111(11):1945-1956. [DOI] [PubMed] [Google Scholar]
- 102.Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004;80(6):1658-1664. [DOI] [PubMed] [Google Scholar]
- 103.Gerbitz A, Schultz M, Wilke A, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103(11):4365-4367. [DOI] [PubMed] [Google Scholar]
- 104.Gorshein E, Wei C, Ambrosy S, et al. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2017;31(5):e12947. [DOI] [PubMed] [Google Scholar]
- 105.Mehta A, Rangarajan S, Borate U. A cautionary tale for probiotic use in hematopoietic SCT patients-Lactobacillus acidophilus sepsis in a patient with mantle cell lymphoma undergoing hematopoietic SCT. Bone Marrow Transplant. 2013;48(3):461-462. [DOI] [PubMed] [Google Scholar]
- 106.Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543-547. [DOI] [PubMed] [Google Scholar]
- 107.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim SG, Becattini S, Moody TU, et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature. 2019;572(7771):665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klemashevich C, Wu C, Howsmon D, Alaniz RC, Lee K, Jayaraman A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr Opin Biotechnol. 2014;26:85-90. [DOI] [PubMed] [Google Scholar]
- 110.Swimm A, Giver CR, DeFilipp Z, et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood. 2018;132(23):2506-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Drekonja D, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection: a systematic review. Ann Intern Med. 2015;162(9):630-638. [DOI] [PubMed] [Google Scholar]
- 112.DeFilipp Z, Peled JU, Li S, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2(7):745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taur Y, Coyte K, Schluter J, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018;10(460):eaap9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohn’s Colitis. 2014;8(3):252-253. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108(8):1367. [DOI] [PubMed] [Google Scholar]
- 116.Chang BW, Rezaie A. Irritable bowel syndrome-like symptoms following fecal microbiota transplantation: a possible donor-dependent complication. Am J Gastroenterol. 2017;112(1):186-187. [DOI] [PubMed] [Google Scholar]
- 117.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043-2050. [DOI] [PubMed] [Google Scholar]
- 118.Peled JU, Devlin SM, Staffas A, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol. 2017;35(15):1650-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pasolli E, Asnicar F, Manara S, et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176(3):649-662.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Integrative HMP (iHMP) Research Network Consortium The Integrative Human Microbiome Project. Nature. 2019;569(7758):641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Michonneau D, Latis E, Curis E, et al. Metabolomics analysis of human acute graft-versus-host disease reveals changes in host and microbiota-derived metabolites. Nat Commun. 2019;10(1):5695. [DOI] [PMC free article] [PubMed] [Google Scholar]