Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
In addition to thrombotic complications, bleeding is a significant cause of morbidity in patients with COVID-19.
D-dimer elevation at admission was predictive of bleeding, thrombosis, critical illness, and death in patients with COVID-19.
Abstract
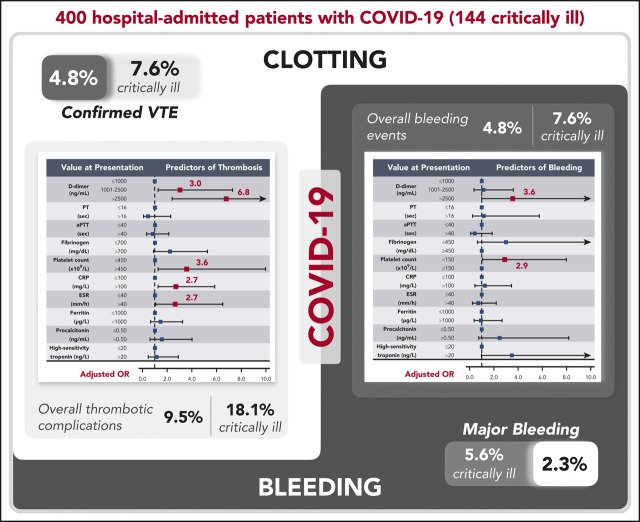
Patients with coronavirus disease 2019 (COVID-19) have elevated D-dimer levels. Early reports describe high venous thromboembolism (VTE) and disseminated intravascular coagulation (DIC) rates, but data are limited. This multicenter retrospective study describes the rate and severity of hemostatic and thrombotic complications of 400 hospital-admitted COVID-19 patients (144 critically ill) primarily receiving standard-dose prophylactic anticoagulation. Coagulation and inflammatory parameters were compared between patients with and without coagulation-associated complications. Multivariable logistic models examined the utility of these markers in predicting coagulation-associated complications, critical illness, and death. The radiographically confirmed VTE rate was 4.8% (95% confidence interval [CI], 2.9-7.3), and the overall thrombotic complication rate was 9.5% (95% CI, 6.8-12.8). The overall and major bleeding rates were 4.8% (95% CI, 2.9-7.3) and 2.3% (95% CI, 1.0-4.2), respectively. In the critically ill, radiographically confirmed VTE and major bleeding rates were 7.6% (95% CI, 3.9-13.3) and 5.6% (95% CI, 2.4-10.7), respectively. Elevated D-dimer at initial presentation was predictive of coagulation-associated complications during hospitalization (D-dimer >2500 ng/mL, adjusted odds ratio [OR] for thrombosis, 6.79 [95% CI, 2.39-19.30]; adjusted OR for bleeding, 3.56 [95% CI, 1.01-12.66]), critical illness, and death. Additional markers at initial presentation predictive of thrombosis during hospitalization included platelet count >450 × 109/L (adjusted OR, 3.56 [95% CI, 1.27-9.97]), C-reactive protein (CRP) >100 mg/L (adjusted OR, 2.71 [95% CI, 1.26-5.86]), and erythrocyte sedimentation rate (ESR) >40 mm/h (adjusted OR, 2.64 [95% CI, 1.07-6.51]). ESR, CRP, fibrinogen, ferritin, and procalcitonin were higher in patients with thrombotic complications than in those without. DIC, clinically relevant thrombocytopenia, and reduced fibrinogen were rare and were associated with significant bleeding manifestations. Given the observed bleeding rates, randomized trials are needed to determine any potential benefit of intensified anticoagulant prophylaxis in COVID-19 patients.
Visual Abstract
Introduction
Coronavirus disease 2019 (COVID-19), first identified in Wuhan, China in December of 2019, has become a worldwide pandemic with widespread illness and mortality and a profound impact on society, culture, and the global economy. Although respiratory compromise is the cardinal feature of the disease, early studies have suggested that elevated circulating D-dimer levels are associated with mortality,1,2 suggesting a distinct coagulation disorder associated with COVID-19. In support of this hypothesis are recent autopsy studies of COVID-19 patients demonstrating the presence of fibrin thrombi within distended small vessels and capillaries and extensive extracellular fibrin deposition.3
Given the ongoing global pandemic, there is an urgent need to understand the rate of bleeding and thrombotic manifestations associated with COVID-19 coagulopathy, as well as the clinical utility of abnormal coagulation testing to predict risk for bleeding, thrombosis, and severity of illness. In addition to D-dimer, a prolonged prothrombin time (PT) has been associated with decreased survival and increased need for critical care.4,5 On the basis of laboratory findings, disseminated intravascular coagulation (DIC) has been reported to develop in >70% of patients who succumb to the infection.5 Other retrospective studies have reported thrombotic rates in excess of 20% to 30%, but use of prophylactic anticoagulation was not consistent between studies.6,7
The high venous thromboembolism (VTE) rates reported in these early studies have prompted some investigators to recommend empiric escalation of anticoagulation doses used for prophylaxis in COVID-19 patients.7 A recently published expert opinion guidance statement did not reach a consensus on whether standard prophylactic dose or escalated anticoagulation was optimal to prevent thrombotic events.8 Despite the lack of a clear picture of thrombotic risk or any studies describing bleeding manifestations, several institutions have recently released guidance statements, both internal only and externally published,9 recommending the use of low molecular weight heparin (LMWH) at intermediate or therapeutic doses or unfractionated heparin (UFH) infusions in COVID-19 patients with elevated D-dimer levels but no known thrombotic complications. Other commentaries have argued against empiric escalation of anticoagulation.10
Therefore, the primary goal of this study was to describe the rate of bleeding and thrombotic complications in a large multicenter cohort of critically ill and noncritically ill COVID-19 patients. In addition, given the previously demonstrated associations between coagulation and inflammatory parameters and mortality in COVID-19 patients, we investigated these markers as predictors of thrombosis and bleeding and examined the relationship between inflammation and coagulopathic complications in COVID-19.
Methods
Patients and data collection
This study was approved by the Institutional Review Board of Partners Healthcare (approval PHS/2020P000994). All patients aged ≥18 years with confirmed COVID-19 (defined as a positive SARS-CoV-2 reverse-transcriptase polymerase chain reaction test by nasopharyngeal/oropharyngeal swab or sputum specimen) from 1 March 2020 through 5 April 2020 who had a D-dimer test performed on initial presentation with COVID-19 were identified using the Research Patient Data Registry at Partners Healthcare, a large multi-institutional patient data registry. The D-dimer test was used to identify a population of interest for this study because participating institutions had instituted routine D-dimer testing at initial evaluation in all admitted COVID-19 patients during the early days of the pandemic. Data were obtained retrospectively from patients treated at 5 Partners Healthcare institutions (Massachusetts General Hospital, Brigham and Women’s Hospital, North Shore Medical Center, Newton-Wellesley Hospital, and Brigham and Women’s Faulkner Hospital) by manual chart review of the electronic medical record with a data cutoff date of 8 April 2020. Patient data collected included demographics, relevant comorbidities, need for hospital admission, need for endotracheal intubation, hospital length of stay, completion of hospitalization (hospital discharge and death), bleeding events, arterial and venous thrombotic events, anticoagulation administered, and coagulation and inflammatory laboratory parameters.
Bleeding and thrombotic events
The incidence of bleeding and thrombotic events in COVID-19 patients was assessed. Bleeding events were graded according to the modified World Health Organization (WHO) grading system.11,12 Pulmonary embolism (PE) and deep vein thrombosis (DVT) were confirmed radiographically. Synchronously diagnosed DVT and PE in the same patient were considered 1 VTE event. Also collected were cases of presumed VTE unable to be confirmed radiographically (because of an inability to perform the necessary test secondary to diagnostic limitations imposed by this infection) but for whom all of the following criteria were satisfied: (1) clinical evidence consistent with VTE on vital signs, physical examination, hemodynamic monitoring, or electrocardiogram; (2) a strong clinical suspicion on the part of the treating attending physician; and (3) therapeutic anticoagulation was initiated as a result of high clinical suspicion. Cases meeting these criteria underwent independent validation by 2 chart reviewers (H.A.-S. and R.P.R.); unless stated otherwise, they were included in VTE analyses. Myocardial infarction was diagnosed utilizing clinical criteria plus biomarker elevation and electrocardiographic changes; biomarker elevation in the absence of other clinical criteria was not considered a myocardial event.
Clinically significant nonvessel thrombotic complications potentially representative of a systemic hypercoagulable state were also collected. These include ≥2 occurrences of intraluminal central venous catheter or arterial line clotting necessitating line replacement to a new site or ≥2 occurrences of clotting of the continuous veno-venous hemofiltration (CVVH) circuit in a 24-hour period in patients requiring renal replacement therapy that was deemed sufficiently problematic to initiate therapeutic systemic anticoagulation.
Analysis of coagulation and inflammatory parameters
Results of 6 routinely drawn coagulation-based laboratory parameters (PT, international normalized ratio [INR], activated partial thromboplastin time [PTT], D-dimer, fibrinogen, and platelet count), 4 laboratory measures of inflammation (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], ferritin, and procalcitonin), and high-sensitivity cardiac troponin were evaluated and compared between patients with thrombotic complications (composite of venous thromboembolism, arterial thromboembolism, and clinically significant nonvessel thrombotic complications), patients with bleeding complications, and patients without bleeding or thrombotic complications. Specific assay methodology and manufacturer information are listed in supplemental Table 2, available on the Blood Web site. Initial values on presentation, minimum values, and peak values for each parameter were compared in patients with thrombotic complications and patients without coagulation-associated complications, as well as in patients with bleeding complications and patients without coagulation-associated complications, using the Mann-Whitney U test. Using these data, correlations between peak values of D-dimer and inflammatory markers for each patient (irrespective of the timing of measurement) were assessed with Spearman correlation coefficients.
Predictors of bleeding events, thrombotic events, critical illness, and death
Univariable and multivariable logistic regression models were used to evaluate whether coagulation or inflammatory parameters drawn during initial clinical evaluation were predictive of bleeding events or thrombotic events diagnosed during hospitalization. These analyses were also performed to evaluate whether these parameters were predictive of critical illness or death. The thrombotic, bleeding, and critical illness models included all patients, and the mortality models included only patients reaching completion of hospitalization (discharge or death). Each parameter was evaluated in a univariable (unadjusted) model and a multivariable (adjusted) model controlling for age, sex, body mass index (BMI), baseline anticoagulation intensity (mechanical thromboprophylaxis only/standard prophylactic anticoagulation [supplemental Table 1], or intermediate/full-dose anticoagulation), and clinical risk factors previously demonstrated to impart a high risk for severe COVID-19: chronic lung disease, cardiovascular disease, immunocompromise, diabetes mellitus, chronic kidney disease requiring dialysis, chronic liver disease, and residence in a long-term care facility (see supplemental Table 3 for additional details regarding these risk factors). Laboratory parameters were not adjusted for one another. Thresholds used in models for each biomarker were selected based on a combination of biological relevance, laboratory reference ranges, and the distribution of the data.
Following these primary analyses, which included all thrombotic complications or bleeding events, any coagulation or inflammatory parameters significantly predictive of thrombotic complications or bleeding were evaluated further in sensitivity analyses. The thrombosis sensitivity analysis included only patients with radiographically confirmed DVT, PE, or arterial thrombosis, as well as myocardial infarction; the bleeding sensitivity analysis included only major bleeding events (WHO grade 3 or 4).
Critical illness was defined throughout the study as a requirement for endotracheal intubation and mechanical ventilation; this included patients for whom intubation was clinically indicated but who chose to forego it (those with a “do not intubate” status).
Statistical analysis software
Statistical analysis was performed, and graphs for figures were prepared, using Stata version 14.2 (StataCorp LLC, College Station, TX), GraphPad Prism 7 (GraphPad, Inc., San Diego, CA), and Microsoft Excel 360 (Microsoft Corp., Seattle, WA). Missing data were not imputed. Any results above the upper limit of the assay were entered as 1 unit higher than the assay upper limit value for all analyses using continuous variables. The threshold for statistical significance (P < .05) was not adjusted for multiple comparisons.
Results
Patient characteristics
Among 429 patients with a positive SARS-CoV-2 reverse-transcriptase polymerase chain reaction test and a D-dimer test obtained on initial evaluation, 400 were admitted to the hospital and included in the study (supplemental Figure 1). Table 1 lists baseline patient characteristics by severity group (noncritically ill and critically ill). The study included a total of 3226 patient-days (461 patient-weeks) analyzed, including 1608 patient-days (230 patient-weeks) in noncritically ill patients and 1618 patient-days (231 patient-weeks) in critically ill patients.
Table 1.
Baseline characteristics of patients included in the study (N = 400)
| Characteristic | Admitted, not critically ill (n = 256) | Critically ill (n = 144) |
|---|---|---|
| Age, mean (range), y | 60 (23-99) | 65 (32-97) |
| Female, % | 47.3 | 35.4 |
| Race | ||
| White | 148 (57.8) | 95 (66.0) |
| Black | 43 (16.8) | 17 (11.8) |
| Asian or Pacific islander | 13 (5.1) | 6 (4.2) |
| Other | 42 (16.4) | 15 (10.4) |
| Not specified | 10 (3.9) | 11 (7.6) |
| Ethnicity, Hispanic or Latino | 74 (28.9) | 42 (29.2) |
| Hospitalization status | ||
| Discharged | 205 (80.1) | 18 (12.5) |
| Still admitted at end of study period | 49 (19.1) | 99 (68.75) |
| Deceased | 2 (0.8) | 27 (18.75) |
| Hospital length of stay, mean (range), d | ||
| Hospitalization complete | 6 (1-24) | 9 (2-23) |
| Hospitalization ongoing | 8 (2-18) | 12 (3-33) |
| Baseline anticoagulation | ||
| None (mechanical thromboprophylaxis only) | 9 (3.5) | 2 (1.4) |
| Standard prophylactic anticoagulation* | 230 (89.8) | 124 (86.1) |
| Intermediate or full-dose anticoagulation† | 17 (6.6) | 18 (12.5) |
| BMI, kg/m2 | ||
| Underweight (<18.5) | 3 (1.2) | 0 (0.0) |
| Normal weight (18.5-24.9) | 55 (21.5) | 28 (19.5) |
| Overweight (25.0-29.9) | 95 (37.1) | 54 (37.5) |
| Class I obesity (30.0-34.9) | 56 (21.9) | 29 (20.1) |
| Class II obesity (35.0-39.9) | 29 (11.3) | 20 (13.9) |
| Class III obesity (≥40.0) | 18 (7.0) | 13 (9.0) |
| Risk factors for severe COVID-19 | ||
| Cardiovascular disease | 82 (32.0) | 43 (29.9) |
| Chronic lung disease | 53 (20.7) | 25 (17.4) |
| Diabetes mellitus | 65 (25.4) | 58 (40.3) |
| Immune compromise or suppression | 29 (11.3) | 20 (13.9) |
| Chronic kidney disease on dialysis | 4 (1.6) | 6 (4.2) |
| Chronic liver disease | 13 (5.1) | 6 (4.2) |
| Reside in nursing home or long-term care facility | 21 (8.2) | 7 (4.9) |
Unless otherwise noted, data are n (%).
Details are in supplemental Table 1.
Includes patients continuing on previously prescribed oral anticoagulants and patients with BMI <30 kg/m2 given subcutaneous enoxaparin at higher-than-standard dosing (eg, 40 mg, twice daily).
Thrombotic events and event rates
Venous thromboembolism
The rate of radiographically confirmed VTE was 4.8% (95% confidence interval [CI], 2.9-7.3, including 19 events in 19 patients), or 4.13 per 100 patient-weeks. This included a rate of 3.1% (95% CI, 1.4-6.1), or 3.49 per 100 patient-weeks, in noncritically ill patients and 7.6% (95% CI, 3.9-13.3), or 4.76 per 100 patient-weeks, in critically ill patients. The overall VTE rate (including suspected VTEs unable to be confirmed radiographically that met criteria for inclusion detailed in "Methods") was 6.0% (95% CI, 3.9-8.8; 24 events in 24 patients), or 5.22 per 100 patient-weeks. This included a rate of 3.5% (95% CI, 1.6-6.6), or 3.91 per 100 patient-weeks, in noncritically ill patients and 10.4% (95% CI, 5.9-16.6), or 6.49 per 100 patient-weeks, in critically ill patients.
Events confirmed on imaging included 4 patients with proximal lower extremity DVT, 2 with line-associated internal jugular DVT, 2 with extensive superficial venous thrombosis, 1 with extensive upper extremity DVT, 6 with proximal (lobar or segmental) PE, 1 with distal (subsegmental) PE, and 3 with both DVT and PE. Suspected VTE events included 2 patients with suspected DVT and 3 patients with suspected PE. All but 1 of the patients were receiving anticoagulation with standard prophylactic doses (supplemental Table 1) of UFH or LMWH at the time of the event; 1 patient was receiving therapeutic-dose apixaban at the time of the event. Two patients with VTE also had arterial thrombotic events. There were no fatal VTE events.
Arterial thrombosis
The arterial thrombosis rate was 2.8% (95% CI, 1.4-4.9; including 11 events in 11 patients), or 2.39 per 100 patient-weeks. This included a rate of 1.2% (95% CI, 0.2-3.4), or 1.30 per 100 patient-weeks, in noncritically ill patients and 5.6% (95% CI, 2.4-10.7), or 3.46 per 100 patient-weeks, in critically ill patients. Events included 9 patients with non-ST elevation myocardial infarction, 1 patient with unstable angina, and 1 patient with bilateral line–associated radial artery thromboses. All patients were receiving anticoagulation with prophylactic doses of UFH or LMWH at the time of the event. There were no fatal arterial thrombotic events.
Clinically significant nonvessel thrombotic complications
Of 12 critically ill patients placed on CVVH, 8 had recurrent clotting of the CVVH circuit while receiving prophylactic-dose anticoagulation, resulting in a change to therapeutic-dose heparin infusion; 2 had continued recurrent clotting of the circuit despite therapeutic-dose heparin infusion. Of note, 5 of these 8 patients also had venous or arterial thrombotic events. Of the 4 patients receiving CVVH who did not have recurrent clotting of the CVVH circuit, 3 were on a therapeutic-dose heparin infusion for other indications at the time the CVVH was initiated.
Two additional critically ill patients had recurrent thrombosis of central venous catheters and arterial lines necessitating repeat replacement of lines for ongoing care. There were no fatal complications of nonvessel thrombosis.
Overall thrombotic complication rate and management
The overall thrombotic complication rate was 9.5% (95% CI, 6.8-12.8; 45 events in 38 patients), or 9.78 per 100 patient-weeks. This included a rate of 4.7% (95% CI, 2.4-8.0), or 5.22 per 100 patient-weeks, in noncritically ill patients and a rate of 18.1% (95% CI, 12.1-25.3), or 14.29 per 100 patient-weeks, in critically ill patients. Forty-one patients (10%) were transitioned from prophylactic-dose to therapeutic-dose anticoagulation during admission to manage thrombotic complications and/or new-onset atrial fibrillation. Four patients with thrombotic complications also developed bleeding complications.
Bleeding events and event rates
Bleeding events
The overall bleeding rate was 4.8% (95% CI, 2.9-7.3; 21 bleeding events in 19 patients), or 4.57 per 100 patient-weeks. This included a rate of 3.1% (95% CI, 1.4-6.1), or 3.49 per 100 patient-weeks, in noncritically ill patients and 7.6% (95% CI, 3.9-13.3), or 5.63 per 100 patient-weeks, in critically ill patients. The major bleeding (WHO grade 3-4) rate was 2.3% (95% CI, 1.0-4.2), or 1.96 per 100 patient-weeks. All but 1 major bleed occurred in the critically ill, for a rate of 5.6% (95% CI, 2.4-10.7), or 3.46 per 100 patient-weeks. Events are described in detail in Table 2. One bleeding event, an intracranial hemorrhage, was fatal.
Table 2.
Bleeding events
| Patient sex | Age, y | Critically ill | Type(s) of bleeding | Platelet count at bleed(s), ×109/L | Anticoagulation at bleed(s) | WHO grade(s) | Comments |
|---|---|---|---|---|---|---|---|
| Male | 67 | Yes | GI | 47 | Therapeutic (UFH) | 2 | On anticoagulation for pulmonary embolus and clotting of CVVH circuit at time of bleed |
| Male | 59 | No | Hemoptysis | 134 | None | 1 | Recurrent |
| Male | 62 | Yes | GI | 242 | Prophylactic (UFH) | 3 | |
| Male | 37 | No | Hemoptysis | 426 | Prophylactic (enoxaparin) | 2 | |
| Male | 68 | Yes | Oral mucosa bleeding | 227 | Prophylactic (enoxaparin) | 2 | Recurrent |
| Male | 56 | No | Epistaxis | 208 | Prophylactic (enoxaparin) | 2 | Recurrent |
| Male | 49 | Yes | Epistaxis; bleeding from multiple cannulation sites | 115; 184 | Prophylactic (enoxaparin) for both events | 3; 1 | Epistaxis recurrent and required otolaryngology consult and prolonged packing; DIC |
| Male | 59 | Yes | Bleeding from multiple cannulation sites; intracranial hemorrhage | 155; 257 | Therapeutic (UFH) for both events | 1; 4 | Fatal intracranial hemorrhage; DIC |
| Female | 65 | Yes | Internal bleeding | 177 | Prophylactic (UFH) | 3 | Recurrent; strong clinical suspicion of internal bleeding due to rapidly declining hemoglobin (>2 g/dL drop each time requiring transfusion) with no other cause (unable to scan) |
| Male | 69 | Yes | Oropharyngeal bleeding from tongue mass | 414 | Prophylactic (UFH) | 2 | Recurrent; required consult from otolaryngology and prolonged oral packing. Tongue mass was a suspected, but not a known, cancer. |
| Male | 34 | No | Hemoptysis | 142 | Therapeutic (UFH) | 1 | |
| Female | 44 | Yes | Pulmonary hemorrhage | 47 | Therapeutic (UFH) | 3 | Anticoagulated with impella in place; DIC |
| Male | 79 | Yes | GI | 297 | Therapeutic (UFH) | 3 | |
| Male | 67 | No | Spontaneous right kidney hematoma | 157 | Prophylactic (enoxaparin) | 2 | |
| Male | 59 | Yes | GI | 1 | None | 3 | Developed COVID-19–associated immune thrombocytopenia 3 d prior to bleed |
| Male | 83 | No | GI | 46 | Warfarin | 2 | INR: 6.5 at time of bleed |
| Male | 84 | No | GI | 66 | Warfarin | 2 | INR: 1.5 at time of bleed |
| Male | 70 | No | GI | 262 | Clopidogrel | 3 | |
| Male | 65 | Yes | GI | 59 | Prophylactic (enoxaparin) | 3 |
GI, gastrointestinal.
Disseminated intravascular coagulation
Three patients were diagnosed with DIC on the basis of clinical and laboratory evidence of DIC13; all had grade 3 or 4 bleeding events (Table 2). Seven patients (1.8%) had fibrinogen <200 mg/dL at any point during their hospitalization; of these, 5 had a fibrinogen nadir between 100 and 150 mg/dL, and 2 had a nadir <100 mg/dL. One of the patients with a fibrinogen nadir <100 mg/dL had known liver cirrhosis, not DIC, and was not critically ill.
Thrombocytopenia
Forty-one patients (10.3%) and 10 patients (2.5%) had a platelet count <100 × 109/L and <50 × 109/L during their hospital course, respectively. Four with a platelet count <50 × 109/L had grade ≥2 bleeding events (Table 2).
Coagulation and inflammatory parameter analyses
Table 3 lists the initial, peak, and minimum values for the 11 evaluated markers in patients with thrombotic complications, bleeding complications, or neither type of complication. Compared with patients without bleeding or thrombotic complications, the thrombotic complications group had consistently higher D-dimer, fibrinogen, CRP, ferritin, and procalcitonin, whereas the bleeding complications group had higher procalcitonin and peak D-dimer and lower platelet counts.
Table 3.
Coagulation markers, inflammatory markers, and high-sensitivity cardiac troponin by patient group
| Marker | No thrombotic or bleeding complication (n = 347)* | Thrombotic complication (n = 38)† | P (no complication vs thrombotic complication)‡ | Bleeding complication (n = 19)§ | P (no complication vs bleeding complication)‡ |
|---|---|---|---|---|---|
| D-dimer, ng/mL | |||||
| Initial | 891 (568-1503) | 1538 (953-3288) | .0002 | 1189 (788-2577) | .083 |
| Minimum | 760 (494-1189) | 1336 (833-1681) | .0006 | 928 (605-1620) | .17 |
| Peak | 1377 (818-3052) | 4001 (2896-8821) | <.0001 | 3625 (2135-4783) | .0004 |
| PT, s¶ | |||||
| Initial | 13.9 (13.1-14.8) | 13.8 (13.2-14.6) | .99 | 14.0 (13.3-14.8) | .44 |
| Minimum | 13.6 (12.9-14.3) | 13.5 (12.9-14.3) | .80 | 13.9 (13.0-14.3) | .51 |
| Peak | 14.4 (13.5-15.8) | 16.0 (14.7-16.8) | .0001 | 16.3 (14.6-17.4) | .011 |
| INR | |||||
| Initial | 1.1 (1.0-1.2) | 1.1 (1.0-1.2) | .94 | 1.1 (1.0-1.2) | .58 |
| Minimum | 1.1 (1.0-1.1) | 1.1 (1.0-1.1) | .68 | 1.1 (1.0-1.2) | .43 |
| Peak | 1.1 (1.1-1.3) | 1.3 (1.2-1.4) | .0002 | 1.3 (1.2-1.4) | .017 |
| PTT, s|| | |||||
| Initial | 34.3 (30.8-39.1) | 34.3 (31.5-38.5) | .88 | 36.5 (30.4-38.4) | .72 |
| Minimum | 32.8 (29.6-36.3) | 30.6 (29.1-38.7) | .61 | 31.6 (28.3-37.3) | .80 |
| Peak | 37.0 (32.5-43.5) | 38.1 (34.0-47.8) | .56 | 47.5 (39.1-60.8) | .059 |
| Fibrinogen, mg/dL | |||||
| Initial | 579 (481-696) | 696 (535-849) | .0045 | 682 (405-838) | .50 |
| Minimum | 549 (463-663) | 669 (528-753) | .0028 | 486 (312-712) | .61 |
| Peak | 662 (504-760) | 828 (666-976) | .0001 | 703 (497-1081) | .19 |
| Platelet count, ×109/L | |||||
| Initial | 188 (153-233) | 206 (161-274) | .083 | 157 (132-220) | .072 |
| Minimum | 163 (130-210) | 179 (149-238) | .091 | 124 (95-154) | .0005 |
| Peak | 270 (197-366) | 283 (243-385) | .12 | 267 (153-353) | .23 |
| CRP, mg/L | |||||
| Initial | 63.3 (25.6-139.3) | 124.7 (55.5-163.1) | .0011 | 46.6 (15.5-219.1) | .91 |
| Minimum | 35.4 (12.7-73.7) | 94.2 (46.2-134.7) | <.0001 | 21.4 (4.9-120.3) | .64 |
| Peak | 130.3 (54.2-216.0) | 277.7 (150.3-338.4) | <.0001 | 148.4 (88.6-301.0) | .18 |
| ESR, mm/h | |||||
| Initial | 38 (23-58) | 47 (38-63) | .020 | 21 (14-52) | .22 |
| Minimum | 36 (21-56) | 43 (34-59) | .079 | 21 (10-44) | .068 |
| Peak | 56 (35-97) | 91 (54-124) | .0077 | 65 (21-115) | .87 |
| Ferritin, µg/L | |||||
| Initial | 504 (253-1007) | 825 (594-1249) | .015 | 739 (289-1305) | .32 |
| Minimum | 453 (235-834) | 750 (554-1128) | .0056 | 696 (289-901) | .49 |
| Peak | 707 (348-1358) | 1182 (697-2081) | .0020 | 1075 (533-1722) | .13 |
| Procalcitonin, ng/mL | |||||
| Initial | 0.13 (0.08-0.26) | 0.23 (0.13-0.43) | .0040 | 0.20 (0.17-0.58) | .0046 |
| Minimum | 0.13 (0.07-0.22) | 0.22 (0.12-0.43) | .0033 | 0.20 (0.17-0.58) | .0031 |
| Peak | 0.15 (0.08-0.37) | 0.55 (0.16-2.72) | <.0001 | 0.58 (0.19-3.48) | .0002 |
| High-sensitivity cardiac troponin, ng/L | |||||
| Initial | 10 (0-23) | 16 (8-29) | .051 | 14 (11-41) | .022 |
| Minimum | 9 (0-19) | 16 (8-24) | .021 | 13 (8-39) | .033 |
| Peak | 6 (13-32) | 54 (18-118) | <.0001 | 35 (16-77) | .0003 |
Bold P values represent statistical significance.
All data are median (interquartile range). Four patients had bleeding and thrombotic complications and were included in each group. For additional details regarding assays and reference ranges, see supplemental Table 2.
Of 347 patients, initial data were available for D-dimer in all patients; platelet count data were available for all patients; CRP data were available for 343 patients, high-sensitivity cardiac troponin data were available for 342 patients, ferritin data were available for 320 patients, procalcitonin data were available for 314 patients, ESR data were available for 262 patients, PT data were available for 260 patients, INR data were available for 260 patients, PTT data were available for 173 patients, and fibrinogen data were available for 151 patients. Peak and minimum values are included only for patients with initial values to ensure that the same population of patients contributed to each measure. Twenty-three patients were excluded from PT and INR analyses because of the use of warfarin or direct oral anticoagulants during all measurements, and 20 patients were excluded from PTT analyses because of the use of direct oral anticoagulants or heparin infusion during all measurements.
Of 38 patients, initial D-dimer data were available for all patients, platelet count data were available for all patients, CRP data were available for 35 patients, high-sensitivity cardiac troponin data were available for 35 patients, ferritin data were available for 35 patients, procalcitonin data were available for 34 patients, PT data were available for 34 patients, INR data were available for 34 patients, PTT data were available for 32 patients, fibrinogen data were available for 29 patients, and ESR data were available for 27 patients. Peak and minimum values are included only for patients with initial values to ensure that the same population of patients contributed to each measure. Two patients were excluded from PT, INR, and PTT analyses because of the use of apixaban during all measurements.
Mann-Whitney test.
Of 19 patients, initial data were available for D-dimer, platelet count, CRP, ferritin, and high-sensitivity cardiac troponin in all patients; procalcitonin data were available for 17 patients; PT data were available for 18 patients; INR data were available for 18 patients; fibrinogen data were available for 16 patients; PTT data were available for 15 patients; and ESR data were available for 15 patients. Peak and minimum values are included only for patients with initial values to ensure that the same population of patients contributed to each measure.
Individual measurements taken while receiving warfarin or direct oral anticoagulants were excluded from analysis.
Individual measurements taken while receiving heparin infusion or direct oral anticoagulants were excluded from analysis.
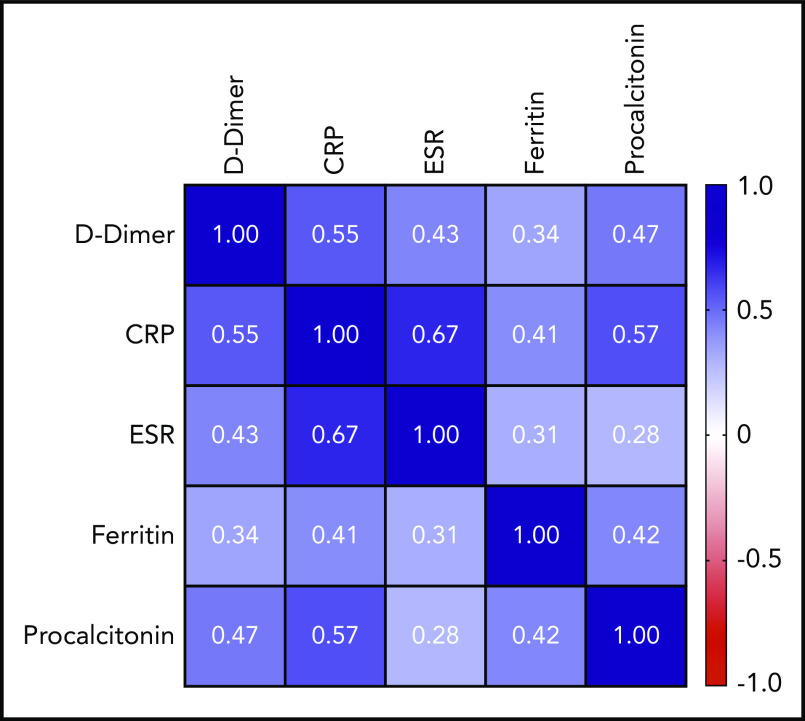
Peak D-dimer was moderately positively correlated with peak CRP (r = +0.55; 95% CI, 0.47-0.62; P < .0001), peak ESR (r = +0.43; 95% CI, 0.33-0.52; P < .0001), and peak procalcitonin (r = +0.47; 95% CI, 0.38-0.55; P < .0001) and weakly positivelylated with peak ferritin (r = +0.34; 95% CI, 0.25-0.43; P < .0001). A complete correlation matrix for these parameters is illustrated in Figure 1.
Figure 1.
Correlation matrix showing the strength of correlation between peak values of D-dimer and evaluated inflammatory parameters. Values in cells are Spearman correlation coefficients. All correlations were statistically significant (P < .0001). 95% CIs for each correlation coefficient can be found in supplemental Table 6.
Predictors of bleeding events, thrombotic events, critical illness, and death
Elevations in D-dimer, platelet count, CRP, and ESR at initial presentation were predictive of thrombotic complications during hospitalization (Figure 2); in multivariable analysis, D-dimer of 1001 to 2500 ng/mL had an odds ratio (OR) for thrombotic complications of 3.04 (95% CI, 1.26-7.31), and a D-dimer >2500 ng/mL had an OR of 6.79 (95% CI, 2.39-19.30; P < .001). Thrombocytopenia (platelet count <150 × 109/L) and elevations in D-dimer >2500 ng/mL at initial presentation were also predictive of bleeding complications during hospitalization (Figure 3) (in multivariable analysis, for platelet count <150 × 109/L: OR, 2.90; 95% CI, 1.05-7.99; and for D-dimer >2500 ng/mL: OR, 3.56; 95% CI, 1.01-12.66). Elevations in D-dimer, CRP, ESR, ferritin, procalcitonin, and high-sensitivity cardiac troponin at initial presentation were predictive of critical illness during hospitalization in multivariable analysis (Figure 4). Elevations in D-dimer, PT, activated PTT, fibrinogen, CRP, ESR, and procalcitonin at initial presentation were predictive of death during hospitalization in multivariable analysis (Figure 5).
Figure 2.
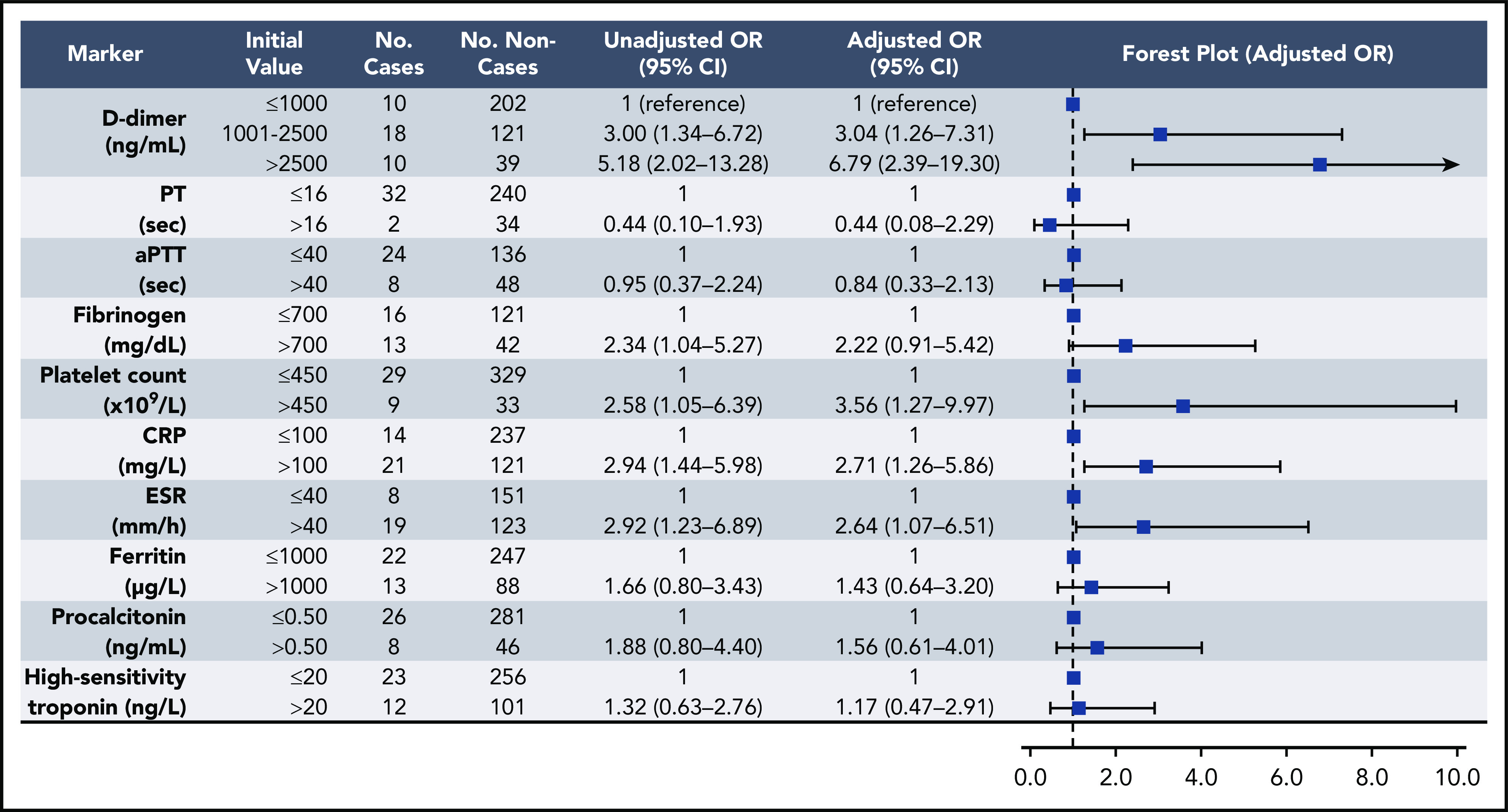
Association of coagulation and inflammatory parameters at initial presentation with thrombotic complications during hospitalization in univariable and multivariable analyses. aPTT, activated PTT.
Figure 3.
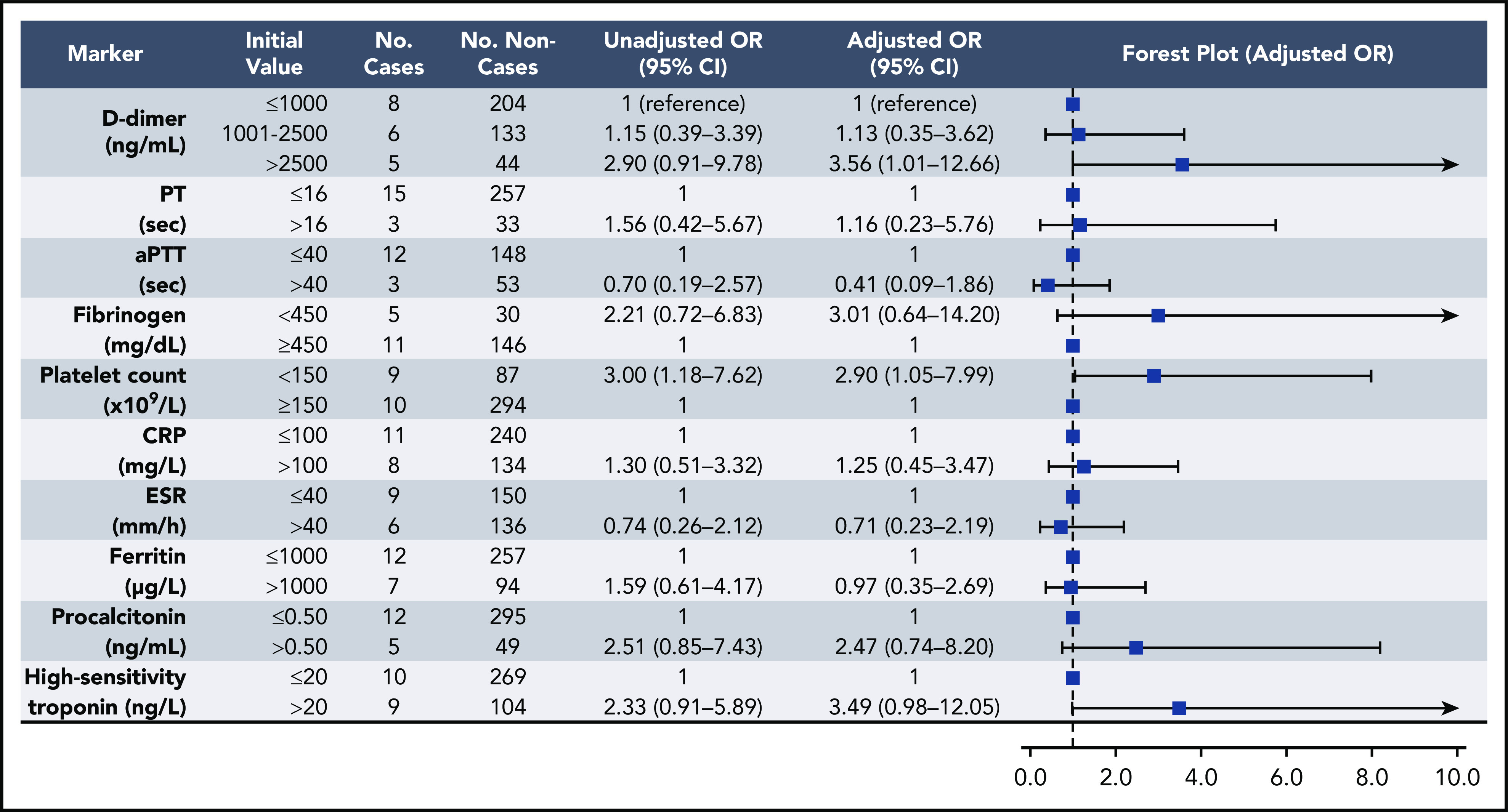
Association of coagulation and inflammatory parameters at initial presentation with bleeding events during hospitalization in univariable and multivariable analyses. aPTT, activated PTT.
Figure 4.
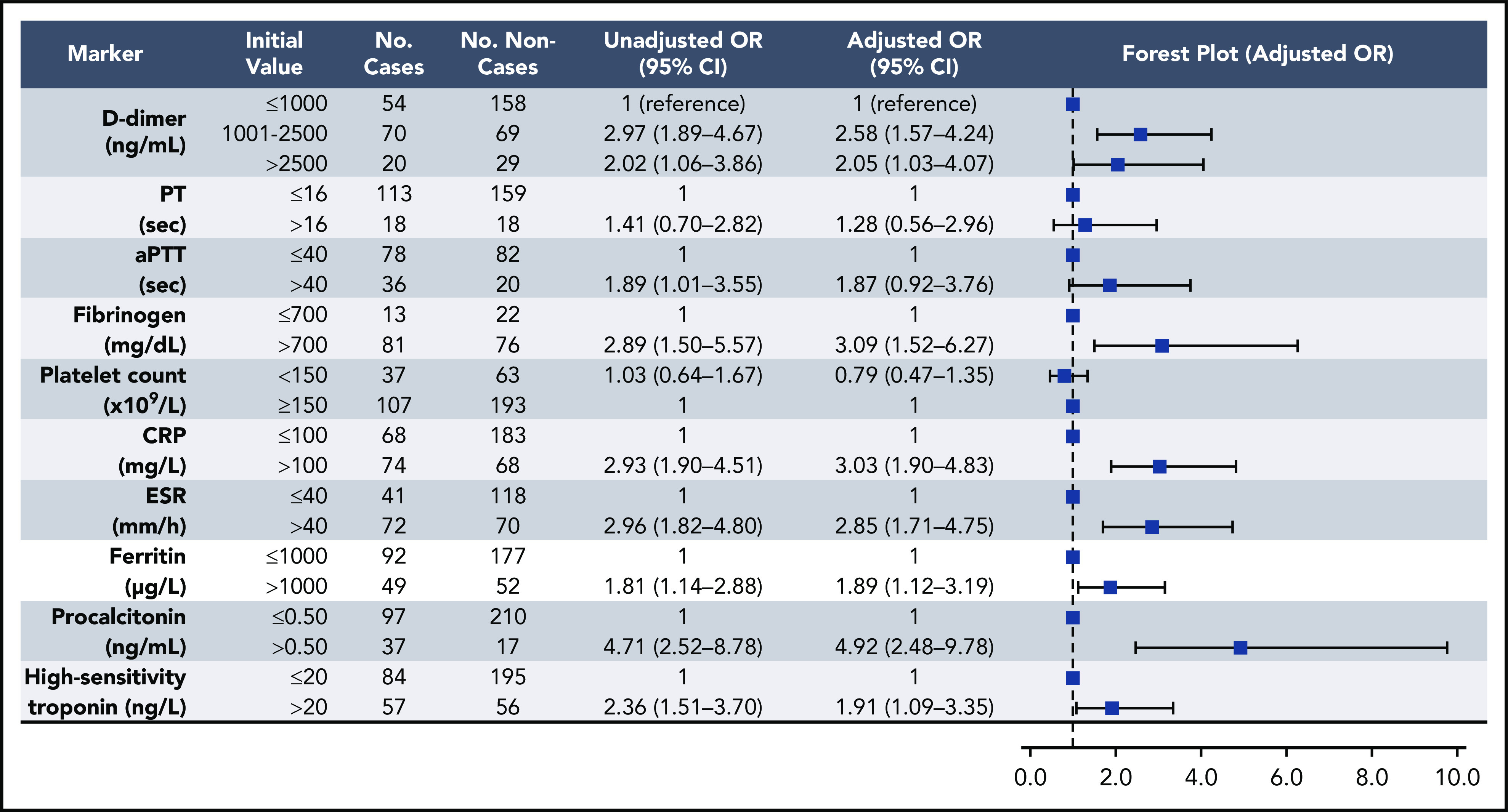
Association of coagulation and inflammatory parameters at initial presentation with critical illness in univariable and multivariable analyses. aPTT, activated PTT.
Figure 5.
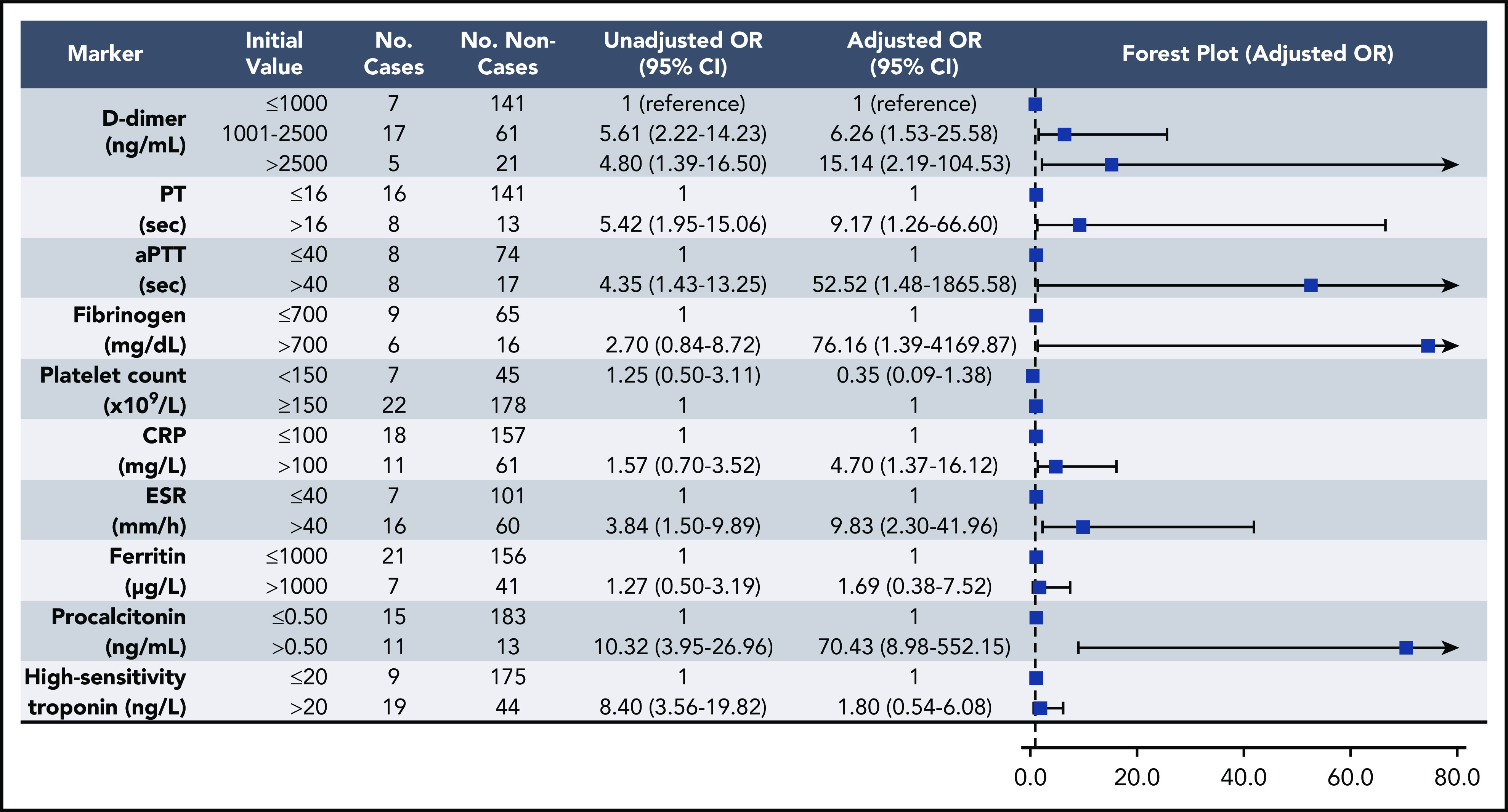
Association of coagulation and inflammatory parameters at initial presentation with mortality during hospitalization in univariable and multivariable analyses. Analysis was limited to those patients reaching a terminal end point (hospital discharge or death) by the end of the study period (n = 252). aPTT, activated PTT.
In the thrombosis sensitivity analysis, D-dimer, platelet count, and CRP elevations on presentation were similarly predictive of thrombotic complications in multivariable analysis (supplemental Table 4). In the bleeding sensitivity analysis, when limiting cases to major bleeds only, thrombocytopenia at presentation was still predictive of bleeding in multivariable analysis but D-dimer >2500 ng/mL was no longer a statistically significant predictor (OR, 4.73; 95% CI, 0.85-26.21; P = .076) (supplemental Table 5).
Discussion
In this multicenter study, we report the hemostatic manifestations and bleeding and thrombotic complications of 400 COVID-19 patients. In a population managed with standard doses of prophylactic anticoagulation, we found a radiographically confirmed venous thromboembolic rate of 4.8% (7.6% in critically ill patients), far lower than other published studies from China6 or Europe7 and more consistent with another study from the United States.14 Including events unconfirmed on imaging but with objective clinical findings that were managed as VTE, arterial thrombotic events, and clinically significant nonvessel thrombotic complications, such as CVVH circuit thrombosis, we found an overall thrombotic complication rate of 9.5%. In contrast, the observed overall bleeding rate was 4.8% (7.6% in the critically ill), with a major bleeding rate of 2.3% (5.6% in the critically ill, including 1 fatal bleed). Although occult thromboembolic events may be possible or even likely in critically ill COVID-19 patients who are unable to undergo diagnostic imaging, given the observed bleeding rates, our data suggest that empiric intensification of anticoagulation in even critically ill COVID-19 patients, beyond that of the general standard of care, should be pursued with caution. Our findings suggest that any potential benefit of anticoagulation doses beyond the general standard of care in patients with COVID-19 is best evaluated in a randomized study. The one current exception suggested by our findings are those patients receiving renal replacement therapy with CVVH who experience multiple episodes of circuit failure due to coagulation. Several randomized studies comparing the intensity of prophylactic anticoagulation in patients with COVID-19 have been listed on clinicaltrials.gov, and several (NCT04359277, NCT04362085, NCT04345848, NCT04366960) have begun enrollment. In addition to thrombosis prevention, outcomes under evaluation include all-cause mortality and incidence of other complications, such as respiratory failure, shock, and renal injury.
Contrary to the findings of at least one other published study of COVID-19 patients,5 we found a very low rate of DIC (2% of critically ill patients) using a combination of clinical assessment and laboratory parameters, as defined by the International Society for Thrombosis and Haemostasis.13 D-dimer levels were increased far out of proportion to any abnormalities in the PT/INR, activated PTT, fibrinogen level, or platelet count (Table 3); these findings are uncharacteristic of DIC as currently understood.13,15 It is worth noting that elevated levels of D-dimer are common in hospitalized and critically ill patients more generally16-18 and are not routinely measured and scrutinized on a daily basis in other patients as is currently being done in patients with COVID-19. We observed that clinically relevant thrombocytopenia was rare, and reductions in fibrinogen below the assay reference range were extremely rare. However, when these hemostatic defects did occur, major bleeding was common (Table 2).
We additionally found that elevations in D-dimer on admission predicted critical illness and death, as well as bleeding and thrombotic complications. Inflammatory markers, including CRP and ESR, were also associated with thrombosis (Figure 2), and elevations in several coagulation and inflammatory markers were associated with critical illness and mortality (Figures 4-5), albeit with a high degree of uncertainty in the mortality models, as manifested by very wide confidence intervals. Acute inflammation, as measured by elevations in these markers, has previously been associated with increased thrombotic and bleeding risk in patients without COVID-1919,20; indeed, we found significant correlations between D-dimer levels and each measured inflammatory marker (Figure 1). Given previously reported 14-day cumulative VTE rates of 7% to 8% in critically ill patients without COVID-19 receiving standard heparin-based thromboprophylaxis,21 our observed rate of 7.6% in critically ill patients receiving similar prophylactic treatment over a median follow-up of 10 days was comparable. Likewise, to contextualize our major bleeding rate of 5.6% in the critically ill, a large prospective study of bleeding events in 3746 critically ill patients without COVID-19 identified major bleeding (defined similarly as in our study) in 208 patients (5.6%).22 Given these findings, the question arises as to whether the underlying cause of the elevated D-dimer levels and bleeding and thrombotic manifestations are due to a pathophysiologically distinct viral coagulopathy or simply related to coagulation system activation in the setting of severe inflammation.
The limitations of this retrospective observational study are evident. Without a uniform protocol to image all patients with suspected VTE, thrombotic events may have been missed. Conversely, despite relatively stringent criteria for inclusion, the included suspected VTE events that were not confirmed radiologically may not have been true VTE events. Low-grade bleeds considered relatively trivial may not have been documented in the medical record. In the absence of routine bronchoscopy, pulmonary bleeding, including focal or diffuse alveolar hemorrhage, could have been missed and misinterpreted as worsening pulmonary infection or inflammation. Although the study included 400 patients with confirmed COVID-19, bleeds and thrombotic events occurred in a small number of patients, resulting in wider confidence intervals for the ORs describing associations with these outcomes. Laboratory data were not uniformly available for every evaluated laboratory parameter and were not collected in each patient according to a standardized timing or protocol.
In conclusion, we observed that COVID-19 was associated with similar rates of thrombosis and bleeding as seen in hospitalized patients with similar degrees of critical illness. Elevated D-dimer levels at initial presentation predicted bleeding complications, thrombotic complications, critical illness, and death. Beyond D-dimer, thrombosis was primarily associated with inflammatory markers rather than coagulation parameters. Randomized clinical trials are necessary to determine the optimal dose and course of thromboprophylaxis in patients with COVID-19.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Elizabeth Van Cott for providing laboratory test information found in supplemental Table 2.
H.A.-S. is the recipient of the National Hemophilia Foundation-Shire Clinical Fellowship Award, the Harvard Catalyst Medical Research Investigator Training Award, and the American Society of Hematology Scholar Award.
Footnotes
For original data, please contact Hanny Al-Samkari (hal-samkari@mgh.harvard.edu).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.A.-S. conceived and designed the study, collected and analyzed data, created the tables and figures, and wrote the first draft of the manuscript; W.H.D. and D.J.K. conceived and designed the study; J.C.T.C. designed the visual abstract; R.P.R. conceived and designed the study and collected and analyzed data; and all authors critically reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: H.A.-S. has acted as a consultant for Agios, Dova, and Moderna and has received research funding from Agios, Dova, and Amgen. D.J.K. has received research funding from Protalex, Bristol-Myers Squibb, Rigel, Bioverativ, Agios, Syntimmune, Principia, and Alnylam and has acted as a consultant for ONO, Pfizer, 3SBios, Eisai, GlaxoSmithKline, Genzyme, Shire, Amgen, Shionogi, Rigel, Syntimmune, MedImmune, Novartis, Alexion, Bioverativ, Argenx, Zafgen, Fujifilm, Principia, Kyowa Kirin, Takeda, and Platelet Disorders Support Association. R.P.R. has acted as a consultant for Bristol-Myers Squibb, Dova, Janssen, and Portola and has received research funding from Bristol-Myers Squibb and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Hanny Al-Samkari, Division of Hematology Oncology, Massachusetts General Hospital, Zero Emerson Pl, Suite 118 Office 112, Boston, MA 02114; e-mail: hal-samkari@mgh.harvard.edu.
REFERENCES
- 1.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans [published online ahead of print 27 May 2020]. Lancet Respir Med. doi:10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online ahead of print 10 April 2020]. Thromb Res. doi:10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up [published online ahead of print 15 April 2020]. J Am Coll Cardiol. doi:10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obi AT, Barnes GD, Wakefield TW, et al. Practical diagnosis and treatment of suspected venous thromboembolism during COVID-19 pandemic [published online ahead of print 17 April 2020]. J Vasc Surg Venous Lymphat Disord. doi:10.1016/j.jvsv.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? [published online ahead of print 29 April 2020]. Thromb Haemost. doi:10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207-214. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. ; AABB . Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205-213. [DOI] [PubMed] [Google Scholar]
- 13.Taylor FB Jr., Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) . Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327-1330. [PubMed] [Google Scholar]
- 14.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City [published online ahead of print 17 April 2020]. N Engl J Med. doi:10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toh CH, Hoots WK; SSC on Disseminated Intravascular Coagulation of the ISTH . The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007;5(3):604-606. [DOI] [PubMed] [Google Scholar]
- 16.Clark CL, Shams AH, Chang AM, et al. D-dimer in acute medically ill adults and current thromboprophylaxis: a multicenter observational study evaluating the prevalence of elevated D-dimer in acute medically ill, hospitalized adults and current thromboprophylaxis trends; the DAMIACT Study, initial data analysis. Res Pract Thromb Haemost. 2017;1(suppl 1):567-568. [Google Scholar]
- 17.Goldhaber SZ. The perils of D-dimer in the medical intensive care unit. Crit Care Med. 2000;28(2):583-584. [DOI] [PubMed] [Google Scholar]
- 18.Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121(4):1262-1268. [DOI] [PubMed] [Google Scholar]
- 19.Grimnes G, Isaksen T, Tichelaar YIGV, Brox J, Brækkan SK, Hansen JB. C-reactive protein and risk of venous thromboembolism: results from a population-based case-crossover study. Haematologica. 2018;103(7):1245-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamanaka Y, Sotomi Y, Hirata A, et al. Persistent systemic inflammation is associated with bleeding risk in atrial fibrillation patients. Circ J. 2020;84(3):411-418. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Zhang Z, Mi J, et al. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore). 2019;98(23):e15833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauzier F, Arnold DM, Rabbat C, et al. Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis. Intensive Care Med. 2013;39(12):2135-2143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.