Abstract
Background
Until June 23th 2020, 9,195,635 laboratory-confirmed cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection have been reported worldwide, including 473,127 deaths. Bacterial infection is the main cause of sepsis, however, sepsis caused by virus is often ignored. Increased awareness, early recognition of viral sepsis, rapid administration of appropriate antiviral drugs, and urgent treatment can significantly reduce deaths of viral sepsis.
Objectives
Given the rapid global spread of novel Corona Virus Disease (COVID-19), coupled with the high rate of missed diagnosis of viral sepsis caused by SARS-CoV-2 infection, it is urgent to evaluate the multiple organ failure score and viral sepsis in COVID-19 patients, so as to determine the clinical characteristics of viral sepsis more accurately and reveal the risk factors related to mortality.
Methods
Here we provide a full description of three cases of viral sepsis and subsequent multiple organ dysfunction (MODS) caused by SARS-CoV-2 infection imported to Guiyang from Wuhan.
Results
We analyzed complete laboratory examination, imaging data and treatment methods for the patients and assessed Sepsis-related Organ Failure Assessment score (SOFA score) and Multiple organ dysfunction scores (MOD score) daily, aimed to elucidate the clinical feature of viral sepsis and MODS and to attract enough attention by clinicians.
Conclusions
Therefore, we strongly suggest to daily evaluate SOFA score and MOD score in severe and critically-ill COVID-19 patients, so as to early diagnose and prevention of sepsis and MODS.
Given the rapid global spread of novel Corona Virus Disease (COVID-19), coupled with the high rate of missed diagnosis of viral sepsis caused by SARS-CoV-2 infection, it is urgent to evaluate the multiple organ failure score and viral sepsis in COVID-19 patients, so as to determine the clinical characteristics of viral sepsis more accurately and reveal the risk factors related to mortality. Here we provide a full description of three cases of viral sepsis and subsequent multiple organ dysfunction (MODS) caused by SARS-CoV-2 infection imported to Guiyang from Wuhan. We analyzed complete laboratory examination, imaging data and treatment methods for the patients and assessed Sepsis-related Organ Failure Assessment score (SOFA score) and Multiple organ dysfunction scores (MOD score) daily, aimed to elucidate the clinical feature of viral sepsis and MODS and to attract enough attention by clinicians. Therefore, we strongly suggest to daily evaluate SOFA score and MOD score in severe and critically-ill COVID-19 patients, so as to early diagnose and prevention of sepsis and MODS.
Keywords: Viral sepsis, Septic shock, Multiple organ dysfunction, Corona Virus Disease, Severe Acute Respiratory Syndrome Coronavirus 2, Sepsis-related Organ Failure Assessment
Highlights
-
•
Given the rapid global spread of COVID-19, coupled with the high rate of missed diagnosis of viral sepsis caused by SARS-CoV-2 infection, it is essential to evaluate viral sepsis, septic shock and organ functions in COVID-19 patients. Daily evaluate SOFA score and MOD score is suggested so as to early diagnose and prevention of sepsis and MODS.
1. Introduction
A novel Corona Virus Disease (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has occurred in Wuhan, Hubei, China since December 2019 [1]. SARS-CoV-2 virus is a novel beta coronavirus based on gene sequencing [2]. By June 23th 2020, there are 9,195,635 laboratory or clinical confirmed cases in more than 100 countries, 473,127 people have lost their lives. Compared with the 10% death rate of SARS-CoV [3] and 37% death rate of MERS-CoV [4], SARS-CoV-2 has a lower death rate of 2% in China [5]. More and more evidences show that COVID-19 spreads from person to person in hospital and family settings [[6], [7], [8]], the WHO had announced that COVID-19 was a global pandemic. The main clinical symptoms of COVID-19 patients are fever, cough, fatigue or myalgia, sputum production, headache, diarrhea and haemoptysis were less common symptoms. About 50% patients developed dyspnea, among which one third were admitted to ICU [9], while the severe patients often have dyspnea after one week, which rapidly progress to acute respiratory distress syndrome (ARDS), sepsis and multiple organ dysfunction (MODS) [10].
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [11]. Sepsis can be caused by a variety of pathogens. Bacterial infection is the main cause of sepsis. However, as high as 42% of sepsis patients showed culture negative, suggesting a non-bacterial cause [12]. Although almost any virus can lead to sepsis in susceptible patients, the clinical diagnosis of viral sepsis is very rare. Increased awareness, early recognition of viral sepsis, rapid administration of appropriate antiviral drugs, and urgent treatment can significantly reduce deaths of viral sepsis [13].
Given the rapid global spread of COVID-19, coupled with the high rate of missed diagnosis of viral sepsis caused by SARS-CoV-2, it is urgent to evaluate viral sepsis and multiple organ failure score in COVID-19 patients, so as to determine the clinical characteristics of viral sepsis more accurately and reveal the risk factors related to mortality. In our study, we collected fifteen confirmed cases of COVID-19 caused by SARS-CoV-2 infection imported to Guiyang from Wuhan, among which, three cases (20%) were severe or critically-ill patients with viral sepsis. We provided a full description of laboratory examinations, imaging data and treatment methods of the patients, aim to attract enough attention by clinicians and provide treatment experience of viral sepsis.
2. Methods
2.1. Patient and procedures
Fifteen patients were admitted to the Affiliated Hospital of Guizhou Medical University and Renmin Hospital of Guizhou province. They were confirmed to be infected with SARS-CoV-2 by Guizhou Center for Disease Control and Prevention (CDC). The study was performed in accordance with guidelines approved by the Ethics Committees from the Affiliated Hospital of Guizhou Medical University and Renmin Hospital of Guizhou province, and verbal informed consents were obtained from all patients or patients' family members.
2.2. Data collection
Laboratory test were collected upon hospitalization, including blood gas analysis (arterial oxygen pressure / oxygen index, PO2 / FiO2), standard blood counts (white blood cells, lymphocytes, platelet), blood biochemistry (bilirubin, creatinine, electrolyte and etc.), coagulation function (prothrombin time, fibrinogen), procalcitonin, C-reactive protein, erythrocyte sedimentation rate, and myocardial enzyme spectrum (Creatine kinase, creatine kinase isoenzyme and etc.). To determine the percentage of peripheral T cells positive for CD4 or CD8, flow cytometry was used in the detection. Additional data collected included Glasgow coma scale score, computed tomographic (CT) scans and treatment regimens.
2.3. Evaluation of sepsis and MODS
Sepsis is defined as an acute change in total Sepsis-related Organ Failure Assessment (SOFA) score greater than or equal to 2 points consequent to the infection. Septic shock is identified by persisting hypotension requiring vasopressors to maintain mean arterial pressure (MAP) above 65 mmHg and serum lactate level > 2 mmol/L despite adequate volume resuscitation [11]. MOD scores are evaluated each hospitalization day. Briefly, pulmonary, renal, hepatic, neurologic, cardiac and hematologic were scored from 0 to 4 every day. The MOD scores ranged from 0 to 4 and the total score from 0 to 24 (six organs). Failure of organ function was considered as three or more points longer than two consecutive days [14].
3. Results
3.1. General information and imaging examinations
Fifteen patients were all diagnosed as COVID-19 as detected of SARS-CoV-2 virus nucleic acid by RT-PCR. The primers of CoV were as follows: F: 5′-ACTTCTTTTTCTTGCTTTCGTGGT-3′; R: 5′-GCAGCAGTACGCACACAATC-3′. Conditions for the amplifications were: 50 °C for 15 min, 95 °C for 3 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s. All patients had lived, traveled to Wuhan city or had contacted with others came back from Wuhan. Among the fifteen patients, three were severe or critically-ill patients (20.0%). Case 01 was a 83-year-old man who had basic diseases including hypertension, chronic obstructive pulmonary disease (COPD) and tuberculous pleurisy. He returned to Guiyang from Wuhan by air on Jan. 21st and was admitted on Feb. 7th after showing initial symptoms of fever and fatigue. The patient was cured and discharged on Feb 24th. Case 02 was a 61-year-old woman with type 2 diabetes and hypertension. She was admitted with cough, fever and diarrhea as initial symptoms after contacting her sister who returned to Guiyang from Wuhan on Jan. 23rd. The patient was cured and discharged on Feb 24th. Case 03 was a 33-year-old man without basic disease. He came to Guiyang from Wuhan on Jan. 15th by air and was admitted with cough, fever, asthenia, body ache, shortness of breath and dyspnea as initial symptoms on Jan. 21st. He was admitted to a county-level hospital on Jan. 22nd. Due to the aggravation of dyspnea and the oxygenation decrease, the patient was transferred to the Affiliated Hospital of Guizhou Medical University on Jan. 31st for further treatment. The patient died on Feb. 5th, due to respiratory failure and septic shock. Imaging examinations of all 3 cases showed typical features of viral pneumonia. CT scan of Case 01 and Case 02 were obtained on 2nd day and 14th day post admission Case 03 only had a bedside chest radiograph (Fig. 1 ).
Fig. 1.
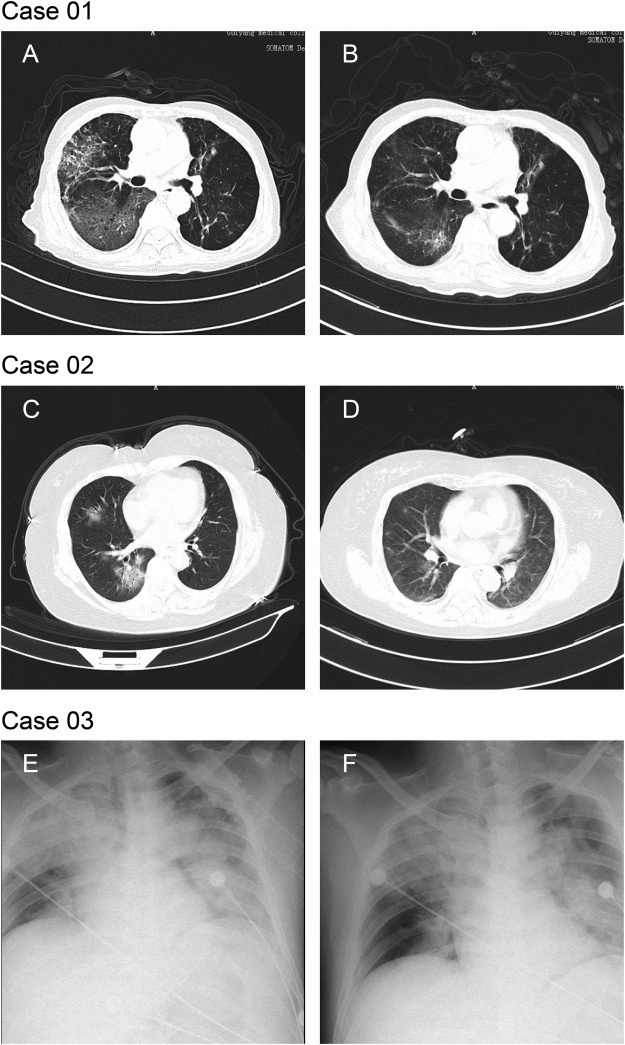
Chest CTs and x-rays of three patients
Case 01: Chest CTs was obtained on 2nd and 14th day post admission. CT findings of Case 01 obtained on 2nd day post admission showed multiple mottling and groundglass opacities (GGO) in bilateral lungs, predominantly involving right lobes. Septal thickening and extensive consolidation in the right middle and lower lobes presented a “paving stone like” reticulation (1A). Chest CT images for 14th day post admission showed improved status with bilateral ground-glass opacity, whereas partial consolidation had been resolved (1B).
Case 02: Chest CTs was obtained on 2nd and 14th day post admission. CT scan of Case 02 obtained on 2nd day post admission showed mixed multifocus GGO and consolidation in basal segment of right lower lobe, and GGO with minimal reticulation in right middle lobe (1C). CT scan for 14th day post admission showed healing of the consolidation and GGO, remaining linear opacities (1D).
Case 03: Chest x-rays was obtained on the first day post admission. The brightness of both lungs was diffusely decreased and extensive patchy shadows were observed, edges were blurred and the heart shadow enlarged slightly. Right diaphragmatic surface was light and smooth, costal diaphragmatic angle was sharp, while left diaphragmatic surface and costal diaphragmatic angle blunted (1E and 1F).
3.2. Treatment regimen
All patients were given mask oxygen inhalation and ventilator to assist breathing immediately after admission to improve the situation of hypoxia. Abidol hydrochloride tablets, interferon alfa-2b, ribavirin, Lianhuaqingwen combined with lopinavir plus ritonavir or chloroquine phosphate were given as antiviral therapy. Thymalfasin and γ-immunoglobulin were administered to enhance immunity. Methylprednisolone and Xuebijing was intravenously dripped based on disease severity to suppress cytokine storm according to the fifth edition guidelines for new coronavirus pneumonia. Meanwhile, empirical antibiotic agents were applied to prevent secondary bacterial and fungal infections. Case 03 was given adjusted dose of heparin to maintain activated clotting time (ACT) of whole blood at about 200 s because the use of extracorporeal membrane oxygenation (ECMO). In addition, on 3rd day of admission, low doses of norepinephrine was intermittently used to maintain circulatory stability, and therapeutic plasma exchange was performed twice to remove inflammatory factors caused by cytokine storm. On the 5th day of admission, ultrasound showed massive hemorrhage in the left chest of the patient. Thus heparin were stopped and leukocyte-removing red blood cells, fresh frozen plasma, cryoprecipitated coagulation factors, platelets and recombinant activated factor VIIa were given to improve coagulation status.
3.3. SOFA scores and sepsis
A SOFA score of 2 or above identified a 2- to 25-fold increased risk of death compared with a SOFA score less than 2 [15]. All the three patients met the criteria of sepsis with SOFA scores over or equal to 2. Case 01 and Case 02 were two severe cases of COVID-19. The number of days in hospital for Case 01 is 17 days, among which 12 days had a SOFA score greater than or equal to 2 points. The number of days in hospital for Case 02 was 25 days, of which 18 days had a SOFA score greater than or equal to 2 points. Case 03 was a critical-ill case of COVID-19, his daily SOFA score was all above or equal to 5. During the last four days post admission, the SOFA score had increased to 9 and septic shock was diagnosed because of persisting hypotension requiring vasopressors to maintain MAP >65 mmHg and having a serum lactate level > 2 mmol/L despite adequate volume resuscitation. The patient began to use ECMO to provide extracorporeal respiration on the first day of admission. From the 3rd to 5th day post admission, 0.1 μg/kg noradrenaline was given to maintain blood pressure. The concentration of lactate in peripheral blood was 3.0 mmol/L. On the 6th day post admission, the PaO2/FiO2 was lower than 100 mmHg even when treated with ventilator. 3.0μg/kg noradrenaline was given while the MAP was still lower than 65 mmHg, the patient eventually died of septic shock and MODS (Fig. 2 ).
Fig. 2.
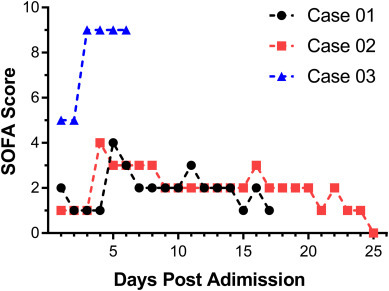
Daily SOFA scores of three patients during admission
Daily SOFA score of the three patients was assessed during admission to elucidate the clinical feature of viral sepsis.
3.4. Multiple organ dysfunction scores
MOD score above or equal to 4 represented marked functional dysfunction and a mortality rate of more than or equal to 50% [16]. Data were collected daily to calculate three patients' MOD score. Among the 17-admission-days of Case 01, the MOD scores did not exceed 2. On the 5th and 6th days post admission, the MOD score was 2 (1 point for bilirubin and 1 point for creatinine) (Fig. 3 ). From the 11th to 19th days post admission, the MOD score of Case 02 was 4 (3 points for PaO2/FiO2 and 1 point for bilirubin). When the patient was discharged on 25th day post admission, the MOD score was reduced to 0 (Fig. 3). Case 03 was a critical-ill case of COVID-19, the MOD score of the first two days post admission was 5 (3 points for PaO2/FiO2 and 1 point for creatinine). From the 3rd to 5th days post admission, the MOD point increased to 7 (3 points for PaO2/FiO2, 1 point for creatinine and 2 points for CNS Glasgow Coma Scale). At 12:40 pm on 5th day post admission of Case 03, the patient had a sudden increase of heart rate, decrease of blood pressure, progressive decrease of hemoglobin. After dilatation and transfusion, 0.3μg/kg noradrenaline was given. Bedside ultrasound indicated that there was a large amount of blood in the left thorax. Non-coagulated blood was drawn out by thoracic puncture, so heparinization treatment of ECMO and pressurized blood transfusion were stopped immediately. Coagulation test showed that thrombin time (TT) and activated partial thromboplastin time (APTT) were significantly prolonged (TT: 240 s, APTT: 206.3 s). At 1:30 am on the 6th day post admission, the blood pressure and heart rate decreased gradually. After rescue, chest compression and intravenous injection of noradrenaline, unfortunately, the patient died on the 6th day post admission (16 days after initial symptoms) with coagulation, respiratory, circulatory and renal dysfunction.
Fig. 3.
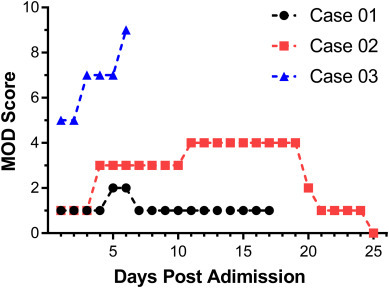
Daily MOD scores of three patients during admission
Daily MOD score of the three patients was assessed during admission to elucidate the clinical feature of multiple organ dysfunction caused by SARS-CoV-2 infection.
3.5. Analysis of immune cell populations
Patients who died of COVID-19 had significantly reduced lung immune cells and reduced peripheral blood lymphocytes. Meanwhile, lymphocytes are over-activated because of the increase in highly pro-inflammatory CCR4+ CCR6+ Th17 cells [5]. The number of peripheral blood lymphocytes was significantly lower in severe patients when admitted to the hospital than mild patients. The T cells and CD4+ T cell subsets of severe patients continued to decrease compared to mild patients [17]. Consistent with these studies, the percentage of peripheral blood T lymphocytes all decreased in the three patients post admission. Respectively for cases 01 and 02, the percentage of CD3+ T lymphocyte was 64.24% and 55.8% for the 7th day post admission, a slightly lower than normal reference range (65%–75%). The percentage of CD3+ CD8+ suppressor T cells was 27.58% and 14.78% (normal reference range 20%–30%), that of CD3+ CD4+ helper T cells was 27.58% and 47.65% (normal reference range 35%–55%). The percentage of CD3+ T lymphocyte of Case 03 was significantly decreased to 35.68% on the first day of admission. The percentage of suppressor T cells and helper T cells were 18.48% and 16.91% on the first day of admission, much lower than normal reference range. However, the percentage of B cells all increased to 19.30%, 26.35% and 29.66% for three patients (normal reference range 5.9%–19.2%).
4. Discussion
Although there have been big progress in the research of sepsis recent years, sepsis is still one of the leading cause of death in intensive care units (ICU) [18,19]. Bacterial infections represent the majority of sepsis cases. Sepsis caused by virus is often ignored [20]. At present, the viral sepsis caused by SARS-CoV-2 virus has a relatively high risk of sepsis and multiple organ failure [21]. Moreover, research data showed that, severe COVID-19 patients often combined with bacterial or fungal infection. Many patients have organ dysfunction, 4% of them have septic shock [10]. Given the rapid global spread of COVID-19, coupled with the high rate of missed diagnosis of SARS-CoV-2 induced viral sepsis, it is urgent to evaluate the SOFA score of sepsis and multiple organ failure score in COVID-19 patients, so as to determine the clinical characteristics of viral sepsis more accurately and reveal the risk factors related to mortality.
According to Huang et al. [9], 13 out of 41 COVID-19 patients (31.7%) were admitted to intensive care unit (ICU). In our study, 3 out of 15 COVID-19 patients (20%) eventually developed sepsis and/or MODS and were admitted to ICU. Case 01 was diagnosed as sepsis for a SOFA score more than 2 in 12 admission days. Case 02 was diagnosed as sepsis and MODS, because of both respiratory and liver dysfunction for 9 days during the admission. Case 03 was a critically ill patient, diagnosed with septic shook and MODS. All patients were given mask oxygen inhalation and ventilator to assist breathing immediately after admission to improve the situation of hypoxia. Abidol hydrochloride tablets, interferon alfa-2b, ribavirin, Lianhuaqingwen combined with lopinavir plus ritonavir or chloroquine phosphate were given as antiviral therapy. Case 03 was given low doses of norepinephrine was intermittently used to maintain circulatory stability, and therapeutic plasma exchange was performed twice to remove inflammatory factors caused by cytokine storm. This patient died of multiple organ dysfunction, including coagulation, respiratory, circulatory and renal dysfunctions.
According to the anatomy results of COVID-19 patients, flow cytometry revealed a decrease in the number of CD4+ and CD8+ T lymphocytes, but lymphocytes were over-activated, as evidenced by the higher double-positive ratio of HLA-DR (CD4: 3.47%) and CD38 (CD8: 39.4%) [10]. In addition, pro-inflammatory CCR4+ CCR6+ Th17 cells are increased. The results show that the increase of Th17 and the high cytotoxicity of CD8+ T cells as the major causes of severe immune damage in patients with T cell over-activation. Consistent with these studies, the percentage of peripheral blood T lymphocytes all decreased in three patients of our study. Moreover, The percentage of CD3+ CD8+ suppressor T cells and CD3+ CD4+ helper T cells were all significantly decreased. The decrease of immune cells and the non-response of immune cells are closely related to the rapid deterioration of multiple organ failure, which is consistent with the characteristics of sepsis immuno-suppression, indicating that SARS-CoV-2 viral sepsis patients might have immuno-suppression.
A considerable proportion of COVID-19 patients developed multiple organ dysfunction. ICU becomes the main department for the treatment of severe and critically-ill patients. Specialized scientific monitoring, life support and treatment of ICU will be an important measure to reduce mortality. However, in COVID-19 epidemic area, a large number of critically ill patients resulted in ICU overcrowding and medical staff overloading. After the epidemic, many countries need to reconsider the expansion and increasing the fund of ICU in public hospitals. In the months after hospital discharge for sepsis, management should focus on (1) identifying COVID-19 caused physical, mental and cognitive problems and referring for appropriate intervening, (2) reviewing and adjusting long-term medications, and (3) long term evaluation of patients' immune status to avoid re-infection [22].
Conflict of interests
All authors declare no competing financial interests.
CRediT author statement
L. Z., J. J. and J. D. designed the study; D. L., Q. W., H. Z., L. C., J. L. S., L. G., S. J., J. W., J. Z., Q. C., F. S., drafted the manuscript and performed data analysis; J. J. and J. D. reviewed and revised the manuscript; F. S., Y. C. recruited patients.
Acknowledgments
Acknowledgements
We greatly appreciate all the people, particularly thousands of clinicians, nurses and scientists who are heroically battling to eradicate the epidemic of COVID-19 for days and nights.
Funding
This study was supported by national natural science funds of China (81571892 and 81660317), project of trauma, burns and combined injury state key laboratory (SKLYQ201901 and SKLKF201802), the funds of clinical research projects of the Military Medical University (2018XLC3057), the funds for Chongqing science and technology talents, and the training plan of innovation ability of military medical frontier research (2019CXJSB014).
References
- 1.Li Q., Guan X., Wu P. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao R., Li J. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 4.Niu P., Zhao G., Deng Y., Sun S., Wang W., Zhou Y. A novel human mAb (MERS-GD27) provides prophylactic and postexposure efficacy in MERS-CoV susceptible mice. Sci China Life Sci. 2018;61:1280–1282. doi: 10.1007/s11427-018-9343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modeling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phua J., Ngerng W., See K. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17:R202. doi: 10.1186/cc12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinhart Konrad, Daniels Ron, Kissoon Niranjan, Machado Flavia R., Schachter Raymond D., Finfer Simon. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 14.Marshall J.C., Cook D.J., Christou N.V., Bernard G.R., Sprung C.L., Sibbald W.J. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Seymour C.W., Liu V.X., Iwashyna T.J. Assessment of Clinical Criteria for Sepsis. JAMA. 2016;315:762. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauaia A., Moore E.E., Johnson J.L., Ciesla D.J., Biffl W.L., Banerjee A. Validation of postinjury multiple organ failure scores. Shock. 2009;31:438–447. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Li SM, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheralblood of SARS-CoV-2 infected patients. medRxiv. 2020; doi: 10.1101/2020.02.16.20023671. [DOI] [PMC free article] [PubMed]
- 18.Kempker J.A., Martin G.S. A global accounting of sepsis. Lancet. 2020;395:168–170. doi: 10.1016/S0140-6736(19)33065-X. [DOI] [PubMed] [Google Scholar]
- 19.Rudd K.E., Johnson S.C., Agesa K.M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:201–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin G., McGinley J.P., Drysdale S.B., Pollard A.J. Epidemiology and immune pathogenesis of viral sepsis. FRONT IMMUNO. 2018;9:2147. doi: 10.3389/fimmu.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O. The Continuing 2019-nCoV Epidemic Threat of Novel Coronaviruses to Global Health - The Latest 2019 Novel Coronavirus Outbreak in Wuhan. China Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prescott H.C., Angus D.C. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]


