Abstract
Pulmonary embolisms (PEs) in coronavirus disease 2019 (COVID-19) have increasingly been reported in observational studies. However, limited information describing their clinical characteristics and outcomes exists. Our study aims to describe clinical features and risk stratification strategies of hospitalized COVID-19 patients with PE. We retrospectively analyzed 101 hospitalized patients with COVID-19 infection and acute PE. Clinical outcomes measured were intensive care unit admission, mechanical ventilation, bleeding and transfusion events, acute kidney injury (AKI) and mortality. Pulmonary severity index (PESI) scores were used for risk stratification. The most common comorbidities were hypertension (50%), obesity (27%) and hyperlipidemia (32%) among this cohort. Baseline D-dimer abnormalities (4,647.0 ± 8,281.8) were noted on admission with a 3-fold increase at the time of PE diagnosis (13,288.4 ± 14,917.9; p <0.05). Five (5%) patients required systemic thrombolysis and 12 (12%) patients experienced moderate to severe bleeding. Thirty-one (31%) patients developed AKI and 1 (1%) patient required renal replacement therapy. Twenty-three (23%) patients were admitted to intensive care unit, of which 20 (20%) patients received mechanical ventilation. The mortality rate was 20%. Most patients (65%) had Intermediate to high risk PESI scores (>85), which portended a worse prognosis with higher mortality rate and length of stay. In conclusion, this study provides characteristics and early outcomes for hospitalized patients with COVID-19 and acute pulmonary embolism. PESI scores were utilized for risk stratifying clinical outcomes. Our results should serve to alert the medical community to heighted vigilance of this VTE complication associated with COVID-19 infection.
INTRODUCTION
The novel 2019 coronavirus disease (COVID-19) has led to a global pandemic with a spectrum of clinical manifestations among infected patients.1 Cardiopulmonary injury related to this viral infection is now recognized as a common feature resulting in high mortality.2 Prior cardiovascular comorbidities, such as obesity, diabetes, hypertension, and coronary artery disease, portend a poor prognosis amongst these patients.3 An association of COVID-19 infection with venous thromboembolic events (VTEs) has increasingly been recognized by observational studies outside the United States.4 , 5 This was further corroborated by high incidences of VTE findings, such as clinically overlooked pulmonary embolism, on autopsy.6 COVID-19 creates a hypercoagulable milieu due to exaggerated inflammatory response and, thus, leads to a coagulation disarray, as evidenced by elevated inflammatory markers including D-dimer.7, 8, 9 However, such a correlation has not been systemically studied or published to date. We present our experience, including demographics, clinical characteristics, and outcomes, in COVID-19 patients who also developed confirmed pulmonary embolisms during the course of their initial COVID-19 illness.
Methods
This study was conducted at North Shore University Hospital and Long Island Jewish Hospital, 2 tertiary medical centers within the Northwell Health system. Both hospitals are located in Queens and Long Island area with catchment areas encompassing Brooklyn and Queens, NY, an epicenter for COVID-19 in March and April of 2020. Institutional review board approval was obtained via the COVID-19 Consortium at Northwell Health. Hospitalized patients with confirmed COVID-19 positive results on polymerase chain reaction testing, as well as confirmed PE by computed tomography angiogram were included. All studied patients were admitted between March 1, 2020, and April 30, 2020. Clinical outcomes were monitored until May 14, 2020, the final date of follow-up. The presenting characteristics of 5,700 patients from Northwell were presented previously.10 This cohort presents a further in-depth assessment of clinical features and outcomes in COVID-19 positive patients with concurrent PE not presented in the prior article.
Data was retrospectively collected from the enterprise electronic health record (Sunrise Clinical Manager; Allscripts). Patients’ demographic information, comorbidities and laboratory tests at the time of COVID-19 diagnosis were recorded through chart review. Obesity was defined as having a body mass index (BMI) >30. Hypertension, hyperlipidemia, and diabetes status was assessed through clinical chart documentation and medication reconciliation. Vital signs, electrocardiogram, laboratory tests, and relevant imaging were obtained at the time of PE diagnosis. These clinical parameters were used to calculate individual PESI scores. RV strain by CT was defined as a right ventricle to left ventricle size ratio of 0.9 or greater. RV dysfunction on transthoracic echocardiography was diagnosed through qualitative and quantitative RV dilation or RV systolic dysfunction as assessed by a board certified echocardiographer.
Clinical outcomes recorded were the use of systemic thrombolysis, mechanical ventilation, intensive care unit (ICU) admission, stroke, deep venous thromboembolism (DVT), bleeding events, transfusion event, acute kidney injury (AKI), renal replacement therapy, and mortality. Bleeding events were classified by the GUSTO (global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries) bleeding criteria.11 Acute kidney injury, based on the kidney disease: improving global outcomes (DKIGO) definition, was identified as an increase in serum creatinine to 1.5 times or more baseline within the prior 7 days compared with the preceding 1 year of data, or an increase in serum creatinine by 0.3 mg/dL or more within 48 hours.12 Additionally, D-dimer measurements at COVID-19 diagnosis and the time of PE diagnosis were recorded, both within 24 hours of events. Similar analyses were made to study vital sign variations from the time of admission to the time of PE diagnosis. Finally, we risk stratified outcomes based on PESI score into low risk (PESI ≤85) and intermediate to high risk (PESI >85) for comparison.
Statistical analysis was performed by means of Fisher's exact test in categorical variables, pertaining mostly to patient clinical outcomes. Within group comparisons of continuous variables, that is comparison of d-dimer values at times of COVID-19 and PE diagnoses, were performed with the paired-sample t test. Continuous data are presented as mean ± standard deviation and sample proportions are reported as percentages. P values were reported as 2-sided, and a p value < 0.05 was considered statistically significant. All statistical analyses were performed with the use of SPSS software version 23 (IBM, Armonk, New York).
Results
A total of 101 patients were included in this study (Figure 1 ). Basic demographics including mean age, gender and ethnicity distributions are presented in Table 1 . The most common comorbidities were hypertension, obesity, hyperlipidemia, diabetes mellitus, smoking history and previous VTE, which was similar to the larger Northwell experience.10 All hospitalized patients were on prophylactic anticoagulation as per health system policy in patients with COVID-19 infection, with 12 patients continued on their home anticoagulation regimen.
Figure 1.
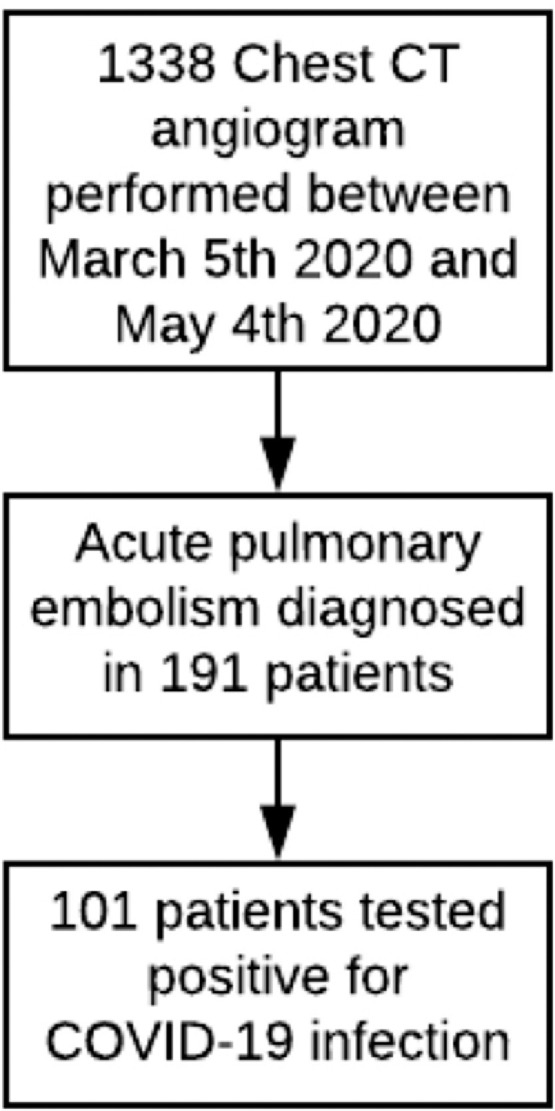
Flowchart of the study enrollment.
Table 1.
Baseline characteristics of COVID-19 patients with acute PE diagnosis*
| Variable | N = 101 |
|---|---|
| Mean age (years) | 62 (± 15) |
| Men | 74 (73%) |
| Women | 27 (27%) |
| White | 28 (28%) |
| Black | 33 (33%) |
| Asian | 17 (17%) |
| Other, multiracial | 23 (23%) |
| Current smoker | 18 (18%) |
| Obesity† | 27 (27%) |
| Diabetes mellitus | 18 (18%) |
| Hypertension | 51 (50%) |
| Hyperlipidemia‡ | 32 (32%) |
| Cancer | 13 (13%) |
| Asthma | 6 (6%) |
| Chronic obstructive pulmonary disease | 4 (4%) |
| Coronary arterial disease/peripheral vascular disease/cerebrovascular accident | 15 (15%) |
| Atrial fibrillation | 6 (6%) |
| Heart failure | 5 (5%) |
| Venous Thromboembolism | 11 (11%) |
| Anti-platelet, anticoagulation therapy | 21 (21%) |
Values presented as no. (%) or mean (± standard deviation) where appropriate. Age refers to the age at study enrollment.
Obesity defined as body mass index greater than or equal to 30.0 kg/m2.
Assessed based on a diagnosis of hyperlipidemia/dyslipidemia in medical history by ICD-10 coding.
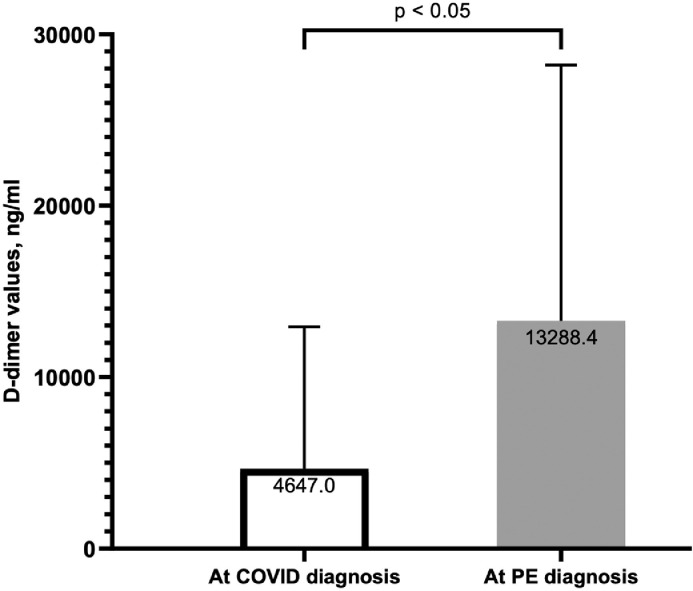
At the time of COVID-19 diagnosis on admission, 53 (52%) patients exhibited bilateral patchy opacities on presenting chest x-ray, consistent with viral pneumonia infection (Table 2 ). Significant elevation in inflammatory markers was noted on admission. The baseline D-dimer increased by 3-fold (13,288 ± 14,918 ng/ml) within 24 hours of PE diagnosis. Intra-group comparison among patients who had both measurements demonstrated significant rise (p <0.05) from the time of COVID-19 diagnosis to the time of acute PE diagnosis (Figure 2 ). Additionally, 34 patients had elevated troponin on admission (27.7 ± 25 ng/L) and this number rose to 48 at the time of PE diagnosis (49.1 ± 141 ng/L).
Table 2.
Findings at times of COVID-19 and pulmonary embolism diagnoses*
| Variable | N = 101 | Reference Ranges |
|---|---|---|
| On admission | ||
| Bilateral patchy opacity (X-ray) | 53 (52%) | |
| White blood cell (109/L) | 9.5 (± 4.5) | 3.8 – 10.5 |
| Lymphocyte (109/L) | 1.0 (± 0.9) | 1.0 – 3.3 |
| Platelet (109/L) | 265.0 (± 98.0) | 150 – 450 |
| Sodium (mmol/L) | 135.5 (± 5.8) | 135 – 145 |
| Creatinine (mg/dL) | 1.1 (± 0.5) | 0.5 – 1.2 |
| Aspartate aminotransferase (U/L) | 56.0 (± 44.0) | 10 – 40 |
| Alanine aminotransferase (U/L) | 51.0 (± 46.0) | 10 – 45 |
| Lactate dehydrogenase (U/L) | 558.5 (± 283.8) | 50 – 242 |
| Ferritin (ng/mL) | 1467.2 (± 1314.0) | 15 – 400 |
| C-reactive protein (mg/dL) | 66.5 (± 90.6) | 0 – 10 |
| International normalized ratio | 1.3 (± 0.2) | 0.9 – 1.2 |
| D-dimer (ng/ml) | 4647.0 (± 8281.8) | 0 – 229 |
| At the time of PE diagnosis | Definitions | |
| Tachycardia Hypotension Hypoxia Fever |
36 (36%) 3 (3%) 71 (70%) 8 (8%) |
Heart rate > 100 BPM Systolic < 90 mmHg O2 saturation < 90% Body temp > 38 °C |
| Sinus tachycardia Supraventricular tachycardia Right bundle branch block Right ventricular strain pattern S1Q3T3 pattern |
38 (38%) 5 (5%) 17 (17%) 15 (15%) 9 (%) |
|
| Pulmonary Computed Tomography Angiography: Saddle embolism Bilateral PE Right ventricular strain |
2 (2%) 47 (47%) 47 (47%) |
|
| Transthoracic echocardiography†: Ejection fraction (%) Right ventricle dysfunction Left ventricular dysfunction Pulmonary arterial systolic pressure (mmHg) Intracardiac thrombus |
48.8 (± 26.3) 14 (34%) 5 (12%) 37.1 (± 18.7) 3 (7%) |
|
| Pulmonary Embolism Class: Low risk Sub-massive Massive |
23 (23%) 75 (75%) 3 (3%) |
|
| D-dimer (ng/ml) | 13288.4 (± 14917.9) | 0 - 229 |
| High sensitivity troponin (ng/L) | 49.1 (± 141.0) | <6 – 14 |
| Creatine kinase (U/L) | 221.0 (± 319.0) | 25 – 200 |
| Brain-type natriuretic peptide (pg/mL) | 1890.5 (± 2875.0) | 0 – 99 |
Values are presented as no (%) or mean (± standard deviation) where applicable.
Transthoracic studies performed in 41 patients. Calculated percentages for right and left ventricular dysfunction, as well as Ejection fraction was based on this number of patients. Pulmonary arterial systolic pressure was only able to be estimated on 29 patients as tricuspid regurgitation velocity envelope were not well visualized in the other cases.
Figure 2.
D-dimer rise between COVID diagnosis and PE diagnosis. Average D-dimer level was >18x the upper limit of normal (ULN) at the time of COVID-19 diagnosis on admission (4,647.0 ng/ml ± 8,281.8) with a significant 3-fold increase at the time of PE diagnosis to >57x ULN (13,288.4 ng/ml ± 14,917.9; p <0.05).
Average time from hospital admission to PE diagnosis was 5 days (± 6.1). At the time of PE diagnosis, tachycardia, hypotension, hypoxia, and fever were most frequently reported physical exam findings (Table 2). EKG rhythm at time of PE diagnosis was most commonly sinus tachycardia followed by atrial fibrillation or atrial flutter. The McGinn-White sign of S1Q3T3 pattern was observed in 9 patients (9%).13 RV strain on CT was noted in approximately half of this cohort with an estimated mean pulmonary artery systolic pressure (PASP) of 37.1 mmHg. Based on imaging findings, vital signs and biomarker measurements, 75 patients (75%) were diagnosed with sub-massive PE and 3 patients (3%) were diagnosed with massive PE.
At the final study follow-up date, all 101 patients (100%) received full dose anticoagulation therapy (Table 3 ). Twenty-three patients (23%) were admitted to ICU, with 20 (20%) ultimately requiring invasive mechanical ventilation. Five (5%) patients received systemic thrombolysis. Indication for thrombolytic therapy was hemodynamic instability in 2 patients and presence of intra-cardiac thrombus with clot in transit in another 3 patients. No patient received catheter-directed thrombolysis or thrombectomy. A total of 12 patients experienced moderate or severe bleeding incidents: there were 7 severe cases, including 3 intracranial (IC) hemorrhages and one of which was fatal. Within this group, another patient had severe gastrointestinal bleeding in the context of previous systemic thrombolytic use. Additionally, there were 5 moderate bleeding incidents, of which 2 patients required blood transfusions. Thirteen (13%) patients were found to have concomitant DVT, although only 36 patients underwent venous ultrasound study largely due to selection bias. Overall mean length of stay (LOS) was 13 days. Six patients remained in the hospital at the end of the study, 2 of whom were in the ICU on mechanical ventilation.
Table 3.
Clinical intervention and outcomes based on PESI risk stratification*
| Variable | Total (N = 101) | PESI ≤ 85 (n = 35) | PESI > 85 (n = 66) | P value |
|---|---|---|---|---|
| Anticoagulation: Heparin Enoxaparin Argatroban Direct oral anticoagulant |
25 (25%) 69 (69%) 4 (4%) 3 (3%) |
4 (11%) 29 (83%) 1 (3%) 1 (3%) |
21 (31%) 40 (60%) 4 (5%) 3 (4%) |
0.084 |
| Thrombolytic use: Full dose Half dose |
3 (3%) 2 (2%) |
1 (3%) 0 |
2 (3%) 2 (3%) |
0.794 |
| Transfusion | 9 (9%) | 3 (9%) | 6 (9%) | 0.621 |
| Renal replacement therapy | 1 (1%) | 0 | 1 (2%) | 0.653 |
| Intensive care unit admission | 23 (23%) | 4 (11%) | 19 (29%) | 0.038 |
| Mechanical Ventilation | 20 (20%) | 4 (11%) | 16 (24%) | 0.099 |
| Length of hospital stay, days† | 13.0 (± 10.2) | 9.7 (± 5.4) | 14.3 (± 11.6) | 0.030 |
| Acute kidney injury | 31 (31%) | 7 (20%) | 24 (36%) | 0.069 |
| Bleeding: Severe Moderate |
7 (7%) 5 (5%) |
2 (6%) 1 (3%) |
5 (8%) 4 (5%) |
0.893 |
| Deep Venous Thromboembolism | 13 (13%) | 3 (9%) | 10 (15%) | 0.347 |
| Stroke‡ | 3 (3%) | 0 | 3 (4%) | 0.257 |
| Mortality | 20 (20%) | 2 (6%) | 18 (27%) | 0.007 |
Values are presented as no (%) or mean (± standard deviation) where applicable.
At the final study follow-up date, 6 patients remain admitted in the hospital.
All 3 patients had acute hemorrhagic stroke and, thus, were also accounted for under severe bleeding events.
Intermediate to high risk PESI score patients (Class 3, 4 and 5) had worse outcomes compared to patients with low risk PESI scores (Class 1 & 2), resulting in higher percentage ICU admission (29% vs 11%;p = 0.038), longer LOS (14.3 vs 9.7 days; p = 0.030) and higher mortality rate (27% vs 6%;p = 0.007). There was no significant variation between intermediate and high risk groups with respect to mechanical ventilation use, AKI, bleeding events, thromboembolic incidents including stroke and DVT (p >0.05).
Discussion
To our knowledge, this cohort represents the largest collection of COVID-19 patients with concurrent pulmonary embolism. As reported in prior COVID-19 studies, our patients were more likely to have cardiovascular disease risk factors such as hypertension, hyperlipidemia, obesity, diabetes mellitus, and smoking history.14 Only a small percentage of our patients had previous VTEs or malignancies. Direct effects of COVID-19 or its sequela, via severe inflammatory response and hypoxia, are postulated to be the main drivers of pulmonary thrombotic events in our patients.15 , 16
Compare with the larger Northwell series,10 our cohort had higher rates of ICU admission, mechanical ventilation, AKI and mortality, suggesting the addition of pulmonary embolism to COVID-19 exacerbates morbidity and mortality. Additionally, the majority of this cohort had PESI scores >85, which has previously been shown to predict higher short-term adverse events and mortality.17 When we applied such risk predictor to this COVID-19 population, high risk patients required significantly more ICU level care, increased LOS in the hospital, and experienced a higher mortality rate. To our knowledge, our study is the first to suggest that high PESI scores represent a poor prognostic indicator in COVID-19 patients who develop acute PE.
We report an overall low incidence of DVT and ischemic stroke in our studied population, although diagnostic testing was limited in an effort to minimize personnel exposure and viral transmission. This observation may suggest that localized in situ pulmonary thrombosis, rather than embolism, occurs among these COVID-19 patients. Similar findings were reported by Susan et al in their case series of 22 patients with confirmed PE and COVID-19.18 Lung pathology studies corroborate this finding, with reports of widespread vascular thrombosis with microangiopathy and intussusceptive angiogenesis.19 COVID-19 is now widely considered as a pro-thrombotic disease with systemic inflammation,20 , 21 as demonstrated in our cohort by inflammatory marker elevation. The average D-dimer level in our patients upon initial COVID-19 diagnosis was >18x the upper limit of normal, with a significant 3-fold increase at the time of PE diagnosis to a mean D-dimer of >57x ULN in this cohort. This finding coincides with prior data, which reported elevated D-dimer levels correlate with the presence of VTE in COVID-19 patients as well as the severity of infection.8 , 22 , 23 Furthermore, Tang et al reported that patients with higher D-dimer (>6x ULN) have an increasing mortality benefit with anticoagulation.22 Our study suggests a benefit to trend D-dimer levels from initial admission to aid in early recognition of PE and subsequent prompt initiation of anticoagulation for maximal therapeutic benefit.22 , 24
Most patients (75%) in this cohort were categorized as having sub-massive PE, with either positive cardiac biomarkers or RV dysfunction on imaging. It has been shown that COVID-19 and its sequela of ARDS managed with high positive end expiratory pressure can exert detrimental effects on the myocardium and right ventricular function.25 It is also worth noting these findings predict adverse short- and long-term outcomes in the PE population.26 Given the high rate of sub-massive PE in our cohort, we emphasize the importance of increased clinical awareness, as well as early imaging testing for expeditely diagnosing PE in COVID-19 patients. All patients require therapeutic anticoagulation, when PE diagnosis is highly suspected based on clinical context, D-dimer values, and imaging results, with escalation to potential thrombolytic therapy when indicated clinically. Based on this practice strategy, we report an overall small number of bleeding events in our group. Understanding the potential adverse effects of severe hemorrhage and mortality associated, the benefits of anticoagulation should still be balanced with bleeding risks on an individual basis
Our study is limited by its inherent retrospective nature. Only patients who were stable enough to undergo CTA were analyzed, resulting in unavoidable selection bias. Similarly, patients with acute kidney injury, which commonly co-exists in COVID-19, were excluded in an effort to prevent contrast nephropathy. In an attempt to curtail viral transmission, many of these patients underwent alternative diagnostic testing such as point of care ultrasound to evaluate right ventricle dilatation or dysfunction. Such testing results are frequently ambiguous and difficult to capture for our cohort. For all the aforementioned reasons, PE was likely underestimated in our institutional dataset.
In conclusion, acute pulmonary embolism is increasingly being recognized as a complication of active COVID-19 infection. Our results suggest the use of PESI scores and D-dimer measurements for risk stratification and management guidance in this patient population.
Authors’ contributions
Hai Xu, MD: Data curation, Methodology, Formal analysis, Investigation, Writing – Original draft preparation; Angel Martin, MD: Data curation, Writing – Original draft preparation; Avneet Singh, MD: Methodology, Writing – Reviewing and Editing; Mangala Narasimhan, DO: Writing – Reviewing and Editing; Joe Lau, MD, FACC: Methodology, Writing – Reviewing and Editing; Mitchell Weinberg, MD: Conceptualization; Rajiv Jauhar, MD: Supervision, Writing – Reviewing and Editing; Gaurav Rao, MD: Supervision, Conceptualization, Methodology, Writing – Original draft preparation.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to acknowledge the Northwell COVID-19 Research Consortium for their support and resources.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. China medical treatment expert group for clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 March 25 doi: 10.1001/jamacardio.2020.0950. https://jamanetwork.com/journals/jamacardiology/fullarticle/2763524 ([E-pub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schroder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Puschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020 May 6 doi: 10.7326/M20-2003. https://www.acpjournals.org/doi/10.7326/M20-2003 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, and the Northwell C-RC. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Investigators G An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 12.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 13.Petruzzelli S, Palla A, Pieraccini F, Donnamaria V, Giuntini C. Routine electrocardiography in screening for pulmonary embolism. Respiration. 1986;50:233–243. doi: 10.1159/000194933. [DOI] [PubMed] [Google Scholar]
- 14.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 March 27 doi: 10.1001/jamacardio.2020.1017. https://jamanetwork.com/journals/jamacardiology/article-abstract/2763845 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quere I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erkens PM, Gandara E, Wells PS, Shen AY, Bose G, Le Gal G, Rodger M, Prins MH, Carrier M. Does the pulmonary embolism severity index accurately identify low risk patients eligible for outpatient treatment? Thromb Res. 2012;129:710–714. doi: 10.1016/j.thromres.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S. Lille ICUHC-g. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;120 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 19.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, Hlh Across Speciality Collaboration UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors J, Levy J. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK, American Heart Association Council on Cardiopulmonary CCP, Resuscitation, American Heart Association Council on Peripheral Vascular D, American Heart Association Council on Arteriosclerosis T, Vascular B Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]