Highlights
-
•
An extensive review on diverse bioactive components of buckwheat.
-
•
Versatile beneficial phytochemicals are abundant in buckwheat.
-
•
Buckwheat has a wide range of pharmacological and beneficial health effects.
-
•
Huge research scope on Fagopyrum cymosum to identify the beneficial phytochemicals.
Keywords: Buckwheat, D-chiro-inositol, Flavonoids, Nutritional value, Rutin
Abstract
Buckwheat is a gluten-free crop under the family Polygonaceae abundant with beneficial phytochemicals that provide significant health benefits. It is cultivated and adapted in diverse ecological zones all over the world. Recently its popularity is expanding as a nutrient-rich healthy food with low-calories. The bioactive compounds in buckwheat are flavonoids (i.e., rutin, quercetin, orientin, isoorientin, vitexin, and isovitexin), fatty acids, polysaccharides, proteins, and amino acids, iminosugars, dietary fiber, fagopyrins, resistant starch, vitamins, and minerals. Buckwheat possesses high nutritional value due to these bioactive compounds. Additionally, several essential bioactive factors that have long been gaining interest because these compounds are beneficial for healing and preventing several human diseases. The present review demonstrates an overview of the recent researches regarding buckwheat phytochemicals and particularly focusing on the distinct function of bioactive components with their health benefits.
1. Introduction
Buckwheat is an ancient pseudocereal crop under Polygonaceae family and genus Fagopyrum which occupy a crucial part of the human diet, consumed globally (Kwon et al., 2018, Park et al., 2019, Sinkovic et al., 2020). Buckwheat is broadly cultivated in Asia, Europe, and the Americas but originated from mountainous provinces of southern China (Ji et al., 2019, Sinkovic et al., 2020). Buckwheat has numerous ecological adaptabilities, so it can be cultivated in high altitude regions with low rainfall and temperature (Ge and Wang, 2020, Liu et al., 2015). Common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) are the most widely grown and consumed species throughout the world (Ahmed et al., 2013, Kwon et al., 2018). The world production of buckwheat was more than 2.90 million tonnes in 2018. The world-leading buckwheat producing countries are China, Russia, France, Ukraine, Poland, the United States and Kazakhstan (FAOSTAT, 2020). Moreover, nowadays buckwheat has been becoming more popular in the USA, Canada, and Europe (Giménez-Bastida & Zieliński, 2015) and is mostly consumed as noodles, pancakes, and muffins in different countries, such as China, Ukraine, Japan, Canada, India, and Nepal (Sytar, Brestic, Zivcak, & Tran, 2016). Buckwheat has been confirmed as a good source of various nutritious and bioactive components possessing diverse health and pharmaceutical effects (Christa and Soral-Smietana, 2008, Sinkovic et al., 2020), therefore it drew more attention for being a potentially valuable food source.
Throughout recent years, buckwheat has increased demand and attracted research attention of food scientists because of its identical chemical compounds and highly effective as a functional food with healing effects over chronic diseases, such as anti-oxidative, cardioprotective, anti-cancer, hepatoprotective, anti-hypertension, anti-tumor, anti-inflammatory, anti-diabetic, neuro-protection, cholesterol-lowering, cognition-improving activities, and so on (Ge and Wang, 2020, Kwon et al., 2018, Lv et al., 2017). These health effects have been partially or fully correlated with several bioactive compounds that exist in buckwheat. These bioactive compounds comprise a large number of chemicals, such as flavonoids, polyphenols, carbohydrates, dietary fiber, proteins and amino acids, fatty acids, vitamins, and minerals (Gonḉalves et al., 2016, Ji et al., 2019, Wang et al., 2016a, Zhao et al., 2018). Buckwheat is rich in B group vitamins, including thiamine, riboflavin, and pyridoxine (Beitane & Krumina-Zemture, 2017), and also contains some macroelements and microelements, like sodium, potassium, copper, zinc, magnesium, iron, calcium, and manganese (Krupa-Kozak et al., 2011, Mota et al., 2016). These nutritious substances present buckwheat as a better option to make various food products like bakeries, biscuits, breads, cakes, casseroles, cookies, crepes, porridge, pancakes, pasta-noodles, soups, and other confectionery products (Mohajan et al., 2019, Tien et al., 2018). Therefore, this article aims to review the bioactive compounds isolated from buckwheat with their nutritional and health effects. In this review, we expect to draw the attention of organic product researchers to focus on the unidentified bioactive compounds for further improvement.
2. Bioactive compounds
Several bioactive compounds have been detected from various plant parts (i.e., leaves, seeds, roots, and so on) of different species of buckwheat. These compounds comprise of Flavonoids, Phenolic acids and their derivatives, Tannins, Fagopyrin, Triterpenoids, Steroids, Stilbenes, and so on. The compound names, basic skeleton, examples, and sources of these major bioactive compounds are summarized in Table 1 .
Table 1.
Major polyphenolic compounds, basic skeleton thereof and some examples isolated from different Fagopyrum species. (Dziedzic et al., 2018, Gorniak et al., 2019, Jing et al., 2016; Karamac et al., 2015; Lv et al., 2017, Martin-Garcia et al., 2019, Matsui and Walker, 2019, Melini et al., 2020, Park et al., 2019, Wajid et al., 2015, Zhu, 2019).
| Compounds Name | Basic skeleton | Examples | Source |
|---|---|---|---|
| Flavinoids | |||
| Flavonols | 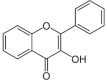 |
Rutin | Fc, Fe, Fh, Ft |
| Kaempferol | Fc, Ft | ||
| Kaempferol-3-O-galactoside | Ft | ||
| Kaempferol-3-O-glucoside | Ft | ||
| Kaempferol-3-O-rutinoside | Ft | ||
| Kaempferol-3-O-sophoroside | Fe | ||
| Kaempferol-3-O-glucoside-7-O-glucoside | Fe | ||
| Myricetin | Fe | ||
| Quercetin | Fc, Fe, Ft | ||
| Isoquercetin | Fc, Fe | ||
| Quercitrin (quercetin-3-O-rhamnoside) | Fc, Ft | ||
| Isoquercitrin | Fe | ||
| Quercetin-3-O-[β-d-xyloxyl-(1–2)-α-l-rhamnoside] | Ft | ||
| Quercetin-3-O-β-d-galactoside | Fe, Ft | ||
| Quercetin-3-O-rutinoside-3′-O-β-glucopyranoside | Fc, Ft | ||
| Quercetin-3-O-rutinoside-7-O-galactoside | Ft | ||
| Rhamnetin | Fc | ||
| Flavones | 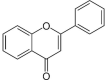 |
Luteolin | Fc |
| Vitexin | Fe, Ft | ||
| Isovitexin | Fe, Ft | ||
| Orientin | Fe, Ft | ||
| Isoorientin | Fe, Ft | ||
| Homoorientin | Fe | ||
| Quercetin-3-O-rutinoside-3′-O-glucoside | Ft | ||
| Quercetin-3-O-rutinoside-7-O-galactoside | Ft | ||
| 3′,4′-methylenedioxy-7-hydroxy-6-isopentenyl flavone | Fc | ||
| Flavanones | 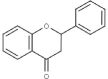 |
Hesperetin 7-rutinoside (hesperidin) | Fe, Ft |
| Hesperetin 7-O-neohesperidoside | Fe, Ft | ||
| Hesperetin O-hexosyl-O-hexoside | Fe, Ft | ||
| Hesperetin 5-O-glucoside | Fe, Ft | ||
| Hesperetin O-malonylhexoside | Fe, Ft | ||
| Naringenin | Fe, Ft | ||
| Naringenin chalcone | Fe, Ft | ||
| Naringenin O-malonylhexoside | Fe, Ft | ||
| Naringenin 7-O-glucoside | Fe, Ft | ||
| Phloretin | Fe, Ft | ||
| Homoeriodictyol | Fe, Ft | ||
| Hesperetin | Fc | ||
| (-)-Liquiritigenin | Ft | ||
| Flavanols/Flavan-3-ols | 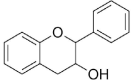 |
Catechin | Fc, Fe, Fh |
| (+)-catechin-7-O-glucoside | Fe | ||
| Catechin hydrate | Ft | ||
| Epicatechin | Fc, Fe, Fh, Ft | ||
| Epicatechin-3-O-(3,4-di-O-methyl)-gallate | Fe, Fh | ||
| (–)-epicatechin-3-O-p-hydroxybenzoate | Fe | ||
| Epigallocatechin | Ft | ||
| Epicatechingallate | Fe, Fh | ||
| Epiafzelechin-(4–6)-epicatechin | Fe, Fh | ||
| Epiafzelechin-(4–8)-epicatechin-p-OH-benzoate | Fe, Fh | ||
| Epiafzelechin-(4–8)-epicatechin-methylgallate | Fe, Fh | ||
| Epicatechin(4–8)-epicatechin-O-(3,4-dimethyl)-gallate | Fe, Fh | ||
| Epiafzelechin-(4–8)-epicatechin(3,4-dimethyl)-gallate | Fe, Fh | ||
| Epiafzelechin-(4–8)-epiafzelechin-(4–8)-epicatechin | Fe | ||
| Epiafzelechin-(4–8)-epiafzelechin-(4–8)-epicatechin-O-(3,4-dimethyl)-gallate | Fe, Fh | ||
| Anthocyanins |  |
Cyanidin 3-O-glucoside | Fe, Ft |
| Cyaniding 3-O-rutinoside | Fe, Ft | ||
| Cyanidin 3-O-galactoside | Fe | ||
| Cyanidin 3-O-galactopyranosyl-rhamnoside | Fe | ||
| Fagopyrins |  |
Fagopyrin A | Fc, Fe, Ft |
| Fagopyrin B | Fc, Fe, Ft | ||
| Fagopyrin C | Fc, Fe, Ft | ||
| Fagopyrin D | Fc, Fe, Ft | ||
| Fagopyrin E | Fc, Fe, Ft | ||
| Fagopyrin F | Fc, Fe, Ft | ||
| Proanthocyanidins |  |
Procyanidin A1 | Fe, Fh |
| Procyanidin A2 | Fe, Ft | ||
| Procyanidin A3 | Fe, Ft | ||
| Procyanidin B2 | Fe, Ft | ||
| Procyanidin B3 | Fe, Ft | ||
| Procyanidin B5 | Fe, Fh | ||
| Procyanidin C1 | Fc | ||
| Isoflavones |  |
6-hydroxydaidzein | Fe, Ft |
| 2′-hydroxydaidzein | Fe, Ft | ||
| Sissotrin | Fe, Ft | ||
| Formononetin (4′-O-methyldaidzein) | Fe, Ft | ||
| Glycitin | Fe, Ft | ||
| Genistein 7-O-glucoside (genistin) | Fe, Ft | ||
| Formononetin 7-O-glucoside (Ononin) | Fe, Ft | ||
| Flavonolignan | 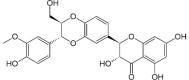 |
Tricin 4′-O-(β-guaiacylglyceryl) ether O-hexoside | Fe, Ft |
| Tricin 7-O-β-guaiacylglycerol | Fe, Ft | ||
| Tricin 4′-O-β-guaiacylglycerol | Fe, Ft | ||
| Tricin 4′-O-syringic acid | Fe, Ft | ||
| Tricin 4′-O-(syringyl alcohol) ether 5-O-hexoside | Fe, Ft | ||
| Tricin 4′-O-(syringyl alcohol) ether 7-O-hexoside | Fe, Ft | ||
| Phenolic acids and their derivatives | |||
| Hydroxybenzoic acids | 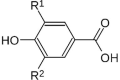 |
Benzoic acid | Fc |
| Gallic acid | Fc, Ft | ||
| 4-hydroxybenzoic acid | Ft | ||
| P-hydroxybenzoic acid | Fc | ||
| P-Hydroxybenzaldehyde | Ft | ||
| Vanillic acid | Ft | ||
| Protocatechuic acid | Fc, Fe, Ft | ||
| Syringic acid | Ft | ||
| Hydroxycinnamic acids | 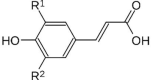 |
P-coumaric acid | Ft |
| O-coumaric acid | Ft | ||
| Caffeic acid | Ft | ||
| Ferulic acid | Ft | ||
| 2,4-dihyroxycinnamic acid | Ft | ||
| Chlorogenic acid | Fe, Ft | ||
| Stilbenes | |||
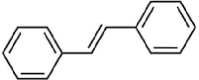 |
Resveratrol | Fe, Ft | |
| Steroids | |||
 |
Hecogenin | Fc | |
| Β-sitosterol | Fc, Ft | ||
| Β-sitosterol palmitate | Ft | ||
| Ergosterol peroxide | Ft | ||
| Daucosterol | Ft | ||
| Β-daucosterol | Fc | ||
| 6-hydroxy stigmasta-4,22-dien-3-one | Fe | ||
| 23S-methylcholesterol | Fe | ||
| Stigmast-5-en-3-ol | Fe | ||
| Stigmast-5,24-dien-3-ol | Fe | ||
| Trans-stigmast-5,22-dien-3-ol | Fe | ||
| Stigmsat-4-ene-3,6-dione | Ft | ||
| Triterpenoids | |||
 |
Ursolic acid | Fc, Ft | |
| Olean-12-en-3-ol | Fe | ||
| Urs-12-en-3-ol | Fe | ||
| Α-thujene | Ft | ||
| Α-terpineol | Ft | ||
| Glutinone | Fc | ||
| Glutinol | Fc | ||
| Tannins | |||
 |
3,3-di-O-galloyl-procyanidin B-2 | Fc | |
| 3-O-galloyl-procyanidin B-2 | Fc | ||
| Phenylpropanoid glycosides | |||
 |
Tatarisides A | Ft | |
| Tatarisides B | Ft | ||
| Tatarisides C | Ft | ||
| Tatarisides D | Ft | ||
| Tatarisides E | Ft | ||
| Tatarisides F | Ft | ||
| Tatarisides G | Ft | ||
| Diboside A | Fc, Ft | ||
| Lapathoside A | Fc | ||
| 3,6-di-p-coumaroyl-1,6′-di-feruloyl sucrose | Ft | ||
| 1,3,6′-tri-feruloyl-6-p-coumaroyl sucrose | Ft | ||
| 1,3,6-tri-p-coumaroyl-6′-feruloyl sucrose | Ft | ||
| 1,3,6,6′-tetra-feruloyl sucrose | Ft | ||
Abbreviations: Fc, Fe, Fh, Ft = Fagopyrum cymosum (F. dibotrys), Fagopyrum esculentum, Fagopyrum homotropicum and Fagopyrum tataricum, respectively.
2.1. Flavonoids
Flavonoids are the prominent group of polyphenol secondary metabolites that bear an aromatic ring holding minimum one hydroxyl group presented particularly in plants and diets (Gorniak et al., 2019, Tungmunnithum et al., 2018, Wang et al., 2018). Various pharmaceutical uses of bioactive flavonoids exist in buckwheat establish it a highly treasured crop. Different types of flavonoids have been detected from the root, flower, fruit, seed, sprouted seed, seedling, seed coat, Seed husk, and processed food of buckwheat (Borovaya and Klykov, 2020, Matsui and Walker, 2019, Park et al., 2017) by using different detection methods (Table 2 ). The content of flavonoids depends on various factors including plant growth stage, organ, cultivated buckwheat species, growing season and area (Matsui & Walker, 2019). Usually, the content of flavonoids in Tartary buckwheat (~40 mg/g) is more than Common buckwheat (~10 mg/g), estimating about 100 mg/g in leaves, stems, and flowers of Tartary buckwheat. (Li, 2019). Moreover, common buckwheat flowers and leaves contain 8.3–10% and 1.2–2.6% flavonoids, respectively (Borovaya & Klykov, 2020). Flavonoids are classified into numerous subgroups namely Flavonols, Flavones, Flavanones, Flavanols, Anthocyanins, Fagopyrins, Proanthocyanidins, Isoflavones, and Flavonolignans.
Table 2.
Name of various methods used by different researchers to detect the bioactive compounds present in buckwheat.
| Category | Compounds | Detection Methods | References |
|---|---|---|---|
| Flavonoids | Isoquercetin, quercetin, and Rutin | HPLC | Gabr et al., 2019, Ge and Wang, 2020, Kalinova et al., 2019 |
| HPLC–ESI–MS and HPLC–UV | Park et al. (2019) | ||
| UPLC-ESI-MS/MS | Li et al. (2019a) | ||
| HPLC–MS | Martin-Garcia et al. (2019) | ||
| Hyperoside and quercetin | RP–UHPLC–ESI-MS | Dziedzic et al. (2018) | |
| UPLC-ESI-MS/MS | Li et al. (2019a) | ||
| HPLC | Kalinova et al. (2019) | ||
| Procyanidin B2 | RP–UHPLC–ESI-MS | Dziedzic et al. (2018) | |
| HPLC–MS | Martin-Garcia et al. (2019) | ||
| HPLC | Kalinova et al. (2019) | ||
| Luteolin | RP–UHPLC–ESI-MS | Dziedzic et al. (2018) | |
| Kaempferol | RP–UHPLC–ESI-MS | Dziedzic et al. (2018) | |
| UPLC-ESI-MS/MS | Li et al. (2019a) | ||
| HPLC | Gabr et al. (2019) | ||
| Catechin | RP–UHPLC–ESI-MS | Dziedzic et al. (2018) | |
| Catechin and epicatechin | HPLC–PAD and LIT–FTICR-MS | Zhu (2016) | |
| Catechin, epicatechin, and epiafzelchin | HPLC–MS | Martin-Garcia et al. (2019) | |
| Catechin, epicatechin, and epicatechin gallate | HPLC | Kalinova et al. (2019) | |
| Fagopyrin A to fagopyrin F | RP–UHPLC–ESI-MS and NMRS | Joshi et al. (2020) | |
| Isovitexin and Vitexin | RP–UHPLC–ESI-MS | Dziedzic et al. (2018) | |
| UPLC-ESI-MS/MS | Li et al. (2019a) | ||
| HPLC | Kalinova et al. (2019) | ||
| Isoorientin, isovitexin, orientin, and vitexin | HPLC | Kalinova et al., 2019, Nam et al., 2018 | |
| HPLC–ESI–MS, HPLC–UV | Park et al. (2019) | ||
| UPLC-ESI-MS/MS | Li et al. (2019a) | ||
| HPLC–MS | Martin-Garcia et al. (2019) | ||
| Anthocyanins | Cyanidin, cyanidin-3-O-glucoside, Cyanidin-O-syringic acid, cyanidin-3-O-glucosyl-malonylglucoside, peonidin, petunidin 3-O-glucoside, and cyanidin-3-O-rutinocide | UPLC-ESI-MS/MS | Li et al. (2019a) |
| Cyanidin-3-O-glucoside and cyanidin-3-O-rutinocide | HPLC-ESI-MS | Zhu (2016) | |
| Anthraquinones | Aloe-emodin, aurantio-obtusin, chryso-phanol, emodin, rhein, and physcion | HPLC–DAD and UPLC–DAD | Zhu (2016) |
| Phenolic acids | Caffeic acid, chlorogenic acid, ferulic acid, gallic acid, 4-hydrobenzoic acid, isovanilic acid, p-coumaric acid, p-hydroxybenzoic, and syringic acids | RP–UHPLC–ESI-MS | Dziedzic et al. (2018) |
| Caffeic acid, chlorogenic acid, ferulic acid, gallic acid, p-coumaric acid, p-hydroxybenzoic, protocatechuic, syringic, and vanillic acids | HPLC, MS, and NMRS | Zhu (2016) | |
| Vanillic acid, vanillin, and protocatechuic acid | HPLC | Kalinova et al. (2019) | |
| Stilbene | Resveratrol | HPLC | Zhu (2016) |
| Fagopyrins | Fagopyrin A–F | NMRS and MS | Joshi et al, (2020) |
| HPLC-UV–vis photometry | Zhu (2016) | ||
| Fagopyritol | Fagopyritol A1 and B1 | GC–MS and NMRS | Wu, Wang, Qiu, & Li (2018) |
| Steroids | β-sitosterol, β-sitosterol palmitate, daucosterol, ergosterol peroxide, stigmsat-4-en-3,6-dione, stigmast-5-en-3-ol | Capillary GC/MS | Zhu (2016) |
| Triterpenoids | Glutinone, glutinol, olean-12-en-3-ol and urs-12-an-3-ol | Capillary GC/MS | Jing et al. (2016) |
| Phenylpropanoid glycosides | Diboside A and tatarisides A–G | HPLC–PDA/LTQ-FTICR-MS, NMRS, and MS | Zhu (2016) |
| Proteins | Amino acid compositions | HPLC | Tien et al. (2018) |
| Carbohydrates | Polysaccharides and monosaccharides | IRS, GC, GC–MS, NMRS, and HPLC | Ji et al. (2019) |
| d-chiro-inositol | HPLC- ELSDs | Zhu (2016) | |
| Fatty acids | Fatty acid compositions | GC | Sinkovic et al. (2020) |
| Free fatty acid compositions | GLC | Tien et al. (2018) | |
| Vitamins | Vitamins B1, B6, and C | HPLC | Kim, Kim, & Park (2004) |
| Carotenoids | Lutein and β-carotene | HPLC-UV-HG-AFS | Tuan, Thwe, Kim, Kim, Lee, & Park (2013) |
Abbreviations:HPLC–High-performance liquid chromatography; HPLC-ESI-MS–High-performance liquid chromatography-electrospray ionization-mass spectrometry; HPLC-UV–High-performance liquid chromatography-UV analyses; UPLC-ESI-MS/MS–Ultra performance liquid chromatography–electrospray ionization–tandem mass spectrometry system; HPLC-MS–High-performance liquid chromatography-mass spectrometry; RP-UHPLC-ESI-MS–Reverse-phase ultra-performance liquid chromatography electrospray ionization-mass spectrometry; HPLC-PAD–High performance liquid chromatography with photo-diode array detector; LIT-FTICR-MS–Linear ion trap Fourier transform ion cyclotron resonance hybrid mass spectrometry; NMRS–Nuclear magnetic resonance spectroscopy; HPLC-DAD–High-performance liquid chromatography with diode array detector; UPLC–DAD- Ultra performance liquid chromatography with diode array detector; MS–Mass spectrometry; GC–MS–Gas chromatography-mass spectrometry; HPLC-PDA/LTQ-FTICR-MS–High performance liquid chromatography photo-diode array detector/linear ion trap Fourier transform ion cyclotron resonance hybrid mass spectrometry; IRS–Infrared spectroscopy; GC–Gas chromatography; HPLC-ELSDs–High-performance liquid chromatography-evaporative light-scattering detectors; GLC–Gas-liquid chromatography; HPLC-UV-HG-AFS–High performance liquid chromatography-UV irradiation-hydride generation-atomic fluorescence spectrometry.
2.1.1. Flavonols
Flavonols are the principal bioactive compound of buckwheat. Numerous flavonols have been detected from various Fagopyrum species. Rutin is the main flavonol in buckwheat where it comprises 90% of the total phenolics (Sytar, Biel, Smetanska, & Brestic, 2018a). Rutin and quercetin were isolated from different organs such as leaves, flowers, sprouts, and seeds of F. esculentum, F. tataricum, and F. cymosum (Jing et al., 2016). The content of rutin and quercetin is more in Tartary buckwheat than common buckwheat (Borovaya and Klykov, 2020, Zhu, 2016). Rutin content is higher in common buckwheat flowers (47–63 mg/g), followed by stems (6–14 mg/g) and roots (3–8 mg/g) (Borovaya & Klykov, 2020). Rutin also presents in immature seeds but its quantity reduces during seed ripening. Usually, rutin content in common buckwheat is lower in seeds [0.01–0.02% of (DW)] compared to other organs, whereas during germination, sprouts (cotyledons) may contain up to 0.6% of DW (Taguchi, 2016). Kalinova, Vrchotova, & Jan Triska (2019) observed 0.283 g/kg rutin in embryo axis with the cotyledons of common buckwheat. The concentration of rutin quickly increases from 0.1 mg/g DW in ungerminated seed to 3 mg/g DW in 27 days aged plant in green and etiolated seedlings (Borovaya & Klykov, 2020).
Seeds of Tartary buckwheat have higher amount of rutin (14.1 mg/g DW) compared to common buckwheat (0.2 mg/g DW) (Zhu, 2016). Similarly, rutin content is higher in groats of Tartary buckwheat compare to common buckwheat, while sprouts of Tartary buckwheat contain 2.2 folds more rutin than sprouts of common buckwheat (Li, 2019) and rutin content increased 4 times after 7 days of germination (Zhu, 2016). The highest amount of rutin present in Tartary buckwheat sprouts could be up to 109 mg/100 g FW (Joshi et al., 2020). Maximum rutin content recorded in flowers and leaves during seed development and the flowering stage. Rutin acts as a vital element for the protection of common buckwheat and Tartary buckwheat plants against solar UV radiation, chilling injury, drought, and insects. Extremely high rutin content and activity of rutinoside makes very strong bitterness, which prevents animals to graze the common buckwheat particularly Tartary buckwheat (Kreft et al., 2020, Joshi et al., 2020). Recently, thirty seven flavonols i.e., syringetin 3-O-hexoside, dihydromyricetin, kaempferol 3-O-robinobioside, kaempferol 3-O-rutinoside, methyl quercetin O-hexoside, quercetin 4′-O-glucoside, kaempferol 3-O-galactoside, quercetin 3-alpha-l-arabinofuranoside, kaempferide, isorhamnetin 5-O-hexoside, dihydroquercetin, isorhamnetin O-hexoside, myricetin, quercetin, kaempferol, kumatakenin, 3-Hydroxyflavone, myricetin 3-O-galactoside, rutin, isorhamnetin 3-O-neohesperidoside, fustin, kaempferol 3,7-dirhamnoside, quercetin 3-O-glucoside, quercetin 7-O-β-d-Glucuronide, quercetin O-acetylhexoside, kaempferol 3-O-glucoside, kaempferol 3-O-rhamnoside, aromadedrin, kaempferol 7-O-rhamnoside, kaempferol 3,7-O-diglucoside 8-prenyl derivative, laricitrin, morin, syringetin, isorhamnetin, di-O-methylquercetin, 7-O-methxyl quercetin, and 3,7-di-O-methylquercetin were identified from leaves of three varieties of common buckwheat and one variety of Tartary buckwheat (Li et al., 2019a). Moreover, kaempferol, quercitrin (quercetin-3-O-rhamnoside) and quercetin-3-O-rutinoside-3′-O-β-glucopyranoside have been identified from various organs (i.e., leaf, root, stem, flower, and seed) of F. tataricum and F. cymosum, while quercetin-3-O-β-d-galactoside found in F. esculentum and F. tataricum. Similarly, isoquercitrin detected from F. esculentum and isoquercetin detected from F. cymosum (Jing et al., 2016).
2.1.2. Flavones
Flavones are another major subgroup of flavonoids. Flavones broadly exist in seeds, leaves, sprouts, flowers, grains, and hulls of Fagopyrum species. Zielinska, Turemko, Kwiatkowski, & Zielinski (2012) have identified the existence of orientin, vitexin, homoorientin, and isovitexin in common buckwheat seeds. In contrary, the accumulation of vitexin, isovitexin, orientin, and isoorientin has been reported in cotyledons and seeds of common buckwheat (Matsui & Walker, 2019), while these flavones noted in F. cymosum (Jing et al., 2016). Four flavones including orientin, isoorientin, vitexin and isovitexin have been identified from common buckwheat sprouts (Kwon et al., 2018), while these flavones isolated from seeds, sprouts, and germinated seeds of common and Tartary buckwheat (Zhu, 2016). Common buckwheat hull extracts contain orientin, vitexin, isoorientin, and isovitexin, whereas Tartary buckwheat hull extracts contain isoorientin (Park et al., 2019). On the other hand, orientin, vitexin, isoorientin, and isovitexin have been isolated from buckwheat grain and hulls, while buckwheat seeds contain isovitexin (Giménez-Bastida et al., 2018, Kwon et al., 2018). Common buckwheat has a higher amount of vitexin, isovitexin, orientin and isoorientin than Tartary buckwheat (Sytar et al., 2018a). The concentration of these flavones increased during seed germination in common buckwheat, whereas, the concentration remains unchanged (0.1–0.6 mg/g DW) till 9 days of germination (Zhu, 2016). Beside these flavones, quercetin-3-O-rutinoside-3′-O-glucoside and quercetin-3-O-rutinoside-7-O-galactoside have been found in F. tataricum and 3′,4′-methylenedioxy-7-hydroxy-6-isopentenyl flavone and Luteolin have been identified from F. cymosum (Jing et al., 2016).
2.1.3. Flavanones
Li et al. (2019a) have isolated a total of twenty four flavanones from leaves of three varieties of common buckwheat and one variety of Tartary buckwheat and those are afzelechin (3,5,7,4′-Tetrahydroxyflavan), eriodictyol O-malonylhexoside, hesperetin 7-rutinoside (hesperidin), hesperetin 7-O-neohesperidoside, naringenin 7-O-glucoside, naringenin O-malonylhexoside, naringenin, isoliquiritigenin, xanthohumol, hesperetin O-hexosyl-O-hexoside, hesperetin 5-O-glucoside, naringenin 7-O-neohesperidoside, hesperetin O-malonylhexoside, eriodictyol, isosakuranetin-7-neohesperidoside, naringenin chalcone, butein, phloretin, homoeriodictyol, hesperetin, 7-O-Methyleriodictyol, 4′-Hydroxy-5,7-dimethoxyflavanone, isosakuranetin, and pinocembrin. Besides, flavanone, (−)-liquiritigenin has been detected from Tartary buckwheat roots (Lv et al., 2017). Buckwheat honey is a good reservoir of hesperetin that regulates liver and DNA damage in mice caused by carbon tetrachloride (Bose et al., 2018, Joshi et al., 2020).
2.1.4. Flavanols/ Flavan-3-ols (catechins)
Flavan-3-ols are derivatives of flavans that comprises of different compounds including catechin, epicatechin, epigallocatechin, gallocatechin, proanthocyanidins, epiafzelechin, and so on. Epicatechin and catechin have been detected from different botanical parts (i.e., leaf, root, stem, seeds, and sprout) of various Fagopyrum species (Zhu, 2016). Four flavanols namely (−)-epicatechin (25.70 mg/kg), (−)-epicatechin 3-O-p-hydroxybenzoate, (−)-epicatechin 3-O-(3,4-di-O-methyl) gallate (61.27 mg/kg), and (+)-catechin-7-O-glucoside have been observed in different parts like seeds, fruits, leaves, husks, and so on of common buckwheat, while (−)-catechins and (−)-epicatechin were isolated from F. cymosum (Jing et al., 2016, Lv et al., 2017, Kalinova et al., 2019, Borovaya and Klykov, 2020). Moreover, (−)-epicatechin has been found in F. esculentum, F. tataricum, and F. cymosum (Jing et al., 2016). Furthermore, catechin (6–66 mg/kg), epicatechin (23–110 mg/kg), epicatehin-3-O-dimethylgallate (1–11 mg/kg), epicatechin(4–8)-epicatechin-O-(3,4-dimethyl)-gallate (1–6 mg/kg), epiafzelechin-(4–6)-epicatechin (3–9 mg/kg), epiafzelechin-(4–8)-epicatechin-methylgallate (1–3 mg/kg), epiafzelechin-(4–8)-epicatechin-p-OH-benzoate (0–9 mg/kg), epiafzelechin-(4–8)-epiafzelechin-(4–8)-epicatechin (0–4 mg/kg), epiafzelechin-(4–8)-epicatechin(3,4-dimethyl)-gallate (17–57 mg/kg), epiafzelechin-(4–8)-epiafzelechin(4–8)-epicatechin-O-(3,4-dimethyl)-gallate (8–34 mg/kg), and epicatechingallate (4–22 mg/kg) were identified from different plant parts of eight genotypes of common buckwheat (Zhu, 2019). On the contrary, Kalinova et al. (2019) observed 161.41 mg/kg catechin in embryo axis with the cotyledons and 257.60 mg/kg epicatechin, 118.6 mg/kg procyanidin B2, and 61.27 mg/kg epicatechin gallate, 45.55 mg/kg catechin were determined in seed coat of common buckwheat.
2.1.5. Anthocyanins
Anthocyanins are color-producing pigments expressed in flowers, fruits, and plants. Li et al. (2019a) identified a total of eighteen anthocyanins namely cyanidin 3,5-O-diglucoside, cyanidin 3-O-glucosyl-malonylglucoside, delphinidin 3-O-rutinoside, delphinidin 3-O-glucoside, pelargonin, malvidin 3,5-diglucoside, cyanidin 3-O-rutinoside, petunidin 3-O-glucoside, pelargonidin 3-O-beta-d-glucoside, malvidin 3-O-galactoside, malvidin 3-O-glucoside, peonidin O-hexoside, rosinidin O-hexoside, cyanidin, peonidin, cyanidin O-syringic acid, cyanidin 3-O-glucoside, and peonidin O-malonylhexoside from leaves of three common buckwheat and one Tartary buckwheat species. Moreover, four anthocyanins such as cyanidin 3-O-glucoside, cyaniding 3-O-rutinoside, cyanidin 3-O-galactoside, and cyanidin 3-O-galactopyranosyl-rhamnoside were detected in the sprout of common buckwheat (Zhu, 2016, Kwon et al., 2018) and petals of flowers (Borovaya & Klykov, 2020). The presence of anthocyanins is higher in sprout of Tatary buckwheat than common buckwheat that reduce the possibility of heart disease (Kwon et al., 2018). Moreover, cyanidin-3-O-glucoside and cyanidin-3-O-rutinocide were identified from Tartary buckwheat leaves, stems, and sprouts (Borovaya and Klykov, 2020, Jing et al., 2016, Zhu, 2016), while cyanidin-3-O-rutinocide content was more compared to cyanidin-3-O-glucoside (Zhu, 2016). In three days of seedlings, the accumulation of anthocyanins is high in cotyledons and hypocotyls of buckwheat under light condition (Kwon et al., 2018). In contrary, anthocyanins content is higher in hypocotyls of buckwheat sprouts compared to cotyledons (Borovaya & Klykov, 2020). In buckwheat, anthocyanins mainly deposit at the bottom of the stem, which gradually pales in color from the bottom to the top of the stem. The expression of genes responsible for anthocyanin synthesis occurs in the roots of buckwheat. Therefore, anthocyanins may be carried from the bottom of the stem or root to the apex of the stem of buckwheat (Matsui & Walker, 2019). Still, more research is required to explain the process.
2.1.6. Fagopyrin
Fagopyrin is another type of flavonoid, which stores mainly in buckwheat seeds with low density and difficult to extract. Several fagopyrins were identified from three species of Fagopyrum (F. esculentum, F. tataricum, and F. cymosum). Six fagopyrin derivatives were identified i.e., fagopyrin A–F (Joshi et al., 2020). Stojilkovski, Glavac, Kreft, & Kreft (2013) observed the highest fagopyrin content in F. cymosum flower was 20.7 mg/g. They also determined a considerable amount of fagopyrin ≤ 4.83 mg/g and 0.322–2.3 mg/g from common buckwheat flowers and leaves, respectively. During seed germination concentration of fagopyrin is the highest and light is important to transform protofagopyrins to fagopyrins as the increase of fagopyrin is concentrated by supporting the light condition (Joshi et al., 2020). Due to consuming a large quantity of buckwheat, it may provoke fagopyrism. Fagopyrism indicates photosensitization which causes irritation of the skin, oedema and a serous exudate (Zhu, 2016). The amount of fagopyrin is lower than other antioxidative compounds, and maybe its presence in grain cannot affect human health negatively (Dziedzic et al., 2018). Moreover, more advancement of analytical approaches is required for further fagopyrin study.
2.1.7. Proanthocyanidins
Zhu (2019) isolated procyanidin B2 (3–13 mg/kg) and procyanidin B5 (4–11 mg/kg) from various parts of eight genotypes of common buckwheat, whereas procyanidin A1, A2, A3, B2, and B3 were identified by Li et al. (2019a) from leaves of one Tartary buckwheat and three common buckwheat species.
2.1.8. Isoflavones
Isoflavones, sissotrin, 2′-Hydroxydaidzein, glycitin, 6-Hydroxydaidzein, genistein 7-O-Glucoside, formononetin, and formononetin 7-O-glucoside were isolated from leaves of three common buckwheat and one Tartary buckwheat species (Li et al., 2019a).
2.1.9. Flavonolignan
Li et al. (2019a) identified several flavonolignan namely tricin 4′-O-(β-guaiacylglyceryl) ether O-hexoside, tricin 7-O-β-guaiacylglycerol, tricin 4′-O-β-guaiacylglycerol, tricin 4′-O-syringic acid, tricin 4′-O-(syringyl alcohol) ether 5-O-hexoside, and tricin 4′-O-(syringyl alcohol) ether 7-O-hexoside from leaves of one Tartary buckwheat and three common buckwheat varieties.
2.2. Phenolic acids and their derivatives
Several researchers have been identified different phenolic acids and their derivatives from Fagopyrum species. The phenolic acids isolated from every milled sample (i.e., hull, coarse bran, fine bran, and light flour) of Tartary buckwheat including chlorogenic, caffeic, ferulic, gallic, p-hydroxybenzoic, protocatechuic, syringic, p-coumaric, and vanillic acids (Li, 2019, Zhu, 2016). The concentration of phenolic acids is highest in the brans, as in the free form and comparatively lower in the bound form. In bran, the most abundant phenolic acids are p-hydroxybenzoic acid (up to 3.6 mg/g fine bran), caffeic acid (0.38 mg/g fine bran), chlorogenic acid (0.21 mg/g fine bran), and protocatechuic acid (0.18 mg/g fine bran) (Li, 2019, Zhu, 2016). On the other hand, protocatechuic acid (54 mg/100 g dry weight) is the most abundant phenolic acid noted in hulls (Li, 2019, Zhu, 2016). Moreover, ferulic acid, p-cumaric and protocatechuic acid have been identified in a lower amount from different parts (i.e., leaves, stems, roots, seeds, and so on) of common buckwheat, whereas benzoic acid, protocatechuic acid, p-hydroxybenzoic acid, succinic acid, protocatechuic acid methyl ester, and syringic acid were isolated from F. cymosum (Wajid et al., 2015). Still, more comparative investigation is required regarding the phenolic acid composition of Tartary buckwheat and common buckwheat.
2.3. Stilbenes
Buckwheat contains only one stilbene called resveratrol. It remains in trans- and cis-forms, where trans-form is more stable than cis-form. Trans-resveratrol was identified from leaves, seeds and hulls of common and Tartary buckwheat (Jing et al., 2016, Zhu, 2016). Tartary buckwheat seeds contain 3.43–3.50 mg/kg trans-resveratrol and leaves contain 0.19–0.20 mg/kg trans-resveratrol. On the contrary, common buckwheat leaves and seeds contain 1.81–1.82 mg/kg and 0.98–1.68 mg/kg trans-resveratrol, respectively. Hence, Tartary buckwheat showed a good source of trans-resveratrol compared to common buckwheat (Zhu, 2016).
2.4. Steroids
From seed oil of common buckwheat, five steroids were isolated namely stigmast-5-en-3-ol, stigmast-5,24-dien-3-ol, trans-stigmast-5,22-dien-3-ol, 23S-methylcholesterol, and 6-hydroxy stigmasta-4,22-dien-3-one (Jing et al., 2016). Similarly, from Tartary buckwheat seeds, the other five steroids specifically daucosterol, β-sitosterol palmitate, stigmsat-4-en-3,6-dione, ergosterol peroxide, and β-sitosterol were identified (Jing et al., 2016). Other steroids were isolated as β-sitosterol, hecogenin and β-daucosterol from F. cymosum roots (Wajid et al., 2015).
2.5. Triterpenoids
Seven triterpenoids have been isolated from three Fagopyrum species (F. esculentum, F. tataricum, and F. cymosum). Triterpenoid, olean-12-en-3-ol and urs-12-an-3-ol were reported from the seed oil of common buckwheat, whereas α-thujene and α-terpineol were isolated from Tartary buckwheat and ursolic acid from both F. tataricum and F. cymosum (Lv et al., 2017). Similarly, glutinol and glutinone were identified from F. cymosum rhizomes (Jing et al., 2016).
2.6. Tannins
Tannins are astringent phenolic compounds present in buckwheat. Tannins may protect against various biotic and abiotic stresses. Because of this, buckwheat bran is consumed in a higher amount for nutritional or medicinal purposes. Usually, throughout the development of buckwheat seedlings, tannin concentration gradually increases (Joshi et al., 2020). 3,3-di-O-galloyl-procyanidin B-2 and 3-O-galloyl-procyanidin B-2 were identified from rhizomes of F. cymosum (Jing et al., 2016, Joshi et al., 2020).
2.7. Phenylpropanoid glycosides
A total of thirteen phenylpropanoid glycosides have been isolated from different Fagopyrum species. Among these, tatarisides A–G and diboside A were isolated from roots of Tartary buckwheat (Jing et al., 2016, Zhu, 2016), while diboside A also extracted from rhizomes of F. cymosum (Jing et al., 2016). Four compounds namely 3,6-di-p-coumaroyl-1,6′-di-feruloyl sucrose, 1,3,6′-tri-feruloyl-6-p-coumaroyl sucrose, 1,3,6-tri-p-coumaroyl-6′-feruloyl sucrose, and 1,3,6,6′-tetra-feruloyl sucrose were identified from seed of Tartary buckwheat (Jing et al., 2016, Zhu, 2016). Another phenylpropanoid glycosides lapathoside A was detected from F. cymosum rhizomes (Jing et al., 2016).
2.8. Other compounds
Fagopyrum species also contain some other phytochemicals like alkaloids, anthraquinones, coumarins, and carbohydrate derivatives. Squalene, n-butyl-β-d-fructopyranoside, γ-tocopherol, and (3-Methoxyphenyl)-2-piperidinemethanol were isolated from Tartary buckwheat, while (3-Methoxyphenyl)-2piperidinemethanol also detected from F. cymosum (Jing et al., 2016). Similarly, N-trans-feruloyltyramine, 3,4-dihydroxy benzamine, 5,5′-di-α-furaldehyde dimethyl ester, uracil, and 5-hydroxymethyl-2-furoic acid were isolated from Tartary buckwheat (Jing et al., 2016, Zhu, 2016). Moreover, emodin, emodin-8-O-β-d-glucopyranoside, glycerol mono palmitate, n-trans-coumaroyltyramine, shakuchirin, succinic acid, and 7-hydroxycoumarin were found in F. cymosum (Jing et al., 2016, Lv et al., 2017, Wajid et al., 2015). Among these compounds N-trans-feruloyltyramine exhibited neuro-protective functions (Zhu, 2016), whereas emodin showed several anti-virus activities particularly against novel coronavirus (2019-nCoV/ SARS-CoV-2) (Zhou, Hou, Shen, Huang, Martin, & Cheng, 2020). As most of these identified compound’s health effect is yet unexplored, further research is required to know the pharmaceutical effects of these compounds.
During the last decade, many researchers have been identified different kinds of bioactive compounds in buckwheat species with their pharmacological and beneficial health effects including anti-oxidative, anti-cancer, anti-inflammatory, neuro-protection, cholesterol-lowering, neuroprotective, antidiabetic, cardioprotective, and so on (Table 3 ).
Table 3.
Significant health effects of different bioactive compounds of buckwheat.
| Bioactive compounds | Effect | References |
|---|---|---|
| Polysaccharides, procyanidin dimer, quercetin, and tannins | Anti-tumor | Ji et al., 2019, Jing et al., 2016, Joshi et al., 2020, Lv et al., 2017, Wang et al., 2016b |
| Catechins, coumarins, curcuminoids, mandelic acid, lignans, polysaccharides, phenolic acids, quercetin, rutin, stilbenes, and tannins | Anti-oxidant | Bose et al., 2018, Ji et al., 2019, Jing et al., 2016, Joshi et al., 2020, Lv et al., 2017, Tungmunnithum et al., 2018, Wang et al., 2018, Wang et al., 2016b |
| Apigenin, chrysin, hispidulin, hesperidin, isoorientin, isovitexin, luteolin, polysaccharides, quercetin, and rutin | Anti-inflammatory | Bose et al., 2018, Giménez-Bastida and Zieliński, 2015, Ji et al., 2019, Tungmunnithum et al., 2018, Wang et al., 2018 |
| Apigenin, naringenin, polysaccharides, quercetin, quercitrin, rutin, and silymarin | Hepatoprotective | Bose et al., 2018, Ji et al., 2019, Jing et al., 2016, Joshi et al., 2020, Zhang et al., 2015 |
| Chlorogenic acid, epicatechin, hydroxybenzoic acid, luteolin, kaempferol, quercetin, quercitrin, and rutin | Anti-bacterial | Bose et al., 2018, Jing et al., 2016, Joshi et al., 2020, Tungmunnithum et al., 2018, Wang et al., 2018 |
| Quercetin and esperidin | Anti-fungal | Bose et al. (2018) |
| Apigenin, catechin, dihydroquercetin, emodin, hesperidine, morin, quercetin, and rutin | Anti-viral | Bose et al., 2018, Wang et al., 2018, Zhou et al., 2020 |
| Kaempferol, quercetin, and rutin | Anti-ulcer | Bose et al. (2018) |
| Globulin | Anti-fatigue | Jing et al. (2016) |
| Polysaccharides | Hypolipidemic | Ji et al., 2019, Wang et al., 2016b |
| Apigenin, hesperidin, luteolin, polysaccharides, and quercetin | Immunoregulatory | Ji et al., 2019, Tungmunnithum et al., 2018, Wang et al., 2016b |
| Galangin, kaempferol, myricetin, N-trans-feruloyltyramine, polysaccharides, and rutin | Neuroprotective | Giménez-Bastida and Zieliński, 2015, Ji et al., 2019, Jing et al., 2016, Wang et al., 2016b, Wang et al., 2018, Zhu, 2016 |
| d-chiro-inositol, isoquercetin, polysaccharides, quercetin, and rutin | Anti-diabetic | Bose et al., 2018, Ji et al., 2019, Joshi et al., 2020, Lv et al., 2017, Wang et al., 2018, Zhang et al., 2015 |
| Apigenin, cinnamic acid, ferulic acid, gallic acid, isorhamnetin, kaempferol, luteolin, naringenin, quercetin, resveratrol, rutin, and syringic acid | Cardioprotective | Bose et al., 2018, Gorniak et al., 2019, Joshi et al., 2020, Tungmunnithum et al., 2018, Wang et al., 2018 |
| Apigenin, naringenin, nobiletin, phenylpropanoid glycosides, procyanidin dimer, quercetin, and tangeretin | Anti-cancer | Giménez-Bastida and Zieliński, 2015, Gorniak et al., 2019, Jing et al., 2016, Lv et al., 2017, Tungmunnithum et al., 2018, Wang et al., 2018 |
| Quercetin | Anti-atherosclerosis | Bose et al., 2018, Gorniak et al., 2019 |
| Fagopyritol A1 and rutin | Blood glucose and cholesterol lowering | Giménez-Bastida and Zieliński, 2015, Joshi et al., 2020, Wu et al., 2018, Lv et al., 2017 |
| Catechins, fisetin, genistein, hydrobenzoic acids, kaempferol, synigric acid, toxifolin, and vanillic acid | Anti-neoplastic | Bose et al., 2018, Joshi et al., 2020 |
| Catechins, galangin, kaempferol, and myricetin | Anti-aging | Joshi et al., 2020, Wang et al., 2018 |
| Kaempferol, myricetin, rutin, and quercetin | Anti-thrombotic | Bose et al., 2018, Lv et al., 2017 |
3. Biosynthesis pathway of flavonoids present in buckwheat
The flavonoid biosynthesis pathway is a part of the phenylpropanoid synthesis pathway, which is indifferent within the plant species, although their encoding genes and enzymes can be different (Matsui & Walker, 2019).
3.1. Flavonols and their O-glycosides
Various flavonol-O-glycosides, including quercitrin, rutin, isoquercitrin, quercetin 3-O-robinobioside, kaempferol 3-O-rutinoside and so on present in buckwheat (Kiprovski et al., 2015, Taguchi, 2016), therefore buckwheat was used as an ideal plant to investigate the biosynthesis of flavonoids particularly in the old days (Taguchi, 2016). Flavonols and their O-glycosides have a well-organized biosynthesis pathway in different higher plants (Martin and Li, 2017, Perez de Souza et al., 2020). The rutin biosynthesis initiates from phenylalanine which is deaminated by phenylalanine ammonia-lyase (PAL) to form cinnamic acid, followed by a series of reactions catalyzed by cinnamate 4-hydroxylase (C4H), 4-coumarate CoA ligase (4CL), chalcone synthase (CHS) and chalcone isomerase (CHI) (Davies et al., 2020, Matsui and Walker, 2019, Taguchi, 2016, Yonekura-Sakakibara et al., 2019). This condensation reaction is a major action in the pathway directing to the synthesis of flavonoids (Holton & Cornish, 1995). Naringenin is the main substance, from which various types of flavonoids are synthesized (Lehka, Eichenberger, Bjorn-Yoshimoto, Vanegas, Buijs, & Jensen, 2017). CHS mainly occurs to form flavonoid pigments and isoflavonoids. CHS gene is important for rutin biosynthesis due to its down-regulation in tomato fruits by RNA interference (RNAi) of CHS. As a result, CHS reduces flavonoids accumulation (Chauhan, Gupta, Sharma, Rana, Sharma, & Jana, 2010). In this state, Flavone 3-hydroxylase (F3H) and flavonoid 3′-hydroxylase (F3′H) catalyze the reactions to form dihydrokaempferol and dihydroquercetin, respectively, from Naringenin. Then kaempferol and quercetin are produced from dihydrokaempferol and dihydroquercetin by flavonol synthase (FLS), respectively (Matsui et al., 2018, Taguchi, 2016, Yonekura-Sakakibara et al., 2019, Zhao et al., 2015). The last step of rutin synthesis is the glycosylation of quercetin by quercetin 3-O-glucosyltransferase (F3GT) to form isoquercitrin, this isoquercitrin converted to rutin by rhamnosyl transferase (RT) (Davies et al., 2020, Taguchi, 2016). The activity of F3GT was linked with the rutin accumulation, which was refined partly from the common buckwheat cotyledons (Suzuki et al., 2005, Taguchi, 2016).
The rutin-degrading enzymes are the catalyzer for the degradation of rutin in buckwheat, which hydrolyze the glycosidic bonds of rutin (Taguchi, 2016). Unfortunately, the physiological functions of those enzymes that degrade rutin in planta have yet not been interpreted thoroughly. Several researches have been done to identify the rutin accumulation and biosynthetic gene expression patterns in several developmental stages of buckwheat. The flowering stage of buckwheat contains the maximum amount of rutin and some important enzymes are associated with the biosynthesis of flavonoids such as PAL, CHS, CHI, and FLS (Gupta, Sharma, Rana, & Chauhan, 2011). During the process of germination, the content of rutin and the expression of biosynthetic enzymes genes (e.g., PAL, 4CL, and F3H) raised in sprouts. However, in common buckwheat, those genes expressed mostly in stem and root, while the flavonoids synthesis was higher in leaves and flowers, which seems that flavonols might be transferred from roots and stems toward leaves and flowers. The biosynthesis of rutin and anthocyanin of buckwheat is also affected by light irradiation (Taguchi, 2016). In common buckwheat, rutin synthesis was induced in sprouts by light irradiation, particularly by the UV-B light, which was 1.6 times greater compared to the amount under dark conditions (Tsurunaga et al., 2013). A similar observation was found for the accumulation of anthocyanin (Taguchi, 2016).
A few studies were done until now regarding flavonoid biosynthesis in buckwheat in the aspect of molecular breeding. In Arabidopsis, flavonoid biosynthesis is positively regulated by the transcription factor AtMYB12, which also increases the effect of flavonoids biosynthetic genes and the amount of rutin of common buckwheat hairy root culture due to its overexpression (Matsui and Walker, 2019, Park et al., 2012). Luo et al. (2020) revealed that transcription factor TrMYB4 showed a significant regulatory effect in flavonoids, particularly in rutin biosynthesis of F. cymosum. But they suggested, further studies are required to completely analyze the base of rutin biosynthesis regulatory network under this transcription factor for molecular function. Another transcription factor FeMYBF1 regulating the biosynthesis of flavonols was identified by Rapid amplification of cDNA end (RACE) and genome walking (Matsui et al., 2018, Matsui and Walker, 2019). Flavonoid biosynthetic gene, FeFLS1, is particularly expressed in flowers and buds, while FeFLS2 is mainly expressed in leaves, stems, and roots of seedlings and also in seeds. But gene FeFLS is highly expressed in flowers (Matsui et al., 2018). The whole sequences of FeFLS1 and FeFLS2 are yet unknown (Matsui & Walker, 2019). Matsui et al. (2018) isolated a gene named FeMYBF1, which encodes an R2R3-MYB TF regulating flavonol especially rutin and other flavonoids biosynthesis in buckwheat as well. On the contrary, Bai et al. (2014) identified two transcription factor genes, namely FtMYB1 and FtMYB2, from Tartary buckwheat, which were expressed mostly in the flowers compared to other organs during flavonol biosynthesis. On the other hand, the accumulation of proanthocyanidins was significantly increased by the overexpression of FtMYB1 and FtMYB2. The R2R3-MYB transcription factors such as FtMYB1, FtMYB2, FtMYB123L, FtMYB11, FtMYB13, FtMYB14, FtMYB15, and FtMYB16 were identified as essential repressors for the biosynthesis of flavonoids in buckwheat (Bai et al., 2014, Li et al., 2019, Zhang et al., 2018, Zhou et al., 2017). These transcription factors regulate the expression of genes (i.e., CHS, CHI, F3H and FLS) associated with the flavonoid biosynthesis pathway (Luo et al., 2020, Matsui et al., 2018, Taguchi, 2016, Zhou et al., 2017). Therefore, the analysis of the transcription factors focusing on those genes involved in flavonoids biosynthesis in buckwheat would be needed. Moreover, many unknown regulators associated with the flavonoid biosynthesis of buckwheat require to be identified.
3.2. C-Glucosylflavones
The C-glucosylflavones are another important flavonoid present in different cereals and pseudocereals such as buckwheat, rice, maize, and wheat (Brazier-Hicks et al., 2009, Casas et al., 2014). Buckwheat contains mainly four C-glucosylflavones including vitexin, isovitexin, orientin, and isoorientin. Common buckwheat seeds and cotyledons accumulate most of the C-glucosylflavones (Matsui and Walker, 2019, Taguchi, 2016). At the anomeric carbon, the sugars belonging to C-glycosidic flavonoids are directly bound to the aglycon of the flavonoids by a C—C bond (Gan et al., 2019, Nagatomo et al., 2014). The C-glycosides are more stable than other common glycosides like O-glycosides since their C—C bonds show resistance to glycosidase or acid and alkali hydrolysis (Gan et al., 2019, Nagatomo et al., 2014).
Intensive experiments have been conducted to identify the molecular mechanisms of C-glucosylflavones biosynthesis in various crops like rice (Brazier-Hicks et al., 2009), maize (Ferreyra, Rodriguez, Casas, Labadie, Grotewold, & Casati, 2013) and buckwheat (Nagatomo et al., 2014). In the 1980s, Kerscher & Franz (1987) first proposed the biosynthesis pathway of C-glucosylflavones in buckwheat and purification of C-glucosyltransferase (CGT) was partially done by Kerscher & Franz (1988). The biosynthesis of vitexin and isovitexin begins from the intermediate compound of flavonol synthesis (Naringenin), which was hydroxylated by F2H at the 2-position of flavanone and produces 2-hydroxyflavanone. This 2-hydroxyflavanone is in equilibrium with its another open-ring form (dibenzoylmethane form), which is C-glucosylated by using C-glucosyltransferase (CGT) (Gan et al., 2019, Nagatomo et al., 2014, Taguchi, 2016, Vanegas et al., 2018). Two closed-circular forms of C-glucoside are 2-hydroxyflavanone 6-C-glucoside and 8-C-glucoside which are also in equilibrium with produced C-glucoside to form two flavone C-glycoside (vitexin and isovitexin) by dehydration after the sugar moiety conjugation (Vanegas et al., 2018). The dehydration process seems to be done enzymatically, but the enzymes are still missing (Gan et al., 2019, Nagatomo et al., 2014, Taguchi, 2016).
Nagatomo et al. (2014) reported that the FeCGT genes mostly exist in developing cotyledons of common buckwheat, recommending that FeCGT genes expressed mainly in cotyledons during seed germination. Like rutin biosynthesis, the accumulation of C-glucosylflavones was not influenced by light irradiation (Taguchi, 2016). Therefore, advanced researches will be needed to identify the causes of C-glucosylflavones accumulation occurred particularly in cotyledons during seed germination of buckwheat.
4. Nutritional constituents
Besides numerous bioactive compounds buckwheat is rich in high-quality carbohydrates, protein and amino acid, fatty acid, vitamins, and minerals. Compared to other major cereal grains, buckwheat has superior nutritional value (Table 4 ).
Table 4.
Comparison of nutritional compounds with their concentration among buckwheat, rice, wheat, and maize. (Joshi et al., 2019, Sindhu and Khatkar, 2019).
| Nutrients | Buckwheat | Rice | Wheat | Maize |
|---|---|---|---|---|
| Proximate composition (g/100 g grain) | ||||
| Energy (Kcal) | 355 | 345 | 346 | 365 |
| Total Carbohydrates (g) | 72.9 | 78.2 | 71.2 | 74.3 |
| Total fiber (%) | 17.8 | 4.5 | 12.5 | 7.5 |
| Crude protein (%) | 12 | 6.8 | 11.8 | 9.4 |
| Moisture (%) | 11 | 13.7 | 12.8 | 10.4 |
| Fat (g) | 7.4 | 1.5 | 2.5 | 4.7 |
| Essential amino acids (% of total protein) | ||||
| Leucine | 6.7 | 8.2 | 6.3 | 13 |
| Lysine | 5.9 | 3.8 | 2.6 | 1.9 |
| Valine | 4.7 | 5.9 | 4.5 | 5.0 |
| Phenylalanine | 4.2 | 5.7 | 4.4 | 4.5 |
| Methionine | 3.7 | 3 | 3.5 | 3.2 |
| Isoleucine | 3.5 | 4.5 | 3.4 | 3.8 |
| Threonine | 3.5 | 3.8 | 2.8 | 3.9 |
| Histidine | 2.2 | 2.4 | 2.3 | 2.4 |
| Cystine | 2.2 | 2.2 | 1.8 | 2.2 |
| Tryptophan | 1.4 | 1 | 1.2 | 0.6 |
| Minerals and trace elements (mg/100 g grain) | ||||
| Potassium | 450 | 268 | 284 | 287 |
| Magnesium | 390 | 65 | 138 | 127 |
| Phosphorus | 330 | 160 | 298 | 210 |
| Calcium | 110 | 10 | 30 | 7 |
| Iron | 4 | 0.7 | 3.5 | 2.7 |
| Manganese | 3.4 | 0.5 | 2.3 | 1.9 |
| Zinc | 0.8 | 1.3 | 2.7 | 2.3 |
| Vitamins (mg/100 g grain) | ||||
| Niacin | 18 | 1.9 | 5.5 | 3.6 |
| Riboflavin | 10.6 | 0.06 | 0.2 | 0.2 |
| Thiamine | 3.3 | 0.06 | 0.5 | 0.4 |
| Choline | 440 | – | – | – |
| Tocopherols | 40 | – | – | – |
4.1. Carbohydrate
Carbohydrate is the maximum considerable constituent in buckwheat accounting for as much as 73% of the overall dry weight (Acanski et al., 2015, Li, 2019, Zhu, 2016). Buckwheat flour includes 70–91% carbohydrate relying on the milling process (Yilmaz et al., 2020). The amount of amylose in starch is the basis for the presence of degenerated starch throughout the hydrothermal processing of food materials (Skrabanja & Kreft, 2016). Nevertheless, nowadays it's far becoming greater and extra vital food source due to its gluten-free attribute (Acanski et al., 2015). Buckwheat grain contains 0.65–0.76% lowering sugars, 0.79–1.16% oligosaccharides and 0.1–0.2% non-starchy polysaccharides (Ahmad, Ahmad, Dar, Bhat, Mushtaq, & Shah, 2018). Buckwheat groats showed a low glycemic index (GI = 34.7) that indicates it does not create unhealthy spikes in blood sugar levels (Rozanska, Mikos, & Regulska-Ilow, 2020).
4.1.1. Polysaccharides
In buckwheat, polysaccharides mostly accumulate in the seed coat, hull, and cells of the aleurone layer (Skrabanja & Kreft, 2016). Buckwheat polysaccharides comprise different compounds and monosaccharide content, resulting in structural differences that may show different biological activities (Ji et al., 2019). Tartary buckwheat flour (3.16%) contains 1.28-fold more polysaccharide than Tartary buckwheat bran, that decreased by 76.4% in Tartary buckwheat bran and 59.6% in Tartary buckwheat flour after baking (Ge & Wang, 2020). The buckwheat polysaccharides also aid the excretion of cellular factors, such as TNF-α, NO, IL-2 and IL-1β, in macrophages and exhibit significance for leukemia treatment. On digestion in the intestine, buckwheat polysaccharides create short-chain fatty acids on digestion in the intestine, which produce mucosal resistance through connected with intestinal epithelial cells and may cause apoptosis of tumor cells by entering in the blood circulatory system (Ji et al., 2019).
Dietary fiber is another analogous carbohydrate that prevents digestion and absorption within the human short intestine, but it partially or fully fermented via micro-flora inside the large intestine. The amount and proportion of dietary fiber depends on the nature of processing technology used for the production of buckwheat groats, which affects its functional properties (Ahmed et al., 2013, Dziedzic et al., 2012). For nutritional purposes, total dietary fiber is categorized as insoluble and soluble. In buckwheat grains, the total dietary fiber contains 8.4%, whereas the soluble fiber is 0.2%, and the insoluble fiber is 8.2%. On the other hand, buckwheat seeds and groats constitute about 10.9% and 7.3% dietary fiber, respectively, indicating that dietary fiber is mainly present in the outer seed cover including seed coat and hull (Li, 2019). On contrary, the total dietary fiber counted 6.7–9.1%, while the soluble fiber is 4.3–6.5%, and the insoluble fiber is 2.3–3.2% in whole buckwheat flour (Suzuki, Noda, Morishita, Ishiguro, Otsuka, & Brunori, 2020).
Resistant starch (RS) is another type of dietary fiber. Resistant starches are smaller in buckwheat (2–15 μm) compared with other crops like 12.2 μm in maize, 18 μm in tapioca and 30.5 μm in potato (Ahmed et al., 2013, Li, 2019). The starch contains 20–30% amylose and 70–85% amylopectin in buckwheat, which depends on the species and variety (Suzuki et al., 2020). Common buckwheat flours contain higher amount of resistant starch compared to Tartary buckwheat flours (Zhu, 2016). Dietary fiber can protect from diabetes (particularly type 2 diabetes), hyperglycaemia, hypercholesterolaemia, heart disease, obesity and some kinds of cancer, particularly colon cancer (Ahmed et al., 2013, Dziedzic et al., 2018, Suzuki et al., 2020).
4.1.1.1. Iminosugar
Amezqueta, Galan, Fuguet, Carrascal, Abian, & Torres (2012) first isolated iminosugar, named d-fagomine, from common buckwheat seed, and they also detected its diastereomers namely 3-epi-fagomine and 3,4-di-epi-fagomine in buckwheat groats, bran, leaves, and flour. Usually, common buckwheat contains higher amount of d-fagomine compared to Tartary buckwheat (Joshi et al., 2020). The most leading amount of d-fagomine and 3,4-di-epifagomine was 44 mg/kg and 43 mg/kg, respectively in buckwheat groats (Ahmed et al., 2013, Joshi et al., 2020). Iminosugars has gained more interest as a glycosidase inhibitor because of its high bioactivities. Iminosugars is used as a dietary supplement or functional food composition, d-fagomine may lessen the chance of increasing insulin resistance, sucrose‐induced hypertension, F2‐IsoPs (markers of oxidative stress), anti-hyperglycaemic effect and suffering from an excess of potentially pathogenic bacteria, urine uric acid, steatosis, and liver DAGs, without extensively affecting perigonadal fat deposition, and impaired glucose tolerance (Dziedzic et al., 2018, Joshi et al., 2020, Ramos-Romero et al., 2020).
4.1.1.2. d-chiro-inositol
The d-chiro-inositol is a soluble carbohydrate, an epimer of myoinositol which engaged in insulin signaling pathways (Li, 2019, Zhu, 2016). Buckwheat is the most abundant source of d-chiro-inositol. Farinetta is a specific type of buckwheat flour that contains high levels of d-chiro-inositol when it milled especially (Martin-Garcia et al., 2019). Fagopyritols are an important derivative of d-chiro-inositol which accumulates mainly in aleurone tissue and embryo of buckwheat seeds. The fagopyritols present in Tartary buckwheat is 11-fold of free d-chiro-inositol (Wu et al., 2018). In buckwheat, six fagopyritols have been identified namely A1, A2, A3, B1, B2, and B3 (Suzuki et al., 2020), among these A1 and B1 are the principal fagopyritols (Wu et al., 2018, Zhu, 2016). Fagopyritols act as an insulin mediator so it is beneficial for treating Non-Insulin Dependent Diabetes Mellitus (NIDDM) disorder and Polycystic Ovarian Syndrome (PCOS) (Jing et al., 2016, Suzuki et al., 2020). Furthermore, d-chiro-inositol could have capabilities to reduce blood pressure, glucose concentration, and plasma triglycerides in humans (Zhu, 2016, Li, 2019). It also may improve polycystic ovarian syndrome (PCOS), a condition that can reason of infertility (Suzuki, et al., 2020). Besides, Wu et al. (2018) recorded the enhancement of insulin-stimulated phosphorylated PI3K and AKT by d-chiro-inositol and fagopyritol A1, fagopyritol B1 at the 0.5 mM dose.
4.2. Protein and amino acid
Buckwheat contains high nutritive value due to presence of balanced amino acid compositions. Different kinds of amino acid mainly lysine is present in buckwheat protein (Skrabanja & Kreft, 2016). This is especially important due to the fact human bodies cannot produce lysine (a building block of protein), therefore we must obtain it from food. Threonine is the first and methionine is the second limiting amino acids for both common and Tartary buckwheat (Li, 2019). The protein content in common and Tartary buckwheat ranges from 6.4 to 13.15% depending on the species and other external factors (i.e., environment) during plant growth (Li, 2019). Buckwheat proteins composed of 12–20% glutelin, 50–60% albumin and globulin, 1–7% prolamin and 5–10% other components (Joshi et al., 2020). From buckwheat, a gene had cloned, coding a specific protein that contained 2% methionine and 6% lysine (Chrungoo, Dohtdong, & Chettry, 2016). Legumin genes in maximum angiosperm like Pisum sativum occupy three introns with exactly fixed locus (Maraccini, Deshayes, Petiard, & Rogers, 1999), while the buckwheat legumin gene possesses two-intron structure.
This two-intron structure of the legumin gene was first confirmed from buckwheat which played a key role in gaining advanced information for methionin enriched legumins in lower angiosperms (Chrungoo, et al., 2016). Buckwheat grain contains a balanced amino acid composition with high amount of essential amino acids includes leucine and lysine (6.92, 5.84, and 7.11, 6.18 g/100 g of protein in common and Tartary buckwheat, respectively), but the protein content is low (10.6 and 10.3 g/100 g of dry weight in common and Tartary buckwheat, respectively) (Skrabanja & Kreft, 2016). Ge & Wang (2020) observed the low ratio of lysine/arginine (0.79) and methionine/glycine (0.22) in Tartary buckwheat which shows a significant cholesterol-lowering effect. Moreover, Sytar et al. (2018a) observed the highest quantity of leucine and lysine (1.25 g/100 g and 1.03 g/100 g, respectively) in the seeds of F. tataricum var. Rotundatum, while Krumina-Zemture, Beitane, & Gramatina (2016) reported lysine content (0.56–0.68 g/100 g) in buckwheat flours. For making the well-balanced amino acid composition of buckwheat, albumins and globulins contain a higher amount of lysine (Skrabanja and Kreft, 2016, Zhou et al., 2016). Buckwheat protein shows the gluten-free nature due to the presence of a small proportion of prolamines and absence of α-gliadin protein in buckwheat. This is the main speciality of buckwheat protein compare with protein of other cereals and pseudocereals crops like barley, oats and wheat (Joshi et al., 2020). The life-long adherence to a gluten-free diet is the only available remedy for coeliac disease but in most cases, these products are poor in minerals, vitamins and/or proteins (Sytar et al., 2016, Sytar et al., 2018b). As buckwheat is gluten-free, it is an ideal food material for patients with celiac disease (Skrabanja and Kreft, 2016, Giménez-Bastida and Zieliński, 2015, Zhou et al., 2016). Buckwheat proteins particularly lysin can decrease the concentration of cholesterol (Ge and Wang, 2020, Ji et al., 2019, Li, 2019), increase the fecal excretion of steroids, removal of bile acid, obstruct gallstone formation, may slow mammary carcinogenesis, and suppress colon carcinogenesis (Skrabanja and Kreft, 2016, Zhou et al., 2016).
4.3. Fatty acid
Fatty acid comprises a small portion of buckwheat seed, but they have a vital role to determine the food quality (Ge and Wang, 2020, Subedi, 2018). Generally, buckwheat fat presents as monounsaturated and polyunsaturated fat which are considered as healthy fat. The fatty acids present in Tartary buckwheat compose of palmitic, stearic, oleic, linoleic, linolenic, and eicosenoicacids (Ge & Wang, 2020). Common buckwheat and Tartary buckwheat show similar fatty acid composition, about 3.8% (Li, 2019). The lipid content in Tartary buckwheat ranges from 2.5 to 2.8% (Ahmed et al., 2013) and 2.45% (Xiao, Liu, Wei, Shen, & Wang, 2017), while the lipid content in common buckwheat ranges from 1.6 to 2.9% (Sindhu, 2016), and 3.16% (Xiao et al., 2017). The total lipid content in the whole grain of Common and Tartary buckwheat varies from 1.5 to 4.0% and 1.2–4.3%, respectively (Subedi, 2018). Ge & Wang (2020) determined unsaturated fatty acid 84% in Tartary buckwheat bran and 83% in Tartary buckwheat flour. Furthermore, buckwheat oil has 16–25% of saturated and 74.79% of unsaturated fatty acids. The dominant fatty acids are palmitic acid (15–20%), oleic acid (30–45%), and linoleic acid (31–41%) among other fatty acids (Ge & Wang, 2020). The embryo contains the highest amount of lipids and hull contains the lowest amount of lipids ranges from 7 to 14% and 0.4 to 0.9%. in common buckwheat, respectively (Ahmad et al., 2018). A high level of saturated fatty acids contains in the hull, whereas the unsaturated fatty acids present mostly in the embryo. Buckwheat seed coat contains highest amount of Linoleic acid (essential fatty acid) compared to other organs. The lipid content is different among buckwheat seed parts ranges from 9.6 to 19.7%, 2.0–3.0% and 0.4–0.7% in embryo, endosperm, and hulls, respectively (Subedi, 2018). After baking, total fatty acid decrease in Tartary buckwheat at the rate of 69.6% in bran and 17.3% in flour. Buckwheat has been exhibited to lower the possibility of myocardial infarction because of its Polyunsaturated fatty acids (Ge & Wang, 2020).
4.4. Vitamins and minerals
Buckwheat is rich in thiamine (vitamin B1), niacin (vitamin B3), vitamin B6, vitamin K, and choline but it does not contain vitamin A (Subedi, 2018). In buckwheat seeds, thiamine (vitamin B1) strongly adhered to thiamine-binding proteins. Most of the vitamin E exists as γ-tocopherol (117.8 μg/g), δ-tocopherol (7.3 μg/g) and α-tocopherol (2.1 μg/g) in buckwheat seeds (Li, 2019). Zhou et al. (2015) recorded an increase in the amount of some vitamins, i.e., vitamins B1 and C because of germination. Different types of vitamin present in Tartary buckwheat, such as vitamins B (B1, B2, and B6), vitamin C, and vitamin E (Zhu, 2016, Yiming et al., 2015). The buckwheat flour is the more reliable source of vitamin B than the maize and rice flour. Generally, Tartary buckwheat has a higher amount of B-group vitamins than common buckwheat, while common buckwheat contains higher amount of vitamin E compared to Tartary buckwheat (Joshi et al., 2020).
Different minerals are present in various parts of the buckwheat seed such as hull; aleurone tissues and embryo contain most of the minerals (Subedi, 2018). Mineral elements are very abundant in buckwheat, particularly, trace elements including K, P, Cu, Ca, Se, Mg, Ba, B, I, Fe, Pt, Zn, and Co. These trace elements are excessively present in the outer membrane of buckwheat seeds and seed coat. Buckwheat contains significantly higher amount of trace elements including Ca, Fe, Mg, K, Zn, Cr, Co as well as other elements compared to other cereals (Li, 2019). The mineral present in buckwheat seeds and their morphological proportion ranges from 2.0 to 2.5%, 1.8–2.0%, 2.2–3.5%, 0.80–9% and 3.4–4.2% in the whole grains, kernel, dehulled grains, flour, and hulls, respectively (Christa & Soral-Smietana, 2008). Tartary buckwheat seeds contain relatively higher proportion of Co, Fe, Ni, Se, and Zn than common buckwheat (Zhu, 2016). Common buckwheat contains 70.14 mg/ 100 g Ca, while common and Tartary buckwheat contains 3.4–6.4, 2.47–21.5 mg/100 g Fe, respectively (Subedi, 2018).
The ash content in Common buckwheat varies from 1.4% to 2.5% and in Tartary buckwheat it ranges from 1.8% to 2.3% (Thakur, Kumar, Awasthi, Madan, & Verma, 2017). Vitamins like thiamin assist the body to transform carbs into energy, vitamin B6 helps in brain development and function, folate is crucial for making red blood cells and niacin used in digestive system, skin, and nerves. On the other hand, some trace minerals such as phosphorus, used in the formation of teeth and bones, zinc is essential for the immune system, copper assists the body to produce collagen and absorbs iron and manganese helps to build up bones and connective tissue. Magnesium helps to maintain muscle health (Gilbert, Witt, & Hasjim, 2013). Magnesium also can regulate the functions of the myocardium and the nervous system, resist arteriosclerosis and myocardial infarction and treat and prevent hypertension (Li, 2019).
5. Conclusions and future perspectives
Buckwheat has been gaining more attention across current years, due to its gluten-free attribution, well-balanced amino acid compositions, and health-supporting bioactive flavonoids. Flavonoid compounds present in buckwheat are significantly important to improve human health and to prevent and heal different diseases. However, the safety and toxicity profiles of the roots, leaves, and hulls of buckwheat have not been completely analyzed until now. Some studies reported overeating the seeds of buckwheat can harm the digestive function. So, further researches are needed to investigate the toxicity and eventually harmful effects of different parts of buckwheat and also required further study for clinical trials to investigate the health benefits and pharmaceutical effects of bioactive compounds of those health effects are still unknown. In spite of investigating the synthesis of flavonoids in buckwheat extensively, various biosynthetic genes and transcription factors responsible for flavonoids biosynthesis pathway have yet to be ascertained. Therefore, more studies are needed to isolate the unexplored genes, transcription factors involved in the biosynthesis pathway as well as illustration of the regulation systems of flavonoid biosynthesis in buckwheat. Moreover, among buckwheat species, F. cymosum (also named F. dibotrys) contains the highest number of bioactive compounds, however, limited studies were carried out on this species. Therefore, intensive research should be done to explore the bioactive compound that exists in this species for proper utilization as a medicinal source.
CRediT authorship contribution statement
Md. Nurul Huda, Shuai Lu, Tanzim Jahan, Mengqi Ding, Rintu Jha, Kaixuan Zhang and Wei Zhang wrote the manuscript. Milen I. Georgiev, Sang Un Park and Meiliang Zhou: designed and managed the project. Meiliang Zhou: reviewed and finalized the manuscript. All authors read and approved the paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the National Key R&D Program of China (2017YFE0117600) and National Natural Science Foundation of China (31911540469) (To Meiliang Zhou), the European Union’s Horizon 2020 research and innovation programme, project PlantaSYST (SGA No 739582 under FPA No. 664620), and the BG05M2OP001-1.003-001-C01 project, financed by the European Regional Development Fund through the “Science and Education for Smart Growth” Operational Programme (To Milen I. Georgiev).
References
- Acanski M., Pastor K., Psodorov D., Vujic D., Razmovski R., Kravic S. Determination of the presence of buckwheat flour in bread by the analysis of minor fatty acid methyl esters. Advanced Technologies. 2015;4(2):86–92. [Google Scholar]
- Ahmad M., Ahmad F., Dar E.A., Bhat R.A., Mushtaq T., Shah F. Buckwheat (Fagopyrum esculentum) – a neglected crop of high altitude cold arid regions of ladakh: biology and nutritive value. International Journal of Pure & Applied Bioscience. 2018;6(1):395–406. [Google Scholar]
- Ahmed A., Khalid N., Ahmad A., Abbasi N.A., Latif M.S.Z., Randhawa M.A. Phytochemicals and biofunctional properties of buckwheat: A review. Journal of Agricultural Science. 2013;152(3):349–369. [Google Scholar]
- Amézqueta S., Galán E., Fuguet E., Carrascal M., Abián J., Torres J.L. Determination of D-fagomine in buckwheat and mulberry by cation exchange HPLC/ESI-Q-MS. Analytical and Bioanalytical Chemistry. 2012;402(5):1953–1960. doi: 10.1007/s00216-011-5639-2. [DOI] [PubMed] [Google Scholar]
- Bai Y.C., Li C.L., Zhang J.W., Li S.J., Luo X.P., Yao H.P. Characterization of two Tartary buckwheat R2R3-MYB transcription factors and their regulation of proanthocyanidin biosynthesis. Physiologia Plantarum. 2014;152(3):431–440. doi: 10.1111/ppl.12199. [DOI] [PubMed] [Google Scholar]
- Beitane I., Krumina-Zemture G. Evaluation of nutritional quality of raw and roasted buckwheat (Fagopyrum Esculentum M.) fluor. Journal of International Scientific Publications. 2017;5(1):687–695. [Google Scholar]
- Borovaya S.A., Klykov A.G. Some aspects of flavonoid biosynthesis and accumulation in buckwheat plants: Review. Plant Biotechnology Reports. 2020;14:213–225. doi: 10.1007/s11816-020-00614-9. [DOI] [Google Scholar]
- Bose S., Sarkar D., Bose A., Mandal S.S. Natural flavonoids and its pharmaceutical importance. The Pharma Review. 2018:61–75. [Google Scholar]
- Brazier-Hicks M., Evans K.M., Gershater M.C., Puschmann H., Steel P.G., Edwards R. The C-glycosylation of flavonoids in cereals. Journal of Biological Chemistry. 2009;284(27):17926–17934. doi: 10.1074/jbc.M109.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M.I., Duarte S., Doseff A.I., Grotewold E. Flavone-rich maize: An opportunity to improve the nutritional value of an important commodity crop. Frontiers in Plant Science. 2014;5:440. doi: 10.3389/fpls.2014.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan R.S., Gupta N., Sharma S.K., Rana J.C., Sharma T.R., Jana S. Genetic and genome resources in buckwheat–present status and future perspectives. The European Journal of Plant Science and Biotechnology. 2010;4(Special Issue1):33–44. [Google Scholar]
- Christa K., Soral-Smietana M. Buckwheat grains and buckwheat products-nutritional and prophylactic value of their components-a review. Czech Journal of Food Sciences. 2008;26(3):153–162. [Google Scholar]
- Chrungoo N.K., Dohtdong L., Chettry U. Diversity in seed storage proteins and their genes in buckwheat. In: Zhou M.-.L., Kreft I., Woo S.-.H., Chrungoo N., Wieslander G., editors. Molecular Breeding and Nutritional Aspects of Buckwheat. Academic Press is an imprint of Elsevier; UK: 2016. pp. 387–400. [Google Scholar]
- Davies K.M., Jibran R., Zhou Y., Albert N.W., Brummell D.A., Jordan B.R. Plant Science. 2020;11(7) doi: 10.3389/fpls.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic K., Górecka D., Kucharska M., Przybylska B. Influence of technological process during buckwheat groats production on dietary fibre content and sorption of bile acids. Food Research International. 2012;47:279–283. [Google Scholar]
- Dziedzic K., Górecka D., Szwengiel A., Sulewska H., Kreft I., Gujska E. The content of dietary fibre and polyphenols in morphological parts of buckwheat (Fagopyrum tataricum) Plant Foods for Human Nutrition. 2018;73:82–88. doi: 10.1007/s11130-018-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT (2020). Production/yield quantities of buckwheat in World + (Total) 2018. http://www.fao.org/faostat/en/#data/QC/visualize, Accessed date: February 6, 2020.
- Ferreyra M.L.F., Rodriguez E., Casas M.I., Labadie G., Grotewold E., Casati P. Identification of a bifunctional maize C- and O-glucosyltransferase. Journal of Biological Chemistry. 2013;288:31678–31688. doi: 10.1074/jbc.M113.510040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabr A.M.M., Sytar O., Ghareeb H., Brestic M. Accumulation of amino acids and flavonoids in hairy root cultures of common buckwheat (Fagopyrum esculentum) Physiology and Molecular Biology of Plants. 2019;25(3):787–797. doi: 10.1007/s12298-019-00669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, R.-Y., Chan, C.-L., Yang, Q.-Q., Li, H.-B., Zhang, D., Ying-Ying Ge, Y.-Y., et al. (2019). Bioactive compounds and beneficial functions of sprouted grains. In H. Feng, B. Nemzer, & J. W. DeVries (Eds.), Sprouted grains: nutritional value, production, and applications (pp. 191–246). Elsevier Inc. in cooperation with AACC International.
- Ge R.H., Wang H. Nutrient components and bioactive compounds in Tartary buckwheat bran and flour as affected by thermal processing. International Journal of Food Properties. 2020;23(1):127–137. doi: 10.1080/10942912.2020.1713151. [DOI] [Google Scholar]
- Gilbert R.G., Witt T., Hasjim J. What is being learned about starch properties from multiple- level characterization. Cereal Chemistry. 2013;90(4):312–325. [Google Scholar]
- Giménez-Bastida J.A., Zieliński H. Buckwheat as a functional food and its effects on health. Journal of Agricultural and Food Chemistry. 2015;63(36):7896–7913. doi: 10.1021/acs.jafc.5b02498. [DOI] [PubMed] [Google Scholar]
- Giménez-Bastida J.A., Laparra-Llopis J.M., Baczek N., Zielinski H. Buckwheat and buckwheat enriched products exert an anti-inflammatory effect on the myofibroblasts of colon CCD-18Co. Food and function. 2018;9(6):3387–3397. doi: 10.1039/c8fo00193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonḉalves F.M.F., Debiage R.R., Gonḉalves da Silva R.M., Porto P.P., Yoshihara E., de Mello Peixoto E.C.T. Fagopyrum esculentum Moench: A crop with many purposes in agriculture and human nutrition. African Journal of Agriculture Research. 2016;11(12):983–989. doi: 10.5897/AJAR2015.10747. [DOI] [Google Scholar]
- Gorniak I., Bartoszewski R., Kroliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Reviews. 2019;18:241–272. [Google Scholar]
- Gupta N., Sharma S.K., Rana J.C., Chauhan R.S. Expression of flavonoid biosynthesis genes vis-à-vis rutin content variation in different growth stages of Fagopyrum species. Journal of Plant Physiology. 2011;168(17):2117–2123. doi: 10.1016/j.jplph.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Holton T.A., Cornish E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7:1071–1083. doi: 10.1105/tpc.7.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Han L., Liu F., Yin S., Peng Q., Wang M. A mini-review of isolation, chemical properties and bioactivities of polysaccharides from buckwheat (Fagopyrum Mill) International Journal of Biological Macromolecules. 2019;127:204–209. doi: 10.1016/j.ijbiomac.2019.01.043. [DOI] [PubMed] [Google Scholar]
- Jing R., Li H., Hu C., Jiang Y., Qin L., Zheng C. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. International Journal of Molecular Sciences. 2016;17(4):589. doi: 10.3390/ijms17040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D.C., Chaudhari G.V., Sood S., Kant L., Pattanayak A., Zhang K. Revisiting the versatile buckwheat: Reinvigorating genetic gains through integrated breeding and genomics approach. Planta. 2019;250(3):783–801. doi: 10.1007/s00425-018-03080-4. [DOI] [PubMed] [Google Scholar]
- Joshi D.C., Zhang K., Wang C., Chandora R., Khurshid M., Li J. Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnology Advances. 2020;39 doi: 10.1016/j.biotechadv.2019.107479. [DOI] [PubMed] [Google Scholar]
- Kalinova J.P., Vrchotova N., Triska J. Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. Journal of Cereal Science. 2019;85:243–248. doi: 10.1016/j.jcs.2018.12.012. [DOI] [Google Scholar]
- Kerscher F., Franz G. Biosynthesis of vitexin and isovitexin: Enzymic synthesis of the C-glucosylflavones vitexin and isovitexin with an enzyme preparation from Fagopyrum esculentum M. seedlings. Zeitschrift für Naturforschung C. 1987;42:519–524. [Google Scholar]
- Kerscher F., Franz G. Isolation and some properties of an UDP-glucose: 2-hydroxyflavanone-6(or 8)-C-glucosyltransferase from Fagopyrum esculentum M. cotyledons. Journal of Plant Physiology. 1988;132(1):110–115. [Google Scholar]
- Kim S.L., Kim S.K., Park C.H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Research International. 2004;37:319–327. [Google Scholar]
- Kiprovski B., Mikulic-Petkovsek M., Slatnar A., Veberic R., Stampar F., Malencic D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chemistry. 2015;185:41–47. doi: 10.1016/j.foodchem.2015.03.137. [DOI] [PubMed] [Google Scholar]
- Kreft I., Zhou M.-L., Golob A., Germ M., Likar M., Dziedzic K. Breeding buckwheat for nutritional quality. Breeding Science. 2020;70:67–73. doi: 10.1270/jsbbs.19016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumina-Zemture G., Beitane I., Gramatina I. Amino acid and dietary fibre content of pea and buckwheat flours. Research for Rural Development. 2016;1:84–90. [Google Scholar]
- Krupa-Kozak U., Wronkowska M.M., Soral-Śmietana M. Effect of buckwheat flour on microelements and proteins contents in gluten-free bread. Czech Journal of Food Science. 2011;29(2):103–108. [Google Scholar]
- Kwon S.J., Roy S.K., Choi J., Park J., Cho S., Sarker K. Recent research updates on functional components in Buckwheat. Journal of Agricultural Science-Chungbuk National University. 2018;34(1):1–8. [Google Scholar]
- Lehka B.J., Eichenberger M., Bjorn-Yoshimoto W.E., Vanegas K.G., Buijs N., Jensen N.B. Improving heterologous production of phenylpropanoids in Saccharomyces cerevisiae by tackling an unwanted side reaction of Tsc13, an endogenous double-bond reductase. FEMS Yeast Research. 2017;17(1) doi: 10.1093/femsyr/fox004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Buckwheat. In: Wang J., Sun B., Tsao R., editors. Bioactive Factors and Processing Technology for Cereal Foods. Springer Nature Singapore Pte Ltd.; 2019. pp. 137–150. [Google Scholar]
- Li J., Yang P., Yang O., Gong X., Ma H., Dang K. Analysis of flavonoid metabolites in buckwheat leaves using UPLC-ESI-MS/MS. Molecules. 2019;24:1310. doi: 10.3390/molecules24071310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang K., Meng Y., Li Q., Ding M., Zhou M.-L. FtMYB16 interacts with Ftimportin-a1 to regulate rutin biosynthesis in Tartary buckwheat. Plant Biotechnology Journal. 2019;17(8):1479–1481. doi: 10.1111/pbi.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Lv M., Peng Q., Shan F., Wang M. Physicochemical and textural properties of tartary buckwheat starch after heat-moisture treatment at different moisture levels. Starch. 2015;67(34):276–284. doi: 10.1002/star.201400143. [DOI] [Google Scholar]
- Luo Q., Li J., Wang C., Cheng C., Shao J., Hui J. TrMYB4 transcription factor regulates the rutin biosynthesis in hairy roots of F. cymosum. Plant Science. 2020;294 doi: 10.1016/j.plantsci.2020.110440. [DOI] [PubMed] [Google Scholar]
- Lv L., Xia Y., Zou D., Han H., Wang Y., Fang H. Fagopyrum tataricum (L.) Gaertn.: A review on its traditional uses, phytochemical and pharmacology. Food Science and Technology Research. 2017;23(1):1–7. [Google Scholar]
- Maraccini P., Deshayes A., Petiard V., Rogers W.J. Molecular cloning of the complete 11S seed storage protein gene of Coffea arabica and promoter analysis in transgenic tobacco plants. Plant Physiology and Biochemistry. 1999;37(4):273–282. [Google Scholar]
- Martin C., Li J. Medicine is not health care, food is health care: Plant metabolic engineering, diet and human health. New phytologist. 2017;216(3):699–719. doi: 10.1111/nph.14730. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia B., Pasini F., Verardo V., Gomez-Caravaca A.M., Marconi E., Caboni M.F. Distribution of Free and Bound Phenolic Compounds in Buckwheat Milling Fractions. Foods. 2019;8(12):670. doi: 10.3390/foods8120670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K., Walker A.R. Biosynthesis and regulation of flavonoids in buckwheat. Breeding Science. 2019;1–11 doi: 10.1270/jsbbs.19041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K., Oshima Y., Mitsuda N., Sakamoto S., Nishiba Y., Walker A.R. Buckwheat R2R3 MYB transcription factor FeMYBF1 regulates flavonol biosynthesis. Plant science. 2018;274:466–475. doi: 10.1016/j.plantsci.2018.06.025. [DOI] [PubMed] [Google Scholar]
- Melini V., Melini F., Acquistucci R. Phenolic compounds and bioaccessibility thereof in functional pasta: Review. Antioxidants. 2020;9(343) doi: 10.3390/antiox9040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajan S., Munna M.M., Orchy T.N., Hoque M.M., Farzana T. Buckwheat flour fortified bread. Bangladesh Journal of Science and Industrial Research. 2019;54(4):347–356. [Google Scholar]
- Mota C., Nascimento A.C., Santos M., Delgado I., Coelho I., Rego A. The effect of cooking methods on the mineral content of quinoa (Chenopodium quinoa), amaranth (Amaranthus sp.) and buckwheat (Fagopyrum esculentum) Journal of Food Composition and Analysis. 2016;49:57–64. [Google Scholar]
- Nagatomo Y., Usui S., Ito T., Kato A., Shimosaka M., Taguchi G. Purification, molecular cloning and functional characterization of flavonoid C-glucosyltransferases from Fagopyrum esculentum M. (buckwheat) cotyledon. The Plant Journal. 2014;80(3):437–448. doi: 10.1111/tpj.12645. [DOI] [PubMed] [Google Scholar]
- Nam T.-G., Lim Y.J., Eom S.H. Flavonoid accumulation in common buckwheat (Fagopyrum esculentum) sprout tissues in response to light. Horticulture, Environment, and Biotechnology. 2018;59(1):19–27. doi: 10.1007/s13580-018-0003-5. [DOI] [Google Scholar]
- Park B.I., Kim J., Lee K., Lim T., Hwang K.T. Flavonoids in common and Tartary buckwheat hull extracts and antioxidant activity of the extracts against lipids in mayonnaise. Journal of Food Science and Technology. 2019;56(5):2712–2720. doi: 10.1007/s13197-019-03761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Yeo H.J., Park Y.J., Morgan A.M.A., Arasu M.V., Al-Dhabi N.A. Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules. 2017;22(3) doi: 10.3390/molecules22030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N.I., Li X., Thwe A.A., Lee S.Y., Kim S.G., Wu Q. Enhancement of rutin in Fagopyrum esculentum hairy root cultures by the Arabidopsis transcription factor AtMYB12. Biotechnology Letters. 2012;34(3):577–583. doi: 10.1007/s10529-011-0807-1. [DOI] [PubMed] [Google Scholar]
- Perez de Souza L., Garbowicz K., Brotman Y., Tohge T., Fernie A.R. The acetate pathway supports flavonoid and lipid biosynthesis in Arabidopsis. Plant Physiology. 2020;182:857–869. doi: 10.1104/pp.19.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Romero S., Hereu M., Atienza L., Amézqueta S., Casas J., Muñoz S. The Buckwheat Iminosugar d-Fagomine Attenuates Sucrose-Induced Steatosis and Hypertension in Rats. Molecular Nutrition & Food Research. 2020;64(1) doi: 10.1002/mnfr.201900564. [DOI] [PubMed] [Google Scholar]
- Rozanska D., Mikos K., Regulska-Ilow B. Assessment of the glycemic index of groats available on the polish food market. Roczniki Panstwowego Zakładu Higieny. 2020;71(1):81–87. doi: 10.32394/rpzh.2020.0101. [DOI] [PubMed] [Google Scholar]
- Sindhu R. Composition and functional properties of common buckwheat (Fagopyrum esculentum Moench) flour and starch. International Journal of Innovative Research and Advanced Studies. 2016;3(7):154–159. [Google Scholar]
- Sindhu R., Khatkar B.S. Pseudocereals nutritional composition functional properties and food applications. In: Deka S.C., Seth D., Hulle N.R.S., editors. Food Bioactives: Functionality and Applications in Human Health. Apple Academic Press; U.S.A.: 2019. pp. 129–148. [Google Scholar]
- Sinkovic L., Kokalj D., Vidrih R., Meglic V. Milling fractions fatty acid composition of common (Fagopyrum esculentum Moench) and tartary (Fagopyrum tataricum (L.) Gaertn) buckwheat. Journal of Stored Products Research. 2020;85 doi: 10.1016/j.jspr.2019.101551. [DOI] [Google Scholar]
- Skrabanja V., Kreft I. Nutritional value of buckwheat proteins and starch. In: Zhou M.-.L., Kreft I., Woo S.-.H., Chrungoo N., Wieslander G., editors. Molecular Breeding and Nutritional Aspects of Buckwheat. Academic Press is an imprint of Elsevier; UK: 2016. pp. 169–176. [Google Scholar]
- Stojilkovski K., Glavac N.K., Kreft S., Kreft I. Fagopyrin and flavonoid contents in common, tartary, and cymosum buckwheat. Journal of Food Composition and Analysis. 2013;32(2):126–130. [Google Scholar]
- Subedi, N. (2018). Changes in phytochemical properties of buckwheat varieties on malting (Thesis). Department of Food Technology, Tribhuvan University, Nepal.
- Suzuki T., Kim S.J., Yamauchi H., Takigawa S., Honda Y., Mukasa Y. Characterization of a flavonoid 3-O-glucosyltransferase and its activity during cotyledon growth in buckwheat (Fagopyrum esculentum) Plant Science. 2005;169(5):943–948. [Google Scholar]
- Suzuki T., Noda T., Morishita T., Ishiguro K., Otsuka S., Brunori A. Present status and future perspectives of breeding for buckwheat quality. Breeding Science. 2020;1–19 doi: 10.1270/jsbbs.19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytar O., Biel W., Smetanska I., Brestic M. Bioactive compounds and their biofunctional properties of different buckwheat germplasms for food processing. In: Zhou M.-.L., Kreft I., Suvorova G., Tang Y., Woo S.H., editors. Buckwheat germplasm in the world. Academic Press; Chennai: 2018. pp. 191–204. [Google Scholar]
- Sytar O., Brestic M., Zivcak M., Tran L.P. The contribution of buckwheat genetic resources to health and dietary diversity. Current Genomics. 2016;17(3):193–206. doi: 10.2174/1389202917666160202215425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytar O., Chrenková M., Ferencová J., Polačiková M., Rajský M., Brestič M. Nutrient capacity of amino acids from buckwheat seeds and sprouts. Journal of Food and Nutrition Research. 2018;57(1):38–47. [Google Scholar]
- Taguchi G. Flavonoid biosynthesis in buckwheat. In: Zhou M.-.L., Kreft I., Woo S.-.H., Chrungoo N., Wieslander G., editors. Molecular breeding and nutritional aspects of buckwheat. Academic Press is an imprint of Elsevier; UK: 2016. pp. 377–386. [Google Scholar]
- Thakur R., Kumar S., Awasthi C.P., Madan L., Verma M.L. Biochemical evaluation of tartary buckwheat (Fagopyrum tataricum gaertn.) genotypes of cold desert of himachal pradesh. Biosciences Biotechnology Research Asia. 2017;14(2):821–825. [Google Scholar]
- Tien N.N.T., Trinh L.N.G., Inoue N., Morita N., Hung P.V. Nutritional composition, bioactive compounds, and diabetic enzyme inhibition capacity of three varieties of buckwheat in Japan. Cereal Chemistry. 2018;95(5):615–624. doi: 10.1002/cche.10069. [DOI] [Google Scholar]
- Tsurunaga Y., Takahashi T., Katsube T., Kudo A., Kuramitsu O., Ishiwata M. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food chemistry. 2013;141(1):552–556. doi: 10.1016/j.foodchem.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Tuan P.A., Thwe A.A., Kim J.K., Kim Y.B., Lee S., Park S.U. Molecular characterisation and the light–dark regulation of carotenoid biosynthesis in sprouts of Tartary buckwheat (Fagopyrum tataricum Gaertn.) Food Chemistry. 2013;141(4):3803–3812. doi: 10.1016/j.foodchem.2013.06.085. [DOI] [PubMed] [Google Scholar]
- Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 2018;5(3):93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas K.G., Larsen A.B., Eichenberger M., Fischer D., Mortensen U.H., Naesby M. Indirect and direct routes to C-glycosylated flavones in Saccharomyces cerevisiae. Microbial Cell Factories. 2018;17:107. doi: 10.1186/s12934-018-0952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajid M., Aslam M.S., Uzair M. Genus Fagopyrum: Phytochemical and Ethnopharmacological Review. Indian Research Journal of Pharmacy and Science. 2015;2(1):1–14. [Google Scholar]
- Wang L., Tian X., Wei W., Chen G., Wu Z. Fingerprint analysis and quality consistency evaluation of flavonoid compounds for fermented Guava leaf by combining high-performance liquid chromatography time-of-flight electrospray ionization mass spectrometry and chemometric methods. Journal of Separation Science. 2016;39(20):3906–3916. doi: 10.1002/jssc.201600552. [DOI] [PubMed] [Google Scholar]
- Wang T., Li Q., Bi K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate: Review. Asian Journal of Pharmaceutical Science. 2018;13(1):12–23. doi: 10.1016/j.ajps.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.T., Zhu Z.Y., Zhao L., Sun H.Q., Meng M., Zhang J.Y. Structural characterization and inhibition on alpha-D-glucosidase activity of non-starch polysaccharides from Fagopyrum tartaricum. Carbohydrate polymers. 2016;153:679–685. doi: 10.1016/j.carbpol.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Wu W., Wang L., Qiu J., Li Z. The analysis of fagopyritols from Tartary buckwheat and their anti-diabetic effects in KK-Ay type 2 diabetic mice and HepG2 cells. Journal of Functional Foods. 2018;50:137–146. [Google Scholar]
- Xiao Y., Liu H., Wei T., Shen J., Wang M. Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment. LWT. 2017;86:285–292. [Google Scholar]
- Yilmaz M., Kantarjian H., Wang X., Khoury J.D., Ravandi F., Jorgensen J. The early achievement of measurable residual disease negativity in the treatment of adults with Philadelphia-negative B-cell acute lymphoblastic leukemia is a strong predictor for survival. American Journal of Hematology. 2020;95(2):144–150. doi: 10.1002/ajh.25671. [DOI] [PubMed] [Google Scholar]
- Yiming Z., Hong W., Linlin C., Xiaoli Z., Wen T., Xinli S. Evolution of nutrient ingredients in tartary buckwheat seeds during germination. Food Chemistry. 2015;186:244–248. doi: 10.1016/j.foodchem.2015.03.115. [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K., Higashi Y.Y., Nakabayashi R. The origin and evolution of plant flavonoid metabolism. Frontiers in Plant Science. 2019;10:1–16. doi: 10.3389/fpls.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Logacheva M.D., Meng Y., Hu J., Wan D., Li L. Jasmonate-responsive MYB factors spatially repress rutin biosynthesis in Fagopyrum tataricum. Journal of Experimental Botany. 2018;69(8):1955–1966. doi: 10.1093/jxb/ery032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huang H., Zhao X., Lv Q., Sun C., Li X. Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. Journal of Functional Foods. 2015;14:144–153. [Google Scholar]
- Zhao J., Jiang L., Tang X., Peng L., Li X., Zhao G. Chemical composition, antimicrobial and antioxidant activities of the flower volatile oils of Fagopyrum esculentum, Fagopyrum tataricum and Fagopyrum cymosum. Molecules. 2018;23(1):182. doi: 10.3390/molecules23010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Park C.H., Li X., Kim Y.B., Yang J., Sung G.B. Accumulation of rutin and betulinic acid and expression of phenylpropanoid and triterpenoid biosynthetic genes in mulberry (Morus alba L.) Journal of Agricultural and Food Chemistry. 2015;63(38):8622–8630. doi: 10.1021/acs.jafc.5b03221. [DOI] [PubMed] [Google Scholar]
- Zhou, M.-L., Kreft, I., Woo, S. H., Chrungoo, N., & Wieslander, G. (2016). Bioactive compounds in buckwheat sprouts. In M.-L. Zhou, I. Kreft, S.-H. Woo, N. Chrungoo, & G. Wieslander (Eds.), Molecular Breeding and Nutritional Aspects of Buckwheat (pp. 151–159). Academic Press is an imprint of Elsevier, UK.
- Zhou M.-L., Sun Z., Ding M., Logacheva M.D., Kreft I., Wang D. FtSAD2 and FtJAZ1 regulate activity of the FtMYB11 transcription repressor of the phenylpropanoid pathway in Fagopyrum tataricum. The New phytologist. 2017;216(3):814–828. doi: 10.1111/nph.14692. [DOI] [PubMed] [Google Scholar]
- Zhou X., Hao T., Zhou Y., Tang W., Xiao Y., Meng X. Relationships between antioxidant compounds and antioxidant activities of Tartary buckwheat during germination. Journal of Food Science and Technology. 2015;52(4):2458–2463. doi: 10.1007/s13197-014-1290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discovery. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F. Chemical composition and health effects of Tartary buckwheat. Food Chemistry. 2016;203:231–245. doi: 10.1016/j.foodchem.2016.02.050. [DOI] [PubMed] [Google Scholar]
- Zhu F. Proanthocyanidins in cereals and pseudocereals. Critical Reviews in Food Science and Nutrition. 2019;59(10):1521–1533. doi: 10.1080/10408398.2017.1418284. [DOI] [PubMed] [Google Scholar]
- Zielińska D., Turemko M., Kwiatkowski J., Zieliński H. Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and tartary buckwheat plants. Molecules. 2012;17(8):9668–9682. doi: 10.3390/molecules17089668. [DOI] [PMC free article] [PubMed] [Google Scholar]


