ABSTRACT
Introduction
The 21st century has seen a series of viral pandemics that have collectively infected millions of individuals. To understand factors that may contribute to viral spread and address long-term health sequelae for survivors, it is important to review evidence regarding viral presence in semen, sexual transmission potential, and possible effects on fertility.
Aim
To review the current literature regarding the sexual transmissibility of recent viral pandemics and their effects on semen parameters and fertility. We review evidence for the following viruses: Ebola, Zika, West Nile, pandemic influenza, severe acute respiratory syndrome (SARS), and SARS-corona virus-2 (SARS-CoV-2).
Methods
A literature search was conducted to identify relevant studies. Titles and abstracts were reviewed for relevance. References from identified articles were searched and included, if appropriate.
Main Outcome Measures
The main outcome measure of this study was reviewing of peer-reviewed literature.
Results
Both the Ebola virus and Zika virus are present in semen, but only the Zika virus shows consistent evidence of sexual transmission. Current evidence does not support the presence of the West Nile virus, pandemic influenza, SARS, and SARS-CoV-2 in semen. The Zika virus appears to alter semen parameters in a way that diminishes fertility, but the effect is likely time limited. The West Nile virus and SARS have been associated with orchitis in a small number of case reports. Viruses that cause febrile illness, such as pandemic influenza, SARS, and SARS-CoV-2, are associated with decreased sperm count and motility and abnormal morphology. SARS and SARS-CoV-2 may interact with angiotensin-converting enzyme 2 receptors present in the testes, which could impact spermatogenesis.
Conclusions
We have reported the presence in semen, sexual transmission potential, and fertility side effects of recent viral pandemics. Overall, semen studies and fertility effects are highly understudied in viral pandemics, and rigorous study on these topics should be undertaken as novel pandemics emerge.
Keywords: Viral Pandemic, Semen, Sexual Transmission, Fertility, Ebola, Zika
Introduction
The 21st century has seen a series of viral pandemics that have affected the health of millions of individuals and touched every corner of the globe.1 As one potential means of controlling viral spread, it is necessary to understand what viruses are present in semen, how long they remain detectable, and what their potential is for sexual transmission from male to female partners. In addition, with so many survivors of viral pandemics alive today, knowledge is needed about how these viruses affect the male genital tract and what implications they may have for future fertility.2 Despite the importance of these questions, to our knowledge, these topics have not yet been the subject of systematic review. Here, we detail several important foundational principles that affect viral infection in the testes and comment on the differences in immune response seen between men and women. Then, we present the state of current research regarding presence in semen, sexual transmission, and fertility effects for the Zika virus (ZIKV), Ebola virus (EBOV), West Nile virus (WNV), pandemic influenza, severe acute respiratory syndrome (SARS), and SARS-coronavirus-2 (SARS-CoV-2) (Table 1).
Table 1.
Viral presence in semen, evidence of sexual transmission, and effects on semen parameters
| Virus | Present in semen, maximum detection (d) | Evidence of sexual transmission | Effects on semen parameters |
|---|---|---|---|
| Zika | Yes, 370 | Yes | Transient reduction in sperm count and alteration in morphology (recovered at 120 d)33; impaired motility at 1 y43 |
| Ebola | Yes, 965 | Rare instances | No known effects |
| West Nile | No | No | Single case report of orchitis74 |
| Influenza | No | No | Transient reduction in sperm count (recovered at 79 days) and motility (recovered at 58 days), increased DNA fragmentation index8 |
| SARS | No | No | Gross testicular atrophy,93,94 possible autoimmune orchitis94 |
| SARS-CoV-2 | No | No | Possible autoimmune orchitis102 |
Methods
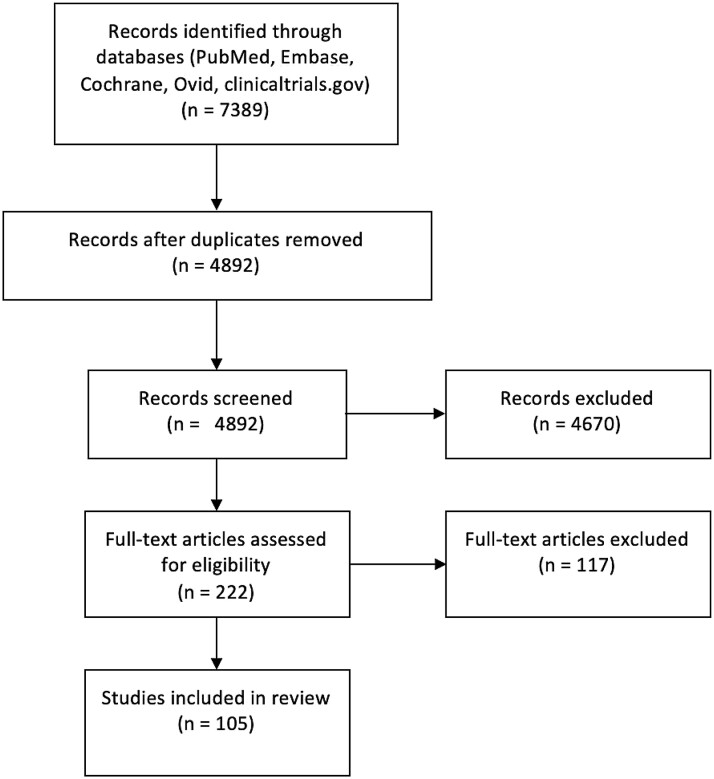
A literature search of PubMed, Embase, Cochrane, Ovid, and clinicaltrials.gov was conducted by 2 independent authors, K.P. and P.K. The literature search was limited to English publications or publications translated into English, and databases were searched from dates of their inception to June 2020. Articles with only abstracts available were included. To review the immune status of the testes, searches were conducted using the following keywords: testes, immune privilege, and blood-testes barrier. To review the difference in immune response between men and women, searches were conducted with the following keywords: gender difference in immune response and sex difference in immune response. Searches were also conducted for each of the recent viral pandemics with the following key words separated by “OR”: genitalia, testis, orchitis, prostate, reproductive, semen, sperm, hormones, endocrine, luteinizing hormone, follicle-stimulating hormone, gonadotropins, testosterone, fertility, fecundity, sexual transmission, erectile function, erectile dysfunction, sexual function, sexual dysfunction, and libido. Case studies, case series, reviews, and meta-analyses were included. Both human, animal, and basic science data were included. For each viral pandemic, articles were selected if they met any of the following inclusion criteria: discussion of semen parameters, identification of virus location within the genital tract, discussion of sexual transmission, or discussion of fertility related processes including spermatogenesis, libido, and sexual function. Article titles and abstracts were reviewed by K.P. and P.K. to identify 222 relevant studies. Full-text articles were then reviewed to determine if they met the stated selection criteria, yielding 105 total articles (Figure 1).
Figure 1.
Flow diagram of literature review.
Testes as an Immune-Privileged Site
In any consideration of viral infection of testicular tissue and seminal fluid, it is important to review the function of the blood-testis barrier (BTB). The BTB provides protection from autoimmune cell destruction, making the testes an immune-privileged site. Evolving understanding of the BTB indicates that it comprises both a physical component and an immune regulatory component.3
The physical component of the BTB comprises a layer of Sertoli cells connected by tight junctions, separating undifferentiated spermatogonia from differentiating germ cells in the form of spermatocytes, spermatids, and spermatozoa.4 Local immunosuppression is carried out by constitutive expression of anti-inflammatory factors by testicular cells, most prominently transforming growth factor beta.5,6
Testosterone plays an additional role in establishing immune privilege by regulating the numbers of testicular macrophages and lymphocytes and downregulating their expression of proinflammatory transcription factors, cytokines, and adhesion molecules.4 An in vitro study that treated macrophages with testosterone demonstrated decreased expression of Toll-like receptor 4 and reduced sensitivity of Toll-like receptor 4 to lipopolysaccharide, a key antigenic molecule for identifying foreign pathogens.7 Furthermore, a study of rats pretreated with estrogen to suppress testosterone production showed a much more rapid rejection of an intratesticular allograft than untreated controls, underscoring testosterone’s immune-modulating effects.8
Despite its immune-privileged nature, the testicular environment is still capable of mounting an effective inflammatory response. In fact, the testes are of equal susceptibility to infection as any other tissue type.9 Given the suppression of adaptive immune mechanisms, it is simply more reliant on the innate immune mechanisms of macrophages, natural killer cells, and antimicrobial secretory products such as interferons and defensins.2,10,11 Nonetheless, there is evidence that the testes can act as a reservoir for pathogens that are no longer detectable systemically.12 Jenabian et al13 have found that in the testicles of men with HIV, HIV viral RNA has remained detectable in the testes even when levels were undetectable in the blood secondary to antiviral therapy (although this is not known to cause increased transmission risk). This becomes especially important in the study of viruses such as the Ebola and Zika, where asymptomatic individuals may still be capable of sexual transmission of the virus.
Sex-Based Differences in Immune Response
Sex-based differences in immune response present an additional consideration for viruses that affect the reproductive tracts. Increasing evidence suggests that the intensity, severity, and mortality of viral disease differs between genders.14 Literature examining this link has generally observed that women mount a stronger immune response to antigens, infections, and vaccines than do men. This effect is most widely observed in the cytokine response to antigens. On inoculation with viruses or direct stimulation of immune Toll-like receptors 3, 7, 8, and 9, men exhibit lower levels of proinflammatory interferon alfa and higher amounts of anti-inflammatory interleukin 10.15 Helper T cells in men also demonstrate lower cluster of differentiation 3 (CD3), CD4, and CD4:CD8 ratio than those in women.14
The X chromosome contains many important genes for immune function, including TLR7, CD40 ligand (CD40L), and forkhead box P3 (FOXP3).16 Notably, it also contains the most microRNAs of all chromosomes, which regulate protein-coding genes and modulate cellular activities. The Y chromosome, by contrast, uniquely contains none.17 And, although X inactivation equalizes gene expression for most of the X chromosome, genes within non-recombining regions may be expressed more in women. In addition, other immune-regulatory genes present on both the X and Y chromosomes have unequal expression levels, and tissue distribution between the sexes is perhaps due to sex hormones.18,19
The first randomized controlled trial to separate the effect of sex hormones and chromosomes was conducted by Palaszynski et al20 in 2005, using mice in which the sex-determining region Y (SRY) gene was moved from the Y chromosome to an autosome. This allowed separation of sex chromosome complement (XX or XY) from gonadal type (ovaries or testes). The authors reported that among ovariectomized (-SRY) mice, those with XY had greater immune response than those with XX, suggesting that the male complement was immune stimulatory. This effect was not detected when comparing intact males (+SRY) with XX and XY complements, suggesting that testosterone tempers the effect of the XY complement. Supporting this, when testosterone was added to the ovariectomized female (-SRY) XY mice, their elevated immune response was suppressed.
In 2011, Robinson et al21 similarly found that the increased immune response of female mice to influenza A was eliminated by removal of the gonads. Although they did not find any immune response to testosterone or dehydroepiandrosterone in male mice, administration of high-dose estradiol attenuated the induction of tumor necrosis factor alpha and C-C motif chemokine ligand by 10 times and resulted in increased rates of survival compared with no or low-dose estradiol. Building on this, administration of 17β-estradiol to female mice resulted in less secretion of tumor necrosis factor alpha and interferon gamma as well as decreased neutrophil recruitment, improving disease tolerance.
Taken as a whole, current evidence suggests women exhibit stronger immune response to viral infections than men. This response typically manifests as better long-term outcomes and to subsequent reexposure but also as more severe disease acutely. The size of the effect can be substantial; in the H1N1 pandemic, infected women suffered more than 2-fold risk of death than men.22,23 Further exploration of the mechanisms behind the sex-based differences may allow for modulation of the immune response with hormone treatments.
Zika Virus
History of the ZIKV
The ZIKV was first recognized in 1947 in a sentinel monkey from Zika forest, Uganda. The first suspected human case followed in 1954, but the virus did not demonstrate mosquito-borne transmission until 1962. In 2013, the ZIKV emerged in Brazil during preparation for its upcoming international sporting events, creating a large epidemic through the Americas. It entered the continental United States in 2016 with cases in Florida and Texas, peaking in 2017. No new cases have been reported since 2017. However, similarities to other Flaviviruses such as dengue fever have made the virus difficult to recognize clinically, with several epidemics first noted after rises in associated conditions such as congenital microcephaly and Guillain-Barre syndrome.24
The ZIKV in Semen
The ZIKV was first suspected to be in semen following case reports of travel-associated transmission to sexual partners in non-endemic areas.25 Since then, multiple studies have confirmed the presence of ZIKV RNA in semen by reverse transcription polymerase chain reaction (RT-PCR).26–30 A 2018 prospective study by Mead et al27 found that semen was positive with ZIKV in 33% of men, including 61% of those tested within 30 days of symptom onset. Infectious ZIKV was detected in 15% of RNA-positive semen samples collected within 30 days of symptom onset, but 0% of samples obtained after 30 days of disease onset were infectious. Evidence from Joguet et al28 and Matusali et al31 suggests that the virus is particularly infectious to spermatocytes, but Matusali et al31 also demonstrated in testis explants that the virus can infect Sertoli cells and release infectious particles on the adluminal side of the BTB.
Although the reported persistence of the ZIKV varies widely from days to months after the onset of symptoms, it is widely agreed that viral RNA persists longer in semen than in other bodily fluids.26 This reflects a potential reservoir in the male genital tract, perhaps because of the immune privilege of the testes. Supporting this, a systemic analysis by Counotte et al29 reported a median duration of ZIKV RNA in semen of 40 days with a maximum of 370 days, vs 7 days in saliva and 14 days in the female genital tract. The persistence of ZIKV RNA in men with vasectomies further indicates that the virus may also replicate in distal genital tract tissues, such as the bulbourethral glands, prostate, and seminal vesicles.27
Several factors are noted to influence persistence of the viral RNA in semen. In the analysis from Counotte et al,29 increased persistence was associated with increased age, absence of joint pain, conjunctivitis, and less frequent ejaculation. Conversely, men who ejaculated more than 4 times a week cleared viral RNA more than 21 days earlier, and for men who reported vasectomies, although the rate of viral RNA detection was similar, the viral load was decreased from 5.6 to 3.6 log10 RNA copies per milliliter.
Evidence of Sexual Transmission
The first suspected case of sexual transmission of the ZIKV occurred in 2008, when a male patient returned home to the United States from Senegal.25 He developed symptoms of arthralgia, rash, and hematospermia shortly after returning, followed by his wife 4 days later. Serologic results confirmed Zika infection, although only convalescent phase samples were positive in the wife, and semen samples were not collected. Although it is possible that this transmission occurred via other bodily fluids such as saliva, no other family members were affected.
After the subsequent Zika outbreaks, in a systematic review Moreira et al32 compiled demonstrated cases of sexual transmission from men to women, women to men, and men to men. Among the cases examined, sexual transmission was confirmed with positive serum antibodies, Zika RNA in semen, and semen cultures. In addition, they demonstrated that ZIKV RNA was detected in semen as late as 188 days and infectious virus in semen up to 69 days after symptom onset. Notably, 3 transmissions were from entirely asymptomatic patients, who represent the majority of Zika cases.
Further complicating the control of Zika transmission, a prospective cohort study by Paz-Bailey et al30 demonstrated that the virus exhibits intermittent shedding, making it difficult to determine when an individual has cleared the virus. The number of days between symptom onset and positive samples was up to 142 in serum, 204 in urine, and 36 in semen. Taken together, these data represent potential challenges in preventing sexual transmission of the virus, which is of particular concern in non-endemic areas such as the continental United States where mosquito-borne transmission is rare.24 Although mathematical modeling suggests that sexual transmission alone is not likely to drive or sustain a ZIKV outbreak,33 serial semen screenings may be effective in preventing the Zika-associated congenital microcephaly and Guillain-Barre syndrome in patients who are or wish to become pregnant.
The ZIKV and Fertility
Several case reports have described genitourinary symptoms in male Zika cases, although their impact on the male reproductive system is as of yet unclear. In a cohort study, Paz-Bailey et al30 noted evidence of hematospermia (4.7%), painful ejaculation (4.3%), and penile discharge (1.7%) in patients with the ZIKV, suggestive of local inflammation and tissue damage. Although fever cannot be excluded as a cause, mouse models suggest direct tissue damage may be involved.34–36 Examining this effect, Huits et al37 microscopically analyzed semen samples from patients with symptoms of the ZIKV in 2 cohort studies, demonstrating macrohematospermia and microhematospermia in 11 of 15 patients tested and transient oligospermia in 8 of 14 patients. Building on these results, Joguet et al28 monitored the reproductive hormones as well as sperm count, motility, viability, and morphology of patients with the ZIKV on days 7, 11, 20, 30, 60, 90, and 120 after symptom onset. They detected that total and motile sperm counts were about 50% lower on day 60 compared with day 7 but recovered by day 120. The long-term effect on sperm morphology is less clear. In the same study, morphology characteristics recovered to normal by day 120. Results also reflected an early increase of follicle-stimulating hormone and inhibin B, which recovered over time. By contrast, in a cross-sectional study after the 2016 epidemic in Brazil, Avelino-Silva et al38 examined serum hormones and semen samples in 6 patients 1 year after symptoms. In all patients, serum hormones were normal, and semen RT-PCR RNA was negative, but impaired motility was seen in all 3 samples tested and low count was noted in 1 of 5 patients. Given the recency of the Zika outbreaks, the long-term effects of the virus on fertility characteristics should continue to be monitored.
Ebola
History of the EBOV
Ebola virus disease (EVD) first emerged in 1976 with 2 simultaneous outbreaks in Zaire (in a village near the Ebola River) and in South Sudan, where it is thought to have originated in African fruit bats.39 Recurrent outbreaks took place in 1994 in Cote d’Ivoire and in 1995 in Kikwit, Zaire.39 Most recently, 2014–2015 saw a West African outbreak of unprecedented scale in Sierra Leone, Liberia, and Guinea with greater than 28,500 cases and 11,000 deaths.40 The large number of EVD survivors means that understanding its persistence in semen, its potential for sexual transmission, and its fertility implications is a matter of considerable importance for the control of viral spread.
EBOV in Semen
Across multiple studies, it has been shown that the cross-sectional percentage of EBOV survivors of the 2014–2015 West African epidemic with a positive RT-PCR for EBOV RNA in the semen ranges from 8.1 to 9.8%.41–45 With more living EBOV survivors from this epidemic than ever before, this number represents a considerable pool of individuals. While disease modeling has shown that 50% of individuals will clear the virus from semen at 115 days and 90% will clear it at 294 days, viral EBOV RNA has been detected in the semen for as long as 965 days after initial infection.45,46 It has also been cultured in the semen up to 82 days after initial infection.47
As expected, the percentage of individuals with a positive semen RT-PCR for EBOV RNA has been demonstrated to decrease in a predictable manner over time. Deen et al48 showed in a cohort of 210 male adult survivors of EVD in Sierra Leone that at 2–3 months, 100% of individuals had positive RT-PCR results in semen; at 4–6 months, 65% had positive results; and at 7–9 months, 26% had positive results. These results were echoed by Keita et al41 in a study including 277 EVD survivors in Guinea where 93.02% of individuals had positive RT-PCR in semen at 3 months, 60.12% at 6 months, 27.68% at 9 months, 10.32% at 12 months, 0.96% at 18 months, and 0.06% at 24 months. Interestingly, limited data indicate that older men may be more likely to have a longer length of time in which semen is positive for EBOV RNA. Soka et al42 showed that men older than 40 years of age were more likely to have a positive semen test than men younger than 40 years of age.
Animal models have been used to further characterize the presence of filoviruses in the gonadal tissue and have shown viral infiltration of the testicular interstitium and the seminiferous tubules. In situ hybridization (ISH) and immunohistochemistry performed on non-human primates (NHPs) infected with the Marburg virus localized the virus to the interstitium between the seminiferous tubules.49 In NHPs infected with EBOV, Perry et al50 similarly showed viral replication in the interstitial tissues of the reproductive tract, including the seminal vesicles, epididymis, prostate, and testis. Perry et al50 also identified the Marburg virus in the seminiferous tubules of NHPs.51 Indeed, the virus was found to be most prominently localized to Sertoli cells, leading to breakdown of the BTB and inflammatory cell invasion into the testicular environment. Another study in NHPs found EBOV RNA to be localized to the tubular lumen of the epididymis of 1 of twelve rhesus monkey sampled but did not find any EBOV localized to the testis.52 In the lumen of the epididymis, macrophages were found to be the EBOV reservoir.52 Overall, it appears that viral persistence may be established in the interstitial tissues of the reproductive tract, where it is transmitted by tissue macrophages across the BTB and into the seminal fluid.
Sexual Transmission of the EBOV
Although the EBOV in the semen is clearly demonstrated, it remains far less certain what potential the virus has for sexual transmission. Since the first known outbreak of a filovirus (the 1967 Marburg virus), there have been 9 documented events of suspected human-to-human filovirus sexual transmission.53 The first case of suspected EBOV transmission occurred during the 1995 Kikwit outbreak, wherein in a 20-year-old woman was shown to have weakly positive IgM antibodies to IgM 52 days after exposure from her convalescent partner. The individual was later negative for IgG antibodies on a serum sample, making it difficult to confirm whether she was in fact asymptomatically infected.47
In the West African outbreak, the first confirmed sexual transmission occurred in March 2015. A man transmitted the virus to his sexual partner 179 days after onset of EVD, which was confirmed by whole viral genome analysis of his semen compared with an acute blood sample of his female partner where the samples were found to differ by only 1 nucleotide.54 In October 2015, a suspected sexual transmission event occurred in Conakry, Guinea.55 A man presenting with EVD was found to have an EBOV genome that did not match the country’s current strain of transmission but instead differed by only 6 nucleotide submissions from a sample of his brother-in-law’s blood collected during prior acute infection. The man’s sister was positive for EBOV IgM at the time of his infection; however, the transmission could not be confirmed as her husband’s semen was negative for the EBOV at the time a sample was collected. It was thought that the transmission from the male survivor occurred at 250 days after disease onset.
The longest recorded convalescent period before sexual transmission occurred 470 days after disease in N’Zerekore, Guinea in March 2016.43 The acute blood sample and semen sample from the male survivor differed by 5 mutations from a female sexual partner with acute EVD and 4 subsequent cases from the disease cluster. In total, the sexual transmission resulted in a cluster of 13 cases with 4 deaths. Sexual transmission from the EBOV, while possible, remains a rare event. The World Health Organization in their guide to clinical care for survivors of EVD recommends abstinence or barrier protection during sexual activity for 12 months after the onset of EVD.56
The EBOV and Fertility
Given the extremely high mortality associated with EVD and the limited number of laboratories with the capability to study such a pathogenic virus, there is much that remains unanswered about the potential reproductive sequelae of EVD.57 In rhesus monkeys, it has been shown that although Marburg infection had a deleterious effect on Sertoli cells, the overall reproductive function of the Sertoli cells was unaffected. Spermatogenesis was unaffected, and tissue morphology was normal.51 Further studies are needed to determine if semen parameters or reproductive capacity are altered in male survivors of EVD.
Sexual health complaints are commonly reported among EVD survivors. A health clinic for the management of EVD survivors in Sierra Leone reported findings from a group of 246 patients from 2015 to 2016. Of 98 men, 5.1% reported erectile dysfunction and 10.2% reported loss of sexual desire.58 An EVD survivors’ clinic in Liberia reported on 329 EVD survivors from 2015 to 2017. In this cohort, which included 135 men, 8% reported erectile dysfunction, whereas 12% reported decreased libido.59 The causal mechanism for these complaints remains to be determined. Although physiologic conditions can play a role, it is also likely that psychosocial factors including residual stress, trauma, stigma, and grief contribute as well.
West Nile Virus
The WNV was first recognized in North America in New York City during the summer of 1999.60 The virus was previously known to cause small human outbreaks in Africa and the Middle East but has now established itself as a major worldwide seasonal pandemic.61 Between 1999 and 2018, there have been 50,830 documented cases of the WNV in the United States, with particularly high-intensity seasonal outbreaks in 2002 and 2003.62 The most concerning sequelae of the WNV is neuroinvasive disease, which occurs in less than 1% of infected individuals but carries a 10% fatality rate.63
The WNV in Semen
There is very little evidence to suggest that WNV is present in semen. While WNV infection in blood, cerebrospinal fluid, and urine has been well studied, to date, there is only 1 study that describes the evaluation of the WNV in semen. Gorchakov et al64 examined 4 semen samples from 3 patients for presence of WNV RNA. Samples were collected a median of 20.5 days after onset of viral illness symptoms. One of the 4 samples was positive.
The WNV has been further studied in a cultured line of human Sertoli cells. When the WNV was introduced to these cells, it was noted that the Sertoli cells supported a robust WNV infection with titers comparable with that of the ZIKV, a pathogen known to have high infectivity in semen.65 In a small case series presenting postmortem autopsy results for individuals deceased from neuroinvasive WNV, the WNV was identified by immunofluorescence in the testes and prostate in 1 of 4 men examined.66 This individual was 43 years of age and on chronic immunosuppression secondary to a kidney transplant, raising the possibility that immunosuppression could contribute to wider systemic dissemination of the disease.
Owing to the limited nature of currently available evidence, further studies are needed to assess larger sample size populations before any definitive conclusions can be drawn about the presence of the WNV in semen. Nevertheless, the studies presented suggest that the WNV in semen is a possibility that bears further investigation. Analysis of semen during the period of acute illness is also needed to assess semen infectivity at that time.
Sexual Transmission of the WNV
Given the poorly established evidence that the WNV is even present in semen, it follows that the possibility of sexual transmission is even less well characterized. There is only a single case report of male-to-female possible sexual transmission of WNV when a previously healthy middle-aged woman developed viral meningoencephalitis 2 weeks after unprotected intercourse.67 Her husband had serologically confirmed WNV. Although no mosquito bite was reported, the women lived in a mosquito endemic area. Interestingly, the Food and Drug Administration recommends that all donor semen for assistive reproductive technology be tested for the WNV using a nucleic acid test in any donation made between June 1 and October 31 each year, the season when the virus is most active.68 Further work is needed to determine if this type of testing should be made more routinely available to men with confirmed WNV desiring to father a pregnancy.
The WNV and Fertility
The only available report of a possible compromise in fertility from the WNV comes from a case report of an autopsy on a 43-year-old man deceased from the WNV.69 In this case, thickening of the tubular basement membrane was observed with frequent foci of tubular necrosis and absence of spermatogenesis. Immunohistochemical staining did not identify WNV antigen in testicular tissue; however, transmission electron microscopy (TEM) of formalin-fixed testicular tissue did reveal enveloped particles fitting the size, structure, and location of Flavivirus particles, leading the authors to speculate that this was likely WNV causing orchitis. No studies could be identified reporting on semen parameters during or after acute WNV infection, and no studies could be identified detailing possible changes in sexual function. More evidence is required to substantiate a link between the WNV and reduced fertility.
H1N1/Influenza
History
The influenza virus is among the most common infectious illnesses worldwide. The virus came into prominence among humans and pigs in the 1918 pandemic, shortly after mutations of avian strains enabled transferal to humans and pigs.70 Amplified by the movement and proximity of World War I soldiers, the virus soon infected 500 million people and killed 50 million, more than the military and civilian deaths of World War I combined. Since then, type A influenza has produced pandemic outbreaks in 1957, 1968, and most recently in 2009. These events are triggered by reassortment in avian or swine reservoirs of genes for key hemagglutinin (H) and neuraminidase (N) surface glycoproteins, by which the virus is subtyped. The H1N1 and H3N2 strains of type A influenza currently circulate among humans, which along with influenza B cause seasonal flu (WHO 2020). Notably, in the 2009 H1N1 pandemic, young people were particularly at risk, whereas 1 of 3 older than the age of 60 years had antibodies, likely from a historical infection or vaccination with a similar strain.71
Influenza in Semen
The presence of influenza in semen has not been reported. However, the virus demonstrates extrapulmonary symptoms in many major organ systems, a large number of which have since been found contain to functional influenza receptors.72,73 No studies to date have explored if those receptors are present in the human genital tract, but such tropism has been noted in turkeys.74,75 Given the variability of viral tropism among animals, further work is need to explore whether such tropism exists among humans or is reflected by the presence of viral particles and RNA in semen.
Sexual Transmission of Influenza
There is a similar dearth of information on sexual transmission of influenza, possibly because of other, more likely routes of infection. Evidence suggests that while infection by direct contact is possible, it is not likely to be a significant mechanism compared with transmission via aerosol and respiratory droplets.76 These airborne routes are both particularly infectious within close contact and should deter high-risk populations from sex during the duration of viral shedding.
Influenza and Fertility
As a pyrogenic virus, influenza affects sperm quality. MacLeod77 noted that medical students who presented with febrile illness had decreased sperm count, motility, and morphology parameters. Morphology and motility recovered at 4 weeks, as did sperm count at 8 weeks.78 Buch and Havlovec78 reported a case of a semen donor who suffered a 24-hour febrile viral illness, who then had decreased sperm count and reduced egg penetration ability 6 and 7 weeks later. Other studies have found similar effects from non-febrile heating such as saunas or laptops.79,80
A 2007 case study by Sergerie et al81 first detailed the timeline of genital tract effects following febrile influenza. After 2 days of high fever due to the virus (39 –40 C), a 47-year-old man had decreased total sperm count at days 15, 37, and 58 before normalizing at day 79. Motility percentile was similarly decreased but recovered sooner at day 58. The authors measured the DNA fragmentation index and found it to increase from 9% before the fever to 24%, 35%, 15% and 8% at 15, 37, 58, and 79 days, respectively. Sperm DNA fragmentation by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay increased from 17% before fever to 23% at day 15, but this was not statistically significant (P < .05). Evenson and Jost82 found that underlying DNA changes included decreased free SH groups and alteration of nuclear protein composition, causing latent effects on sperm chromatin structure and stability.
Murine models suggest that the viral particulates are also damaging. Sharma et al83 first intraperitoneally inoculated mice with influenza A2 and recorded a significant increase in numerical and structural alteration of meiotic chromosomes, particularly aneuploidy. Subsequent mouse studies noted similar DNA damage was induced by both inactivated and purified influenza indicating a possible direct cytotoxicity by the viral particles.84 Therefore, while evidence argues against a local inflammatory response,3,85 the transient sperm changes observed are likely due to a combination of systemic fever and direct DNA damage resulting in apoptosis and a transient decrease in fertility.
Severe Acute Respiratory Syndrome
History
SARS is respiratory disease caused by a novel coronavirus that first appeared in the Guangdong province of southern China in 2002. The virus rapidly became an epidemic and spread globally to 26 countries and 8,000 people before being contained in 2003. Subsequent research uncovered a natural reservoir within horseshoe bats and civets acting as an intermediate host in local meat markets.86 Although no cases have been reported since 2004, the presence of this animal reservoir, the high reported case-fatality rate (7%), and a single reappearance of the virus have driven continued monitoring of the virus near its site of origin.87
SARS in semen investigation into the effect of novel coronaviruses on fertility began after the discovery that angiotensin-converting enzyme 2 (ACE-2), which is expressed in the testis, acts as a functional receptor for the SARS virus.88,89 Zhao et al90 first reported detection of the virus within testicular epithelial cells and Leydig cells through combined ISH and TEM. However, no other research teams have been able to mirror these results.91–93 Ding et al91 examined all tissues that express ACE-2 in 4 autopsies of patients with SARS and were surprised to find that the testes were uniformly negative for the SARS RNA and signature N protein despite high levels of ACE-2 expression in the testes. Similarly, Gu et al92 noted focal testicular atrophy in all 8 autopsies with confirmed SARS but found no evidence of SARS in parenchymal tissue by ISH and TEM. Therefore, although semen has not been directly examined for SARS, indirect evidence suggests that it is unlikely to be a reservoir.
Sexual Transmission of SARS
Similar to other respiratory viruses, SARS spreads most readily through respiratory droplets, fomites, and aerosol.86 To date, no sexual transmissions of SARS have been reported. Given that most studies do not find SARS in genital tissues, any transmission of SARS after intercourse is more likely due to direct contact and close proximity rather than through genital secretions.
SARS and Fertility
A mechanism for genital injury from SARS was furnished in 2006 by Xu et al.93 They analyzed the pathologic changes in testicular autopsy specimens from 6 patients with SARS compared with those of 4 controls. All 6 patients with SARS demonstrated testicular atrophy grossly. The SARS samples exhibited extensive germ cell destruction with increased apoptosis on TUNEL assay. Histologically, basement membrane thickening, peritubular fibrosis, leukocytes, and vascular congestion indicative of local inflammation were noted. ISH that produced positive readings in the lungs of the patients with SARS was negative in the testes. However, extensive deposits of IgG were detected within the seminiferous epithelium, interstitium, Sertoli cells, and some germ cells of patients with SARS compared with controls. This potentially represents an autoimmune orchitis secondary to the immune response to SARS.
Further research is needed to explore this possibility and the persistence of orchitis. In addition, data are needed to ascertain whether the germ cell destruction in SARS-associated orchitis is reflected in semen characteristics.
Severe Acute Respiratory Syndrome–Coronavirus-2
History
SARS-CoV-2 is a novel coronavirus known to cause the disease “coronavirus disease-19” or “COVID-19”. It emerged in late 2019 in Wuhan, Hubei Province, China, and likely has its origin in bat populations.94 On March 11, 2020, the World Health Organization declared COVID-19 to be a worldwide pandemic.95 It has been estimated to cause severe disease in 16% of cases.96 Older individuals are particularly susceptible to severe illness, with 80% of deaths occurring in individuals aged 65 years and older.94
SARS-CoV-2 in Semen
Given the recent and novel nature of the COVID-19 outbreak, data remain limited. Thus far, there are mixed findings regarding the presence of SARS-CoV-2 in semen. Pan et al97 have reported a case series of 34 adult Chinese men diagnosed with COVID-19 who showed no SARS-CoV-2 in semen at a median follow-up of 31 days. Unpublished data from 12 Chinese patients in recovery from COVID-19 showed no SARS-CoV-2 in RT-PCR of semen.98 Testicular biopsy from 1 deceased individual in this study likewise was negative for SARS-CoV-2 viral RNA. In addition, Paoli et al99 showed negative RT-PCR in both urine and semen samples of an individual who tested positive for SARS-CoV-2 by nasopharyngeal swab. In contrast, Li et al100 reported that 6 of 38 (15.8%) patients in a cohort of patients in Shangqiu, China, had virus detected in their semen by RT-PCR. Of them, 4 were in the acute stage of infection, and 2 were recovering from the virus. In providing analysis and commentary on these findings, Paoli et al101 nevertheless recommended that the small caseload and recency of infection in patients should give readers caution before drawing sharp, fatalistic conclusions. Further studies will be essential to confirm these findings.
Sexual Transmission of SARS-CoV-2
Although SARS-CoV-2 is not known to be present in semen, precluding the ability for sexual transmission, the intimate nature of physical sexual contact still dictates caution to prevent viral spread. Given the presence of SARS-CoV-2 in respiratory droplets, saliva, mucus, and fecal matter, as well as current recommendations to maintain 6 feet of distance between individuals to prevent the spread of infection, physical sexual intimacy presents a high-risk scenario for viral transmission, particularly for non-monogamous partners who do not live with one another.94,102
SARS-CoV-2 and Fertility
In the Chinese case series reported by Pan et al,97 6 individuals (19% of the cohort) described scrotal discomfort around the time of onset of viral illness, suggesting SARS-COV-2 may induce a viral orchitis. Studies on semen quality from COVID-19 survivors have not yet been reported. Chen et al103 have proposed a theoretical link between COVID-19 and reduced fertility based on the virus’ strong interaction with angiotensin-converting enzyme 2 (ACE2). Molecular modeling conducted by the authors showed the virus have a unique structure that allows it to penetrate deep into the hydrophobic pocket of ACE2. ACE2 is highly expressed in the renal tubular cells, the intestines, Leydig cells, and the seminiferous ducts in the testes, raising the possibility that the virus may have a deleterious testicular effect.104 By contrast, Pan et al97 demonstrate with single-cell transcriptome data that ACE2 RNA is sparsely expressed in the testis and argue that this is an unlikely medium of viral entry into target host cells. Even so, it is still unknown exactly what function ACE2 serves in the testis.104 Corona et al105 analyzed these studies and recommended that those of reproductive age consider andrological consultation and evaluation before safely pursuing reproduction. Larger studies will be imperative to understand currently conflicting evidence and to deduce what potential fertility SARS-CoV-2 may have.
Discussion
In this article, we have reviewed the presence in semen, possibility of sexual transmission, and fertility implications of each of the major recent viral pandemics: Zika, Ebola, West Nile, pandemic influenza, SARS, and SARS-CoV-2. The ZIKV has been reported in semen up to 370 days after disease onset but appears to be present for a median time of 40 days for most individuals. Sexual transmission of the ZIKV has been repeatedly documented, even among entirely asymptomatic individuals, and is of particular concern given its ability to microcephaly in a developing fetus. Short-term fertility may be negatively affected by the ZIKV based on several reports of reduced sperm count, altered morphology, and impaired mobility in semen samples. Fortunately, this effect appears time limited.
The EBOV has also been detected in semen, with studies indicating its presence for an average of 115 days, with a maximum reported duration of 965 days. Reports of sexual transmission remain rare, with only 9 suspected cases documented in the history of the virus. Semen parameters do not appear altered, although a proportion of survivors decreased sexual function and diminished libido.
The WNV has only been reported in semen or to be transmitted sexually in isolated case reports. Likewise, only a single autopsy report suggests the possibility of WNV-induced orchitis. Influenza, a respiratory pathogen, has not been found in semen or been shown to be sexually transmissible. Research demonstrating its impact on fertility has focused on the effects of febrile illness on semen parameters, showing that febrile episodes are linked to transiently decreased sperm count, motility, and morphology.
The SARS virus has not been shown to be present in semen, but several small case series of autopsy reports have found testicular damage and atrophy in individuals deceased from SARS, which may be linked to secondary autoimmune orchitis. SARS-Cov-2, the most recent viral pandemic included in this review, is expected to behave similar to SARS virus, but further data are required to validate these assumptions. Current evidence from small case series shows that it is not present in semen and thus is unlikely to be sexually transmitted. For both SARS and SARS-CoV-2, there is speculation that the viruses’ interaction with ACE2, which is present in the Leydig cells and the seminiferous ducts of the testes, could have implications for spermatogenesis. Further studies are needed to explore this possibility.
Conclusion
We have reported the presence in semen, sexual transmission potential, and fertility side effects of recent viral pandemics. Overall, semen studies and fertility effects are highly understudied in viral pandemics, and rigorous study on these topics should be undertaken as novel pandemics emerge. Specifically, further semen studies and fertility studies are needed to understand the transmission potential and fertility side effects of the novel SARS-CoV-2.
Statement of authorship
Kelly Payne: Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing, Funding Acquisition; Peter Kelly: Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing, Funding Acquisition; Jason Scovell: Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing, Funding Acquisition; Kajal Khodamoradi: Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing, Funding Acquisition; Ranjith Ramasamy:Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing, Funding Acquisition.
Funding
None.
Contributor Information
Kelly Payne, Scott Department of Urology, Baylor College of Medicine, Houston, TX, USA.
Peter Kenny, Scott Department of Urology, Baylor College of Medicine, Houston, TX, USA.
Jason M. Scovell, Scott Department of Urology, Baylor College of Medicine, Houston, TX, USA; Department of Urology, Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH, USA.
Kajal Khodamoradi, Department of Urology, Miller School of Medicine, University of Miami, Miami, FL, USA.
Ranjith Ramasamy, Department of Urology, Miller School of Medicine, University of Miami, Miami, FL, USA.
References
- 1. Madhav N., Oppenheim B., Gallivan M.et al. . Pandemics: risks, impacts, and mitigation. Disease control priorities: improving health and reducing poverty. 3rd ed. Washington, DC: World Bank, 2017. 315–345. [Google Scholar]
- 2. Dejucq N., Lienard M.O., Guillaume E.et al. . Expression of interferons-alpha and -gamma in testicular interstitial tissue and spermatogonia of the rat. Endocrinology 1998;139: 3081–3087. [DOI] [PubMed] [Google Scholar]
- 3. Le Tortorec A., Matusali G., Mahe D.et al. . From Ancient to emerging infections: the Odyssey of viruses in the male genital tract. Physiol Rev 2020;100: 1349–1414. [DOI] [PubMed] [Google Scholar]
- 4. Fijak M., Bhushan S., Meinhardt A.. Immunoprivileged sites: the testis. Methods Mol Biol 2011;677: 459–470. [DOI] [PubMed] [Google Scholar]
- 5. Pollanen P., von Euler M., Jahnukainen K.et al. . Role of transforming growth factor beta in testicular immunosuppression. J Reprod Immunol 1993;24: 123–137. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe M., Kashiwakura Y., Kusumi N.et al. . Adeno-associated virus-mediated human IL-10 gene transfer suppresses the development of experimental autoimmune orchitis. Gene Ther 2005;12: 1126–1132. [DOI] [PubMed] [Google Scholar]
- 7. Rettew J.A., Huet-Hudson Y.M., Marriott I.. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008;78: 432–437. [DOI] [PubMed] [Google Scholar]
- 8. Head J.R., Billingham R.E.. Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation 1985;40: 269–275. [DOI] [PubMed] [Google Scholar]
- 9. Meinhardt A., Hedger M.P.. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol 2011;335: 60–68. [DOI] [PubMed] [Google Scholar]
- 10. Dejucq N., Lienard M.O., Jegou B.. Interferons and interferon-induced antiviral proteins in the testis. J Reprod Immunol 1998;41: 291–300. [DOI] [PubMed] [Google Scholar]
- 11. Com E., Bourgeon F., Evrard B.et al. . Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod 2003;68: 95–104. [DOI] [PubMed] [Google Scholar]
- 12. Chakradhar S. Puzzling over privilege: how the immune system protects-and fails-the testes. Nat Med 2018;24: 2–5. [DOI] [PubMed] [Google Scholar]
- 13. Jenabian M.A., Costiniuk C.T., Mehraj V.et al. . Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS 2016;30: 2777–2786. [DOI] [PubMed] [Google Scholar]
- 14. Ghosh S., Klein R.S.. Sex drives Dimorphic immune responses to viral infections. J Immunol 2017;198: 1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torcia M.G., Nencioni L., Clemente A.M.et al. . Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS ONE 2012;7: e39853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Libert C., Dejager L., Pinheiro I.. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010;10: 594–604. [DOI] [PubMed] [Google Scholar]
- 17. Pinheiro I., Dejager L., Libert C.. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays: News Rev Mol Cell Developmental Biol 2011;33: 791–802. [DOI] [PubMed] [Google Scholar]
- 18. Ditton H.J., Zimmer J., Kamp C.et al. . The AZFa gene DBY (DDX3Y) is widely transcribed but the protein is limited to the male germ cells by translation control. Hum Mol Genet 2004;13: 2333–2341. [DOI] [PubMed] [Google Scholar]
- 19. Szappanos D., Tschismarov R., Perlot T.et al. . The RNA helicase DDX3X is an essential mediator of innate antimicrobial immunity. Plos Pathog 2018;14: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palaszynski K.M., Smith D.L., Kamrava S.et al. . A Yin-Yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology 2005;146: 3280–3285. [DOI] [PubMed] [Google Scholar]
- 21. Robinson D.P., Lorenzo M.E., Jian W.et al. . ELevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathogens 2011;7: e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Randolph A.G., Vaughn F., Sullivan R.et al. . Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics 2011;128: e1450–e1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zarychanski R., Stuart T.L., Kumar A.et al. . Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 2010;182: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Zika Virus: Centers for Disease Control and Prevention 2017. Available at: http://www.cdc.gov/zika/index.html. . Accessed June 1, 2020 [Google Scholar]
- 25. Foy B.D., Kobylinski K.C., Foy J.L.C.et al. . Probable non–Vector-borne transmission of Zika virus, Colorado, USA. Emerging Infect Dis 2011;17: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurscheidt F.A., Mesquita C.S.S., Damke G.M.Z.F.et al. . Persistence and clinical relevance of Zika virus in the male genital tract. Nat Rev Urol 2019;16: 211–230. [DOI] [PubMed] [Google Scholar]
- 27. Mead P.S., Duggal N.K., Hook S.A.et al. . Zika virus shedding in semen of symptomatic infected men. New Engl J Med 2018;378: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 28. Joguet G., Mansuy J.-M., Matusali G.et al. . Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 2017;17: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 29. Counotte M.J., Kim C.R., Wang J.et al. . Sexual transmission of Zika virus and other flaviviruses: a living systematic review. PLOS Med 2018;15: e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paz-Bailey G., Rosenberg E.S., Doyle K.et al. . Persistence of Zika virus in body fluids - Final report. N Engl J Med 2018;379: 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matusali G., Houzet L., Satie A.-P.et al. . Zika virus infects human testicular tissue and germ cells. J Clin Invest 2018;128: 4697–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreira J., Peixoto T.M., Siqueira A.M.et al. . Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect 2017;23: 296–305. [DOI] [PubMed] [Google Scholar]
- 33. Yakob L., Kucharski A., Hue S.et al. . Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis 2016;16: 1100–1102. [DOI] [PubMed] [Google Scholar]
- 34. Arévalo Romero H., Vargas Pavía T.A., Velázquez Cervantes M.A.et al. . The dual role of the immune response in reproductive organs during Zika virus infection. Front Immunol 2019;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uraki R., Hwang J., Jurado K.A.et al. . Zika virus causes testicular atrophy. Sci Adv 2017;3: e1602899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Govero J., Esakky P., Scheaffer S.M.et al. . Zika virus infection damages the testes in mice. Nature 2016;540: 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huits R., De Smet B., Ariën K.K.et al. . Zika virus in semen: a prospective cohort study of symptomatic travellers returning to Belgium. Bull World Health Organ 2017;95: 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Avelino-Silva V.I., Alvarenga C., Abreu C.et al. . Potential effect of Zika virus infection on human male fertility? Rev Inst Med Trop Sao Paulo 2018;60: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Centers for Disease Control and Prevention . Viral Hemorrhagic Fevers (VHFs), Ebola (Ebola Virus Disease): Centers for Disease Control and Prevention. 2018. Available at: https://www.cdc.gov/vhf/ebola/history/summaries.html. . Accessed June 5, 2020. [Google Scholar]
- 40. Vetter P., Fischer W.A. 2nd, Schibler M.et al. . Ebola virus shedding and transmission: review of current evidence. J Infect Dis 2016;214: S177–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Keita A.K., Vidal N., Toure A.et al. . A 40-Month follow-up of Ebola virus disease survivors in Guinea (PostEbogui) reveals long-term detection of Ebola viral Ribonucleic acid in semen and Breast Milk. Open Forum Infect Dis 2019;6: ofz482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soka M.J., Choi M.J., Baller A.et al. . Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health 2016;4: e736–e743. [DOI] [PubMed] [Google Scholar]
- 43. Diallo B., Sissoko D., Loman N.J.et al. . Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 Days. Clin Infect Dis 2016;63: 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Subtil F., Delaunay C., Keita A.K.et al. . Dynamics of Ebola RNA persistence in semen: a report from the Postebogui cohort in Guinea. Clin Infect Dis 2017;64: 1788–1790. [DOI] [PubMed] [Google Scholar]
- 45. Fischer W.A., Brown J., Wohl D.A.et al. . Ebola virus Ribonucleic acid detection in semen more than two Years after Resolution of acute Ebola virus infection. Open Forum Infect Dis 2017;4: ofx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sissoko D., Duraffour S., Kerber R.et al. . Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health 2017;5: e80–e88. [DOI] [PubMed] [Google Scholar]
- 47. Rodriguez L.L., De Roo A., Guimard Y.et al. . Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999;179: Suppl 1: S170–S176. [DOI] [PubMed] [Google Scholar]
- 48. Deen G.F., Broutet N., Xu W.et al. . Ebola RNA persistence in semen of Ebola virus disease survivors - Final report. N Engl J Med 2017;377: 1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cooper T.K., Sword J., Johnson J.C.et al. . New Insights into Marburg virus disease pathogenesis in the rhesus Macaque model. J Infect Dis 2018;218: S423–S433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perry D.L., Huzella L.M., Bernbaum J.G.et al. . Ebola virus Localization in the Macaque reproductive tract during acute Ebola virus disease. Am J Pathol 2018;188: 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coffin K.M., Liu J., Warren T.K.et al. . Persistent Marburg virus infection in the testes of nonhuman primate survivors. Cell Host Microbe 2018;24: 405–416.e3. [DOI] [PubMed] [Google Scholar]
- 52. Zeng X., Blancett C.D., Koistinen K.A.et al. . Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat Microbiol 2017;2: 17113. [DOI] [PubMed] [Google Scholar]
- 53. Schindell B.G., Webb A.L., Kindrachuk J.. Persistence and sexual transmission of filoviruses. Viruses 2018;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mate S.E., Kugelman J.R., Nyenswah T.G.et al. . Molecular evidence of sexual transmission of Ebola virus. N Engl J Med 2015;373: 2448–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keita M., Duraffour S., Loman N.J.et al. . Unusual Ebola virus chain of transmission, Conakry, Guinea, 2014-2015. Emerg Infect Dis 2016;22: 2149–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. GUIDANCE I . Clinical care for survivors of Ebola virus disease. Geneva: World Health Organization, 2016. Available at: https://www.who.int/csr/resources/publications/ebola/guidance-survivors/en/. . Accessed June 1, 2020 [Google Scholar]
- 57. Purpura L.J., Choi M.J., Rollin P.E.. Zika virus in semen: lessons from Ebola. Lancet Infect Dis 2016;16: 1107–1108. [DOI] [PubMed] [Google Scholar]
- 58. Guetiya Wadoum R.E., Samin A., Mafopa N.G.et al. . Mobile health clinic for the medical management of clinical sequelae experienced by survivors of the 2013-2016 Ebola virus disease outbreak in Sierra Leone, West Africa. Eur J Clin Microbiol Infect Dis 2017;36: 2193–2200. [DOI] [PubMed] [Google Scholar]
- 59. de St Maurice A., Ervin E., Orone R.et al. . Care of Ebola survivors and factors associated with clinical sequelae-Monrovia, Liberia. Open Forum Infect Dis 2018;5: ofy239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roehrig J.T. West nile virus in the United States - a historical perspective. Viruses 2013;5: 3088–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chancey C., Grinev A., Volkova E.et al. . The global ecology and epidemiology of West Nile virus. Biomed Res Int 2015;2015: 376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Control CfD . West Nile virus disease cases reported to CDC by state of residence, 1999-2018. 2019. [Google Scholar]
- 63. Petersen L.R., Brault A.C., Nasci R.S.. West Nile virus: review of the literature. JAMA 2013;310: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gorchakov R., Gulas-Wroblewski B.E., Ronca S.E.et al. . Optimizing PCR detection of West Nile virus from body fluid specimens to delineate natural history in an infected human cohort. Int J Mol Sci 2019;20: 1934–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Siemann D.N., Strange D.P., Maharaj P.N.et al. . Zika virus infects human Sertoli cells and modulates the Integrity of the in vitro blood-testis barrier model. J Virol 2017;91: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Armah H.B., Wang G., Omalu B.I.et al. . Systemic distribution of West Nile virus infection: postmortem immunohistochemical study of six cases. Brain Pathol 2007;17: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kelley R.E., Berger J.R., Kelley B.P.. West Nile virus Meningo-encephalitis: possible sexual transmission. J La State Med Soc 2016;168: 21–22. [PubMed] [Google Scholar]
- 68. Food and Drug Administration. Guidance for Industry: Use of nucleic acid tests to reduce the risk of transmission of West Nile Virus from living donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps). Available at: https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/tissue/ucm372084.pdf. Accessed July 21, 2020. [Google Scholar]
- 69. Smith R.D., Konoplev S., DeCourten-Myers G.et al. . West Nile virus encephalitis with myositis and orchitis. Hum Pathol 2004;35: 254–258. [DOI] [PubMed] [Google Scholar]
- 70. The Threat of pandemic influenza: are We Ready? Workshop summary. Knobler S.L., Mack A., Mahmoud A.et al. Institute of medicine (US) Forum on microbial Threats National Academies Press (US), 2005. [PubMed] [Google Scholar]
- 71. Center for Disease Control and Prevention. Influenza (Flu)/Pandemic Influenza; 2019. Available at: https://www.cdc.gov/flu/pandemic-resources/index.htm/. Accessed June 1, 2020. [Google Scholar]
- 72. Sellers S.A., Hagan R.S., Hayden F.G.et al. . The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses 2017;11: 372–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ning Z.-Y., Luo M.-Y., Qi W.-B.et al. . Detection of expression of influenza virus receptors in tissues of BALB/c mice by histochemistry. Vet Res Commun 2009;33: 895. [DOI] [PubMed] [Google Scholar]
- 74. Pantin-Jackwood M., Wasilenko J.L., Spackman E.et al. . Susceptibility of turkeys to pandemic-H1N1 virus by reproductive tract insemination. Virol J 2010;7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sid H., Hartmann S., Winter C.et al. . Interaction of influenza A viruses with Oviduct explants of different avian Species. Front Microbiol 2017;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Killingley B., Nguyen-Van-Tam J.. Routes of influenza transmission. Influenza Other Respir Viruses 2013;7: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Macleod J. Effect of Chickenpox and of Pneumonia on semen quality. Fertil Sterility 1951;2: 523–533. [DOI] [PubMed] [Google Scholar]
- 78. Buch J.P., Havlovec S.K.. Variation in sperm penetration assay related to viral illness. Fertil Sterility 1991;55: 844–846. [DOI] [PubMed] [Google Scholar]
- 79. Brown-Woodman P.D., Post E.J., Gass G.C.et al. . The effect of a single sauna exposure on spermatozoa. Arch Androl 1984;12: 9–15. [DOI] [PubMed] [Google Scholar]
- 80. Mortazavi SaR., Taeb S., Mortazavi S.M.J.et al. . The Fundamental Reasons Why laptop Computers should not be used on Your Lap. J Biomed Phys Eng 2016;6: 279–284. [PMC free article] [PubMed] [Google Scholar]
- 81. Sergerie M., Mieusset R., Croute F.et al. . High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Sterility 2007;88: 970.e1–970.e7. [DOI] [PubMed] [Google Scholar]
- 82. Evenson D., Jost L.. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci 2000;22: 169–189. [DOI] [PubMed] [Google Scholar]
- 83. Sharma G., Polasa H.. Cytogenetic effects of influenza virus infection on male germ cells of mice. Hum Genet 1978;45: 179–187. [DOI] [PubMed] [Google Scholar]
- 84. Pathki V.K., Polasa H.. Dominant lethality in mice--a test for mutagenicity of influenza X-31 virus. Teratog, Carcinog Mutagen 1988;8: 55–62. [DOI] [PubMed] [Google Scholar]
- 85. Pilatz A., Kaiser R., Mankertz A.et al. . 139 Viral epididymitis and orchitis – myth or reality? Eur Urol Supplements 2015;14: e139. [Google Scholar]
- 86. Center for Disease Control and Prevention. Severe Acute Respiratory Syndrome (SARS): Centers for Disease Control and Prevention. 2017; 2019. Available at: https://www.cdc.gov/sars/index.html/. Accessed June 1, 2020. [Google Scholar]
- 87. Peeri N.C., Shrestha N., Rahman M.S.et al. . The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol 2020. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li W., Moore M.J., Vasilieva N.et al. . Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Harmer D., Gilbert M., Borman R.et al. . Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002;532: 107–110. [DOI] [PubMed] [Google Scholar]
- 90. Zhao J-m, Zhou G-d, Sun Y-let al. . [Clinical pathology and pathogenesis of severe acute respiratory syndrome]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2003;17: 217–221. [PubMed] [Google Scholar]
- 91. Ding Y., He L., Zhang Q.et al. . Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 2004;203: 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gu J., Gong E., Zhang B.et al. . Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xu J., Qi L., Chi X.et al. . Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod 2006;74: 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19)): National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html. Accessed June 3, 2020. [PubMed] [Google Scholar]
- 95. Cucinotta D., Vanelli M.. WHO Declares COVID-19 a pandemic. Acta Biomed 2020;91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guan W.J., Ni Z.Y., Hu Y.et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pan F., Xiao X., Guo J.et al. . No evidence of SARS-CoV-2 in semen of males recovering from COVID-19. Fertility and Sterility 2020;113: 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Song C., Wang Y., Li W.et al. . Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod 2020;103: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Paoli D., Pallotti F., Colangelo S.et al. . Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest 2020. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li D., Jin M., Bao P.et al. . Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020;3: e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Paoli D., Pallotti F., Turriziani O.et al. . SARS-CoV-2 Presence in seminal fluid: myth or reality. Andrology 2020. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Aversa A., Jannini E.A.. COVID-19, or the triumph of monogamy? Minerva Endocrinol 2020;45: 77–78. [DOI] [PubMed] [Google Scholar]
- 103. Chen Y., Guo Y., Pan Y.et al. . Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 2020;525: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Douglas G.C., O’Bryan M.K., Hedger M.P.et al. . The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 2004;145: 4703–4711. [DOI] [PubMed] [Google Scholar]
- 105. Corona G., Baldi E., Isidori A.M.et al. . SARS-CoV-2 infection, male fertility and sperm cryopreservation: a position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (Societa Italiana di Andrologia e Medicina della Sessualita). J Endocrinol Invest 2020. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]