Abstract
Although certain risk factors have been associated with increased morbidity and mortality in patients admitted with Coronavirus Disease 2019 (COVID-19), the impact of cardiac injury and high-sensitivity troponin-I (hs-cTnI) concentrations are not well described. In this large retrospective longitudinal cohort study, we analyzed the cases of 1,044 consecutively admitted patients with COVID-19 from March 9 until April 15. Cardiac injury was defined by hs-cTnI concentration >99th percentile. Patient characteristics, laboratory data, and outcomes were described in patients with cardiac injury and different hs-cTnI cut-offs. The primary outcome was mortality, and the secondary outcomes were length of stay, need for intensive care unit care or mechanical ventilation, and their different composites. The final analyzed cohort included 1,020 patients. The median age was 63 years, 511 (50% patients were female, and 403 (40% were white. 390 (38%) patients had cardiac injury on presentation. These patients were older (median age 70 years), had a higher cardiovascular disease burden, in addition to higher serum concentrations of inflammatory markers. They also exhibited an increased risk for our primary and secondary outcomes, with the risk increasing with higher hs-cTnI concentrations. Peak hs-cTnI concentrations continued to be significantly associated with mortality after a multivariate regression controlling for comorbid conditions, inflammatory markers, acute kidney injury, and acute respiratory distress syndrome. Within the same multivariate regression model, presenting hs-cTnI concentrations were not significantly associated with outcomes, and undetectable hs-cTnI concentrations on presentation did not completely rule out the risk for mechanical ventilation or death. In conclusion, cardiac injury was common in patients admitted with COVID-19. The extent of cardiac injury and peak hs-cTnI concentrations were associated with worse outcomes.
Coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has resulted in a global pandemic affecting more than 10 million people worldwide and more than 2.5 million in the United States.1 Although the reported case-fatality rates have been variable,2 in-hospital mortality has been reported to be as high as 21%.3 Several risk factors have been reported to be associated with worse disease and outcomes. Preexisting cardiac comorbid conditions and acute cardiac complications from COVID-19 correlated with more severe disease and higher fatality rates.4 , 5 Small cohort studies have described the association between cardiovascular disease and cardiac injury with outcomes in patients with COVID-19, but data is still limited.6 We present the largest comprehensive study looking at myocardial injury and mortality in patients with COVID-19 in the U.S. This study aims to evaluate outcomes of U.S. patients with cardiovascular comorbidities, determine factors associated with cardiac injury, and examine the association of cardiac injury with the severity of illness in patients with COVID-19.
Methods
Patients admitted to Henry Ford Health System, a tertiary care center in Southeast Michigan, USA, between March 9 and April 15, 2020, was included in the study. Patients selected were ≥18 years of age, diagnosed with SARS-CoV-2, and hospitalized. Patients were excluded if high-sensitivity troponin (hs-cTnI) levels were not obtained, they were transferred to or out of our center, or developed cardiac arrest before presentation. Records were retrospectively reviewed. This study was approved by the Institutional Review Board (IRB# 13774), and informed consent was waived.
SARS-CoV-2 was diagnosed by onsite molecular diagnostic testing for the identification of SARS-CoV-2 RNA using RT-PCR (NeuMoDx assay). This method has been validated against the Centers for Disease Control and Prevention reference method7 to meet or exceed the level of detection required under the Food and Drug Administration and Emergency Use Authorization guidelines.
The epidemiological, clinical, and laboratory data were manually extracted from electronic health records. Symptoms were deemed positive if endorsed within 24 hours of presentation. Comorbid conditions were identified based on admission and discharge diagnoses. Baseline levels refer to initial blood samples collected in the emergency department (ED) or the first values within 24 hours of admission. Patients admitted with COVID-19 had hs-cTnI (Beckman-Coulter) and inflammatory markers such as D-dimer, lactate dehydrogenase, ferritin, and C-reactive protein performed in the ED. These laboratory tests were repeated after 48 hours. Serial testing, however, was performed earlier based on an abnormal initial result at the discretion of the responsible provider. Peak concentrations referred to the highest laboratory value before the corresponding outcome. Imaging findings were described in accordance to Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19.8
Cardiac injury was defined as hs-cTnI concentration >18ng/L, which is >99th percentile of the upper limit of normal. Hs-cTnI, D-dimer, ferritin, lactate dehydrogenase, and C-reactive protein levels were documented at presentation, and then at peak during hospitalization. Acute kidney injury was defined according to the “Kidney Disease: Improving Global Outcomes” criteria for creatinine.9 Acute respiratory distress syndrome (ARDS) was diagnosed, and its severity defined based on the Berlin definition.10
Data collected from electronic medical records were analyzed using Statistical Package for Social Sciences (SPSS, version 25, IBM, Armonk, New York). Descriptive statistical analysis was obtained for all included study variables and are summarized in Table 1 . Categorical variables were described by frequency and percentages, and continuous variables were defined by the median and interquartile range. Patient characteristics were compared using analysis of variance or Kruskal Wallis test for continuous variables based on the normality of the data, and chi-square test or Fisher exact tests for categorical variables. Univariate analysis was first performed to identify the significant variables associated with cardiac injury and mortality on admission. Multivariable and multivariate logistic regression analyses were then performed to identify significant predictors of mortality. Candidate variables for model inclusion included clinically relevant variables associated with COVID-19 mortality 11 , 12 and those with a p-value ≤0.05 on univariable analysis, with model exit criteria p-value ≥0.1. Time-to-event analysis was done using Kaplan-Meier curves for patients with and without cardiac injury stratified by initial hs-cTnI levels, first abnormal hs-cTnI, and maximum hs-cTnI levels. Post-hoc testing was done and a Benjami-Hochberg adjustment was applied to control for type 1 error. Log-rank test was used to identify any difference between the 3 groups of interest. Statistical analyses were considered significant if p ≤ 0.05.
Table 1.
Clinical characteristics of patients on presentation according to cardiac injury
| Variable | Overall | Cardiac injury |
p-value | |
|---|---|---|---|---|
| Yes | No | |||
| Total number of observations | 1020 | 390 (38%) | 630 (6%) | |
| Age (years) | 63 (52–73) | 70 (51–89) | 59 (39–79) | |
| ≥65 | 471 (46%) | 256 (66%) | 215 (34%) | <0.001 |
| Female— No. (%) | 511 (50%) | 161 (41%) | 350 (56%) | <0.001 |
| White race | 403 (40%) | 152 (39%) | 251 (40%) | 0.312 |
| Black race | 463 (45%) | 171 (44%) | 292 (46%) | |
| Other race categories | 154 (15%) | 67 (17%) | 87 (14%) | |
| Body mass index (kg/m2) | 31 (20-42) | 30 (19-41) | 32 (21-43) | <0.001 |
| <18 | 19 (2%) | 2 (0.3%) | 17 (4%) | |
| 18–30 | 419 (41%) | 246 (39%) | 173 (44%) | |
| 30–40 | 398 (39%) | 254 (40%) | 144 (37%) | |
| >40 | 184 (18%) | 128 (20%) | 56 (14%) | |
| Symptoms at Admission | ||||
| Chest pain | 156 (15%) | 46 (12%) | 110 (18%) | 0.021 |
| Fever | 539 (53%) | 176 (45%) | 363 (58%) | <0.001 |
| Cough | 679 (67%) | 226 (58%) | 453 (72%) | 0.001 |
| Myalgias | 250 (25%) | 74 (19%) | 176 (28%) | 0.001 |
| Dyspnea | 686 (67%) | 243 (62%) | 443 (70%) | 0.008 |
| GI symptoms | 366 (36%) | 127 (33%) | 239 (38%) | 0.103 |
| Comorbidities | ||||
| Hypertension | 742 (73%) | 333 (85%) | 409 (65%) | <0.001 |
| Diabetes mellitus | 452 (44%) | 191 (49%) | 261 (41%) | 0.020 |
| Heart failure | 127 (13%) | 97 (25%) | 30 (5%) | <0.001 |
| Coronary artery disease | 123 (12%) | 80 (21%) | 43 (7%) | <0.001 |
| Atrial fibrillation/flutter | 66 (7%) | 48 (12%) | 18 (3%) | <0.001 |
| Any cardiovascular disease | 268 (26%) | 174 (45%) | 94 (15%) | <0.001 |
| Cerebrovascular Disease | 59 (12%) | 39 (20%) | 20 (7%) | <0.001 |
| Chronic kidney disease | 308 (30%) | 197 (51%) | 111 (18%) | <0.001 |
| Smoker | 361 (25%) | 165 (42%) | 199 (31%) | 0.001 |
| COPD | 105 (10%) | 50 (13%) | 55 (9%) | 0.040 |
| Obstructive sleep apnea | 90 (9%) | 45 (12%) | 45 (7%) | 0.025 |
| Asthma | 104 (10%) | 22 (6%) | 82 (13%) | <0.001 |
| Chronic hypoxic respiratory failure | 30 (3%) | 16 (4%) | 14 (2%) | 0.083 |
| Immunosuppression | 155 (15%) | 74 (19%) | 81 (13%) | 0.008 |
| Cirrhosis | 8 (0.8%) | 5 (1%) | 3 (0.5%) | 0.150 |
| Medications | ||||
| Antiplatelet | 327 (32%) | 167 (43%) | 160 (25%) | <0.001 |
| Anticoagulant | 96 (9%) | 64 (16%) | 32 (5%) | <0.001 |
| ACEi/ ARB | 360 (35%) | 170 (44%) | 190 (30%) | <0.001 |
| Beta blocker | 137 (27%) | 87 (44%) | 50 (16%) | <0.001 |
| Calcium channel blockers | 150 (30%) | 76 (38%) | 74 (24%) | 0.001 |
| Statin | 417 (41%) | 183 (47%) | 234 (37%) | 0.007 |
| Diuretic | 99 (20%) | 64 (324%) | 35 (11%) | <0.001 |
| Systemic steroids | 46 (5%) | 14 (4%) | 32 (5%) | 0.260 |
| Immunosuppressant | 41 (4%) | 13 (3%) | 28 (4%) | 0.380 |
| Insulin use | 158 (16%) | 74 (20%) | 84 (14%) | 0.020 |
| Laboratory data | ||||
| Sodium (mmol/L) | 135 (133–138) | 136 (133–139) | 135 (133–138) | 0.08 |
| Potassium (mmol/L) | 3.9 (3.6–4.4) | 4.0 (3.7–4.5) | 3.9 (3.5–4.2) | <0.001 |
| Bicarbonate (mmol/L | 24 (22–26) | 23 (20–25) | 24 (22–26) | <0.001 |
| BUN (mg/dL) | 19 (13–33) | 30 (19–48) | 15 (11–22) | <0.001 |
| Creatinine (mg/dL) | 1.1 (0.9–1.7) | 1.6 (1.2–3.0) | 1.0 (0.8–1.4) | <0.001 |
| GFR (ml/min) | 69 (40–93) | 44 (21–68) | 81 (57–101) | <0.001 |
| WBC (K/µL) | 6.4 (4.7–8.9) | 6.8 (4.9–10.3) | 6.4 (5.0–8.8) | 0.096 |
| Hemoglobin (g/dL) | 13.0 (11.8–14.3) | 12.7 (11.2–14.4) | 13 (12–14) | <0.001 |
| Neutrophil Count (K/µL) | 4.8 (3.3–7.1) | 5.3 (3.7–8.9) | 4.9 (3.3–6.8) | 0.006 |
| Lymphocyte count (K/µL) | 0.90 (0.60–1.20) | 0.8 (0.5–1.2) | 0.9 (0.7–1.3) | <0.001 |
| Platelet count (K/µL) | 200 (154–269) | 197 (146–263) | 216 (169–280) | <0.001 |
| AST (IU/L) | 37 (26–58) | 44 (29–66) | 34 (24–52) | <0.001 |
| ALT (IU/L) | 24 (15–38) | 25 (15–41) | 24 (16–36) | 0.915 |
| Total bilirubin (mg/dL) | 0.6 (0.4–0.8) | 0.6 (0.4–0.9) | 0.6 (0.4–0.8) | 0.002 |
| Albumin (mg/dL) | 3.5 (3.2–3.8) | 3.4 (3.1–3.7) | 3.6 (3.3–3.8) | <0.001 |
| LDH (IU/L) | 350 (264–475) | 400 (287–533) | 334 (254–452) | <0.001 |
| CPK (IU/L) | 183.0 (90–429) | 263 (133–625) | 151 (77–323) | <0.001 |
| CRP (mg/dL) | 9.7 (4.8–15.7) | 12.0 (7.0–19.1) | 8.8 (3.1–14.9) | <0.001 |
| Ferritin (ng/ml) | 540 (263–1078) | 658 (309–1294) | 425 (203–916) | <0.001 |
| D-dimer (µg/ml) | 1.30 (0.7–2.5) | 1.9 (1.1–3.8) | 1.1 (0.6–2.1) | <0.001 |
| High sensitivity troponin (ng/L) | 16.5 (6.2–32.0) | 43 (27-87) | 8.0 (2.3-14) | <0.001 |
| Chest imaging findings | ||||
| Normal | 144 (14%) | 56 (14%) | 88 (14%) | 0.153 |
| Unilateral pneumonia | 135 (13%) | 54 (14%) | 81 (13%) | |
| Bilateral pneumonia | 224 (22%) | 71 (18%) | 153 (24%) | |
| Multi-focal pneumonia | 308 (49%) | 209 (54%) | 517 (51%) | |
Abbreviations: ACEi = Angiotensin Converting Enzyme Inhibitor; ALT = Alanine Aminotransferase; ARB = Angiotensin II Receptor Blockers; AST = Aspartate Aminotransferase; BUN = Blood Urea Nitrogen; COPD = Chronic Obstructive Pulmonary Disease; CPK = Creatine Phosphokinase; CRP = C-Reactive Protein; GFR = Glomerular Filtration Rate; LDH = Lactate Dehydrogenase; WBC = White Blood Cell.
Results
One thousand and forty-four patients met the initial inclusion criteria. Four patients who developed cardiac arrest or intubation before presentation or were transferred to and out of our center were excluded. Twenty patients without hs-cTnI levels were also excluded. A total of 1,020 patients were included in the final analysis. Baseline characteristics included a median age of 63 (52–73) years, body mass index (BMI) of 31 (20–42) kg/m2, 511 (50%) were female, and 403 (40%) were Caucasian. Additional characteristics are detailed in Table 1.
Of those included in the final analysis, 390 (38%) demonstrated cardiac injury on presentation to the ED. In these patients, the median age was 70 (51–89) years, BMI 30 (19–41) kg/m2, 161 (41%) were female, and 152 (39%) were white. Patients with cardiac injury were noted to have a higher burden of cardiac risk factors including hypertension, diabetes mellitus, heart failure, coronary artery disease, atrial fibrillation/flutter, and cerebrovascular disease. Patients in this group were more likely to be smokers or have a history of pulmonary or kidney disease (Table 1). Baseline laboratory data, including inflammatory markers, were compared between both groups. Patients with cardiac injury had higher median levels of lactate dehydrogenase, C-reactive protein, ferritin, and D-dimer. There were no significant differences on chest X-ray or CT chest imaging findings (Table 1).
Using presenting hs-cTnI trends, patients with higher levels by categories ≤18 ng/L, >18–99 ng/L, and ≥100 ng/L were more likely to develop in-hospital complications and adverse events including acute kidney injury, need for renal replacement therapy, mechanical ventilation, ICU transfer, and mortality (Table 2). A nondetectable hs-cTnI on presentation did not rule out significant events (15% risk for the need for mechanical ventilation or death, Table 2).
Table 2.
In-hospital outcomes categorized according to presenting levels of high sensitivity
| Variable | Presenting high sensitivity troponin levels (ng/L) |
p-value | |||
|---|---|---|---|---|---|
| Undetectable <2.3 (n = 80) | 2.3–18 (n = 550) | >18–99 (n = 303) | ≥ 100 (n = 87) | ||
| Hs-cTnI level (ng/L) | 2.3 (2.3–2.3) | 9 (5–16) | 35 (25–51) | 186 (129–334) | <0.001 |
| Acute kidney injury | 21 (26%) | 157 (29%) | 175 (58%) | 49 (56%) | <0.001 |
| Renal replacement therapy | 2 (3%) | 8 (2%) | 18 (6%) | 8 (9%) | <0.001 |
| Intensive care unit transfer | 9 (11%) | 84 (16%) | 76 (25%) | 29 (34%) | <0.001 |
| ARDS | 7 (9%) | 54 (10%) | 62 (21%) | 31 (36%) | <0.001 |
| Mild | 1 (1%) | 8 (2%) | 10 (3%) | 6 (7%) | <0.001 |
| Moderate | 2 (3%) | 25 (5%) | 18 (6%) | 10 (12%) | |
| Severe | 5 (6%) | 23 (4%) | 33 (11%) | 16 (18%) | |
| Mechanical ventilation | 10 (13%) | 59 (11%) | 68 (22%) | 32 (37%) | <0.001 |
| Mortality | 7 (9%) | 45 (8%) | 84 (28%) | 44 (51%) | <0.001 |
| Mechanical ventilation, and death | 12 (15%) | 110 (20%) | 122 (40%) | 57 (66%) | <0.001 |
| ICU, mechanical ventilation, and death | 12 (15%) | 110 (20%) | 303 (40%) | 87 (66%) | <0.001 |
Abbreviations: ARDS = Acute Respiratory Distress Syndrome; Hs-cTnI = High Sensitivity Troponin; ICU = Intensive Care Unit.
Multivariate logistic regression analysis identified the clinical data elements independently predictive of mortality using comorbidities, peak values of laboratory data, and inpatient complications. These independent predictors included age ≥65, atrial fibrillation, cerebrovascular disease, D-dimer quartiles, hs-cTnI categories (≤18, >18–99, and ≥100 ng/L), and ARDS. Clinical data elements included in the multivariate analysis are detailed in Table 3 .
Table 3.
Predictors of inpatient mortality after multivariate regression using patient risk factors, inpatient complications as well as peak serum biomarkers levels
| Variables | Odds ratio (95% confidence interval) | p-value |
|---|---|---|
| Age ≥ 65 (years) | 3.9 (2.2–6.7) | <0.001 |
| Atrial fibrillation/flutter | 2.5 (1.2–5.3) | 0.014 |
| Cerebrovascular disease | 2.5 (1.3–5.0) | 0.008 |
| Peak D-dimer < 1 µg/ml | Reference | 0.013 |
| Peak D-dimer ≥ 1 < 1.8 µg/ml | 1.2 (0.4–3.7) | 0.696 |
| Peak D-dimer ≥ 1.8 < 4 µg/ml | 2.7 (1.0–7.4) | 0.049 |
| Peak D-dimer ≥ 4 µg/ml | 3.3 (1.2–9.0) | 0.017 |
| Peak high sensitivity troponin ≤ 18 ng/L | Reference | <0.001 |
| Peak high sensitivity troponin 19–99 ng/L | 3.0 (1.5–6.0) | 0.002 |
| Peak high sensitivity troponin ≥100 ng/L | 7.7 (3.7–16.0) | <0.001 |
| Acute respiratory distress syndrome | 14.6 (8.2–25.7) | <0.001 |
Variables controlled for:
Risk factors: Age ≥ 65, Gender, Body Mass Index, Hypertension, Coronary Artery Disease, Heart Failure, Atrial Fibrillation/ Flutter, Cerebrovascular Disease, Immunosuppressed State, Chronic Obstructive Pulmonary Disease, Chronic Kidney Disease, Cirrhosis. Inpatient Clinical Data Elements: Peak levels of High Sensitivity Troponin, Lactate Dehydrogenase, C-Reactive Protein, Ferritin and D-dimer levels based on quartiles, Acute Respiratory Distress Syndrome, Acute Kidney Injury
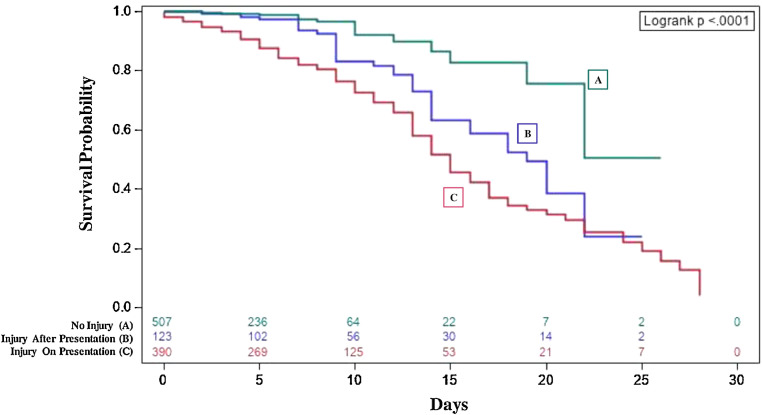
The Kaplan-Meier curves in Figure 1 demonstrate the survival probability between patient groups according to the incidence of cardiac injury. Patients with injury on presentation have a lower survival probability than those who develop cardiac injury later in their stay (adjusted log-rank p=0.012), and both have a lower survival probability than those who never develop cardiac injury (adjusted log-rank p < 0.001 and p=0.003, respectively) (Figure 1).
Figure 1.
Survival probability of patients according to cardiac injury incidence categories. Kaplan Meier survival curves reveal the survival probability of patients according to cardiac injury incidence categories. Patients with injury on presentation (C), (red) have a lower survival probability than those who develop cardiac injury later in their stay (B), (purple) (adjusted log-rank p=0.012), and both have a lower survival probability than those who never develop cardiac injury (A), (green) (adjusted log-rank p <0.001 and p=0.003, respectively). Cardiac injury was defined by a hs-cTnI concentration >99th percentile.
Discussion
This is one of the largest studied cohorts in the U.S. describing cardiac injury and its associated outcomes in patients hospitalized with COVID-19. Patients with cardiovascular risk factors (older age, hypertension, diabetes) and cardiovascular disease (coronary artery disease, heart failure, atrial fibrillation, and cerebrovascular disease) were at an increased risk of developing cardiac injury. Subsequently, those with cardiac injury were associated with a higher risk of intensive care unit admission, mechanical ventilator support, and mortality. These findings are similar to the reports published in Chinese cohorts.2 , 5 , 13 , 14
A notable finding was the higher prevalence of cardiac injury on admission in our patients compared to what was reported in China (38% vs 20%).5 , 15 Our patient cohort also had a higher prevalence of cardiovascular conditions including hypertension (73% vs 31%), diabetes mellitus (44% vs 14%), and cerebrovascular disease (12% vs 5%). These comorbid conditions were associated with cardiac injury (p < 0.05), which likely explains the higher incidence of cardiac injury in our cohort. The higher incidence of cardiac injury may also contribute to the higher mortality rates seen in the U.S. cohort. From the available data, ARDS and cardiac injury are noted to be the strongest predictors of need for mechanical ventilation and mortality in patients with COVID-19.5 Although data from Wuhan showed cardiac injury on presentation was an independent factor associated with mortality, our current U.S. study did not reproduce similar results. However, our study did show that patients with higher peak hs-cTnI levels during admission had significantly higher rates of mechanical ventilation or death. This suggests that the extent of cardiac injury is associated with outcomes including mortality.
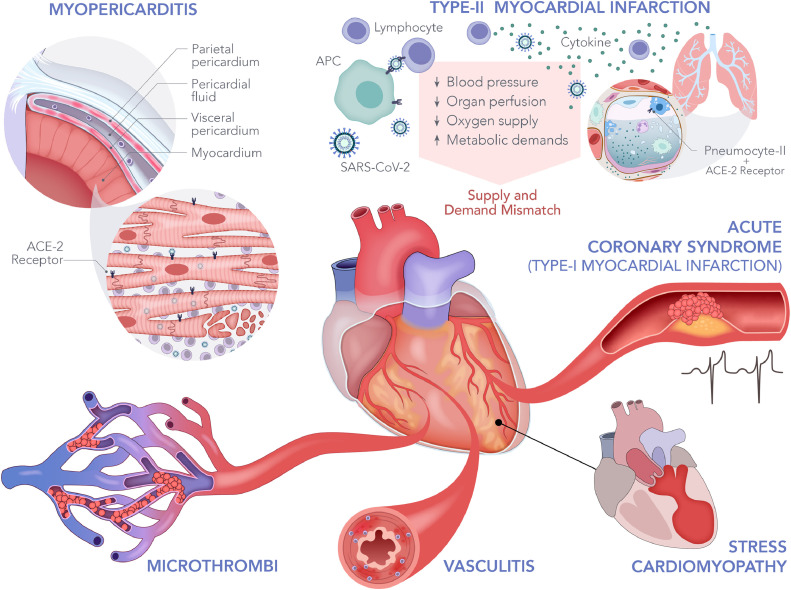
The exact mechanism by which SARS-CoV-2 leads to cardiac injury is not fully elucidated. Figure 2 illustrates the multiple proposed mechanisms. SARS-CoV-2 infection is mediated through the viral surface spike protein to the human angiotensin-converting enzyme 2 (ACE-2) receptor. 16 Systemic infection is likely due to entry through pulmonary alveolar ACE-2 receptors, which are also present on the heart, kidneys, vascular endothelium, and others.16 , 17 During the SARS outbreak, SARS-CoV-1 viral RNA was detected in 35% of hearts on autopsy. 18 In murine models with previous SARS-CoV-1, cardiac viral infection was mediated by an ACE-2-dependent myocardial viral entry 19; it has been proposed that a similar mechanism of cardiac injury exists where SARS-CoV-2 can directly affect cardiac myocytes, inducing myocarditis or pericarditis as supported by case reports of pericardial effusion and tamponade in patients with COVID-19.20 , 21 An early controversy in the treatment of COVID-19 was whether the renin-angiotensin-aldosterone system blockers confer a higher risk of mortality in these patients. This theory is based on the speculation that these drugs may upregulate the expression of ACE-2 receptors. This, however, is not the case in published cohorts,22 including our study. Supply-demand mismatch is another mechanism by which cardiac injury can occur. The severe systemic immune response from infection may lead to increased physiologic stressors resulting in a combination of hypotension, increased metabolic demands, and hypoxia from acute lung injury. This has been historically well described in other disease processes causing sepsis23, 24, 25, and commonly leads to type II myocardial infarction. Sepsis, inflammation and ARDS are well-known entities that cause coagulation disorders.26 Hypercoagulability leading to microthrombi has also been described in patients with COVID-19.27 In this study, patients with cardiac injury were more likely to have elevated D-dimer levels (1.9 vs 1.1 µg/ml, p < 0.001), raising the possibility of microthrombi formation as another mechanism by which cardiac injury occurs. Additionally, new reports of higher incidence of Kawasaki's disease in Italy after the COVID-19 pandemic surge 28 may suggest vascular involvement, which can affect the coronary vessels. Finally, the development of acute coronary syndrome in the setting of infection and heightened cytokine response is possible. A case series from New York identified patients with ST-elevation myocardial infarction in COVID-19 and revealed variability in presentation and presence of obstructive disease, suggesting that myocardial injury could be due to plaque rupture, coronary spasm, or direct endothelial injury.29 Confirmation of these proposed mechanisms will require future post-mortem analysis.
Figure 2.
Potential cardiac injury mechanisms in COVID-19. This figure illustrates the proposed mechanisms of cardiac injury in COVID-19. Systemic infection is likely mediated through angiotensin-converting enzyme 2 (ACE-2) receptors found on multiple cell lineages, including the alveolar and cardiac cells. The proposed mechanisms of injury include myopericarditis through direct viral infection or systemic inflammation, hypercoagulability leading to coronary bed microthrombi, vasculitis, stress cardiomyopathy, acute coronary syndrome, and type-II myocardial infarction from supply-demand mismatch.
There are multiple strengths and limitations to our study. To date, our study is one of the largest to describe patterns of cardiac injury, particularly from the U.S. The main limitation of this study is the retrospective design. Given the retrospective nature of the analysis and changes in contemporary COVID-19 management, the timing of laboratory blood sampling could not be standardized in all patients. Patients who remain admitted were not included, as our primary outcome could not be assessed. Additionally, hs-cTnI is a biomarker, and categorization of the type of injury and mechanism behind hs-cTnI elevation is not easily determined when performing a retrospective analysis. Larger prospective trials with standardized protocols may better verify these results.
In conclusion, the presence of cardiovascular risk factors was associated with an increased risk of developing cardiac injury in patients admitted with COVID-19. The extent of cardiac injury was associated with worse outcomes, including mortality.
Author contributions
Mohamad Raad, MD: Conceptualization, methodology, data curation, data analysis, writing, reviewing and editing of the original and revised drafts. Mohammed Dabbagh, MD: Conceptualization, data curation, writing, reviewing and editing of the original and revised drafts. Sarah Gorgis, MD: Data curation, writing, reviewing and editing of the original and revised drafts.
Jerry Yan, MD: Data curation, writing, reviewing and editing of the original and revised drafts. Omar Chehab, MD, MSc: Data analysis, writing, reviewing and editing of the original and revised drafts. Carina Dagher, MD: Data curation, writing, reviewing and editing of the original and revised drafts. Khaled Jamoor, MD: Data curation, writing, reviewing and editing of the original and revised drafts. Inaya Hajj Hussein, PhD: Illustration, conceptualization, writing, reviewing and editing of the original and revised drafts. Bernard Cook, PhD: Data curation, laboratory information assistance, writing, reviewing and editing of the original and revised drafts. Meredith Van Harn, MS: Data analysis, writing, reviewing and editing of the original and revised drafts. Gurjit Singh, MD: Supervision, conceptualization, methodology, writing, reviewing and editing of the original and revised drafts. James McCord, MD: Supervision, conceptualization, methodology, writing, reviewing and editing of the original and revised drafts. Sachin Parikh, MD: Supervision, conceptualization, methodology, writing, reviewing and editing of the original and revised drafts.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relations that could have appeared to influence the work reported in this study.
Acknowledgments
The authors acknowledge Audrey Bell for her contribution to the illustration in Figure 2.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2020.07.040.
Appendix. Supplementary materials
References
- 1.Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), Johns-Hopkins-University website. July 1, 2020.https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed July 1, 2020.
- 2.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020;7:900–915. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACC CLINICAL BULLETIN COVID-19 Clinical Guidance For the CV Care Team, American College of Cardiology website. February 07, 2020.https://www.acc.org/~/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/2020/02/S20028-ACC-Clinical-Bulletin-Coronavirus.pdf. Accessed July 1, 2020.
- 5.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiology. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC Diagnostic Test for COVID-19, Centers for Disease Control and Prevention website. June 2020.https://www.cdc.gov/coronavirus/2019-ncov/lab/testing.html. Accessed July 1, 2020.
- 8.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt H. Radiological society of North America expert consensus statement on reporting chest CT findings related to COVID-19. endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. Radiology: Cardiothoracic Imaging. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KDIGO KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:19. [Google Scholar]
- 10.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Wu C, Chen X, Cai Y, Ja Xia, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX. China medical treatment expert group for C. cmorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W-j, Z-y Ni, Hu Y, Liang W-h, Ou C-q, He J-x, Liu L, Shan H, Lei C-l, Hui DSC, Du B, Li L-j, Zeng G, Yuen K-Y, Chen R-c, Tang C-l, Wang T, Chen P-y, Xiang J, Li S-y, Wang J-l, Liang Z-j, Peng Y-x, Wei L, Liu Y, Hu Y-h, Peng P, Wang J-m, Liu J-y, Chen Z, Li G, Zheng Z-j, Qiu S-q, Luo J, Ye C-j, Zhu S-y, Zhong N-s. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Peptides. 2012;2012:1–8. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth CM. Clinical features and short-term outcomes of 144 patients with SARS in the greater toronto area. Jama. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 19.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dabbagh MF, Aurora L, D'Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2:1326–1330. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng J-H, Liu Y-X, Yuan J, Wang F-X, Wu W-B, Li J-X, Wang L-F, Gao H, Wang Y, Dong C-F, Li Y-J, Xie X-J, Feng C, Liu L. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020 doi: 10.1007/s15010-020-01424-5. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Zhu L, Cai J, Lei F, Qin J-J, Xie J, Liu Y-M, Zhao Y-C, Huang X, Lin L, Xia M, Chen M-M, Cheng X, Zhang X, Guo D, Peng Y, Ji Y-X, Chen J, She Z-G, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang X-J, Wang X, Touyz RM, Xia J, Zhang B-H, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson P, Frigyesi A. High-sensitivity troponin T is an important independent predictor in addition to the simplified acute physiology score for short-term ICU mortality, particularly in patients with sepsis. J Crit Care. 2019;53:218–222. doi: 10.1016/j.jcrc.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Frencken JF, Donker DW, Spitoni C, Koster-Brouwer ME, Soliman IW, Ong DSY, Horn J, van der Poll T, van Klei WA, Bonten MJM, Cremer OL, de Beer FM, Bos LDJ, Glas GJ, van Hooijdonk RTM, Schouten LRA, Straat M, Witteveen E, Wieske L, van Vught LA, Wiewel M, Hoogendijk AJ, Huson MA, Scicluna B, Schultz MJ, Klein Klouwenberg PMC, van de Groep K, Verboom D. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ: Cardiovasc Qual Outcomes. 2018;11:e004040. doi: 10.1161/CIRCOUTCOMES.117.004040. [DOI] [PubMed] [Google Scholar]
- 25.de Groot B, Verdoorn RCW, Lameijer J, van der Velden J. High-sensitivity cardiac troponin T is an independent predictor of inhospital mortality in emergency department patients with suspected infection: a prospective observational derivation study. Emerg Med J. 2014;31:882–888. doi: 10.1136/emermed-2013-202865. [DOI] [PubMed] [Google Scholar]
- 26.Simmons J, Pittet J-F. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28:227–236. doi: 10.1097/ACO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, Chadow HL, Fishman GI, Reynolds HR, Keller N, Hochman JS. ST-Segment elevation in patients with covid-19 — a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.