Abstract
Background
Insomnia and depression often co-occurr. However, there is lack of effective treatment for such comorbidity. CBT-I has been recommended as the first-line treatment for insomnia; whether it is also effective for comorbidity of insomnia and depression is still unknown. Therefore, we conducted this meta-analysis of randomized controlled trials to assess the clinical effectiveness and safety of CBT-I for insomnia comorbid with depression. Data Sources. Seven electronic databases, including China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science Technology Journal Database, SinoMed Database, PubMed, the Cochrane Library, and EMBASE, as well as grey literature, were searched from the beginning of each database to July 1, 2019. Study Eligibility Criteria. Randomized controlled trials that compared CBT-I to no treatment or hypnotics (zopiclone, estazolam, and benzodiazepine agonist) for insomnia comorbid with depression and reported both insomnia scales and depression scales. Study Assessment and Synthesis Methods. Cochrane Reviewer's Handbook was used for evaluating the risk of bias of included studies. Review Manager 5.3 software was used for meta-analysis. Online GRADEpro was used to assess the quality of evidence.
Results
The pooled data showed that CBT-I was superior to no treatment for insomnia, while it was unsure whether CBT-I was better than no treatment for depression. And the effectiveness of CBT-I was comparable to hypnotics for both insomnia and depression. CBT-I was likely to be safe due to its noninvasive nature. The methodological quality varied across these trials. The evidence quality varied from moderate to very low, and the recommendation level was low.
Conclusions
Currently, findings support that CBT-I seems to be effective and safe for insomnia comorbid with depression to improve the insomnia condition, while it is unsure whether CBT-I could improve depression condition. More rigorous trials are needed to confirm our findings.
1. Introduction
Insomnia is a kind of sleep disorder, and people with insomnia are unsatisfied with their sleep time and sleep quality. Insomnia patients have difficulty initiating, maintaining sleep or returning to sleep [1]. Several studies have shown that 6% to 10% of adults suffered from insomnia [2–4], and 10–15% of insomnia patients tended to develop into chronic course [5]. Insomnia increases the risk of many health problems including suicidal ideation and behavior [6], cardiovascular diseases [7], depressive disorder, arterial hypertension, myocardial infarction [8], chronic heart failure [7, 9], type 2 diabetes [10], and cognitive impairment [11], which would place a heavy burden on society and individuals. Treatments for insomnia included cognitive behavioral therapy, pharmacologic therapy, and complementary and alternative therapy. Pharmacologic therapy for insomnia includes benzodiazepines (triazolam, estazolam, temazepam, flurazepam, and quazepam), nonbenzodiazepine hypnotics (zaleplon, zolpidem, and eszopiclone). Complementary and alternative therapy for insomnia includes acupuncture and Chinese herbal medicine. Moderate-quality evidence showed that CBT-I improved sleep outcomes in the general population, including reduced sleep onset latency and wake after sleep onset, and improved sleep efficiency and sleep quality. And any harm associated with CBT-I is likely to be mild, so CBT-I was recommended as the first-line treatment by the ACP Clinical Guidelines Committee [12, 13].
In fact, comorbid insomnia is commonly seen in clinical practice. And insomnia always combines with depression. Symptoms of depression include depressive mood, decreased interest, lack of motivation, and fatigue [14]. Insomnia and depression are often influenced by each other, and this mutual influence may increase the risk of suicide. A recent study suggests that sleep problems are associated with severe depression, suicidality, and worse outcomes for treatment of depression [15]. And another study shows that early changes in insomnia characteristics may predict long-term depression outcomes [16]. No therapy has been proven to be effective and safe for this comorbidity at present. Sequential approach and concomitant treatment for such comorbidity are both in their preliminary stages; well-designed randomized controlled trials with long-term follow-up are needed to evaluate the effectiveness and safety of these treatments.
Cognitive behavioral therapy is commonly used in clinical practice, which includes cognitive behavioral therapy for insomnia, cognitive behavioral therapy for depression [17], cognitive behavioral therapy for psychosis [18], brief behavioral treatment for insomnia [19], and cognitive behavioral therapy for Parkinson [20]. Among these therapies, cognitive behavioral therapy for insomnia (CBT-I), developed by A.T. Beck in the 1960s, is a branch of CBT and is recommended as first-line treatment for insomnia by the ACP Clinical Guidelines Committee in 2016. The components of CBT-I include cognitive therapy to replace wrong beliefs of sleep; stimulate control to prevent patients from associating with other stimulating activities; sleep restriction to limit time in bed to match perceived sleep duration; sleep hygiene to change habits and physiologic factors and improve sleep; and relaxation to focus on relaxation techniques, such as guided imagery and progressive muscle relaxation (Table 1) [21].Various delivery methods of CBT-I are available, including in-person individual or group therapy, telephone- or Web-based modules, and self-help books. The course of CBT-I varied from 4 sessions to 12 sessions.
Table 1.
Components of CBT-I.
| Component | Description |
|---|---|
| Cognitive therapy | Cognitive therapy aims to identify and replace dysfunctional beliefs and attitudes about sleep. These dysfunctional beliefs include unrealistic expectations of sleep, such as overestimating the consequences of poor sleep. |
| Stimulus control | Stimulus control aims at avoiding patients to associate bed with other stimulating activities such as avoiding nonsleep activities in the bedroom; going to bed only when sleepy; and leaving the bedroom when unable to sleep for15−20 min, returning to bed only when sleepy |
| Sleep restriction | Sleep restriction aims to limit time in bed to match perceived sleep duration in order to increase sleep drive and reduce time awake in bed. Time allowed in bed is initially restricted to the average time perceived as sleep per night and then adjusted to ensure that sleep efficiency remains >85%. |
| Sleep hygiene | Sleep hygiene relates to environmental factors, physiologic factors, and habits that improve sleep, such as regular sleep scheduling, avoiding long daytime naps, and limiting alcohol, caffeine, and nicotine intake especially before bed. |
| Relaxation | Any relaxation technique that the patient finds effective can be used to limit cognitive arousal and reduce muscular tension to improve sleep. Specific relaxation techniques include meditation, mindfulness, progressive muscle relaxation, guided imagery, and breathing techniques. |
We conducted this meta-analysis of randomized controlled trials to assess the clinical effectiveness and safety of CBT-I for insomnia comorbid with depression, compared with no treatment or hypnotics and measured by insomnia outcome measurements, depression outcome measurements, and safety index.
2. Methods
2.1. Protocol and Registration
We made this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We registered this review in PROSPERO (CRD42019145065), http://www.crd.york.ac.uk/PROSPERO/.
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
Participants (aged ≥18 years, regardless of gender and education) met the diagnostic criteria for insomnia and depression, which referred to DSM-5 and CCMD-3.
Intervention of the experimental group was only CBT-I.
Comparison (control group) included no treatment and hypnotic drugs (zopiclone, estazolam, and benzodiazepine agonist). No treatment referred to no treatment for insomnia and depression during observation period. Waitlist control had no treatment for insomnia and depression during observation period, so we also took waitlist control as no treatment. We did not take sleep hygiene education alone as the control for CBT-I contained sleep hygiene education.
Outcome measurements included insomnia scale, depression scale, and adverse events. Insomnia scales included ISI and PSQI. Depression scale included HAMD, HADS-D, BDI, and SDS (primary outcomes) and SCL-90, CES-D, and QIDS-CR16 (secondary outcomes). Adverse events included all adverse events of CBT-I and hypnotics mentioned by these RCTs included in our review.
Study included randomized controlled trials (RCTs) which focused on Chinese language and English language.
2.2.2. Exclusion Criteria
Patients could not be diagnosed with insomnia and depression
Intervention was not only CBT-I, such as CBT-I plus other therapies
Control group was neither no treatment nor hypnotics
Outcomes reported incompletely, such as only reported insomnia scales or only reported depression scales
Trials did not mention RCT or the word “random”
Duplication of the study
Study was mechanism, case report, review, or meta-analysis
Study language was neither Chinese nor English
2.3. Literature Search and Data Extraction
Guiyu Feng and Le Geng independently searched PubMed, the Cochrane Library, EMBASE, SinoMed Database, China National Knowledge Infrastructure (CNKI), Wanfang Database, and China Science Technology Journal Database from the beginning of each database to July 1, 2019 (CNKI: 1915–2019, Wanfang: 1900–2019, VIP: 1989–2019, SinoMed: 1860–2019, PubMed: 1966–2019, EMBASE: 1947–2019, and Cochrane: 1966–2019). The details of search strategy of PubMed were shown in Appendix. Grey literature was also searched to identify potential studies which met the inclusion and exclusion criteria. They focused on languages of English and Chinese. They also searched relevant RCTs from existing systematic review and meta-analysis in the reference list. They downloaded search results for evaluation. They also contacted authors whose RCTs lacked any relevant information. And they also independently screened the literatures and found out suitable RCTs at the same time, according to inclusion and exclusion criteria. After literature selection, they separately extracted data from these suitable RCTs at the same time. Extracted data included author's name, year of publication, sample size, age, intervention, underlying disease, control group, duration of treatment, time point of assessment, and outcomes. If any disagreement happened, they would resolve it in consultation with more experienced author Yingchun Miao. Yingchun Miao conducted the search, Mei Han evaluated the abstract, and Xun Li evaluated the rest of the paper.
2.4. Outcome Measurements
The outcome measurements of this systematic review and meta-analysis included insomnia outcome measurements, depression outcome measurements, and adverse events. More details of outcome measurements are shown in Table 2.
Table 2.
Details of outcome measurements.
| Insomnia outcome measurements | Depression outcome measurements | ||
|---|---|---|---|
| Primary outcomes | Secondary outcomes | Primary outcomes | Secondary outcomes |
| ISI, PSQI | None | HAMD, HADS-D | SCL-90, CES-D |
| BDI, SDS | QIDS-CR16 | ||
Note. ISI: Insomnia Severity Index; PSQI: Pittsburgh Sleep Quality Index; HAMD: Hamilton Depression Scale; HADS-D: Anxiety and Depression Scale-Depression; BDI: Beck Depression Inventory; SDS: Self-Rating Depression Scale; SCL-90: Symptom Checklist 90; CES-D: Centre of Epidemiological Studies Depression Scale (CES-D); and QIDS-CR16: Quick Inventory of Depressive Symptomatology—Clinician Rating.
2.5. Risk of Bias
Guiyu Feng and Le Geng independently evaluated the risk of bias through the Cochrane Handbook for Systematic Reviews of Interventions [22] to evaluate the methodological quality of these included literatures and they performed it via Review Manager 5.3 at the same time. For all evaluation items, the quality of each trial was evaluated using “Yes” (low risk of bias), “No” (high risk of bias), or “Unclear” (unclear risk of bias). Evaluation items included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. If any disagreement happened, they would resolve it in consultation with more experienced author Yingchun Miao.
2.6. Data Synthesis
We used Cochrane collaboration software RevMan (5.3) to pool outcome data. We calculated the risk ratio (RR) with 95% confidence interval (95% CI) for dichotomous variables and mean difference (MD) with 95% CI for continuous outcomes. Outcomes of insomnia and depression were all continuous variables, and we used mean difference (MD) and its 95% confidence interval (CI) to represent them. Heterogeneity was evaluated by the magnitude of Tau2 and I2 statistic. A fixed effect model was performed with minor heterogeneity when the I2 value was below 50%. A random effect model was used with major heterogeneity when the I2 value was above 50%. For patient population with underlying diseases, these underlying diseases were different, such as posttraumatic stress disorder (PTSD), ischemic stroke, hypertension, and nonmetastatic cancer. Heterogeneity was major; so, we also performed the random effect model. We would do subgroup analysis and sensitive analysis if the characteristics of data were allowed. If the number of suitable RCTs was more than 10, we would make an inverted funnel plot to assess the impact of publication bias.
2.7. Evaluating the Quality of Evidence
Guiyu Feng and Le Geng independently used the online GRADEpro to assess the quality of evidence (https://gdt.gradepro.org/app/#) at the same time.
3. Results
3.1. Literature Screening and Its Flow Diagram
We searched 2,101 RCTs according to the search strategy. After duplicated RCTs were deleted, there were 1,641 RCTs left. When we screened the titles and abstracts, 1,387 RCTs which did not meet search criteria were deleted. We then screened the full texts of the remaining 254 RCTs and found 237 RCTs which did not meet search criteria. In the end, 17 RCTs were included in this review. More details of literature screening are shown in Figure 1.
Figure 1.
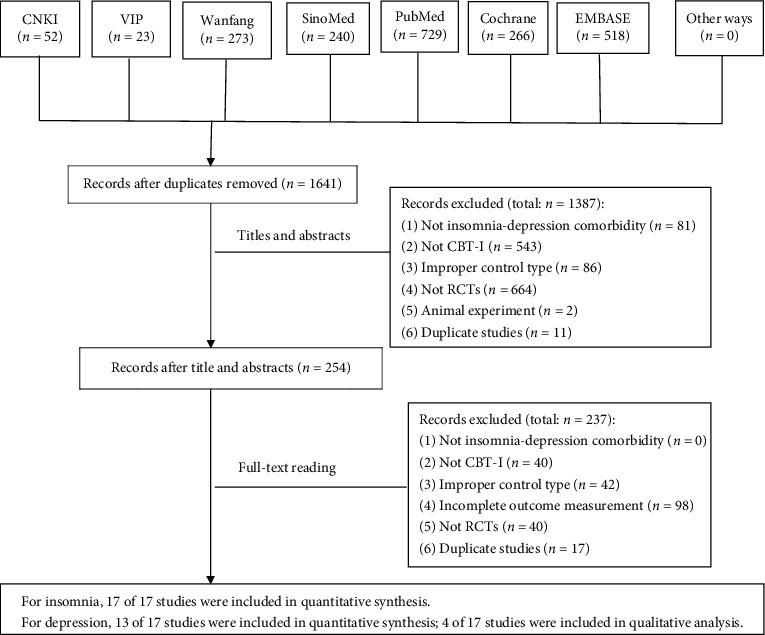
Study flow diagram.
3.2. Characteristics of Included Literature Studies
We finally included 17 RCTs and 1,756 participants (894 participants in the intervention group and 862 participants in the control group). 13 RCTs [23–35] compared CBT-I with no treatment. Four RCTs [36–39] compared CBT-I with hypnotics (zopiclone and estazolam). One RCT [32] reported two types of CBT-I, and the remaining 16 RCTs [23–31, 33–39] all reported one type of CBT-I arms. The mean frequency of CBT-I was about once a week. Two RCTs [36, 37] used zopiclone 3.75~11.25 mg QN, 1 RCT [38] used estazolam 1 mg QN, and 1 RCT [39] did not mention the dosage of benzodiazepine agonist. The mean duration of treatment was about 8 weeks. The mean assessment time point was about week 8. The underlying diseases differed in this review, including nonmetastatic cancer, ischemic stroke, maintain hemodialysis (MHD), hypertension, poststroke fatigue, hearing impairment, and posttraumatic stress disorder (PTSD). More details are shown in Table 3.
Table 3.
Characteristics of included RCTs.
| Study ID | Participants (M/F) | Age (years) | Intervention | Underlying disease | Medicine used for underlying disease | Control | Duration of treatment (I/C) | Time point of assessment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Casault et al. [23] | I: 1/19 C: 2/16 |
I: 56.9 ± 10.8 C: 57.0 ± 9.4 |
CBT-I (self-administered) weekly | Nonmetastatic cancer | None | No treatment | 6 weeks/6 weeks | Week 6 | ISI, HADS-D |
| Daniel et al. [24] | I: 62/13 C: 62/14 |
I: 32.21 ± 7.18 C: 32.67 ± 7.97 |
CBT-I, weekly | None | None | No treatment | 6 weeks/6 weeks | Week 6 | ISI, BDI |
| Shan [25] | I: 26/18 C: 24/20 |
I: 54.22 ± 8.39 C: 54.23 ± 8.42 |
CBT-I, weekly | Ischemic stroke | Aspirin; simvastatin | No treatment | 4 weeks/4 weeks | Week 4 | PSQI,SDS |
| Wang et al. [26] | I: 18/25 C: 18/27 |
I: 57.19 ± 8.51 C: 56.73 ± 8.96 |
CBT-I, weekly | Various types of cancer | Chemotherapy drugs | No treatment | 4 weeks/4 weeks | Week 4 | PSQI,SDS |
| Hou et al. [27] | I: 20/31 C: 22/25 |
I: 54.5 ± 13.8 C: 52.4 ± 14.5 |
CBT-I, weekly | Maintain hemodialysis (MHD) | None | No treatment | 12 weeks/12 weeks | Week 12 | PSQI, SCL-90 |
| Yang et al. [28] | I: 20/30 C: 19/34 |
I: 56.54 ± 9.97 C: 56.73 ± 11.27 |
CBT-I (remote-interactive), more than once a week | Hypertension | Antihypertensive drugs | No treatment | 8 weeks/8 weeks | Week 8 | ISI,PSQI, BDI |
| Sylvia et al. [29] | I: 6/3 C: 5/1 |
I: 47.22 ± 15.21 C: 51.17 ± 10.65 |
CBT-I (individual), weekly | Poststroke fatigue | Medicine (specific drugs not available) | No treatment (waitlist control) | 8 weeks/8 weeks | Week 8 | ISI,PSQI,HADS-D |
| Markus et al. [30] | I: 7/10 C: 5/10 |
I: 57.8 ± 6.6 C: 53.6 ± 10.4 |
CBT-I, weekly | Hearing impairment | Not mentioned | No treatment (waitlist control) | 7 weeks/7 weeks | Week 8 | ISI, HADS-D |
| David et al. [31] | I: 20/82 C: 20/82 |
I: 44.66 ± 11.65 C: 43.75 ± 11.84 |
CBT-I (web-based), weekly | None | None | No treatment (waitlist control) | 6 weeks/6 weeks | Week 7 | ISI,QIDS-CR16 |
| Lancee et al. [32] | I: 6/30 C: 7/20 |
I: 47.47 ± 14.37 C: 49.98 ± 13.71 |
CBT-I (online), weekly | None | None | No treatment (waitlist control) | 12 weeks/12 weeks | Week 12 | ISI, CES-D, adverse event |
| (1) Lancee et al. [33] | I: 4/26 C: 5/25 |
I: 41.2 ± 14.1 C: 45.1 ± 13.7 |
CBT-I (online), weekly | None | None | No treatment (waitlist control) | 12 weeks/12 weeks | Week 12 | ISI, CES-D |
| (2) Lancee et al. [33] | I: 8/22 C: 5/25 |
I: 38.5 ± 13.1 C: 45.1 ± 13.7 |
CBT-I (individual, face to face), weekly | None | None | No treatment (waitlist control) | 12 weeks/12 weeks | Week 12 | ISI, CES-D |
| Lorenz et al. [34] | I: 8/21 C: 9/18 |
I: 41.72 ± 17.31 C: 44.04 ± 20.05 |
CBT-I (web-based), weekly | None | None | No treatment (waitlist control) | 6 weeks/6 weeks | Week 6 | ISI,BDI |
| Talbot et al.[35] | I: 7/22 C :7/9 |
I: 37.1 ± 10.4 C: 37.3 ± 11.0 |
CBT-I (individual), weekly | Posttraumatic stress disorder (PTSD) | Medicine (specific drugs not available) | No treatment (waitlist control) | 8 weeks/8 weeks | Week 8 | ISI,BDI |
| Han and Liu [36] | I: 14/17 C: 14/18 |
I: 37 ± 14 C: 35 ± 14 |
CBT-I, weekly | None | None | Zopiclone 3.75~11.25 mg QN | 8 weeks/8 weeks | Week 8 | PSQI,SCL-90 |
| Huang et al. [37] | I: 35/93 C: 28/85 |
I: 46.78 ± 13.75 C: 45.49 ± 12.83 |
CBT-I (group), weekly | None | None | Zopiclone 3.75~7.5 mg QN | 8 weeks/8 weeks | Week 8 | ISI,HAMD |
| Zhou et al. [38] | 150/160 | None | CBT-I, weekly | None | None | Estazolam 1 mg QN | 6 weeks/6 weeks | Week 6 | PSQI,SDS |
| Lin et al. [39] | I: 4/16 C: 4/17 |
I: 46.5 ± 12.5 C: 45.5 ± 12.4 |
CBT-I (remote-interactive), weekly | None | None | Benzodiazepine agonist | 8 weeks/8 weeks | Week 8 | ISI,PSQI, HAMD |
Note. M: male; F: female; I: intervention; C: control; QN: once a night; MHD: maintain hemodialysis disorder; PTSD: posttraumatic stress; ISI: Insomnia Severity Index; PSQI: Pittsburgh Sleep Quality Index; HAMD: Hamilton Depression Scale; HADS-D: Anxiety and Depression Scale-Depression; BDI: Beck Depression Inventory; SDS: Self-Rating Depression Scale; SCL-90: Symptom Checklist 90; CES-D: Centre of Epidemiological Studies Depression Scale; and QIDS-CR16: Quick Inventory of Depressive Symptomatology—Clinician Rating.
3.3. Methodological Quality Evaluation
In order to evaluate the methodological quality of these included literature studies, we used the Cochrane Handbook for Systematic Reviews of Interventions. The overall methodological quality was not good.
For random sequence generation, 15 RCTs [23–35, 37, 39] used the right methods to produce the random sequence, and we assessed them as ‘low' risk. One RCT [36] did not mention how to produce the random sequence, except the word ‘random,' so we assessed them as ‘unclear' risk. 1 RCT [38] used wrong method to produce the random sequence, for it used enrollment order of the facilities, for example, the first enrolled facility was allocated to the CBT-I group while the second to the control group. We assessed it as ‘high' risk.
For allocation concealment, 5 RCTs [23, 30, 31, 34, 35] mentioned the right methods of allocation concealment, and we assessed them as ‘low' risk. 12 RCTs [24–29, 32, 33, 36–39] did not mention how to make allocation concealment, so we evaluated them as ‘unclear' risk.
For blinding of participants and personnel, due to the characteristics of CBT-I, it was hard to blind doctors and patients. So, we assessed all of them as ‘high' risk. For blinding of outcome assessment, 4 RCTs [23, 31, 34, 35] blinded the outcome assessors, and we assessed them as ‘low' risk. 13 RCTs [24–30, 32, 33, 36–39] did not mention blinding of outcome assessment. We did not know whether they blinded outcome assessors, so we evaluated them as ‘unclear' risk.
For incomplete outcome data, 16 RCTs [23–25, 27–39] reported the drop-outs, lost patients, and the reasons, so we assessed them as ‘low' risk. One RCT [26] did not mention incomplete outcome data. We did not know whether there were incomplete outcome data, so we assessed it as ‘unclear' risk.
For selective reporting, all 17 RCTs [23–39] reported the outcomes of insomnia scales and depression scales, and we assessed all of them as ‘low' risk.
For other biases, we focused on whether the baseline was equal between the intervention group and the control group. There was no statistic difference between the intervention group and the control group in all 17 RCTs [23–39]. We assessed all of them as ‘low' risk.
Details of methodological quality evaluation are shown in Figure 2.
Figure 2.
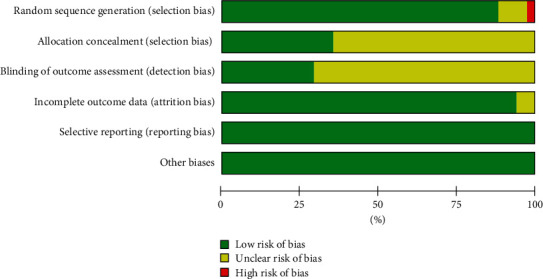
Risk of bias.
3.4. Results of Meta-Analysis
Based on the presence or absence of underlying diseases, patients were divided into 2 groups including patients with underlying diseases and patients without underlying diseases.
3.4.1. Patients with Underlying Diseases
(1) Insomnia Outcome Measurements. In comparison between CBT-I and no treatment, we included 5 RCTs [23, 28–30, 35] (total 229 participants) for ISI and 5 RCTs [25–29] (total 391 participants) for PSQI. The results of meta-analyses showed that CBT-I was more effective than no treatment (MD-4.47, 95% CI [−7.46, −1.48], I2 = 86%; MD-2.57, 95% CI [−3.50, −1.65], I2 = 64% for ISI scores and PSQI scores, respectively). More details of the meta-analysis are shown in Figure 3.
Figure 3.
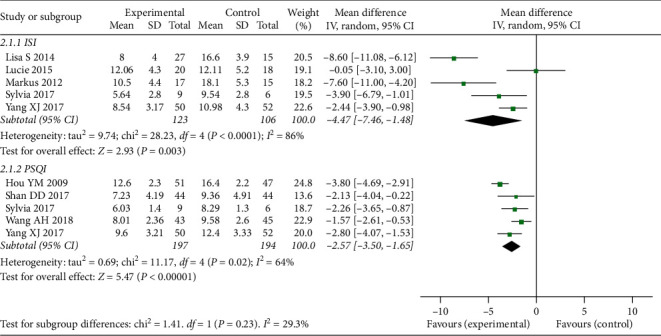
Forest plot of insomnia outcomes (CBT compared to no treatment in patients with underlying diseases).
(2) Depression Outcome Measurements. In comparison between CBT-I and no treatment, we included 2 RCTs [25, 26] (total 176 participants) for SDS scores; 3 RCTs [23, 29, 30] (total 85 participants) for HADS-D scores; 1 RCT [27] (total 98 participants) for SCL-90 scores; and 2 RCTs [28, 35] (total 144 participants) for BDI scores. The results of meta-analyses showed that CBT-I was more effective than no treatment (MD-3.44, 95% CI [−5.83, −1.06], I2 = 0%; MD −3.10, 95%CI [−4.71, −1.50], I2 = 19% for SDS scores and HADS-D scores, respectively). The result of SCL-90 scores showed that CBT-I was more effective than no treatment (MD-0.50, 95% CI [−0.76, −0.24]). However, the result of meta-analysis in BDI scores showed that there was no significant difference between CBT-I and no treatment with MD-2.61, 95% CI [−8.36, 3.14], I2 = 81%. More details of the meta-analysis are shown in Figure 4.
Figure 4.
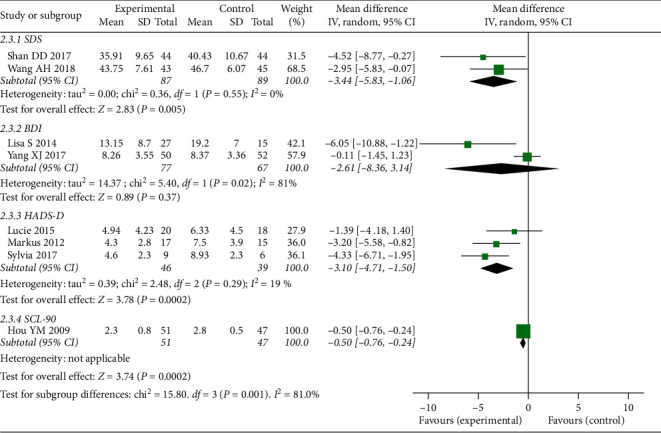
Forest plot of depression outcomes (CBT compared to no treatment in patients with underlying diseases).
3.4.2. Patients without Underlying Diseases
(1) Insomnia Outcome Measurements. In comparison between CBT-I and no treatment, we included 5 RCTs [24, 31–34] (total 455 participants) for ISI. The result of meta-analysis showed that CBT-I was more effective than no treatment with MD-4.88, 95% CI [−5.80, −3.95], I2 = 88%. More details of the meta-analysis are shown in Figure 5.
Figure 5.
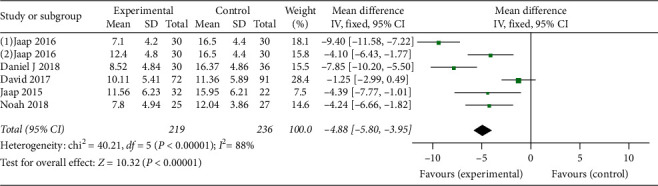
Forest plot of insomnia outcomes (CBT compared to no treatment in patients without underlying diseases).
In comparison between CBT-I and hypnotics, we included 2 RCTs [37, 39] (total 282 participants) for ISI scores and 3 RCTs [36, 38, 39] (total 414 participants) for PSQI scores. The result of meta-analysis in ISI scores showed that CBT-I was superior to hypnotics with MD-2.82, 95% CI [−5.22, −0.41], I2 = 66%. However, the result of meta-analysis in PSQI scores showed that there was no significant difference between CBT-I and hypnotics for insomnia with MD-0.29, 95% CI [−1.21, 0.62], I2 = 52. More details are shown in Figure 6.
Figure 6.
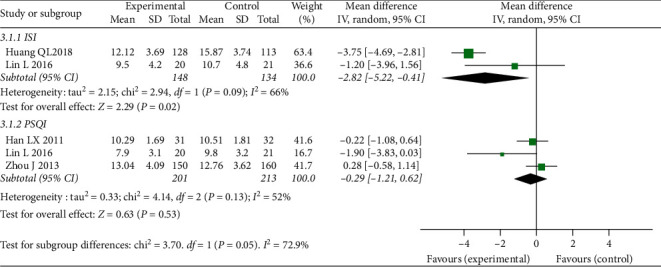
Forest plot of insomnia outcomes (CBT compared to hypnotics in patients without underlying diseases).
(2) Depression Outcome Measurements. In comparison between CBT-I and no treatment, we included 2 RCTs [24, 34] (total 118 participants) for BDI; 2 RCTs [32, 33] (total 174 participants) for CES-D, and 1 RCT [31] (total 163 participants) for QIDS-CR16. The result of meta-analysis showed that CBT-I was more effective than no treatment with MD-9.58, 95% CI [−13.71, −5.45], I2 = 60% in CES-D. The result in QIDS-CR16 illustrated that CBT-I was more effective than no treatment with MD-1.27, 95% CI [−2.25, −0.29]. However, the result of meta-analysis in BDI indicated that there was no significant difference between CBT-I and no treatment for depression with MD-1.19, 95% CI [−4.27, 1.89], I2 = 32%. More details of the meta-analysis are shown in Figure 7.
Figure 7.
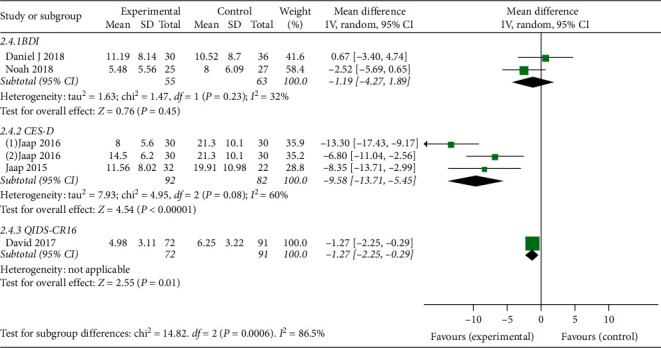
Forest plot of depression outcomes (CBT compared to no treatment in patients without underlying diseases).
In comparison between CBT-I and hypnotics, we included 2 RCTs [37, 39] (total 282 participants) for HAMD scores; 1 RCT [38] (total 310 participants) for SDS scores; and 1 RCT [36] (63 participants) for SCL-90 scores. The result of meta-analysis in HAMD showed that there was no significant difference between CBT-I and hypnotics with MD-1.27, 95% CI [−5.36, 2.82], I2 = 89%. The result of SDS scores showed that CBT-I was more effective than hypnotics with MD-0.86, 95% CI [−1.61, −0.11]. The result of SCL-90 showed that there was no significant difference between CBT-I and hypnotics (zopiclone and estazolam) with MD-0.25, 95% CI [−0.59, 0.09]. More details are shown in Figure 8.
Figure 8.
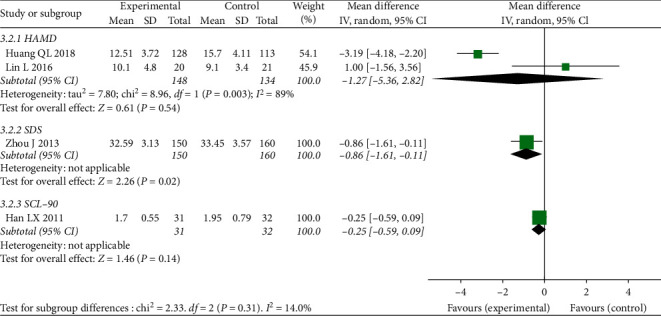
Forest plot of depression outcomes (CBT compared to hypnotics in patients without underlying diseases).
3.4.3. Adverse Event
From these included studies [23–39], only 1 RCT [32], comparing CBT-I with no treatment, reported the item of adverse event, which represented that there was no adverse event which occurred in CBT-I and no treatment.
3.4.4. Publication Bias
For the number of included studies in each of these outcomes was less than 10, we could not make an inverted funnel plot to assess the influence of publication bias of included studies.
3.5. Evaluating the Quality of Evidence
The quality of evidence was evaluated via GRADEpro. The quality of evidence in our review varied from moderate to very low. For patients with underlying diseases, the evidence of insomnia outcome measurements was moderate; the evidence of depression outcome measurements varied from low to very low. For patients without underlying diseases, the evidence of insomnia outcome measurements varied from moderate to low; the evidence of depression outcome measurements varied from low to very low. More details are shown in Table 4.
Table 4.
Summary of findings table.
| Participants (RCTs) | Quality of evidence | Anticipated absolute effects | |
|---|---|---|---|
| Risk difference with intervention (95% CI) | |||
| Patients with underlying diseases | |||
| Insomnia outcome measurements | |||
| CBT-I vs. no treatment | |||
| ISI | 229 (5) | ⊕⊕⊕⃝moderate | MD 4.47 lower (7.46 lower to 1.48 lower) |
| PSQI | 391 (5) | ⊕⊕⊕⃝moderate | MD 2.57 lower (3.5 lower to 1.65 lower) |
| CBT-I vs. hypnotics | None | None | None |
| Depression outcome measurements | |||
| CBT-I vs. no treatment | |||
| SDS | 176 (2) | ⊕⊕⃝⃝low | MD 3.44 lower (5.83 lower to 1.06 lower) |
| BDI | 144 (2) | ⊕⃝⃝⃝very low | MD 2.61 lower (8.36 lower to 3.14 higher) |
| HADS-D | 85 (3) | ⊕⊕⃝⃝low | MD 3.1 lower (4.71 lower to 1.5 lower) |
| CBT-I vs. hypnotics | None | None | None |
| Patients without underlying diseases | |||
| Insomnia outcome measurements | |||
| CBT-I vs. no treatment | |||
| ISI | 455 (6) | ⊕⊕⊕⃝moderate | MD 4.88 lower (5.8 lower to 3.95 lower) |
| CBT-I vs. hypnotics | |||
| ISI | 282 (2) | ⊕⊕⃝⃝low | MD 2.82 lower (5.22 lower to 0.41 lower) |
| PQSI | 414 (3) | ⊕⊕⃝⃝low | MD 0.29 lower (1.21 lower to 0.62 higher) |
| Depression outcome measurements | |||
| CBT-I vs. no treatment | |||
| BDI | 118 (2) | ⊕⃝⃝⃝very low | MD 1.19 lower (4.27 lower to 1.89 higher) |
| CES-D | 174 (3) | ⊕⊕⃝⃝low | MD 9.58 lower (13.71 lower to 5.45 lower) |
| CBT-I vs. hypnotics | |||
| HAMD | 282 (2) | ⊕⊕⃝⃝low | MD 1.27 lower (5.36 lower to 2.82 higher) |
The recommendation level assessed by the GRADE system is based on the factors including the advantages of CBT-I, the evidence quality in our review, the preferences of patients, and the cost of CBT-I. The evidence quality varied from moderate to very low in our review; CBT-I is receptible for the patients due to its noninvasive characteristics. And the cost of CBT-I is not so frightfully expensive. So, the recommendation level of the evidence in our review is low.
4. Discussion
4.1. Summary of Results
Insomnia scale, depression scale, and adverse event were included in our review to assess the effectiveness and safety of CBT-I. Based on the presence or absence of underlying diseases, we divided patients into 2 groups including patients with underlying diseases and patients without underlying diseases. Our results showed that CBT-I was an effective therapy for insomnia, while CBT-I was not an effective therapy for depression in patients suffering from insomnia and depression. And CBT-I was as effective as hypnotics (zopiclone, estazolam, or benzodiazepine agonist) for insomnia, and both CBT-I and hypnotics were not effective for depression. CBT-I was likely to be a safe therapy due to its noninvasive characteristics. The methodological quality was not good enough. The evidence quality varied from moderate to very low, and the recommendation level based on the evidence was low.
4.2. Comparison with the Previous Study
A number of reviews mentioned the effectiveness of CBT for insomnia or depression, respectively. For example, one review [40] assessed the effectiveness of self-help CBT-I for insomnia by comparing it with waiting list control, routine care, or no treatment, and the result showed that self-help CBT-I was significantly more effective than waiting list control, routine care, or no treatment; another review [41] assessed the effectiveness of online cognitive behavioral therapy (OCBT) for postpartum depressive symptomatology by comparing it with waiting list or treatment as usual. And the result identified a moderate significant size effect (d = −0.54, 95% CI [−0.716; −0.423]) of OCBT in reducing postpartum depression. However, few reviews focused on CBT for insomnia comorbidity with depression; therefore, our review assessed the effectiveness of CBT-I for insomnia comorbidity with depression by comparing it with no treatment or hypnotics. And the results of our review showed CBT-I was an effective therapy for patients with insomnia comorbid with depression to some degree. And the clinical effectiveness of CBT-I and hypnotics was familiar with no significant difference between CBT-I and hypnotics.
4.3. Strengths and Limitations
As we mentioned above, a number of reviews focused on the effectiveness of CBT-I for insomnia alone or depression alone. Considering insomnia often co-occurred with depression clinically, there were few guidelines or reviews focused on CBT-I for insomnia comorbidity with depression, so our review assessed the effectiveness of CBT-I for insomnia comorbidity with depression to provide evidence for clinical practice.
Limitations at review level:the quality of evidence varied from moderate to very low; the number of studies in each outcome was less than 10, we could not make publication bias. Limitations at study level: the number of qualified RCTs was insufficient, and the sample size of included studies was small. Limitation at outcome level: only one RCT mentioned the item of adverse event and showed that no harm occurred in both CBT-I and no treatment; more evidence is needed to confirm the safety of CBT-I.
4.4. Implications for Clinical Practice
The evidence of our review supported that CBT-I was effective for insomnia in patients suffering from insomnia and depression. And the effectiveness was comparable to hypnotics. CBT-I was likely to be safe due to its noninvasive nature. The findings suggest that CBT-I confers beneficial effects. Because of the low recommendation level of evidence, practitioners could recommend this therapy to patients and finally make a decision based on the evidence, the experience of doctors, and the preferences of patients.
4.5. Implications for Future Research
(1) Future studies should be conducted according to the Consolidated Standards of Reporting Trials (CONSORT) statement, which is essential to control the risk of bias. For example, considering the characteristics of CBT-I, it is hard to blind the doctors and patients, but we could blind outcome assessors. We could also pay more attention to allocation concealment to improve methodological quality of future studies. (2) Researchers should report every detail of studies according to the CONSORT statement, for example, adverse events in CBT-I should be comprehensively reported, although CBT-I appeared to be safe.
5. Conclusion
CBT-I may be an effective therapy for insomnia, and the effectiveness of CBT-I is comparable to hypnotics, while CBT-I is not an effective therapy for depression, which is the same to hypnotics in patients suffering from insomnia and depression. And CBT-I is likely to be safe. However, the quality and quantity of eligible RCTs are not good enough. And the evidence quality varied from moderate to very low, and the recommendation level of evidence was low (↑?/2). Therefore, more well-designed trials are needed to confirm our findings.
Acknowledgments
The authors sincerely thank Ruixue Hu, Center for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, for helping search RCTs and assessing methodological quality of included RCTs. This review was funded by Beijing ENN Fund for Chinese Medicine (2018-XAJLJJ- 020), Beijing Science and Technology Project (Z141107002514078), National Key Program of China (2011ZX09302-006-01), and Young Talents Project of Beijing University of Chinese Medicine (2011-JYB22-JSY110).
Appendix
#1 Cognitive Behavioral Therapy [MeSH Terms]
#2 Behavioral Therapies, Cognitive [Title/Abstract]
#3 Behavioral Therapy, Cognitive [Title/Abstract]
#4 Cognitive Behavioral Therapies [Title/Abstract]
#5 Therapies, Cognitive Behavioral [Title/Abstract]
#6 Therapy, Cognitive Behavioral [Title/Abstract]
#7 Therapy, Cognition [Title/Abstract]
#8 Therapy, Cognitive Behavior [Title/Abstract]
#9 Cognition Therapy [Title/Abstract]
#10 Cognition Therapies [Title/Abstract]
#11 Therapies, Cognition [Title/Abstract]
#12 Cognitive Psychotherapy [Title/Abstract]
#13 Cognitive Psychotherapies [Title/Abstract]
#14 Psychotherapies, Cognitive [Title/Abstract]
#15 Psychotherapy, Cognitive [Title/Abstract]
#16 Therapy, Cognitive [Title/Abstract]
#17 Cognitive Therapies [Title/Abstract]
#18 Therapies, Cognitive [Title/Abstract]
#19 Cognitive Therapy [Title/Abstract]
#20 Cognitive Behavior Therapy [Title/Abstract]
#21 Behavior Therapies, Cognitive [Title/Abstract]
#22 Cognitive Behavior Therapies [Title/Abstract]
#23 Therapies, Cognitive Behavior [Title/Abstract]
#24 Behavior Therapy, Cognitive [Title/Abstract]
#25#1OR#2OR#3OR#4OR#5OR#6OR#7OR#8OR#9OR#10OR#11OR#12OR#13OR #14OR#15OR#16OR#17OR#18OR#19OR#20OR#21OR#22OR#23OR#24
#26 Depression[MeSH Terms]
#27 Depressions [Title/Abstract]
#28 Depressive Symptoms [Title/Abstract]
#29 Depressive Symptom [Title/Abstract]
#30 Symptom, Depressive [Title/Abstract]
#31 Symptoms, Depressive [Title/Abstract]
#32 Emotional Depression [Title/Abstract]
#33 Depression, Emotional [Title/Abstract]
#34 Emotional Depressions [Title/Abstract]
#35 Depressions, Emotional [Title/Abstract]
#36#26OR#27OR#28OR#29OR#30OR#31OR#32OR#33OR#34OR#35
#37 sleep initiation and maintenance disorders [MeSH Terms]
#38 Disorders of Initiating and Maintaining Sleep [Title/Abstract]
#39 DIMS (Disorders of Initiating and Maintaining Sleep) [Title/Abstract]
#40 Early Awakening [Title/Abstract]
#41 Awakening, Early [Title/Abstract]
#42 Nonorganic Insomnia [Title/Abstract]
#43 Insomnia, Nonorganic [Title/Abstract]
#44 Primary Insomnia [Title/Abstract]
#45 Insomnia, Primary [Title/Abstract]
#46 Transient Insomnia [Title/Abstract]
#47 Insomnia, Transient [Title/Abstract]
#48 Rebound Insomnia [Title/Abstract]
#49 Insomnia, Rebound [Title/Abstract]
#50 Secondary Insomnia [Title/Abstract]
#51 Insomnia, Secondary [Title/Abstract]
#52 Sleep Initiation Dysfunction [Title/Abstract]
#53 Dysfunction, Sleep Initiation [Title/Abstract]
#54 Dysfunctions, Sleep Initiation [Title/Abstract]
#55 Sleep Initiation Dysfunctions [Title/Abstract]
#56 Sleeplessness [Title/Abstract]
#57 Insomnia Disorder [Title/Abstract]
#58 Insomnia Disorders [Title/Abstract]
#59 Insomnia [Title/Abstract]
#60 Insomnias [Title/Abstract]
#61 Chronic Insomnia [Title/Abstract]
#62 Insomnia, Chronic [Title/Abstract]
#63 Psychophysiological Insomnia [Title/Abstract]
#64 Insomnia, Psychophysiological [Title/Abstract]
#65#37OR#38OR#39OR#40OR#41OR#42OR#43OR#44OR#45OR#46OR#47OR#4 8OR#49OR#50OR#51OR#52OR#53OR#54OR#55OR#56OR#57OR#58OR#59OR# 60OR#61OR#62OR#63OR#64
#66#25AND#36AND#65
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Yingchun Miao, Mei Han, and Xun Li contributed to the design of this review. Guiyu Feng and Le Geng separately searched literature studies, selected suitable RCTs, extracted data, assessed the methodological quality of included RCTs, and evaluated the quality of evidence at the same time. Guiyu Feng and Yingchun Miao contributed to data analysis and completed the manuscript. Yingchun Miao, Mei Han, and Xun Li examined the manuscript and gave advice. Guiyu Feng revised the manuscript according to the suggestions of Yingchun Miao, Mei Han, and Xun Li. All the authors read and approved the final manuscript.
References
- 1.American Psychiatric Association. Sleep-wake Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, TX, USA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Morin C. M., Benca R. Chronic insomnia. The Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 3.Insomnia. DynaMed web site. 2015. http://www.dynamed.com/home.
- 4.Buysse D. J. Insomnia. Journal of the American Medical Association. 2013;309(7):706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford D. E., Kamerow D. B. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA: The Journal of the American Medical Association. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 6.Pigeon W. R., Pinquart M., Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. The Journal of Clinical Psychiatry. 2012;73(09):e1160–e1167. doi: 10.4088/jcp.11r07586. [DOI] [PubMed] [Google Scholar]
- 7.Li M., Zhang X.-W., Hou W.-S., Tang Z.-Y. Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. International Journal of Cardiology. 2014;176(3):1044–1047. doi: 10.1016/j.ijcard.2014.07.284. [DOI] [PubMed] [Google Scholar]
- 8.Laugsand L. E., Vatten L. J., Platou C., Janszky I. Insomnia and the Risk of Acute Myocardial Infarction. Circulation. 2011;124(19):2073–2081. doi: 10.1161/circulationaha.111.025858. [DOI] [PubMed] [Google Scholar]
- 9.Laugsand L. E., Strand L. B., Platou C., Vatten L. J., Janszky I. Insomnia and the risk of incident heart failure: a population study. European Heart Journal. 2014;35(21):1382–1393. doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- 10.Anothaisintawee T., Reutrakul S., Van Caute E., Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Medicine Reviews. 2015;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Yaffe K., Falvey C. M., Hoang T. Connections between sleep and cognition in older adults. The Lancet Neurology. 2014;13(10):1017–1028. doi: 10.1016/s1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A., Kansagara D., Forciea M. A., Cooke M., Denberg T. D. Management of chronic insomnia disorder in adults: a clinical practice guideline from the american college of physicians. Annals of Internal Medicine. 2016;165(2):125–133. doi: 10.7326/m15-2175. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington, DC, USA: American Psychiatric Association; 2013. [Google Scholar]
- 14.Wilson S. J., Nutt D. J., Alford C., et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. Journal of Psychopharmacology. 2010;24(11):1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 15.Asarnow L. D., Manber R. Cognitive behavioral therapy for insomnia in depression. Sleep Medicine Clinics. 2019;14(2):177–184. doi: 10.1016/j.jsmc.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bei B., Asarnow L. D., Krystal A., Edinger J. D., Buysse D. J., Manber R. Treating insomnia in depression: insomnia related factors predict long-term depression trajectories. Journal of Consulting and Clinical Psychology. 2018;86(3):282–293. doi: 10.1037/ccp0000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Workers’ Compensation Appeal Tribunal. Major Depressive Disorder in Adults: Diagnosis & Management. Richmond, Canada: Workers’ Compensation Appeal Tribunal; 2013. [Google Scholar]
- 18.Burns A. M. N., Erickson D. H., Brenner C. A. Cognitive-behavioral therapy for medication-resistant psychosis: a meta-analytic review. Psychiatric Services. 2014;65(7):874–880. doi: 10.1176/appi.ps.201300213. [DOI] [PubMed] [Google Scholar]
- 19.Gebara M. A., Dinapoli E. A., Lederer L. G., et al. Brief behavioral treatment for insomnia in older adults with late-life treatment-resistant depression and insomnia: a pilot study. Sleep and Biological Rhythms. 2019;17(3):287–295. doi: 10.1007/s41105-019-00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okai D., Askey-Jones S., Samuel M, et al. Trial of CBT for impulse control behaviors affecting Parkinson patients and their caregivers. Neurology. 2013;80(9):792–799. doi: 10.1212/wnl.0b013e3182840678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trauer J. M., Qian M. Y., Doyle J. S., Rajaratnam S. M. W., Cunnington D. Cognitive behavioral therapy for chronic insomnia. Annals of Internal Medicine. 2015;163(3):191–204. doi: 10.7326/m14-2841. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Hoboken, N J, USA: Wiley; 2011. [Google Scholar]
- 23.Casault L., Savard J., Ivers H., Savard M.-H. A randomized-controlled trial of an early minimal cognitive-behavioural therapy for insomnia comorbid with cancer. Behaviour Research and Therapy. 2015;67:45–54. doi: 10.1016/j.brat.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Daniel J. T., Peterson A. L., Pruiksma K. E., et al. Impact of cognitive behavioral therapy for insomnia disorder on sleep and comorbid Symptoms in military personnel: a randomized clinical trial. Sleep. 2018;41(6) doi: 10.1093/sleep/zsy069. [DOI] [PubMed] [Google Scholar]
- 25.Shan D. Effect of cognitive behavioral intervention on patients with ischemic stroke and insomnia. Chinese Journal of Practical Nervous Diseases. 2017;15(22):97–100. [Google Scholar]
- 26.Wang A., Zhou X., Wang M. Effect of cognitive behavioral therapy on sleep quality in cancer patients during chemotherapy. China Medical Herald. 2018;15(11):154–157. [Google Scholar]
- 27.Hou Y., Hu P., Liang Y., et al. Cognitive behavioral therapy improves insomnia in maintenance hemodialysis patients. Chinese Journal of Mental Health, the 7th Batch of Scientific and Technological Research Projects of Zhanjiang City. 2006;10 [Google Scholar]
- 28.Yang X., Zhang Y., Liu J., et al. Therapeutic effect of remote cognitive behavioral therapy on comorbidity and insomnia in hypertension. Chinese Journal of Medical Officer. 2017;12(4):69–73. [Google Scholar]
- 29.Sylvia N., Wang D., Mckay A., et al. Cognitive behavioural therapy for post-stroke fatigue and sleep disturbance: a pilot randomized controlled trial with blind. assessment. Neuropsychological Rehabilitation. 2017;29(5):723–738. doi: 10.1080/09602011.2017.1326945. [DOI] [PubMed] [Google Scholar]
- 30.Markus J.-F., Linton S. J., Flink I. K., Granberg S., Danermark B., Annika N.-C. Cognitive-behavioral therapy for insomnia co-morbid with hearing impairment: a randomized controlled trial. Journal of Clinical Psychology in Medical Settings. 2012;19(2):224–234. doi: 10.1007/s10880-011-9275-y. [DOI] [PubMed] [Google Scholar]
- 31.David D. E., Buntrock C., Lehr D., et al. Effectiveness of web- and mobile-based treatment of subthreshold depression with adherence-focused guidance: a single-blind randomized controlled trial. Behavior Therapy. 2018;49(1):71–83. doi: 10.1016/j.beth.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Lancee J., Eisma M. C., Van Straten A., Kamphuis J. H. Sleep-Related safety behaviors and dysfunctional beliefs mediate the efficacy of online CBT for insomnia: a randomized controlled trial. Cognitive Behaviour Therapy. 2015;44(5):406–422. doi: 10.1080/16506073.2015.1026386. [DOI] [PubMed] [Google Scholar]
- 33.Lancee J., Van Straten A., Morina N., Kaldo V., Kamphuis J. H. Guided online or face-to-face cognitive behavioral treatment for insomnia: a randomized wait-list controlled trial. Sleep. 2016;39(1):183–191. doi: 10.5665/sleep.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz N., Heim E., Roetger A., Birrer E., Maercker A. Randomized controlled trial to test the efficacy of an unguided online intervention with automated feedback for the treatment of insomnia. Behavioural and Cognitive Psychotherapy. 2018;47(3):1–16. doi: 10.1017/S1352465818000486. [DOI] [PubMed] [Google Scholar]
- 35.Talbot L. S., Maguen S., Metzler T. J., et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han L., Liu X. A comparative study of cognitive behavioral therapy and zopiclone in the treatment of insomnia. Chinese Journal of Health Psychology. 2011;19(10):1166–1167. [Google Scholar]
- 37.Huang Q., Le F., Jiang C., et al. Analysis of the effect of group cognitive behavioral therapy on insomnia. Chinese Journal of Medical Officer. 2018;3(1):224–228. [Google Scholar]
- 38.Zhou J., Meng L, Li C., et al. Observation on the effect of comprehensive psychological counseling on the intervention of insomnia in a certain officer. People’s Military Medical University. 2013;10(8):874–875. [Google Scholar]
- 39.Lin L. Clinical study of chronic CBT-I on chronic insomnia. Third Military Medical University. 2016;10(6):1–57. [Google Scholar]
- 40.Ho F. Y.-Y., Chung K.-F., Yeung W.-F., et al. Self-help cognitive-behavioral therapy for insomnia: A meta-analysis of randomized controlled trials. Sleep Medicine Reviews. 2015;19:17–28. doi: 10.1016/j.smrv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Roman M., Constantin T., Bostan C. M. The efficiency of online cognitive-behavioral therapy for postpartum depressive symptomatology: a systematic review and meta-analysis. Women & Health. 2019;60(1):99–112. doi: 10.1080/03630242.2019.1610824. [DOI] [PubMed] [Google Scholar]


