Abstract
Background
Post-traumatic stress disorder (PTSD) has become an important public health problem. However, the conventional therapeutic strategy, including pharmacotherapy and cognitive behavioral therapy, has limitations. Neurofeedback is a technique that utilizes electroencephalography (EEG) signaling to monitor human physiological functions and is widely used to treat patients with PTSD. The purpose of our study is to assess the efficacy and safety level of neurofeedback treatment in patients with PTSD using quantitative EEG.
Methods
This is a randomized, waitlist-controlled, assessor-blinded, clinical trial. Forty-six patients with PTSD will be randomly assigned at a 1:1 ratio into two groups. The participants in the treatment group will receive neurofeedback treatment for 50 min, twice a week, for 8 weeks (16 sessions). Quantitative EEG will be utilized to monitor the physiological functions and brain waves of the participants. A four-week follow-up period is planned. The participants in the control group will wait for 12 weeks. The primary outcome is the Korean version of PTSD Checklist-5 (PCL-5-K) score. The PCL-5-K scores on week 8 will be compared between the two groups. Anxiety, depression, insomnia, emotions, EEG, quality-of-life, and safety level will be assessed as secondary outcomes.
Discussion
This trial will describe a clinical research methodology for neurofeedback in patients with PTSD. The numerous subjective and objective secondary outcomes add to the value of this trial’s results. It will also suggest a therapeutic strategy for utilizing quantitative EEG in patients with PTSD. Our trial will provide basic evidence for the management of PTSD via an integrative treatment.
Trial registration
Clinical Research Information Service (CRIS): KCT0003271.
Keywords: Randomized controlled trial, Protocol, Neurofeedback, Post traumatic stress disorder, Quantitative electroencephalography
1. Background
Posttraumatic stress disorder (PTSD) is defined as “the complex somatic, cognitive, affective, and behavioral effects of psychological trauma”.1 PTSD occurs in individuals who have been exposed to a traumatic event, such as military combat, criminal victimization, a natural disaster, a severe traffic accident, or sexual abuse.2 According to the World Mental Health Survey, the lifetime prevalence of PTSD is 3.9%, and 5.6% of individuals who experience trauma develop PTSD.3 The symptoms of PTSD include sleep disorders, nightmares, intrusive thoughts, and hypervigilance. Furthermore, PTSD can result in flashbacks to traumatic events, which lead to occupational, social, and interpersonal problems.4 Half of the patients exposed to traumatic events report persistent PTSD symptoms.3 These patients also report suffering from several psychosomatic diseases associated with PTSD, such as hypertension, asthma, obesity, cancer, and gastro-intestinal disease.4 PTSD is becoming an important public health problem, the solution for which will require a novel paradigm and therapeutic strategy.5 Conventionally, PTSD is treated with three main therapeutic techniques: psychoeducation, psychotherapeutic interventions, and psychopharmaceuticals. However, these techniques have several limitations and are associated with a number of adverse events (AEs).5 Psychotherapeutic interventions are typically effective, but their efficacy can be attenuated by patients’ vigilance and anxiety levels.5 Medications such as serotonergic reuptake inhibitors have proven effective in the treatment of PTSD. However, these medications are costly and must be taken for at least six months to prevent symptom reoccurrence or relapse.6 This precludes their utility in low-income countries.2 Additionally, it is well established that these medications can produce various adverse reactions in some individuals.7 In a systematic review, it was found that medications and psychotherapeutic interventions facilitated partial symptom recovery.8 As there is no single, standard treatment for PTSD, the demand for self-regulation strategies is increasing.
Biofeedback is a self-regulation technique used to manipulate human physiological functions in real time, using physiological information acquired via a signal sensor attached to the human body. It has been used to alter heart rates, skin temperatures, skin conductance responses, blood pressure, and signals measurable via electroencephalography (EEG).9 Neurofeedback is a biofeedback technique that utilizes EEG signals to control high brain wave function.10 Via neurofeedback, it is possible to perceive and manipulate autonomic signals and the processes that generate them. By learning to manipulate these signals, an individual can manage stress responses by learning to control their temperature, heart rate, and respiratory rate.10 Neurofeedback is an available treatment for mental and psychological problems, including insomnia, anxiety, depression, and PTSD,11, 12 which is one of the major disorders that is widely treated with neurofeedback.13, 14 As demonstrated by several previous studies, neurofeedback can improve the function and electrical characteristics of damaged brain regions and reduce drug dependency.15, 16, 17, 18, 19
There are several tools used to assess the efficacy of neurofeedback in patients with PTSD. Because these patients have an autonomic imbalance,20 the heart rate variability signal is used to assess the effects of biofeedback.21 It is known that patients with PTSD show structural abnormalities throughout the frontal limbic system.8 Functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies have shown that patients with PTSD display different neuroimaging patterns compared to healthy control groups.22, 23 EEG has shown that abnormal fear responses and information processing, which can occur during attention and working, are reflected in PTSD patients’ unique electrophysiological characteristics.24, 25 Peniston et al. conducted a neurofeedback study, which involved patients with PTSD from the Vietnam war.26 Following its publication, most research in this area began to follow the study’s protocol by focusing on occipital, frontotemporal, and parietal lobe training. According to this protocol, EEG data are reported in an analog form so that EEG signal experts can interpret the data. To overcome limitations, quantitative EEG (QEEG) is widely used, at present. QEEG can be used for the quantitative processing of EEG data, in order to highlight specific waveform components, assess functional connectivity, conduct quantitative comparisons, provide a visual brain map, and elucidate relevant information.27
Several studies have demonstrated the feasibility of neurofeedback for patients with PTSD. However, these studies had several limitations in their clinical study designs and quantitative mechanism assessments.15, 16, 17, 18, 19 To compensate for previous limitations, we will conduct a clinical study featuring a control group, sample size calculations, randomization, and a long-term follow-up. We will conduct a neurofeedback intervention for PTSD patients to determine their physiological responses to the intervention and to evaluate its effectiveness and safety level. We will use QEEG to assess the mechanism of neurofeedback in patients with PTSD. This is the protocol of “Effectiveness and Safety of NeurofeedbacK self-regulAting training in patients with Post Traumatic Stress DisordEr (ESKAPE).”
2. Methods
The study protocol will follow the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist, to maintain a high standard of quality and scientific rigor.28
2.1. Objectives
The purpose of the study is to explore the effectiveness and safety level of a self-regulated neurofeedback intervention in patients with PTSD.
2.2. Trial design and study setting
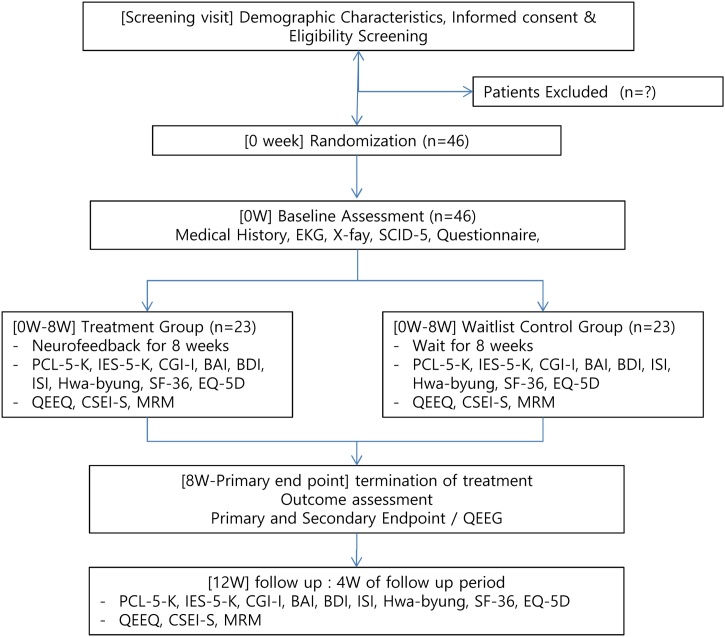
The study is a randomized, waiting list-controlled, single-center, assessor-blinded, open-label trial. Forty-six participants who meet the eligibility criteria will be randomly assigned to the treatment group (Neurofeedback group) or the waiting list control group (waiting for 8 weeks) in a 1:1 ratio. The trial design and study flowchart are shown in Table 1 and Fig. 1.
Table 1.
Study schedule.
| Assessment | Enrollment | Treatment phase |
Follow-up phase | ||
|---|---|---|---|---|---|
| Screening |
Before V1 (0 weeks) |
After V8 (4 weeks) |
After V16 (8 weeks) |
V17 (12 weeks) |
|
| Informed consent | X | ||||
| Demographic characteristics | X | ||||
| Medical history | X | ||||
| Vital signs and physical examination | X | Every visit before intervention | |||
| EKG and X-ray | X | ||||
| Blooda and urine test | X | ||||
| SCID-5 | X | ||||
| Inclusion / exclusion criteria | X | ||||
| Jing Ji and Zheng Chong | X | ||||
| Mibyeong | X | ||||
| KS-15 | X | ||||
| KSRI-SF | X | ||||
| PCL-5-K | X | X | X | X | |
| IES-R-K | X | X | X | X | |
| CGI-I | X | X | X | X | |
| BAI | X | X | X | X | |
| BDI | X | X | X | X | |
| ISI | X | X | X | X | |
| Hwa-byung | X | X | X | X | |
| SF-36 | X | X | X | X | |
| EQ-5D, EQ-VAS | X | X | X | X | |
| Cost | X | X | X | X | |
| CSEI-S | Every visit before and after intervention | ||||
| MRM | Every visit before and after intervention | ||||
| QEEG | X | X | X | ||
| Safety assessment | During trial, including waiting and follow-up period | ||||
aBlood test: red blood cells (RBCs), white blood cell (WBCs), hemoglobin, hematocrit, platelets, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), Gamma-glutamyl transferase (γ-GTP), alkaline phosphatase (ALP), total bilirubin, glucose, blood urea nitrogen (BUN), creatinine, electrolytes (Na, K, Cl), total protein, albumin, and thyroid function test (T3, TSH, Free T4).
Women of childbearing age will be further tested for urine hCG to identify pregnancy before the first treatment. A 3-day window will be allowed for each visit.
If necessary, unscheduled visits will be allowed and recorded in the medical record and care report form.
Abbreviations: EKG, electrocardiogram; KS-15, Korea Sa sang Constitutional Diagnostic Questionnaire-15; KSRI-SF, Korean Sex Role Inventory-Short Form; PCL-5-K, The Posttraumatic Stress Disorder Checklist for DSM-5; IES-R-K, Impact of Event Scale-Revised; CGI-I, The Clinical Global Impression–Improvement scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; ISI, Insomnia Severity Index; SF-36, Short Form Health Survey-36; EQ-5D, EuroQoL-5 Dimension; EQ-VAS, EuroQoL-visual analog scale; CSEI-S, The Core Seven-Emotions Inventory Short Form; MRM, Mentalizing the Rooms of Mind; QEEG, Quantitative Encephalogram.
Fig. 1.
Study flowchart.
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CGI-I, The Clinical GlobalImpression -Improvement scale; CSEI-S, The Core Seven-Emotions Inventory Short Form; EKG, electrocardiogram; EQ-5D,EuroQoL-5 Dimension; IES-R-K, Impact of Event Scale-Revised; ISI, Insomnia Severity Index; MRM, Mentalizing the Rooms of Mind; PCL-5-K, The Posttraumatic Stress Disorder Checklist for DSM-5; QEEG, Quantitative Encephalogram; SF-36, Short Form Health Survey-36.
2.3. Recruitment
Study participants will be patients at the Wonkwang University Sanbon Hospital, Republic of Korea. Clinical trial information will be posted in local newspapers, in online advertisements, on the hospital bulletin board, and on advertisement boards in public spaces. Participants will voluntarily sign a consent form to participate in the clinical trial and will be recruited based on the inclusion/exclusion criteria.
2.4. Eligibility criteria: inclusion criteria
-
1
Male and female participants aged 20–55 years;
-
2
Diagnosis of PTSD based on the Structured Clinical Interview outlined in the DSM-5 (SCID-5) criteria29 (We used the Korean version of SCID-5: a validated, standard, semi-structured interview guide for PTSD diagnosis30);
-
3
Occurrence of a traumatic event more than 6 months prior to recruitment;
-
4
Voluntary participation after explanation of the purpose and process of the clinical trial.
2.5. Eligibility criteria: exclusion criteria
-
1
A severe mental illness, a recent or past delusion or hallucination, at least one manic episode, a history of alcohol abuse or dependency, or a risk of suicide;
-
2
Requirement of continuous administration of a substance that is thought to have an effect on symptom relief, except for concomitant medications;
-
3
Serious diseases, such as those affecting the central nervous system, peripheral nervous system, endocrine system, heart, liver, or kidney as well as congenital or immunological diseases;
-
4
Pregnancy or lactation;
-
5
Participation in other clinical studies during the last month or are current participation in other clinical studies;
-
6
Too much difficulty for the participant in performing and understanding the clinical trial as determined by the principle investigator.
2.6. Participant withdrawal criteria
-
1
Refusal to follow the directions of the investigator;
-
2
Violation of the clinical trial protocol by the participant or investigator;
-
3
A serious adverse reaction (death, life-threatening adverse reactions, hospitalization, disability, permanent damage, congenital anomaly, or birth defect) to any study protocols;
-
4
Withdrawal of consent;
-
5
Request for treatment discontinuation;
-
6
Loss to follow-up.
2.7. Randomization, allocation concealment, and blinding
A randomization table will be created by an independent researcher using the block randomization method, in the Excel program. Allocation to the treatment and control groups will occur at a 1:1 ratio. If a patient meets the eligibility criteria, the CRC will give the random number sequentially to each patient. To conceal allocation, the independent researcher will receive their randomization number and check the allocated group. The allocated group will be sent to the neurofeedback practitioner. Random number table will be kept in a double-locked cabinet that will only accessible by authorized researchers. Consent will be obtained by a traditional Korean Medicine doctor. Because of the waitlist-controlled study design, the participants and practitioners cannot be blinded. For patient-reported outcomes, the assessor cannot be blinded. However, for objective outcomes, including QEEG measures, the assessor will be blinded. The statistician will also be blinded.
2.8. Intervention
2.8.1. Study schedule
The study schedule is presented in Table 1. The clinical trial will include screening, treatment, and follow-up phases. Among the candidates who have agreed to participate in the clinical trial, those who have been diagnosed with PTSD, according to the SCID-5, will be selected. The investigator will collect information on the patients’ demographics, physical examination findings, hematologic findings, urine sample results, thyroid hormone test results, pregnancy test results, chest X-ray findings, electrocardiography findings, medical history, current medications, and concurrent treatment modalities. This information will be used to determine if each individual can participate in the clinical trial. Finally, the baseline questionnaire will be examined for each selected participant. The treatment group will receive neurofeedback training twice a week for 8 weeks (16 sessions in total). The waiting list control group will wait for 8 weeks and their daily lives will go uninfluenced, aside from ongoing status monitoring. A follow-up visit will be scheduled for 12 weeks later.
2.8.2. Neurofeedback procedure
During sessions 1–16, the neurofeedback training procedure will be conducted based on the baseline QEEG analysis of the participant. A ProComp2, 2-Channel EEG System with v6.0 Infiniti Software (Thought Technology Ltd., Quebec, Canada) will be used. Neurofeedback is a form of training based on operant conditioning, during which participants monitor their own brain waves via audio-feedback (eyes-closed state) to learn reinforcement and compensation.31 To reduce PTSD patients’ stress and anxiety, alpha-theta brainwave neurofeedback will be performed according to the protocol developed by Peniston and Kulkosky17 or Smith.15 The purpose of this neurofeedback training is to maintain a relaxed, meditative state with strengthened alpha and theta waves and suppressed beta waves. The participants’ status will be assessed using a 24-electrode array. The training location will be the parietal lobe (PZ). Wavelengths of 8–12 Hz and 4–7 Hz will be categorized as alpha and theta waves, respectively.
The procedure will consist of 3, 10-minute training sessions, broken up by 2, 5-minute rest periods, and ending with a 10-minute rest period, for a total of 50 min. The Neurofeedback session will be conducted by a trained researcher (MJ) with a certification from the Biofeedback Certification International Alliance.
2.8.3. Control group intervention
The waiting list control group will wait for 12 weeks. For safety, the waiting list group will continue their usual treatment and lifestyle management. We also excluded patients with severe mental illness. From weeks 0–8, we will assess changes in anxiety and stress levels by telephone interviews every 2 weeks. The control group participants will visit the site to be evaluated for the outcome variables at weeks 4, 8, and 12. QEEG data will be measured at weeks 0, 8, and 12 in the control group (as in the treatment group). If the control group participants wish to receive neurofeedback treatment, it will begin after the 12th-week assessment. After completion of waiting period for 12 weeks, the control group’s treatment schedule will be the same as that of the treatment group.
2.8.4. Concomitant treatment
In principle, non-pharmaceutical treatments other than neurofeedback training are not allowed during the clinical trial. However, the following non-medicinal treatments and concomitant medication may be allowed.
-
1)In the case of counseling therapy for PTSD, counseling is allowed if stable counseling has been performed for at least 3 months prior to screening;
-
2)In the case of medication use, it is allowed if the dose was stable for 3 months in patients taking hypnotics, antidepressants, antipsychotics, and anxiolytics.
-
3)Drugs for other conditions, such as hypertension, diabetes mellitus, and hyperlipidemia will be allowed, as will analgesics.
-
1)
2.9. Baseline assessment
1) The Jing Ji and Zheng Chong questionnaire,32 2) Mibyeong questionnaire,33 3) Korea Sa Sang Constitutional Diagnostic Questionnaire-15 (KS-15),34 and 4) Korean Sex Role Inventory-Short Form (KSRI-SF)35 will be investigated as covariates on visit 1, before the treatment.
2.10. Outcome measures: primary endpoint
2.10.1. Korean version of PTSD Checklist-5 (PCL-5-K) score
The primary outcome of our study will be the PCL-5-K score. The PCL-5 is a 20-item, 5-point-Likert-scale, self-report questionnaire developed by Weathers et al. to measure the PTSD symptoms in the DSM-536 and to diagnose PTSD. A higher score (out of 80) indicates severe PTSD. The PCL-5-K is a version of the PCL-5, translated into Korean by Kim et al., that has been licensed from the US National PTSD Center.37 In this study, the PCL-5-K will be assessed at baseline, week 4, week 8, and week 12.
2.11. Outcome measures: secondary endpoints
2.11.1. Korean version of the impact of event scale-revised (IES-R-K)
Horowitz et al. developed the initial version of the Impact of Event Scale. Weiss and Marmar revised the modified version in order to evaluate the psychological reaction related to trauma. The scale is a questionnaire composed of 22 items rated on 5-point Likert scales. This study will employ the IES-R-K, which is validated for use in the Korean language.38 Participants will complete the IES-R-K at baseline and at 4, 8, and 12 weeks.
2.11.2. Clinical global impression – improvement scale (CGI-I)
The CGI-I scale assesses whether a patient’s clinical status has improved or worsened after a therapeutic intervention, as compared to their pre-intervention status.39 It is a 7-point Likert scale from 1 (very much improved) to 7 (very much worse). Participants will complete the CGI-I at baseline and at 4, 8, and 12 weeks.
2.11.3. Beck anxiety inventory (BAI)
The BAI is a self-reported anxiety assessment tool developed to focus on differentiating anxiety from depression.40 It consists of 21 items on 4-point Likert scales, ranging from 0 to 3 (total score range: 0–63). Participants will complete the BAI at baseline and at 4, 8, and 12 weeks.
2.11.4. Beck depression inventory (BDI)
The BDI is used to assess depressive symptoms. It is composed of 21 items. Our study will use a version translated into Korean by Lee et al.41 Participants will complete the BDI at baseline and at 4, 8, and 12 weeks.
2.11.5. Insomnia severity index (ISI)
The ISI is a well-validated, sensitive tool for evaluating insomnia.42 It is composed of 7 items (score range: 0–28). An ISI score of more than 15 points indicates clinical insomnia. Participants will complete the ISI at baseline and at 4, 8, and 12 weeks.
2.11.6. Hwa-Byung scale (HBS)
The HBS is a self-report scale developed by Kwon et al. to evaluate the psychological state and personality characteristics of Hwa-Byung patients.43 It is composed of 15 items on 5-point Likert scales (score range: 0–60). Participants will complete the HBS at baseline and at 4, 8, and 12 weeks.
2.11.7. Core seven-emotions inventory short form (CSEI-S)
The CSEI-S is a questionnaire based on the seven-emotions theory of Korean medicine.44 Participants will complete the CSEI-S after every treatment and at 12 weeks. It is composed of 28 items, divided into 7 categories, on 5-point Likert scales. A higher score indicates more emotional problems (score range: 28–140).
2.11.8. Mentalizing the rooms of mind (MRM)
MRM is one of the psychotherapeutic techniques that helps a patient to observe their own mind more objectively by structuring, visualizing, and becoming aware of the rooms of their own mind.45 MRM is known to have clinical value as a tool for objective follow-up observation.46 MRM will be administered after every treatment and at 12 weeks.
2.12. QEEG analysis
The absolute power, relative power, coherence, and localization-low resolution brain electromagnetic tomography (LORETA) analysis of each band range of the QEEG will be performed using the WinEEG (Mitsar, Russia) program. Spectral analysis will use a linked ears reference montage, which is suitable for general analysis, and a local average montage, which has the advantage of minimizing the medication effect and focusing on a localized area. A fast Fourier transform will be used to calculate relative wavelength power (delta, 0.5–4 Hz; theta, 4–8 Hz; alpha, 8–12 Hz; and beta, 12–20 Hz).
QEEG will be measured before the treatment and 8 and 12 weeks after treatment. According to the international 10/20 system, 21 surface electrodes will be attached on the scalp (Electro-cap; Mitsar, Russia). The impedance of each electrode will be less than 5 kΩ. A reference electrode will be attached between A1 and A2. Filter setting will be as follows: high frequency filter (50 Hz), low frequency filter (0.3 Hz), and notch filter (55−65 Hz). The first and last 500 millisecond(ms) will be excluded from analysis. Artifacts will be removed by visual inspection and using algorithms in the WinEEG program. We will obtain absolute power (μV2) and relative power (%) for each frequency band, and we will use normative databases to compute the Z-scores of both values.
2.13. Quality of life
2.13.1. Short form health survey-36 (SF-36)
The SF-36 is composed of 36 items classified into eight categories: physical function, body pain, body role limitations, emotional role limitations, mental health, social function, vitality and fatigue, and general health. This study will employ the calculation method proposed by Ware and Sherboume.47 After transformation, the total score will be 100 points.
2.13.2. EuroQoL-5 Dimension (EQ-5D)
The EQ-5D, developed by the EuroQol Group in 1987, is a utility that measures the health-related quality of life. In this study, we used the Korean version of EQ-5D with cross-cultural adaptation and confirmation process.48 It has five dimensions and scores for each dimension range from 1 to 3. A higher score indicates a lower quality of life, in terms of health.
2.13.3. Visual analog scale (EQ-VAS)
The EQ-VAS is an indicator of overall health status, with 0 being the worst health status and 100 being the best health status.49
2.13.4. Cost measurement
The number and cost of medications and treatments used during the study period will be investigated for economic evaluation.
2.14. Safety assessment
Even though neurofeedback is considered a relatively safe intervention for PTSD patients, several AEs are anticipated such as fatigue, somnolence, and increased sensitivity. All AEs brought to the attention of the research team, or noted by members independently, will be recorded in detail. The frequency of AEs, including those unrelated to the neurofeedback self-regulating training, will also be recorded. According to severity, AEs will be categorized as mild, moderate, or severe. According to causality, AEs will be categorized as definitely related, probably related, possibly related, probably not related, definitely not related, and unknown.
2.15. Data management and quality control
An independent CRC will collect the data on a paper case report form according to the standard operation procedure (SOP). The research team members will be educated about the SOP to ensure accuracy and quality in the clinical trial. All original source documents, including consent forms, questionnaires, medical records, and other relevant records will be stored in a password-protected computer or double-locked cabinet that can be accessed only by authorized researchers.
The data will be stored for 3 years after study completion, according to the applicable laws. Internal monitoring of the research team will be conducted to ensure protection of participant rights, adherence to study protocols, quality of data collection and manipulation, and accurate information on recruitment statuses. All practitioners and researchers will have gone through a Good Clinical Practice (GCP) training course. To enhance the quality of neurofeedback, each session will be administered by a practitioner with more than 3 years of experience, who has an international board certification for neurofeedback practices.
2.16. Sample size calculation
The primary outcome is the difference between the PCL-5-K scores of the intervention and control groups at week 8 (visit 16). The null hypothesis is that there is no difference between the mean PCL-5-K scores, at visit 16, between the neurofeedback group and the waitlist control group. To our knowledge, no study has previously used the PCL-5-K as the primary outcome in a neurofeedback study of PTSD patients.50 Therefore, effect-size-related parameters must be approximated. We will accomplish this by extrapolating from the results of neurofeedback studies of PTSD patients that have measured other outcomes. In a previous neurofeedback trial for PTSD patients, the effect size of the Clinician Administered PTSD Scale was 2.33.51 Because this effect size is considered very high, we adopted a more conservative effect size from clinical research on yoga, which is also a popular complementary and alternative intervention for PTSD. In a recent systematic review of this research, the relevant effect size was 1.1.52 We used PASS 2019 Power Analysis and Sample Size Software (NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass) to calculate the sample size, using a two-sample t-test, with effect size of 1.1.53 The number of patients required for each group was 19, given a 1:1 allocation ratio, a 2-tailed test, and a test power of 90 % (1-β) with a significance level of 5% (α). In actual power calculations, group sample sizes of 19 and 19 achieve 90.97% power to reject the null hypothesis for an effect size of zero given the previously stated parameters and a t-test assuming equal group variances. Considering a 15% dropout rate, we calculated the sample size for each group to be 23 patients per group. A total of 46 patients will be required for our trial.
2.17. Statistical analysis
Categorical variables will be expressed as frequencies and ratios (%). Continuous variables will be expressed as means and standard deviations. According to the outcome of each normality test, parametric or non-parametric tests will be used. If the data are not normally distributed, median and interquartile ranges will be presented.
2.17.1. Primary outcome
The primary outcome is the difference between the final PCL-5-K scores of the two groups (week 8). According to the outcome of the normality test, an independent t-test or a Wilcoxon-Mann-Whitney test will be used to compare the difference between these scores. If there are significant differences between the baseline scores of the two groups, an analysis of covariance will be chosen. The last observation carried forward (LOCF) method will be adopted for missing values.
2.17.2. Secondary outcome
The secondary outcomes are IES-R-K, CGI-I, BAI, BDI, ISI, HBS, CSEI-S, MRM, SF-36, EQ-5D, EQ-VAS, and cost. The final values of each secondary outcome at weeks 8 and 12 will be compared between the two groups. If there are significant differences between the baseline scores of the two groups, an analysis of covariance will be chosen. The LOCF method will be adopted for missing values.
2.17.3. QEEG analysis
Frequency spectrum analysis of the absolute (μV2) and relative power (%) will be conducted on each electrode from baseline to weeks 8 and 12. Both resource mindfulness and stress mindfulness will be analyzed. Source localization will be conducted, using LORETA. According to LORETA, both resource mindfulness and stress mindfulness will be analyzed for each frequency and Brodmann area. Spearman correlation tests will be used to assess the relationship between QEEG absolute power (μV2)/relative power (%) and clinical outcomes (PCL-5-K, ISE-R-K, CGI-I, BAI, BDI, ISI).
2.18. Ethics approval and registration
The Institutional Review Board of Wonkwang University Sanbon Hospital (IRB approval No WMCSB 201802-10) has approved the protocol. Written informed consent will be obtained from all participants. We registered our clinical trial protocol with the clinical research information service (CRIS), which is one of the primary registries of the World Health Organization International Clinical Trials Registry Platform. (CRIS No. KCT0003271; https://cris.nih.go.kr/)
3. Discussion
Our trial will investigate the effectiveness and safety level of neurofeedback treatment for patients with PTSD. The long-term use of pharmacological treatments for PTSD is limited by the potential for these treatments to result in side effects and dependencies.54 Several non-pharmacological interventions are available for the treatment of PTSD. Cognitive and behavioral therapy (CBT) is focused on trauma-focused themes to deal with the psychological meaning of the trauma.55 It consists of prolonged exposure (PE), stress inoculation training, cognitive therapy, cognitive restructuring, eye movement desensitization/reprocessing, and dialectical behavior therapy. Psychoeducation is a conventional intervention that is commonly administered for patients with PTSD. This intervention educates patients on the theories and principles of CBT, to help them understand the reasoning behind the therapy.56 PE refers to a gradual, in vivo, or imaginary (indirect) exposure of the patient to the traumatic event. However, PE can be a very challenging and painful process for patients with PTSD.55 Therefore, PE should not be attempted until a considerable amount of time has passed since the traumatic event. In addition, it is hard for these patients to establish cognitive awareness. Shaw et al. examined the relationship between cognitive awareness and various clinical variables (included those measured in the current study), among people with PTSD. They found that cognitive awareness was negatively related to psychotic symptom severity and positively related to depersonalization/derealization symptoms.57 Due to these limitations, the demand for alternative PTSD treatments is increasing.
Neurofeedback is widely used for various psychiatric diseases, including PTSD. It has an advantage over CBT because it does not re-expose patients with PTSD to the traumatic event. Several studies have reported on the effects of neurofeedback on patients with PTSD. However, most of these studies used a relatively small sample size.11, 58, 59 Additionally, some did not feature a control group and, therefore, could not account for the natural history effect of the disease, regression to mean, and several non-specific effects.15, 16 Some studies did not report the exact location of the electrode.18 Several studies did not report the neurofeedback protocol, which limits their reproducibility and the clinical utility of their results.17, 26
To the best of our knowledge, our study will be the first randomized study with a control group to examine the effect of neurofeedback in patients with PTSD, using simultaneous QEEG feedback, in Korea. Our study has several strengths. First, it features a sample size calculation that accounts for the anticipated drop-out rate and a reasonable effect size. Second, it will explore the continuous effects of neurofeedback over a 4-week follow-up period. Next, we adopted a waitlist-controlled design to exclude several non-specific effects, such as regression to mean, the Hawthorne effect, placebo effects, and others. Furthermore, we will examine various subjective and objective outcomes, including QEEG, to explore the mechanism and therapeutic effects of neurofeedback. Additionally, QEEG enables us to explore the functional connectivity of the brain using the LORETA technique.60 Finally, we will employ a well-established protocol for the neurofeedback procedure that contributes to the reliability of our study. We expect our results to provide basic evidence on the efficacy of neurofeedback as a treatment strategy for PTSD. The results of our study will represent a valuable resource for patients, researchers, physicians, stakeholders, and psychotherapists.
3.1. Trial status
The final protocol version is 1.3, dated July 8, 2018. This trial is currently recruiting participants. Recruitment began on May 2, 2019. We expect the recruitment phase to be completed by December 2022.
Author contribution
Conceptualization: HWK; Data curation: NA; Formal analysis: NA; Funding acquisition: GK; Investigation: HJ; Methodology: JL, MJ, and SY; Project administration: MJ and JK; Resources: HL and HYK; Software: HL and HHK; Supervision: JL; Validation: NA; Visualization: NA; Writing - original draft: JL, SY, and GK; Writing - review & editing: MJ and HWK
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the Korea Health Industry Development Institute (grant# HB16C0021).
Ethical statement
The Institutional Review Board of Wonkwang University Sanbon Hospital has approved the protocol (IRB approval No WMCSB 201802-10). Written informed consent will be obtained from all participants.
Data availability
Not applicable.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.imr.2020.100464.
Contributor Information
Geun-Woo Kim, Email: kgwoo86@hanmail.net.
Hyung Won Kang, Email: dskhw@wku.ac.kr.
Supplementary material
The following are Supplementary data to this article:
References
- 1.van der Kolk B.A., Pelcovitz D. Dissociation, somatization, and affect dysregulation: The complexity of adaptation of trauma. Am J Psychiatry. 1996;153(7 Suppl):83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- 2.Feldman D.B. Posttraumatic stress disorder at the end of life: Extant research and proposed psychosocial treatment approach. Palliat Support Care. 2011;9(4):407–418. doi: 10.1017/S1478951511000435. [DOI] [PubMed] [Google Scholar]
- 3.Koenen K.C., Ratanatharathorn A., Ng L. Posttraumatic stress disorder in the world mental health surveys. Psychol Med. 2017;47(13):2260–2274. doi: 10.1017/S0033291717000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Player M.S., Peterson L.E. Anxiety disorders, hypertension, and cardiovascular risk: A review. Int J Psychiatry Med. 2011;41(4):365–377. doi: 10.2190/PM.41.4.f. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Hu Y.-P., Wang W.-C., Pang R.-Z., Zhang A.-R. Clinical studies on treatment of earthquake-caused posttraumatic stress disorder using electroacupuncture. Evid-Based Complement Altern Med ECAM. 2012;2012 doi: 10.1155/2012/431279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson J., Pearlstein T., Londborg P. Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: Results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry. 2001;158(12):1974–1981. doi: 10.1176/appi.ajp.158.12.1974. [DOI] [PubMed] [Google Scholar]
- 7.Spigset O. Adverse reactions of selective serotonin reuptake inhibitors: Reports from a spontaneous reporting system. Drug Saf. 1999;20(3):277–287. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- 8.Karl A., Schaefer M., Malta L.S., Dörfel D., Rohleder N., Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Thompson M., Thompson L. Association for Applied Psychophysiology and Biofeedback; 2003. The neurofeedback book: An introduction to basic concepts in applied pychophysiology. [Google Scholar]
- 10.Gustafson C. David Haase,md: Healing the Gut and Brain Through Electrophysiology. Integr Med Encinitas Calif. 2016;15(5):26–29. [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter K., Andersen S.B., Carlsson J. Neurofeedback treatment and posttraumatic stress disorder: Effectiveness of neurofeedback on posttraumatic stress disorder and the optimal choice of protocol. J Nerv Ment Dis. 2016;204(2):69–77. doi: 10.1097/NMD.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 12.Hammond D.C. Neurofeedback treatment of depression and anxiety. J Adult Dev. 2005;12(2-3):131–137. [Google Scholar]
- 13.Rubí M.C.M. Neurofeedback around the world. J Neurother. 2007;10(4):63–73. [Google Scholar]
- 14.Othmer S., Othmer S.F. Post traumatic stress disorder—the neurofeedback remedy. Biofeedback. 2009;37(1):24–31. [Google Scholar]
- 15.Smith T.C., Ryan M.A.K., Wingard D.L. New onset and persistent symptoms of post-traumatic stress disorder self reported after deployment and combat exposures: prospective population based US military cohort study. BMJ. 2008;336(7640):366–371. doi: 10.1136/bmj.39430.638241.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluetsch R.C., Ros T., Théberge J. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr Scand. 2014;130(2):123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peniston E.G., Kulkosky P.J. Alpha-theta brainwave neurofeedback for Vietnam veterans with combat-related post-traumatic stress disorder. Med Psychother. 1991;4:47–60. [Google Scholar]
- 18.Pop-Jordanova N., Zorcec T. Child trauma, attachment and biofeedback mitigation. Prilozi. 2004;25(1-2):103–114. [PubMed] [Google Scholar]
- 19.Gapen M., van der Kolk B.A. Pilot study of neurofeedback for chronic PTSD. Appl Psychophysiol Biofeedback. 2016;41(3):251–261. doi: 10.1007/s10484-015-9326-5. [DOI] [PubMed] [Google Scholar]
- 20.Brudey C., Park J., Wiaderkiewicz J., Kobayashi I., Mellman T.A., Marvar P.J. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol. 2015;309(4):R315–321. doi: 10.1152/ajpregu.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blase K.L., van Dijke A., Cluitmans P.J.M., Vermetten E. [Efficacy of HRV-biofeedback as additional treatment of depression and PTSD] Tijdschr Voor Psychiatr. 2016;58(4):292–300. [PubMed] [Google Scholar]
- 22.Bremner J.D., Staib L.H., Kaloupek D., Southwick S.M., Soufer R., Charney D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol Psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pissiota A., Frans O., Fernandez M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci. 2002;252(2):68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- 24.Lobo I., Portugal L.C., Figueira I. EEG correlates of the severity of posttraumatic stress symptoms: A systematic review of the dimensional PTSD literature. J Affect Disord. 2015;183:210–220. doi: 10.1016/j.jad.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Chae J.H., Jeong J., Peterson B.S. Dimensional complexity of the EEG in patients with posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2004;131(1):79–89. doi: 10.1016/j.pscychresns.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Peniston E.G., Kulkosky P.J. Neurofeedback in the treatment of addictive disorders. In: Evans J.R., Abarbanel A., editors. Introduction to quantitative EEG and neurofeedback. Academic Press; 1999. pp. 157–179. [DOI] [Google Scholar]
- 27.Kanda PA de M., Anghinah R., Smidth M.T., Silva J.M. The clinical use of quantitative EEG in cognitive disorders. Dement Neuropsychol. 2009;3(3):195–203. doi: 10.1590/S1980-57642009DN30300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan A.-W., Tetzlaff J.M., Altman D.G. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.First M.B. Structured clinical interview for the DSM (SCID) Encycl Clin Psychol. 2014:1–6. [Google Scholar]
- 30.Kim W.H., Jung Y.E., Roh D. Reliability and validity of the korean version of clinician-administered posttraumatic stress disorder scale for DSM-5. J Korean Med Sci. 2019;34(32):e219. doi: 10.3346/jkms.2019.34.e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chae J.H. Diagnosis and pathophysiology of posttraumatic stress disorder. Korean J Psychopharmacol. 2004;15(1):14–21. [Google Scholar]
- 32.Park D.M., Lee S.R., Kang W., Jung I. Preliminary study to develop the instrument of pattern identification for Jing Ji and zheng chong. Korean Soc Orient Neuropsychiatry. 2010;21(2):1–15. [Google Scholar]
- 33.Lee Y., Baek Y., Park K., Jin H.-J., Lee S. Development and validation of an instrument to measure the health status of healthy but unsatisfied people : Mibyeong index(未病 index) Soc Prev Korean Med. 2016;20(3):45–53. [Google Scholar]
- 34.Baek Y., Jung E.-S., Park K.-H., Yoo J.-H., Lee S. Development and Validation of Brief KS-15 (Korea Sasang Constitutional Diagnostic Questionnaire) Based on Body Shape, Temperament and Symptoms. J Sasang Const Med. 2015;27(2):211–221. doi: 10.7730/JSCM.2015.27.2. [DOI] [Google Scholar]
- 35.Kim J.-H., Ha M.-S., Kim J.-H., Ha M.-S., Kim J.-H. Validation of short form of Korean Sex Role Inventory(KSRI-SF) Korean J Couns Psychother. 2016;17(1):125–147. doi: 10.15703/kjc.17.1.201602.125. [DOI] [Google Scholar]
- 36.Weathers F.W., Litz B.T., Herman D.S., Huska J.A., Keane T.M. Vol. 462. 1993. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. (Annual convention of the International society for traumatic stress studies, San Antonio, TX). San Antonio, TX. [Google Scholar]
- 37.Kim J.W., Chung H.G., Choi J.H. Psychometric properties of the Korean version of the PTSD Checklist-5 in elderly Korean veterans of the Vietnam war. Anxiety Mood. 2017;13(2):123–131. [Google Scholar]
- 38.Lee S., Eun H. A study of reliability and validity on the korean version of impact of event scale. J Korean Neuropsychiatr Assoc. 1999;38(3):501–513. [Google Scholar]
- 39.Guy W. Handbook of psychiatric measures. American Psychiatric Association; 2000. Clinical global impressions (CGI) scale, modified; p. 820. [Google Scholar]
- 40.Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y.H., Song J.Y. A Study of the Reliability and the Validity of the BDI, SDS, and MMPI-D Scales. Korean J Clin Psychol. 1991;10(1):98–113. [Google Scholar]
- 42.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 43.Kwon J.H., Kim J.W., Park D.G., Lee M.S., Min S.G., Kwon J.H.I. Development and validation of the Hwa-Byung Scale. Korean J Clin Psychol. 2008;27(1):237–252. [Google Scholar]
- 44.Lee G., Park B., Moon K., You J., Kang H. A Study on the Development of the Core Emotional Assessment Questionnaire(CEAQ) Based on the Seven Emotions. Korean Soc Orient Neuropsychiatry. 2015;26(2):143–160. [Google Scholar]
- 45.Kim J.H., Sue J.H., Lee G.E. Development of Korean Medical Psychotherapy and Preliminary Clinical Trial for Post Traumatic Stress Disorder. J Orient Neuropsychiatry. 2015;26(1):49–61. [Google Scholar]
- 46.Yu S.Y., Kang H.W. The Study on Quantitative and Qualitative Analysis of Mentalizing the Rooms of Mind. Korean Soc Orient Neuropsychiatry. 2017;28(3):275–286. [Google Scholar]
- 47.Ware J.E., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 48.Kim M.-H., Cho Y.-S., Uhm W.-S., Kim S., Bae S.-C. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2005;14(5):1401–1406. doi: 10.1007/s11136-004-5681-z. [DOI] [PubMed] [Google Scholar]
- 49.Kim S., Won C.W., Kim B.S. EuroQol Visual Analogue Scale (EQ-VAS) as a Predicting Tool for Frailty in Older Korean Adults: The Korean Frailty an Aging Cohort Study (KFACS) J Nutr Health Aging. 2018;22(10):1275–1280. doi: 10.1007/s12603-018-1077-6. [DOI] [PubMed] [Google Scholar]
- 50.Steingrimsson S., Bilonic G., Ekelund A.-C. Electroencephalography-based neurofeedback as treatment for post-traumatic stress disorder: A systematic review and meta-analysis. Eur Psychiatry. 2020;63(1) doi: 10.1192/j.eurpsy.2019.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Kolk B.A., Hodgdon H., Gapen M. A randomized controlled study of neurofeedback for chronic PTSD. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0166752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cramer H., Anheyer D., Saha F.J., Dobos G. Yoga for posttraumatic stress disorder - a systematic review and meta-analysis. BMC Psychiatry. 2018;18(1):72. doi: 10.1186/s12888-018-1650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan T.P. John Wiley & Sons; 2013. Sample size determination and power. [Google Scholar]
- 54.Ipser J.C., Stein D.J. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) Int J Neuropsychopharmacol. 2012;15(6):825–840. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- 55.Kim M. The Cognitive Behavioral Therapy for Posttrumatic Stress Disorder. J Korean Assoc Psychother. 2009;1(1):25–61. [Google Scholar]
- 56.Seo H., Chae J. Recent cognitive behavioral therapy for posttraumatic stress disorder. Cogn Behav Ther Korea. 2006;6(2):117–129. [Google Scholar]
- 57.Yanos P.T., Vayshenker B., Pleskach P., Mueser K.T. Insight among people with severe mental illness, co-occurring PTSD and elevated psychotic symptoms: Correlates and relationship to treatment participation. Compr Psychiatry. 2016;68:172–177. doi: 10.1016/j.comppsych.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lanius R.A., Bluhm R., Lanius U., Pain C. A review of neuroimaging studies in PTSD: Heterogeneity of response to symptom provocation. J Psychiatr Res. 2006;40(8):709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Liberzon I., Abelson J.L., Flagel S.B., Raz J., Young E.A. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 1999;21(1):40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 60.Youn T., Kwon J.S. Clinical applications of quantitative EEG. Sleep Med Psychophysiol. 1995;2(1):31–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.