Abstract
Worldwide, a child dies every two minutes due to malaria with Africa bearing about 90% of all malaria deaths particularly among children. This study aimed to describe malaria prevalence and its associated factors among children aged 6 months to 9 years in Guinea. We conducted a cross-sectional household survey between 02 and 29 August 2014 in children aged 6 months to 9 years in the four natural regions of the country. A five-level cluster sampling using the national database from the national institute of statistics was used to select study participants. A total of 1984 children aged 6 months to 9 years were enrolled. The mean age was 50 months (SD, 27). The rapid diagnostic test showed a high malaria prevalence (44%) countrywide along with regional variation ranging from 38% to 61%. A multivariate analysis showed that living in Forest Guinea (AOR: 2.48; 95% CI: 1.78–3.46), in rural areas (AOR: 1.91; 95% IC: 1.45–2.5) and having a splenomegaly (AOR: 2.66; 95% CI: 1.75–4.04) were highly associated with malaria. This study shows that malaria is still prevalent in Guinea among children aged 6 months to 9 years of age.
Keywords: Malaria, Prevalence, Children, Infection, Guinea
1. Introduction
A child dies every two minutes from malaria around the world (WHO | World Health Organization, 2016a). In 2015, 90% of all malaria death occurred in sub-Saharan Africa, mostly in young children (WHO | World Health Organization, 2016a). Despite all the progresses made during the past ten years in reducing malaria incidence, several African countries still have high malaria mortality and endemicity (WHO | World Health Organization, 2016a; WHO | World Health Organization, 2016b). While in Nigeria malaria prevalence was estimated to be 45% in 2015 among children under five (National Malaria Control Program, 2016), in Chad, malaria accounted for 74 death per 100,000 inhabitants in 2014 (WHO | World Health Organization, 2020). The spatial distribution of asymptomatic malaria infection among children under 5 years old in 24 health districts in Burkina Faso reported a prevalence of asymptomatic malaria of 38%, with a significant variation (ranging from 11% to 79%) between districts. The Burkina Faso study showed that children aged 48 to 59 months, resident of households located at more than 5 km from a health facility, living in localities with inadequate number of nurses, infected in high transmission period were at risk of developing asymptomatic malaria (Ouédraogo et al., 2018). Similar findings in Equatorial Guinea reported a high malaria prevalence of malaria (48%) among children aged 2 months to 15 years old. In addition, factors including inland rural areas, living near a river, a forest and or at low altitude were significantly associated with malaria (Gómez-Barroso et al., 2017). An analysis of seasonal pattern and age distribution of malaria incidence in a Malian district reported a high malaria incidence between August and December along with a two- to nine-fold increase of clinical malaria among children aged 5 to 9 years old compared to those under five years old (Touré et al., 2016).
Guinea is a malaria endemic country with a global prevalence of 44%, and regional malaria prevalence ranging from 39% to 61% (Institut National de la Statistique IM du P et de la CI, 2006; Institut National de la Statistique IM du P et de la CI, 2013). Malaria is the first cause of all outpatient visits (34%) and the first cause of death (28%) among Guinean children (Institut National de la Statistique IM du P et de la CI, 2006; Institut National de la Statistique IM du P et de la CI, 2013). The prevalence and risk factors of malaria infection is poorly documented in Guinea. A single study conducted simultaneously in Guinea, Benin and Gambia reported that malaria was significantly associated with fever or recent history of fever and anemia (Ceesay et al., 2015). In addition of being conducted in 2011, the study was limited to only one district, and covered a four months period.
Given the high burden of malaria in the country, and the dwarf of information on malaria incidence and risk factors, it's crucial to generate baseline data to guide future malaria research and control studies. The aim of this study is to describe malaria prevalence in Guinea and to assess the risk factors associated with malaria infection and clinical episodes in children aged 6 months to 9 years old.
2. Materials and methods
2.1. Study design and population
We conducted a national malaria cross-sectional household survey in August 2014 to assess malaria prevalence and associated risk factors. August corresponds to a high malaria transmission season in Guinea. The study which included children aged 6 months to 9 years old covered all the four natural regions of the country (Lower Guinea, Middle Guinea, Upper Guinea and Forest Guinea). These four regions differ in human, geographical and climate characteristics (Institut National de la Statistique IM du P et de la CI, 2013).
2.2. Sampling
A five-level cluster sampling was used to select study participants. At the first level, all four natural regions were selected to ensure representability. The second level included the random selection of 32 out of 38 Health districts, followed at the third level by the selection of 69 sub-districts including 51 from rural area (74%). At the fourth level, equal number of villages (clusters) were selected in each sub-district and from each of these villages, households were selected. Within these households, statistical units represented by children aged between 6 months to 9 years were selected for clinical and laboratory data collection. The national database used for the sampling was provided by the national institute of statistics.
The sample size was calculated using the formula n = Z2 ∗ (P ∗ Q)/i2, based on the national prevalence of malaria estimated at 34% in 2007 (Programme National de Lutte contre le Paludisme, 2014), a confidence level of 95% and a two-sided margin of error of 5%. A minimum sample size by region of 359 children aged 6 months to 9 years was needed and rounded to 400 to account for non-response rate. Expecting an average of 2 children aged 6 months to 9 years per household, a total of 260 households were required to achieve the sample.
2.3. Data collection
Data were collected in households, using structured and pre-tested questionnaires. Information on sociodemographic characteristics was collected from children's caregivers and included the residence, child's age, caregiver's relationship with the child, caregiver's education level, profession, and marital status. The profession and educational level of caregiver's spouse was also recorded. Clinical and parasitological variables were collected through children's physical and paramedical examination and included illness symptoms and malaria parasite assessment.
The data collection team included trained sociologists, doctors and biologists. Mothers or guardian were interviewed in local languages. Informed consent was obtained from each mother or guardian prior to data collection and the study protocol was approved by the National Ethics Committee for Health Research of Guinea (No. 08/CNERS/13).
2.4. Clinical procedures
Children's clinical examination consisted of measuring their weight, height, and axillar temperature followed by body palpation, and a full physical examination. Splenomegaly was systematically investigated in all participants and was classified according to the Hackett grading system (Laman et al., 2015).
Capillary blood was sampled by fingerpick from each participant. The resulting blood drop was used for malaria parasite diagnosis. The diagnostic methods used included rapid diagnostic tests (RDTs) immediately followed by thick and thin film stained with Giemsa at 10% according to the Standard Operating Procedure (SOP) (World Health Organization, 2015).
We performed RDT besides thick smear test because RDT is the commonest malaria test available and used in Guinea, especially in rural areas where care settings lack electricity and equipped laboratories. We used the SD Bioline Malaria Antigen P.f Rapid diagnostic test (RDT) for Plasmodium falciparum (Histidine Rich Protein 2).
Thick smears were double-blind performed by two certified Malaria microscopist. The two separate readings were compared and if the difference was more than 30%, a third reader was involved. The mean value between the two microscopist was used. These smears were used for the determination of parasite asexual and gametocyte forms density while thin smears were used to identify parasite species. Parasite density was determined using the following formula and a binocular Olympus CX 21 electric microscope:
Parasitemia per microliter = (number of asexual parasites / 300 leukocytes counted) × 7500, with 7500 being the average number of white blood cells in one millimetre of blood of people living in Sub-Saharan Africa (Jeremiah and Adias, 2011). A smear was classified negative when the examination of the whole thick smear revealed no asexual form of Plasmodium. The presence and density of P. falciparum gametocytes was scored independently from asexual forms.
All participants with a rapid diagnostic test (RDT) were treated with artemether-lumefantrine.
2.5. Data management and analysis
Data collected on questionnaires were entered in EpiData software (EpiData Association, Odense, Denmark) and analysed using Stata 13 software (Stata Corporation, College Station, TX, USA). Descriptive variables including demographic, clinical and outcome variables were presented as proportion or mean with standard deviations (SD). The epidemiological facies of malaria across the four natural regions was described based on the following ranges of malaria prevalence: ≤10% (hypoendemic area), 11%–50% (mesoendemic area), 51%–75% (hyperendemic area), and 76%–100% (holoendemic area) (Hay et al., 2008).
Pearson Chi Square, Fischer and Student's tests were used to compare the prevalence of malaria infection across study variables in bivariate analysis. All variables were included a priori in logistic regression models and the unadjusted and adjusted odds ratios were derived. The significance level was set at 5% with a 95% confidence interval and the Hosmer and Lemeshow test was used to assess the goodness of fit of the final model.
3. Results
3.1. Descriptive analysis
3.1.1. Sociodemographic characteristics
A total of 1785 children aged 6 months to 9 years old were included in the study (Table 1). These children were from Lower Guinea (469, 26.3%), Middle Guinea (455, 25.5%), Upper Guinea (377, 21.1%), and Forest Guinea (484, 27.1%). Children had a mean age of 50 months (SD = 27 months). The majority of these children were from rural areas (69%) and were being taken care by their mothers (78.3%). Most of the caregivers were housewives or farmers (66%) with no formal education (53%). Most of the caregivers were married or in union (91.9%). 34% of their husband/partner were farmers and 56% had no formal education.
Table 1.
Sociodemographic characteristics of the surveyed sample (N = 1984) in Guinea in August 2014 (N = 1785).
| Variables | Number | % |
|---|---|---|
| Natural region visited | ||
| Lower Guinea | 469 | 26.3 |
| Middle Guinea | 455 | 25.5 |
| Upper Guinea | 377 | 21.1 |
| Forest Guinea | 484 | 27.1 |
| Mean age (SD) | 50 (27) | |
| Age group (N = 1785) | ||
| Infant | 460 | 25.8 |
| Young child | 595 | 33.3 |
| Child | 730 | 40.9 |
| Residence | ||
| Urban | 588 | 31 |
| Rural | 1306 | 69 |
| Caregiver's relationship with the child (N = 1782) | ||
| Mother | 1395 | 78.3 |
| Other persona | 387 | 21.7 |
| Caregiver's profession (N = 1782) | ||
| Housewife/Farmer | 1177 | 66.0 |
| Other professionb | 605 | 34.0 |
| Caregiver's educational level (N = 1781) | ||
| None | 908 | 53.0 |
| Primary | 240 | 13.5 |
| Secondary or more | 633 | 35.5 |
| Caregiver's marital status (N = 1781) | ||
| Unmarried | 145 | 8.1 |
| Married/In union | 163 | 91.9 |
| Profession of the caregiver's spouse (N = 1704) | ||
| Farmer | 580 | 34.0 |
| Workman | 500 | 29.3 |
| Employee | 250 | 14.7 |
| Trader | 235 | 13.8 |
| Other professionc | 139 | 8.6 |
| Education level of the caregiver's spouse (N = 1699) | ||
| None | 954 | 56.1 |
| Primary | 190 | 11.2 |
| Secondary or more | 555 | 32.7 |
SD: Standard deviation Missingness across variables ≤ 10%.
Infant: ≤24 months; Young child: 25–59 months; Child: ≥60 months.
Father, aunt, uncle, sister, grandparents.
Employee, student, workman/woman, trader.
Student, day labourer, unemployed.
3.1.2. Malaria prevalence and epidemiological facies of malaria in Guinea
Among children tested for malaria countrywide, 35% were positive by thick smear (Fig. 1). The highest prevalence of malaria infection was observed in Forest Guinea (48%), followed by Lower Guinea (32%), Middle Guinea (28%), and Upper Guinea (31%). Plasmodium falciparum was the most common malaria parasite found in children countrywide (94.2%), followed by Plasmodium malariae (2.1%) and Plasmodium vivax (1%). A combination of parasites species was observed in 2.7% of children with positive microscopy (Table 2).
Fig. 1.
Prevalence of malaria in Guinea stratified by natural region (N = 1518), August 2014.
Table 2.
Malaria parasite species and splenic grades in Guinea stratified by natural region (N = 1784), August 2014.
| Variables | Lower Guinea (N = 143) |
Middle Guinea (N = 131) |
Upper Guinea (N = 107) |
Forest Guinea (N = 222) |
Guinea (N = 603) |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Parasite species | |||||
| Plasmodium falciparum | 135 (94.4) | 123 (93,9) | 101 (94.4) | 209 (94.1) | 568 (94.2) |
| Plasmodium malariae | 4 (2.8) | 6 (4.6) | 0 (0) | 3 (1.4) | 13 (2.1) |
| Plasmodium vivax | 1 (0.7) | 1 (0.8) | 3 (2.8) | 1 (0.5) | 6 (1.0) |
| Multiple | 3 (2.1) | 1 (0.8) | 3 (2.8) | 9 (4.1) | 16 (2.7) |
| Grade of splenomegaly (N = 1761) | Lower Guinea (N = 449) | Middle Guinea (N = 399) | Upper Guinea (N = 357) | Forest Guinea (N = 430) | Guinea (N = 1635) |
| No splenomegaly | 449 (98.5) | 399 (88.9) | 357 (95.5) | 430 (89.2) | 1635 (92.8) |
| Grade 1 | 5 (1.1) | 52 (10.2) | 0 (0) | 48 (10.0) | 103 (5.9) |
| Grade 2 | 2 (0.4) | 0 (0) | 13 (3.5) | 4 (0.8) | 19 (1.1) |
| Grade 3 | 0 (0) | 0 (0) | 4 (1.1) | 0 (0) | 4 (0.3) |
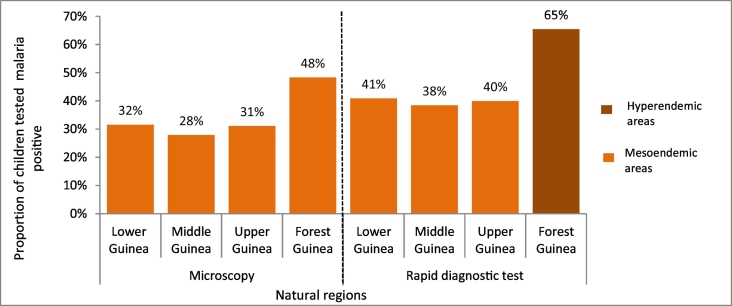
Rapid diagnostic tests showed a higher malaria prevalence of malaria, with 46% countrywide, 65% in Forest Guinea, 41% in Lower Guinea, 40% in Upper Guinea, and 38% in Middle Guinea (Fig. 2). Results from microscopy in children aged 2 to 9 years old showed that malaria was mesoendemic (prevalence between 11% and 50%) across the four natural regions of the country.
Fig. 2.
The average parasite density among children tested malaria positive through thick smear was 6022/μL (SD = 18,923/μL).
Splenomegaly was found in 5.9% of children countrywide, most (5.9%) had grade 1 splenomegaly. The number of children with splenomegaly was higher in Forest Guinea (10.8% overall with 10% with grade 1 splenomegaly), and in Middle Guinea (10.1% overall, all with grade 1 splenomegaly).
3.2. Bivariate and multivariate analysis
Using results from microscopy readings, we found in a bivariate analysis that the natural region, the residence, child's gender, age and weight, caregiver's profession and educational level, the grade of splenomegaly, and the haemoglobin rate were statistically associated with malaria infection in children (Table 3). However, in multivariate analyses after controlling for all exposure variables, only the natural region, residence, age, caregiver's profession, grade of splenomegaly, and haemoglobin mean rate were independently associated with malaria. The odd of having a malaria infection was higher in children living in Forest Guinea compared to those living in Upper Guinea (Adjusted Odd Ratio, AOR: 2.40; 95% CI: 1.7–3.40). In addition, malaria prevalence was higher in children residing in rural areas compared to those residing in urban areas (AOR: 1.82; 95% IC: 1.38–2.42). The odd of having a malaria infection was negatively associated with their haemoglobin rate (AOR: 0.74; 95% CI: 0.68–0.80). Children with splenomegaly had higher odds of having a malaria infection compared to those without splenomegaly (AOR: 2.58; 95% CI: 1.68–3.95).
Table 3.
Factors associated with malaria diagnosed in children aged 6 months to 9 years in Guinea, August 2014.
| Variables | Malaria positive to microscopy (yes) |
Malaria positive to Rapid Diagnostic Test (yes) |
||||
|---|---|---|---|---|---|---|
| N (%) | Crude OR(95% CI) | Adjusted OR (95% CI) | N (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Natural region | ||||||
| Lower Guinea | 144 (30.7) | 1.08 (0.81–1.47) | 1.002 (0.69–1.45) | 184 (39.2) | 1.10 (0.84–1.46) | 1.11 (0.77–1.6) |
| Middle Guinea | 131 (28.8) | 0.99 (0.74–1.34) | 1.34 (0.83–2.16) | 173 (38) | 1 | 1.47 (0.92–2.33) |
| Upper Guinea | 109 (28.9) | 1 | 1 | 139 (36.9) | 1.05 (0.79–1.39) | 1 |
| Forest Guinea | 222 (45.9) | 2.08 (1.57–2.77) | 2.40 (1.7–3.40) | 296 (61.2) | 2.70 (2.04–3.56) | 3.57 (2.52–5.06) |
| Mean age (SD), years | 52 (27) | 1.01 (1–1.01) | 1.21 (1.01–1.02) | 52 (27) | 1.01 (1.01–1.01) | 1.02 (1.02–1.03) |
| Gender | ||||||
| Female | 281 (32.0) | 1 | 1 | 379 (43.22) | 1 | 1 |
| Male | 325 (36.2) | 1.2 (0.99–1.46) | 1.11 (0.89–1.39) | 411 (45.7) | 1.11 (0.92–1.33) | 1 (0.8–1.25) |
| Mean weight (SD) | 16 (5.6) | 1.01 (0.99–1.03) | 0.99 (0.96–1.02) | 16.45 (5.93) | 1.02 (1.004–1.04) | 0.99 (0.95–1.02) |
| Residence | ||||||
| Urban | 114 (22.1) | 1 | 1 | 158 (30.68) | 1 | 1 |
| Rural | 458 (38.6) | 2.2 (1.74–2.81) | 1.82 (1.38–2.42) | 595 (50.13) | 2.23 (1.82–2.83) | 2.07 (1.58–2.73) |
| Caregiver's relationship with the child | ||||||
| Mother | 466 (33.4) | 1 | 1 | 599 (42.94) | 1 | 1 |
| Other person | 140 (36.2) | 1.35 (0.89–1.43) | 0.81 (0.6–1.01) | 192 (49.61) | 1.31 (1.04–1.64) | 0.74 (0.54–1.01) |
| Caregiver's profession | ||||||
| Housewife/ farmer | 436 (37) | 1 | 1 | 554 (47.07) | 1 | 1 |
| Other profession | 170 (28.1) | 0.66 (0.54–0.82) | 0.63 (0.46–0.85) | 237 (39.2) | 0.72 (0.59–0.88) | 0.66 (0.49–0.89) |
| Caregiver's educational level | ||||||
| None | 339 (37.3) | 1 | 1 | 426 (46.92) | 1 | 1 |
| Primary or more | 267 (30.6) | 0.8 (0.66–0.96) | 0.82 (0.59–1.12) | 365 (41.81) | 0.81 (0.67–0.98) | 1.04 (0.74–1.44) |
| Caregiver's marital status | ||||||
| Unmarried | 52 (35.8) | 1 | 1 | 69 (47.6) | 1 | 1 |
| Married/In union | 554 (33.9) | 0.92 (0.64–1.31) | 0.84 (0.43–1.67) | 722 (44.1) | 0.87 (0.62–1.22) | 0.75 (0.4–1.43) |
| Education level of caregiver's spouse | ||||||
| None | 333 (34.9) | – | – | 440 (46.12) | – | – |
| Primary or more | 238 (32) | – | – | 305 (40.9) | – | – |
| Splenomegaly | ||||||
| Yes | 78 (61.9) | 3.48 (2.40–5.1) | 2.58 (1.68–3.95) | 99 (78.6) | 5.11 (3.3–7.91) | 3.68 (2.21–6.14) |
| No | 520 (31.8) | 1 | 1 | 683 (41.8) | 1 | 1 |
| Haemoglobin mean rate (SD) | 10.6 (1.68) | 0.78 (0.73–0.83) | 0.74 (0.68–0.80) | 10.63 (1.70) | 0.74 (0.70–0.79) | 0.66 (0.61–0.72) |
Results from rapid diagnostic test showed the same trend as those observed with microscopy (Table 3).
4. Discussion
This study was conducted to generate the baseline data needed for malaria control in Guinea. The findings suggest that Guinea had a high malaria prevalence in 2014, with all natural regions being meso-endemic per microscopy and the forest region being hyper-endemic per RDT. Plasmodium falciparum was the leading cause of malaria across the four natural regions. Malaria burden was associated with living in the forest, in rural areas, being old in age, and being taken care by a housewife or a farmer. Our findings emphasize the need for more targeted effort to eliminate malaria burden in Guinean children, as prioritized in the 2030 Sustainable Development Goals (SDG) (United Nations. A/RES/70/1, 2015). Despite the progress achieved over the preceding ten years (2003−2013) in malaria control in the country (OMS | Organisation mondiale de la santé, 2016), the findings were similar to what was reported in the 2012 Demographic and Health Survey (DHS) (Institut National de la Statistique IM du P et de la CI, 2013). One reason is that the study was conducted during malaria high transmission season (August).
Malaria burden was significantly higher in Forest Guinea. The previous DHS also reported Forest Guinea as the most malaria prevalent natural region (Institut National de la Statistique IM du P et de la CI, 2006; Institut National de la Statistique IM du P et de la CI, 2013). This region has the highest and longest rainfall (over 6 to 10 month per year) in the country (Institut National de la Statistique IM du P et de la CI, 2013). Our data indicates that the high prevalence of malaria in Upper and Middle Guinea regions could be reduced by the implementation of seasonal malaria chemotherapy (SMC) as recommended by the World Health Organization (WHO). Indeed, most of these regions are SMC eligible and the strategy has been proven effective in similar contexts (WHO | World Health Organization, 2012; World Health Organization, 2017).
Our study suggests that children living in rural areas or being taken care by a housewife or a farmer were the most affected by malaria. One reason may be that these two professions have less or no formal education and have limited means to bring their kids to health clinic. Despite the availability of effective antimalarial drugs (Artemisinin-based combination therapy, ACT) at health facilities free of charge for children, only 1% of children with fever surprisingly received ACT in 2012 (Institut National de la Statistique IM du P et de la CI, 2013). As more than 60% of Guinean live in rural areas, our finding call for strengthened community interventions against malaria in children. Marsh (Marsh, 1998) recommends to get antimalarial drugs out to rural communities with minimum access to health facilities to address the malaria disaster in Africa. Access to and utilization of malaria care might be challenging in rural areas or among poor people because of barriers that might include the lack of transportation means, user fees for comorbidities, unclear information about which health services are free and which ones are not, and misinterpretation of fever illness (Kokwaro, 2009). A constant involvement of community health workers in increasing caregivers‘awareness about health services and care for malaria would help remove some of these barriers and achieve the universal health coverage (Zin et al., 2013). To this end, the free malaria care policy for children should be extended to cover complicated malaria to reduce out of pocket expenditure for caregivers.
Older children were found more prone to malaria infection. Children, as they grow, seem more exposed to mosquito bites and therefore, to malaria. By progressively losing their maternal antibodies they become more vulnerable to the Plasmodium (Simon et al., 2015). In addition, free Malaria treatment is limited to children under five years. Therefore, beyond this age, if parents do not afford to pay for treatment, one might see high prevalence in older ages. Prevention at community level through clean environment and systematic use of mosquito nets should be prioritized. In 2012, despite the household distribution of long lasting insecticidal net (LLINs), 71% of children under 5 years did not sleep inside mosquito nets (Institut National de la Statistique IM du P et de la CI, 2013). Pulford et al. reported discomfort due to heat, perceived low mosquito density and other social factors as reasons for not using available mosquito net in sub-Saharan Africa (Pulford et al., 2011).
As reported by the 2012 Guinean DHS, there was no variation in Plasmodium prevalence across the four natural regions in the country (Institut National de la Statistique IM du P et de la CI, 2013). However, we observed a higher positivity using the RDTs compared to the thick smear, probably due to antigens that can still remain in blood after thick smear negativity. Splenomegaly was associated with higher odds of positivity for Malaria while Haemoglobin rate was negatively associated with malaria positivity, the reason being that malaria infection destroys the red blood cell in infected children, resulting in reduced haemoglobin (Hay et al., 2008; Kokwaro, 2009).
We found an important difference between malaria prevalence reported by thick smear (35%) and RDT (46%). Similar findings were reported by the malaria national survey in 2016, showing a prevalence of 15% from thick smear compared with 21.9% from RDT (Institut National de la Statistique, 2017). This shows a possible difference in the quality of the two malaria tests available in the country. It implies either over estimation of malaria cases in cases RDT quality were poorer, or underestimation of malaria cases in case thick smear quality were poorer. However, it is important to question malaria testing conditions (environment, test performers, conservations, etc.) in the contexts of these surveys that occur in the field.
The cross-sectional design of this study constitutes its main limitation, since it does not allow a comprehensive understanding of factors determining malaria burden in the country. However, this study has the potential to advocate for targeted interventions and guide for priority researches to keep Guinea on the path toward malaria elimination.
5. Conclusion
This study shows all Guinea four regions have a high prevalence of Plasmodium falciparum malaria in children aged 6 months to 9 years old. Although data for a cross-sectional study were used, we found that malaria burden was associated with older children who were being taken care by housewife or a farmer. Moreover, living in Forest Guinea and rural areas were associated to malaria infection. Achieving the sustainable development goals by 2030 will require a targeted intervention approach to better prevent and manage malaria in rural and poor areas along with a qualitative research to inform such efforts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the National Malaria Control Program for their collaboration. The authors wish to thank the data collectors, and families who participated in this study. This work was funded by the European and Developing Countries Trials Partnership (EDTCP-1), (TA_11_40200). This work is dedicated in memory of the late Professor Ogobara K. Doumbo whose guidance and support were instrumental in the grant application and the study completion.
References
- Ceesay S.J., Koivogui L., Nahum A., Taal M.A., Okebe J., Affara M. Malaria prevalence among young infants in different transmission settings, Africa. Emerg. Infect. Dis. 2015;21(7):1114–1121. doi: 10.3201/eid2107.142036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Barroso D., García-Carrasco E., Herrador Z., Ncogo P., Romay-Barja M., Ondo Mangue M.E. Spatial clustering and risk factors of malaria infections in Bata district, Equatorial Guinea. Malar J. 2017;16(1):146. doi: 10.1186/s12936-017-1794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay S., Smith D., Snow R. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect. Dis. 2008;8(6):369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut National de la Statistique . 2017. Enquête de prevalence parasitaire du paludisme et de l’anemie- Guinée 2016. [Malaria Parasite and Anemia Prevalence Survey - Guinea 2016] [Google Scholar]
- Institut National de la Statistique IM du P et de la CI . 2006. Enquête Démographique et de Santé (EDS) III Guinée 2005; p. 457. [Google Scholar]
- Institut National de la Statistique IM du P et de la CI . 2013. Guinea Demographic and Health and Multiple Indicators Survey 2012; pp. 1–530. [Google Scholar]
- Jeremiah Z., Adias T. Subclinical leukopenia in a cross section of Nigerian blood donors. J. Blood Med. 2011;79 doi: 10.2147/JBM.S16214. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokwaro G. Ongoing challenges in the management of malaria. Malar J. [Internet]. 2009;8(Suppl 1):S2. Available from: http://malariajournal.biomedcentral.com/articles/10.1186/1475-2875-8-S1-S2 [DOI] [PMC free article] [PubMed]
- Laman M., Aipit S., Bona C., Siba P.M., Robinson L.J., Manning L. Ultrasonographic assessment of splenic volume at presentation and after anti-malarial therapy in children with malarial anaemia. Malar J. 2015;14(1) doi: 10.1186/s12936-015-0741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K. Malaria disaster in Africa. Lancet. 1998;352(9132):924. doi: 10.1016/S0140-6736(05)61510-3. [DOI] [PubMed] [Google Scholar]
- National Malaria Control Program . 2016. Nigeria Malaria Indicator Survey 2015. [Google Scholar]
- OMS | Organisation mondiale de la santé, editor. Rapport sur le paludisme dans le monde. 2016. (résumé) [Google Scholar]
- Ouédraogo M., Samadoulougou S., Rouamba T., Hien H., Sawadogo J.E.M., Tinto H. Spatial distribution and determinants of asymptomatic malaria risk among children under 5 years in 24 districts in Burkina Faso. Malar. J. 2018;17(1):460. doi: 10.1186/s12936-018-2606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Programme National de Lutte contre le Paludisme . 2014. Plan stratégique national de lutte contre le paludisme 2013-2017. [Google Scholar]
- Pulford J., Hetzel M.W., Bryant M., Siba P.M., Mueller I. Reported reasons for not using a mosquito net when one is available: a review of the published literature. Malaria J. 2011;10 doi: 10.1186/1475-2875-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015;282(1821):20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touré M., Sanogo D., Dembele S., Diawara S.I., Oppfeldt K., Schiøler K.L. Seasonality and shift in age-specific malaria prevalence and incidence in Binko and Carrière villages close to the lake in Selingué, Mali. Malar J. 2016;15(1):0–18. doi: 10.1186/s12936-016-1251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. A/RES/70/1 . 2015. Transforming Our World: the 2030 Agenda for Sustainable Development Transforming Our World: the 2030 Agenda for Sustainable Development Preamble; pp. 1–35. 16301(October) [Google Scholar]
- WHO | World Health Organization, editor. WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub-region in Africa. Vol. 2012. 2012. p. 1. [Google Scholar]
- WHO | World Health Organization, editor. World Malaria Report 2016. 2016. [Google Scholar]
- WHO | World Health Organization, editor. Fact Sheet: World Malaria Day 2016. WHO; 2016. [Google Scholar]
- WHO | World Health Organization, editor. Malaria Death Rate By Country. 2017. https://www.who.int/malaria/publications/world-malaria-report-2017/wmr2017-regional-profiles.pdf?ua=1 [Google Scholar]
- World Health Organization Guidelines for the treatment of Malaria. World Heal Organ. 2015;3:1–313. [PubMed] [Google Scholar]
- World Health Organization . 2017. Malaria Prevention Works. World Malaria Day 2017. [Google Scholar]
- Zin T., Mudin K., Myint T., Naing D.S., Sein T., Shamsul B. Influencing factors for household water quality improvement in reducing diarrhoea in resource-limited areas. WHO South-East Asia J. Public Heal. 2013;2(1):6. doi: 10.4103/2224-3151.115828. [DOI] [PubMed] [Google Scholar]