Abstract
Taraxasterol (TAX), a pentacyclic triterpene, has been reported to exhibit potent antitumor activity. However, the effects and molecular mechanisms of TAX in gastric cancer (GC) remain undocumented. A network pharmacology approach was applied to identify the collective targets of TAX and GC. Nude mice were subcutaneously injected with MKN-28 cells to establish GC subcutaneous xenograft model, which were treated with TAX for 16 days. Tumor volume was then examined every other day. The pathological scoring was assessed by using hematoxylin and eosin (H&E) staining, and the expression levels of Ki-67 and the target genes of TAX were confirmed by immunohistochemistry analysis. Five collective targets of TAX and GC were identified, such as epidermal growth factor receptor (EGFR), matrix metalloproteinase 2 (MMP2), B-Raf proto-oncogene, serine/threonine kinase (BRAF), fibroblast growth factor receptor 2 (FGFR2), and AKT serine/threonine kinase 1 (AKT1). Further investigations showed that, TAX administration repressed xenograft tumor growth and decreased Ki-67 levels, followed by the downregulation of EGFR and AKT1 expression in xenograft tumor tissues as compared with the untreated group. Our findings demonstrated that TAX inhibited the growth of GC by inhibition of EGFR/AKT1 signaling and might provide a novel therapeutic strategy for treatment of GC.
Keywords: gastric cancer, growth, network pharmacology, taraxasterol
Introduction
Gastric cancer (GC) is the fourth most common malignancy and the second leading cause of cancer death worldwide.1 The patients with early-stage GC are asymptomatic, and they are usually diagnosed in an advanced stage. In spite of the popularization of gastroscopy and integrated therapy for GC, the 5-year survival rate of GC is still less than 40% due to tumor metastasis and recurrence.2 In addition, chemotherapy is used to improve the disease outcome, but their drug resistance and side effects limit their application in treatment of GC.3 Therefore, identification of a novel therapeutic strategy for GC is urgently needed.
Taraxasterol (TAX) is a pentacyclic triterpene isolated from Taraxacum officinale. Mounting evidence indicated that TAX possesses a variety of biological activities including anti-inflammatory and antitumor activity.4,5 TAX can suppress in vivo breast carcinogenesis and in vitro cell growth in colorectal cancer, cervical cancer, and melanoma.5,6 Nevertheless, the effects and underlying mechanism of TAX in GC remain unreported.
Network pharmacology is an emerging method incorporating systems biology, bioinformatics, and pharmacology.7 In this study, we applied a comprehensive network pharmacology-based approach to identify the common target genes of TAX and GC, and found that TAX inhibited the growth of GC by inhibition of epidermal growth factor receptor (EGFR)/AKT1 signaling and might provide a novel therapeutic strategy for treatment of GC.
Materials and methods
Materials
TAX was obtained from Reifensis Biotechnology (Chengdu, China). Male, 6-week-old nude mice were supplied by Shanghai Laboratory Animal Center (SLAC, Shanghai, China). The GC cell line (MKN-28) was obtained from our Digestive Disease Laboratory. All antibodies used in this study were purchased from HuaAn Biotechnology (Hangzhou, China).
Identification of candidate targets of TAX
The Canonical SMILES of TAX was obtained from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The targets of TAX were screened from Swiss Target Prediction Database (http://www.swisstargetprediction.ch/), Search Tool for Interactions of Chemicals (STITCH) Database (http://stitch.embl.de/), and PharmMapper Database (http://www.lilab-ecust.cn/pharmmapper/), by which about 190 target genes of TAX were identified.
Identification of candidate targets of GC
The targets of GC were acquired from Online Mendelian Inheritance in Man (OMIM) Database (http://omim.org/), Therapeutic Target Database (http://bidd.nus.edu.sg/group/cjttd/), and PharmGKB Database (https://www.pharmgkb.org/), by which about 198 targets of GC were collected for further analysis.
Protein-protein interaction network construction
The protein-protein interaction network was constructed by Cytoscape software (http://www.cytoscape.org/).
Xenograft tumor model in mice
Male, 6-week-old nude mice were purchased from the Shanghai Laboratory Animal Center (SLAC, Shanghai, China). Our experiments were approved by the Ethics Committee of our Hospital. MKN-28 cells (1 × 106) were resuspended in PBS and injected subcutaneously into the right axilla of nude mice. After a week, each mouse was treated with 6.0–7.0 mL of phosphate buffer saline (PBS) or 25 μg/mL TAX per day5 and the mice were randomly divided into two groups (each, n = 7): GC group and GC + TAX group. Tumor volumes were measured every other day, and the tumor volume was calculated based on the formula: V = 0.5 × L × W2, where L means the maximum length (mm) and W means the minimum width (mm).8 After the treatment for 16 days, the mice were sacrificed, and xenograft tumor tissues were collected for further experiments.
Hematoxylin and Erosion staining
Tumor tissues were separated and longitudinally incised, then fixed on a 4% paraformaldehyde solution for 48 h and embedded in paraffin. Histological examinations were performed by hematoxylin and eosin (H&E) staining after paraffin sections of these tumor tissues.
Immunohistochemistry
According to the previous description,4 immunohistochemistry (IHC) analysis was conducted. The tumor tissues were immune-stained for phospho-EGFR (AF3048), phospho-AKT1 (AF0832), anti-EGFR (ET1604-44), anti-BRAF (ET1608-36), anti-fibroblast growth factor receptor 2 (anti-FGFR2; EM50103), anti-AKT1 (ET1609-47) and anti-matrix metalloproteinase 2 (anti-MMP2; ET1606-4), respectively.
Statistical analysis
SPSS18.0 software was used to analyze the experiment data, and graph presentation was achieved by using the GraphPad Prism 5 Software (GraphPad, San Diego, CA, USA). All quantitative data were expressed as Mean ± standard deviation (SD). The Student’s t-test and analysis of variance were used to compare quantitative variables. P < 0.05 is considered as a statistical significance.
Results
The collective targets of TAX and GC were identified
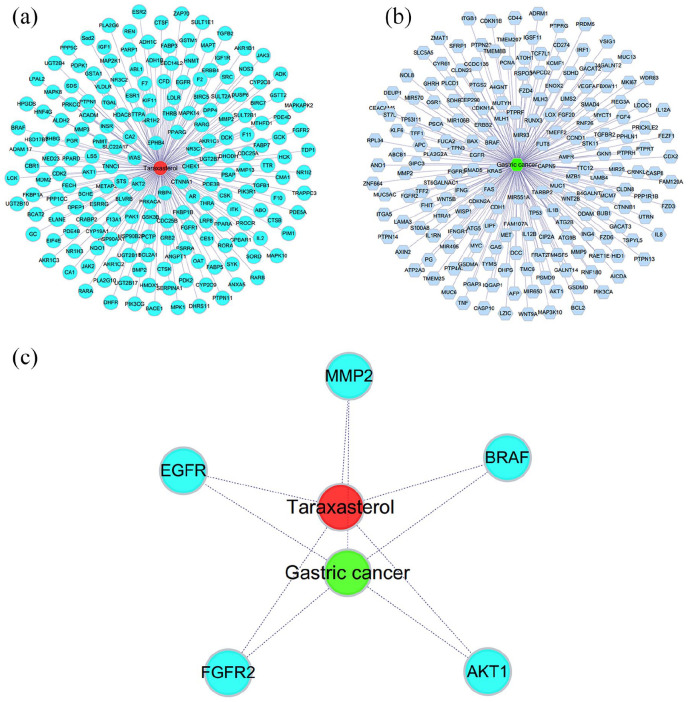
Based on STITCH, PharmMapper databases, and Swiss Target Prediction, 190 target genes of TAX were obtained. They were further used for constructing the protein-protein regulation network including 191 link nodes (Figure 1(a)). OMIM, Therapeutic Targets, and PharmGKB databases were then used to identify 198 targets of GC, which were performed for constructing the protein-protein regulation network consisting of about 199 link nodes (Figure 1(b)). Thus, five collective targets of TAX and GC were acquired (Figure 1(c)).
Figure 1.
(a) About 190 candidate targets of TAX were identified and used to establish the protein-protein regulation network. The green nodes represent the protein targets of TAX, and the red node stands for the TAX. (b) About 198 targets of GC were identified and used to establish the protein-protein regulation network. The blue nodes represent the protein targets, and the green node stands for the GC. (c) About five collective targets of TAX and GC were identified and were used to establish the protein-protein regulation network. The blue nodes represent the protein targets, red node stands for TAX, and the green nod represents GC.
TAX inhibited the xenograft tumor growth
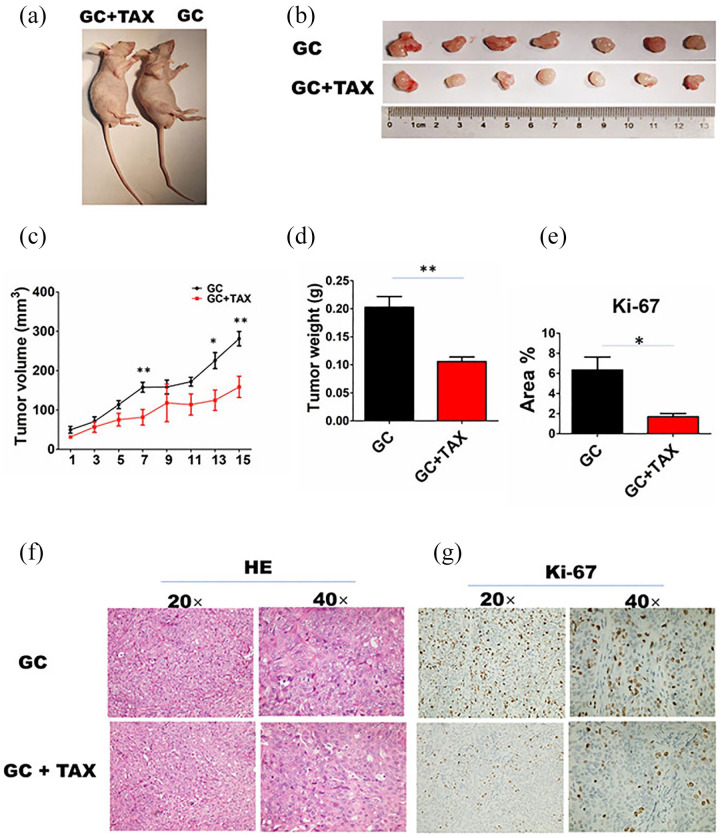
To determine the effects of TAX on GC growth, MKN-28 cells (1 × 106) were injected subcutaneously on the right axilla of nude mice (Figure 2(a)). As shown in Figure 2(b)–(d), TAX significantly inhibited the growth of xenograft tumors and lowered the tumor volumes and weight. H&E staining showed that the amount of proliferating tumor cells was decreased by TAX as compared with the GC group (Figure 2(f)). IHC analysis showed that Ki-67 levels were reduced by TAX as compared with the GC group (Figure 2(e) and (g)).
Figure 2.
TAX inhibited the growth of GC cells in vivo. (a) Representative schematic of the mice in different groups. (b) Macroscopic appearance of the tumor tissues. ((c) and (d)) Comparison of the mean tumor volume and weight between the two groups (n = 7). (f) The histopathological changes in tumor tissues were examined by H&E staining (20×, 40×). ((e) and (g)) The protein expression levels of Ki-67 were verified by IHC analysis in tumor tissues in the two groups. Data are the means ± SEM of two experiments.
*P < 0.05; **P < 0.01.
TAX downregulated the expression of EGFR and AKT1 in GC tumor tissues
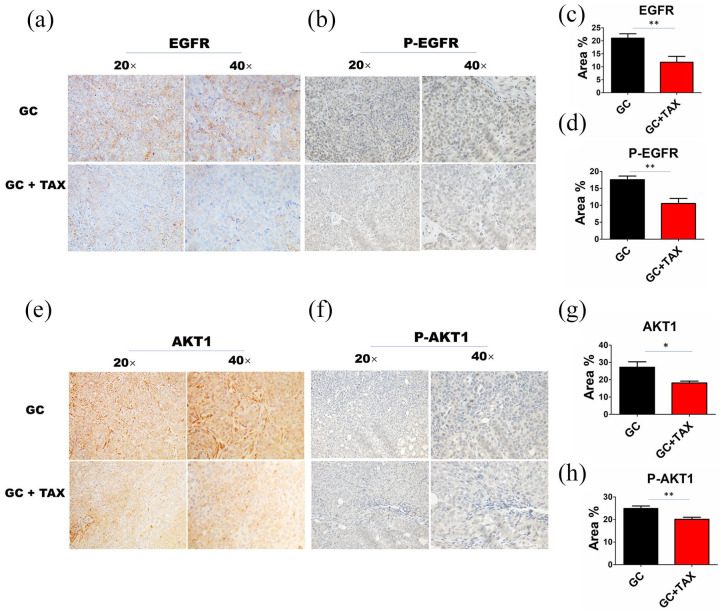
According to the five collective targets (EGFR, MMP2, BRAF, FGFR2, and AKT1) of TAX and GC, IHC analysis revealed that TAX significantly downregulated the levels of EGFR, AKT1, p-EGFR, and p-AKT1 (Figure 3(a)–(h)), but had no effects on MMP2, BRAF, and FGFR2 expression (Supplementary Figure S1) in tumor tissues as compared with the GC group.
Figure 3.
TAX inactivated the EGFR/AKT1 signaling in GC tumor tissues. ((a)–(d)) The protein expression levels of EGFR and p-EGFR were verified by IHC analysis in tumor tissues in two groups. ((e)–(h)) The protein expression levels of AKT1 and p-AKT1 were verified by IHC analysis in tumor tissues in two groups. Data are the means ± SEM of two experiments.
*P < 0.05; **P < 0.01.
Discussion
Previous study indicated that TAX displays anti-inflammatory and antioxidant effects in various kinds of models. Recently, TAX has been reported to act by enhancing the expression of Hint1. In this study, we demonstrated that TAX could inhibit the xenograft tumor growth of GC. These studies suggested that TAX might display an antitumor activity in GC.
Epidermal growth factor receptor (EGFR) consists of four homolog receptors, including EGFR (HER1, ErbB1), HER2 (Neu, ErbB2), HER3 (ErbB3), and HER4 (ErbB4).9 EGFR signaling is involved in promoting the carcinogenesis, including the cell survival, migration, angiogenesis, differentiation, and apoptosis escape. Overexpression of EGFR is associated with poor prognosis in GC.10 Herein, we found that TAX could downregulate the expression of EGFR in GC tumor tissues.
AKT is a downstream protein of PI3K pathway, and its activation serves as a biomarker for predicting the GC metastasis.11 The activation of AKT1 induces cell proliferation and inhibits the intrinsic apoptotic pathway. High expression of p-AKT has been described in GC,10 and is associated with poor prognosis in GC.12 Herein, we found that, TAX decreased AKT1 expression in GC tumor tissues. These studies indicated that, TAX might exert anti-GC effect by inactivating the EGFR/AKT1 signaling. However, GC is a complex disease, and the cell types targeted by TAX and its impact on the invasion of GC cells still need further investigation. In any case, the study indicates the need for further research.
Conclusion
In conclusion, our findings demonstrated that TAX inhibited the growth of GC by inhibition of the EGFR/AKT1 signaling and might provide a therapeutic strategy for GC.
Supplemental Material
Supplemental material, Supplementary_1 for Network pharmacology-based identification of the antitumor effects of taraxasterol in gastric cancer by Wei Chen, Jingwei Li, Chen Li, Hui-Ning Fan, Jing Zhang and Jin-Shui Zhu in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the ethics committee of Shanghai Sixth People’s Hospital (no. 2018-0080). The present study followed Shanghai Sixth People’s Hospital guidelines for humane animal study and complied with relevant legislation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the grants from the National Nature Science Foundation of China (nos 81573747 and 81873143).
ORCID iD: Jing Zhang  https://orcid.org/0000-0002-9412-3567
https://orcid.org/0000-0002-9412-3567
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ashktorab H, Kupfer SS, Brim H, et al. (2017). Racial disparity in gastrointestinal cancer risk. Gastroenterology 153(4): 910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allemani C, Weir HK, Carreira H, et al. (2015) Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385(9972): 977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muto M. (2014) Treatment of gastric cancer. World Journal of Gastroenterology 355(7): 729–735. [Google Scholar]
- 4. Chen W, Da W, Li C, et al. (2019) Network pharmacology-based identification of the protective mechanisms of taraxasterol in experimental colitis. International Immunopharmacology 71: 259–266. [DOI] [PubMed] [Google Scholar]
- 5. Bao T, Ke Y, Wang Y, et al. (2018) Taraxasterol suppresses the growth of human liver cancer by upregulating Hint1 expression. Journal of Molecular Medicine 96(7): 661–672. [DOI] [PubMed] [Google Scholar]
- 6. Dai J, Zhao C, Zhang Q, et al. (2002) Taraxastane-type triterpenoids from Saussurea petrovii. Phytochemistry 58(7): 1107–1111. [DOI] [PubMed] [Google Scholar]
- 7. Zhang GB, Li QY, Chen QL, et al. (2013). Network pharmacology: A new approach for Chinese herbal medicine research. Evidence-Based Complementary and Alternative Medicine 2013: 621423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu J, Ding Y, Chen CH, et al. (2016) A new oridonin analog suppresses triple-negative breast cancer cells and tumor growth via the induction of death receptor 5. Cancer Letters 380(2): 393–402. [DOI] [PubMed] [Google Scholar]
- 9. Casalini P, Iorio MV, Galmozzi E, et al. (2004) Role of HER receptors family in development and differentiation. Journal of Cellular Physiology 200(3): 343–350. [DOI] [PubMed] [Google Scholar]
- 10. Hisamatsu Y, Oki E, Otsu H, et al. (2016) Effect of EGFR and p-AKT overexpression on chromosomal instability in gastric cancer. Annals of Surgical Oncology 23(6): 1986–1992. [DOI] [PubMed] [Google Scholar]
- 11. Ng L, Poon RTP, Pang R. (2013) Biomarkers for predicting future metastasis of human gastrointestinal tumors. Cellular and Molecular Life Sciences 70(19): 3631–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murakami D, Tsujitani S, Osaki T, et al. (2007) Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer 10(1): 45–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_1 for Network pharmacology-based identification of the antitumor effects of taraxasterol in gastric cancer by Wei Chen, Jingwei Li, Chen Li, Hui-Ning Fan, Jing Zhang and Jin-Shui Zhu in International Journal of Immunopathology and Pharmacology