Abstract
Extended-spectrum β-lactamase (ESBL)-positive bloodstream infection (BSI) is on the rise worldwide. The purpose of this study is to evaluate the impact of inappropriate initial antibiotic therapy (IIAT) on in-hospital mortality of patients in the emergency department (ED) with Escherichia coli and Klebsiella pneumoniae BSIs. This retrospective single-center cohort study included all adult patients with E. coli and K. pneumoniae BSIs between January 2007 and December 2013, who had undergone a blood culture test and initiation of antibiotics within 6 h of ED registration time. Multiple logistic regression was used to adjust for bacterial species, IIAT, time to antibiotics, age, sex, quick Sepsis Related Organ Failure Assessment (qSOFA) score ⩾ 2, and comorbidities. A total of 3533 patients were enrolled (2967 alive and 566 deceased, in-hospital mortality rate 16%). The patients with K. pneumoniae ESBL-positive BSI had the highest mortality rate. Non-survivors had qSOFA scores ⩾ 2 (33.6% vs 9.5%, P < 0.001), more IIAT (15.0% vs 10.7%, P = 0.004), but shorter mean time to antibiotics (1.70 vs 1.84 h, P < 0.001). A qSOFA score ⩾ 2 is the most significant predictor for in-hospital mortality; however, IIAT and time to antibiotics were not significant predictors in multiple logistic regression analysis. In subgroup analysis divided by qSOFA scores, IIAT was still not a significant predictor. Severity of the disease (qSOFA score ⩾ 2) is the key factor influencing in-hospital mortality of patients with E. coli and K. pneumoniae BSIs. The time to antibiotics and IIAT were not significant predictors because they in turn were affected by disease severity.
Keywords: bloodstream infection, E. coli, ESBL, inappropriate initial antibiotic therapy, in-hospital mortality, K. pneumoniae
Introduction
The emergence of bacterial resistance is a serious problem of globalization. The extended-spectrum β-lactamase (ESBL) producing organisms are showing an increasing trend in hospital or community infections.1,2 The most common ESBL-producing bacteria are Escherichia coli and Klebsiella pneumoniae.3 Infections caused by ESBL-producing pathogens lead to a rise in mortality and an increase in length of hospital stay and costs.4
Drug resistance should be considered in the selection of empiric antibiotic therapy, especially in critically ill patients as inappropriate initial antibiotic therapy (IIAT) seems to be associated with poor prognosis.5,6 The timing of antibiotic administration appears to be associated with the prognosis; the Surviving Sepsis Campaign (SSC) guidelines suggest that intravenous antibiotics should be given within 1 h, for patients with sepsis and septic shock.7 However, there are some studies that show that the timing of antibiotic administration is not significantly related to the prognosis.8
The severity of the patient with infection affects the choice and timing of administration of antibiotics, as well as the prognosis. We evaluated the severity of the patient with infection with the quick Sepsis Related Organ Failure Assessment (qSOFA) score, and qSOFA scores ≧ 2 represented the more serious conditions and a higher risk of death.9 The aim of our study was to analyze the impact of the timing of appropriate antibiotic administration, qSOFA scores, and ESBL producers on in-hospital mortality.
Methods
Study design
A single-center retrospective observational cohort study was carried out from January 2007 to December 2013, at the Kaohsiung Chang Gung Memorial Hospital, which is a tertiary referral hospital in southern Taiwan with more than 2000 beds. More than 100,000 emergency patients visit the hospital each year. This study was approved by the Institutional Review Committee on Human Research of the Kaohsiung Chang Gung Memorial Hospital. The reference number is 103-0053B.
Study setting and population
Patients with E. coli and K. pneumoniae bloodstream infections (BSIs) were included if they were over 18 years old and had undergone a blood culture test and initiation of antibiotics within 6 h of emergency department (ED) registration time. Only the first episode of bacteremia in each patient was included, while recurrent infections with the same pathogen were excluded.
Data collection
We extracted data from electronic medical records that included age, sex, vital signs, appropriateness and time to antibiotics, comorbidities, infection sites, and clinical outcomes. Information about comorbidities and infection sites is gained by the International Classification of Diseases, Ninth Revision (ICD-9) coding.10 The primary outcome was in-hospital mortality.
Susceptibility testing
The production of ESBL was verified by the double disk diffusion method suggested by the Clinical and Laboratory Standards Institute (CLSI).11 We evaluated the minimum inhibitory concentration of the antibiotics with the Epsilometer test. The results were interpreted in light of the CLSI’s recommendations, except that breakpoints were suggested by the Food and Drug Administration in the United States.12,13
Antibiotics regimen
The initial antibiotic prescription and dosage were based on the diagnosis and the prevailing medical standards. In addition, the infectious disease physicians in our hospital would give advice based on the medical records, within 48 h. The definition of appropriate antibiotics is that at least one antibiotic is active in vitro and is based on the breakpoint for antimicrobial activity of an individual drug against E. coli and K. pneumoniae.14 The time from ED admission to the start of the initial antibiotics regimen was also recorded.
Definition of qSOFA score
The qSOFA was a scoring system recommended by the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) to quickly assess sepsis-related organ failure and screen patients suspected of sepsis.9 In an international prospective cohort study, Freund et al.15 found that qSOFA had a greater accuracy at predicting mortality among patients with suspected infections. Our previous study used the qSOFA score to predict mortality in patients with suspected infections, and the conclusion was that qSOFA scores were also good at predicting mortality.16 The qSOFA score was calculated after determining whether the systolic blood pressure is less than or equal to 100 mmHg, respiratory rate is more than or equal to 22 breaths/minute, and Glasgow Coma Scale (GCS) score is less than 15.9 The result is one point for each of the above conditions, and range of the score is from 0 to 3. We used the first values in the ED for each criterion to calculate the qSOFA score.
Statistical analysis
We used the Statistical Package for the Social Sciences 20.0 (SPSS, Chicago, IL, USA) to perform Statistical analyses. Continuous variables were represented as mean ± standard deviation and analyzed with the application of the t-test. Categorical variables were represented as numbers and percentages and analyzed with the application of the χ2 test or Fisher’s exact test. We used multivariable logistic regression analysis to adjust for bacterial species, IIAT, time to antibiotics, age, sex, qSOFA score ⩾ 2, and comorbidities. We also divided patients by qSOFA scores and ESBL production, to survey the effect of IIAT and time to antibiotics on the in-hospital mortality rate. P values < 0.05 were regarded as statistically significant. To evaluate whether our sample size was sufficient to solve the research problem, we used Power Analysis and Sample Size Software 14.0.7 to calculate the power and sample size. The result was that the sample sizes achieves 80% power at a 0.05 significance level for qSOFA, IIAT, and time to antibiotics = 137, 2787, and 3070 observations and odds ratios = 4.81, 1.64, and 0.87. Our sample size (3533) can achieve > 80% power.
Results
Our study included 3533 patients, of which 2967 patients were alive and 566 were deceased (in-hospital mortality rate was 16%). The difference in the pathogen species was significant between the non-survivors and survivors (P < 0.001) (Table 1). The patients with K. pneumoniae ESBL-positive BSI had the highest mortality rate (18/45 = 40%), and the patients with E. coli ESBL-negative BSI had the lowest mortality rate (309/2384 = 13%). Non-survivors had a significantly higher proportion of older people and men, and they also included more patients with liver cirrhosis, chronic renal insufficiency, malignancy, and respiratory tract infections, than survivors.
Table 1.
Univariate analysis of in-hospital mortality in patients with extended-spectrum beta-lactamase (ESBL)-positive or -negative Escherichia coli and Klebsiella pneumoniae bloodstream infection.
| All patients (N = 3533) | Survivors (n = 2967) | Non-survivors (n = 566) | P value | |
|---|---|---|---|---|
| Pathogen species | ||||
| E. coli ESBL-negative | 2384 (67.5) | 2075 (69.9) | 309 (54.6) | <0.001* |
| E. coli ESBL-positive | 229 (6.5) | 184 (6.2) | 45 (8.0) | |
| K. pneumoniae ESBL-negative | 875 (24.8) | 681 (23.0) | 194 (34.3) | |
| K. pneumoniae ESBL-positive | 45 (1.3) | 27 (0.9) | 18 (3.2) | |
| Age (years) | 66.9 ± 14.4 | 66.6 ± 14.5 | 68.2 ± 14.2 | 0.016* |
| Male | 1530 (43.3) | 1199 (40.4) | 331 (58.5) | <0.001* |
| IIAT | 401 (11.4) | 316 (10.7) | 85 (15.0) | 0.004* |
| Time to antibiotics (hours) | 1.82 ± 1.02 | 1.84 ± 1.02 | 1.70 ± 1.03 | 0.003* |
| qSOFA score | ||||
| qSOFA < 2 | 3061 (86.6) | 2685 (90.5) | 376 (66.4) | <0.001* |
| qSOFA ≧ 2 | 472 (13.4) | 282 (9.5) | 190 (33.6) | |
| Major comorbidities | ||||
| Liver cirrhosis | 505 (14.3) | 356 (12.0) | 149 (26.3) | <0.001* |
| Diabetes mellitus | 1332 (37.7) | 1172 (39.5) | 160 (28.3) | <0.001* |
| Chronic renal insufficiency | 593 (16.8) | 423 (14.3) | 170 (30.0) | <0.001* |
| Congestive heart failure | 108 (3.1) | 85 (2.9) | 23 (4.1) | 0.142 |
| Cerebrovascular disease | 375 (10.6) | 329 (11.1) | 46 (8.1) | 0.037* |
| Malignancy | 823 (23.3) | 559 (18.8) | 264 (46.6) | <0.001* |
| Infection site | ||||
| Respiratory tract | 644 (18.2) | 421 (14.2) | 223 (39.4) | <0.001* |
| Urinary tract | 1762 (49.9) | 1630 (54.9) | 132 (23.3) | <0.001* |
| Skin and soft tissue | 120 (3.4) | 95 (3.2) | 25 (4.4) | 0.162 |
| Intra-abdominal | 881 (24.9) | 748 (25.2) | 133 (23.5) | 0.397 |
| Others or unknown source | 613 (17.4) | 462 (15.6) | 151 (26.7) | <0.001* |
ESBL: extended-spectrum beta-lactamase; IIAT: inappropriate initial antibiotic therapy; qSOFA: quick sepsis-related organ failure assessment; E. coli: Escherichia coli; K. pneumonia: Klebsiella pneumoniae.
Data are presented as n (%) or mean ± standard deviation.
P < 0.05.
Non-survivors had a higher percentage of IIAT than survivors (15.0% vs 10.7%, P = 0.004). The mean time to antibiotics was shorter in non-survivors than survivors (1.70 vs 1.84 h, P < 0.001). Patients with qSOFA scores ⩾ 2 were more among non-survivors (33.6% vs 9.5%, P < 0.001).
Table 2 summarizes the initial empiric antibiotic regimens. The most commonly used antibiotic in patients who received IIAT was ceftriaxone (106/401, 26.4%), followed by quinolones (75/401, 18.7%) and cefuroxime (50/401, 12.5%).
Table 2.
Initial antibiotic regimens administered within 6 h of ED registration.
| Initial empiric regimens | Non-IIAT (n = 3132) | IIAT (n = 401) |
|---|---|---|
| Ertapenem | 273 | 0 |
| Meropenem | 27 | 0 |
| Imipenem | 12 | 0 |
| Doripenem | 4 | 0 |
| Piperacillin/tazobactam | 112 | 4 |
| Cefepime | 58 | 10 |
| Ceftriaxone | 915 | 106 |
| Flomoxef | 530 | 10 |
| Cefuroxime | 380 | 50 |
| Quinolones | 324 | 75 |
| Others | 497 | 146 |
IIAT: inappropriate initial antibiotic therapy; ED: emergency department.
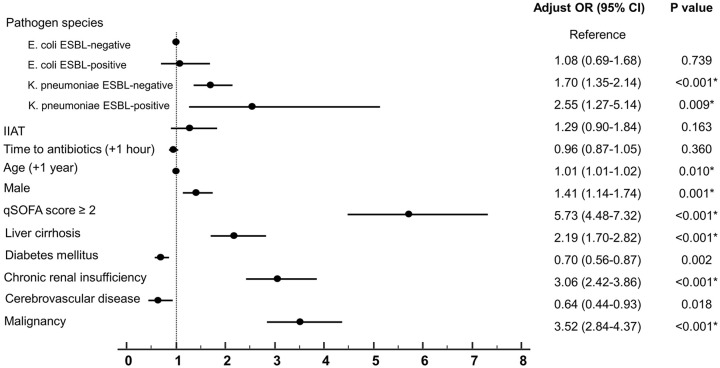
In multiple logistic regression analysis, the most significant predictor for in-hospital mortality was qSOFA score ⩾ 2 (adjusted odds ratio (AOR) = 5.73; 95% confidence interval (CI), 4.48–7.32); P < 0.001) (Figure 1). Age, male sex, and comorbidities such as liver cirrhosis, chronic renal insufficiency, and malignancy were also significant predictors. For evaluating the pathogen species, we used patients with E. coli ESBL-negative BSI as the reference group; patients with K. pneumoniae ESBL-negative BSI (AOR = 1.7; 95% CI, 1.35–2.14; P < 0.001) and K. pneumoniae ESBL-positive BSI (AOR = 2.55; 95% CI, 1.27–5.14; P = 0.009) were significant predictors. IIAT was not a significant predictor for in-hospital mortality (AOR = 1.29; 95% CI, 0.90–1.84; P = 0.163). Time to antibiotics was not a significant predictor for in-hospital mortality (AOR = 0.96; 95% CI, 0.87–1.05; P = 0.360).
Figure 1.
Multivariable logistic regression of risk factors for in-hospital mortality after BSI with ESBL-positive or -negative Escherichia coli and Klebsiella pneumonia.
BSL: bloodstream infection; ESBL: extended-spectrum beta-lactamase; IIAT: inappropriate initial antibiotic therapy; qSOFA: quick sepsis-related organ failure assessment.
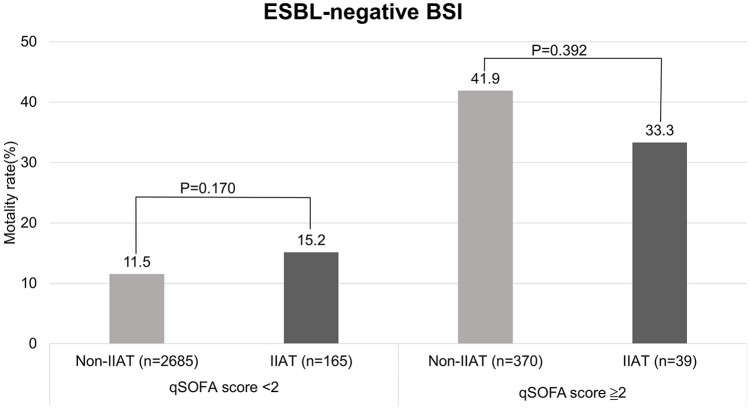
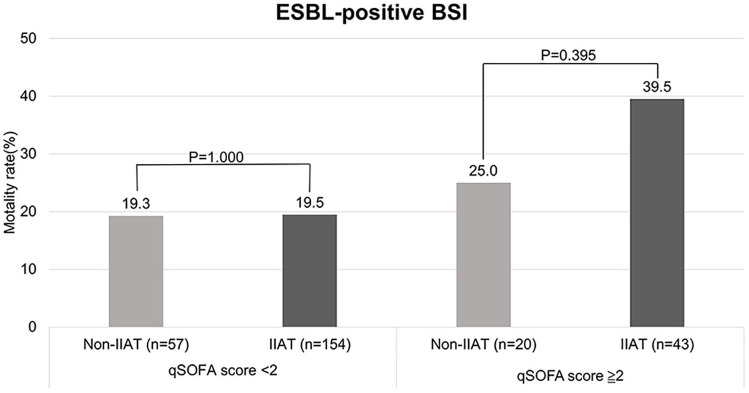
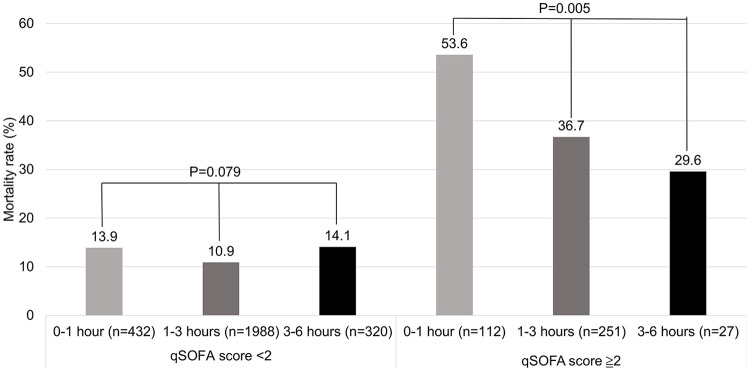
Because the qSOFA score was the most significant, we divided patients by qSOFA scores and ESBL production to see the difference in IIAT and non-IIAT patients, and in patients with varying time to antibiotics. In patients with ESBL-negative BSI and qSOFA score < 2, the mortality rate of patients with IIAT is a little higher than patients with non-IIAT, but not significant (15.2% vs 11.5%; P = 0.170) (Figure 2). In patients with ESBL-positive BSI and qSOFA score ≧ 2, the mortality rate of patients with IIAT is a little higher than patients with non-IIAT, but not significant (39.5% vs 25.0%; P = 0.395) (Figure 3). After exclusion of IIAT patients, we divided the patients with time to antibiotics in the range of 0–1, 1–3, and 3–6 h. We found that in the group of patients with qSOFA score ≧ 2, patients with time to antibiotics in the range of 0–1 h had a significantly higher mortality rate (53.6% vs 36.7% vs 29.6%; P = 0.005) than others (Figure 4).
Figure 2.
The difference in mortality rate among patients with IIAT and non-IIAT with ESBL-negative BSI divided by qSOFA score < 2 and qSOFA score ≧ 2. There is no significant different of mortality between non-IIAT and IIAT patients with ESBL-negative bacteremia qSOFA score < 2 (P = 0.170) and qSOFA score ≧ 2 (P = 0.392).
BSL: bloodstream infection; ESBL: extended-spectrum beta-lactamase; IIAT: inappropriate initial antibiotic therapy; qSOFA: quick sepsis-related organ failure assessment.
Figure 3.
The difference in mortality rate among patients with IIAT and non-IIAT with ESBL positive BSI divided by qSOFA score < 2 and qSOFA score ≧ 2. There was no significant difference in mortality rate between patients with IIAT and non-IIAT with ESBL positive BSI dived by qSOFA score < 2 (P = 1.000) and qSOFA score ≧ 2 (P = 0.395).
BSL: bloodstream infection; ESBL: extended-spectrum beta-lactamase; IIAT: inappropriate initial antibiotic therapy; qSOFA: quick sepsis-related organ failure assessment.
Figure 4.
The difference in mortality rate among patients with time to antibiotics 0–1, 1–3, and 3–6 h divided by qSOFA score < 2 and qSOFA score ≧ 2 (excluding IIAT patients). The mortality rate was not significant different among patients with qSOFA score < 2 (P = 0.079) but there was a significant difference among patients with qSOFA score ≧ 2 (P = 0.005).
qSOFA: quick sepsis-related organ failure assessment.
Discussion
The major findings of this research were that qSOFA score ⩾ 2, comorbidities (liver cirrhosis, chronic renal insufficiency and malignancy), and ESBL production influence the in-hospital mortality significantly in patients with E. coli and K. pneumoniae BSIs. IIAT and time to antibiotics do not seem to be significant predictors for in-hospital mortality.
In previous studies, the findings suggested that ESBL-positive infections were associated with higher mortality.17 However, recent researches have questioned this viewpoint and have shown that there was a lack of adequate controlled studies, particularly in the area of antibiotic treatment.18 In this study, different effects are seen on the prognosis of patients, with the production of ESBL in E. coli and K. pneumoniae after adjustment of antibiotic factors. These findings suggest that we do not yet fully understand the differences between the two groups, such as the factors in epidemiology and pathophysiology. There seems to be a trend of K. pneumoniae infections being more severe than E. coli infections. Leistner et al.19 enrolled 1851 patients with E coli and K. pneumoniae BSIs and found that the in-hospital mortality is higher in K. pneumoniae BSIs (25.0% vs 18.5%, P = 0.006). In a study by Martelius et al.,20 among 2878 patients, K. pneumoniae BSIs (including ESBL positive and negative) were higher than E. coli BSIs (including ESBL positive and negative) in a 28-day mortality period (28%, 14.6% vs 14.3%, 11.9%). There may be confounding factors that are related to the increased mortality of K. pneumoniae BSIs that have not been assessed, and further investigation is needed.
In this study, there was no significant correlation between IIAT and in-hospital mortality compared with non-IIAT. This result is different compared with previous studies, in which IIAT is a significant risk factor for poor prognosis in patients with severe ESBL-producing bacterial infections.21,22 The reason for the difference may be due to comorbidities and severity of the infection. Patients with more severe infections have a higher chance of receiving broad-spectrum antibiotics and non-IIAT treatment. Therefore, compared with the IIAT group, the mortality rate of the non-IIAT group will increase. In patients with E. coli and K. pneumoniae BSIs, the severity of infection (qSOFA score ⩾ 2) and comorbidities have a greater impact on prognosis than IIAT. We have found that severe comorbidities (such as cirrhosis, chronic renal insufficiency, and malignant tumors) are independent risk factors associated with in-hospital mortality.
In an often cited study, Kumar et al.23 included 2154 patients with septic shock between 1989 and 2004 and found that the increase in time between the first episode of hypotension and the use of effective antibiotics was a risk factor for in-hospital mortality. However, only half of the patients with septic shock received effective treatment within 6 h of hypotension. When viewed from the current perspective, the management of sepsis was not performed proactively and the criteria for study patient selection were less strict. In a meta-analysis by Sterling et al., they found no significant improvement in mortality due to early administration of antibiotics. For patients who had received antibiotic treatment more than 3 h after ED triage (reference time less than 3 h), the odds ratio for mortality was only 1.16 (P = 0.21).8
Although the timing of administration of antibiotics seems to affect mortality, the condition in critical ill patients appears to be obscure or vice versa because they are administered antibiotics earlier. Our study selected patients who received antibiotics within 6 h; the delay in antibiotic treatment may be due to uncertain diagnosis and potentially delayed care. Nearly 90% of the patients received antibiotics within 3 h: patients with qSOFA score < 2 (2420/2740 = 88.3%) and patients with qSOFA score ⩾ 2(363/390 = 93.1%). Due to the pathophysiology of sepsis and the resulting organ dysfunction or failure, the effect of single-dose antibiotic therapy on the survival rate of patients cannot be deep and strong.
Our study had some limitations. First, the study is a retrospective single-center design, which belongs to local epidemiology and the criteria for inclusion of patients may be affected by selection bias despite all our efforts. Second, due to incomplete data, we cannot distinguish between community-acquired infections and healthcare-related infections; although the difference is important, ESBL positive strains have an increasing trend in hospital and community settings. Third, there are many sepsis bundle data that cannot be obtained, such as serum lactate, central venous pressure, venous oxygen saturation, and subsequent changes in antibiotic treatment or serum drug concentrations, and are not included and should be the direction of future research.
Conclusion
The importance of using appropriate antibiotics early in patients with infection has been overemphasized. For the in-hospital mortality of patients with E. coli and K. pneumoniae BSIs, the time to antibiotics and IIAT is not enough to have an impact, because the severity of the disease (qSOFA ⩾ 2) is the key factor. Future research needs to consider this result when formulating indicators that improve and evaluate the quality of care.
Acknowledgments
We acknowledge the staff at the Biostatistics Center of the Kaohsiung Chang Gung Memorial Hospital.
Footnotes
Author contributions: F.C.C., Y.N.H., H.H.C., C.H.W., and C.M.S. contributed to the study design. F.C.C., Y.N.H., H.H.C., M.W.C., and C.M.S. participated in the data analysis. All authors contributed to research implementation/data acquisition, manuscript preparation, and revision. The final manuscript was read and approved by all the authors.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from The Institutional Review Committee on Human Research of the Chang Gung Memorial Hospital (APPROVAL NUMBER 103-0053B).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent for this study was waived by The Institutional Review Committee on Human Research of the Chang Gung Memorial Hospital (WAIVER NUMBER 103-0053B).
ORCID iD: Fu-Cheng Chen  https://orcid.org/0000-0001-9211-3990
https://orcid.org/0000-0001-9211-3990
References
- 1. Leistner R, Schröder C, Geffers C, et al. (2015) Regional distribution of nosocomial infections due to ESBL-positive Enterobacteriaceae in Germany: Data from the German National Reference Center for the Surveillance of Nosocomial Infections (KISS). Clinical Microbiology and Infection 21(3): 255.e1–255.e5. [DOI] [PubMed] [Google Scholar]
- 2. Doi Y, Park YS, Rivera JI, et al. (2013) Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clinical Infectious Diseases 56(5): 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musicha P, Cornick JE, Bar-Zeev N, et al. (2017) Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998-2016): A surveillance study. The Lancet Infectious Diseases 17(10): 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leistner R, Gürntke S, Sakellariou C, et al. (2014) Bloodstream infection due to extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: An analysis of the disease burden in a large cohort. Infection 42(6): 991–997. [DOI] [PubMed] [Google Scholar]
- 5. Raman G, Avendano E, Berger S, et al. (2015) Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: Systematic review and meta-analysis. BMC Infectious Diseases 15: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hyle EP, Lipworth AD, Zaoutis TE, et al. (2005) Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: Variability by site of infection. Archives of Internal Medicine 165(12): 1375–1380. [DOI] [PubMed] [Google Scholar]
- 7. Rhodes A, Evans LE, Alhazzani W, et al. (2017) Surviving sepsis campaign—International guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine 43(3): 304–377. [DOI] [PubMed] [Google Scholar]
- 8. Sterling SA, Miller WR, Pryor J, et al. (2015) The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: A systematic review and meta-analysis. Critical Care Medicine 43(9): 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seymour CW, Liu VX, Iwashyna TJ, et al. (2016) Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA: The Journal of the American Medical Association 315(8): 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quan H, Sundararajan V, Halfon P, et al. (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care 43(11): 1130–1139. [DOI] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute (2009) Performance Standards for Antimicrobial Suscep-tibility Testing: 19th Informational Supplement (M100-S119). Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 12. Clinical and Laboratory Standards Institute (2010) Performance Standards for Antimicrobial Suscepti-bility Testing: 20th Informational Supplement (M100-S120-U). Wayne, PA: Clinical and Labo-ratory Standards Institute. [Google Scholar]
- 13. Liu JW, Wang LS, Cheng YJ, et al. (2008) In-vitro activity of tigecycline against clinical isolates of Acinetobacter baumannii in Taiwan. International Journal of Antimicrobial Agents 32(Suppl. 3): S188–S191. [DOI] [PubMed] [Google Scholar]
- 14. Clinical and Laboratory Standards Institute. (2014) Performance Standards for Antimicrobial Susceptibility Testing: 24th Informational Supplement (M100-124). Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 15. Freund Y, Lemachatti N, Krastinova E, et al. (2017) Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA: The Journal of the American Medical Association 317(3): 301–308. [DOI] [PubMed] [Google Scholar]
- 16. Chen FC, Kung CT, Cheng HH, et al. (2019) Quick sepsis-related organ failure assessment predicts 72-h mortality in patients with suspected infection. European Journal of Emergency Medicine 26(5): 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shorr AF. (2009) Review of studies of the impact on gram-negative bacterial resistance on outcomes in the intensive care unit. Critical Care Medicine 37(4): 1463–1469. [DOI] [PubMed] [Google Scholar]
- 18. Rottier WC, Ammerlaan HS, Bonten MJ. (2012) Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae and patient outcome: A meta-analysis. Journal of Antimicrobial Chemotherapy 67(6): 1311–1320. [DOI] [PubMed] [Google Scholar]
- 19. Leistner R, Bloch A, Gastmeier P, et al. (2016) E. coli bacteremia in comparison to K. pneumoniae bacteremia: Influence of pathogen species and ESBL production on 7-day mortality. Antimicrobial Resistance and Infection Control 5: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martelius T, Jalava J, Karki T, et al. (2016) Nosocomial bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae resistant to third-generation cephalosporins, Finland, 1999-2013: Trends, patient characteristics and mortality. Infectious Diseases 48(3): 229–234. [DOI] [PubMed] [Google Scholar]
- 21. Tumbarello M, Sali M, Trecarichi EM, et al. (2008) Bloodstream infections caused by extended-spectrum-beta-lactamase- producing Escherichia coli: Risk factors for inadequate initial antimicrobial therapy. Antimicrobial Agents and Chemotherapy 52(9): 3244–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tumbarello M, Sanguinetti M, Montuori E, et al. (2007) Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: Importance of inadequate initial antimicrobial treatment. Antimicrobial Agents and Chemotherapy 51(6): 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar A, Roberts D, Wood KE, et al. (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Medicine 34(6): 1589–1596. [DOI] [PubMed] [Google Scholar]