Abstract
Mesh was a promising, minimally invasive, and ‘gold standard’ treatment for urinary stress incontinence. Time has shown that complications from these devices can happen early, or even several years, after mesh placement and can be catastrophic. Pain, erosion, voiding dysfunction, infection, recurrent UTIs [urinary tract infections (UTIs)], fistulae, organ perforation, bleeding, vaginal scarring, neuromuscular alterations, LUTS (lower urinary tract symptoms), bowel complications and even immune disorders have been linked to mesh. Various tools, such as imaging, endoscopic and functional studies, are available for diagnosis of mesh complications. Since the spectrum of complications is wide, involvement of other specialties is usually beneficial in the diagnosis and management of these complications. There is still much to learn on the accuracy and utility of diagnostic studies in each type of complication. Evidence on the best diagnostic and treatment pathways for these complications is scarce but continuously growing as information is being reported, and we continue to gain expertise in dealing with patients affected by mesh. Treatment options include conservative and medical management initially and then open or minimally invasive surgical procedure approaches. This article will describe diagnostic and treatment pathways for mesh complications.
Keywords: mesh, incontinence, complications, guidelines, treatment, erosion, pain, voiding dysfunction
Introduction
Stress urinary incontinence (SUI) is the leakage of urine on effort. It has a prevalence of around 6% in women worldwide.1 Treatment options have included bulking agents, which do not last long, and open procedures such as retropubic autologous pubovaginal slings and Burch colposuspension.2 The introduction of the less invasive mid-urethral slings/meshes was taken up by many due to ease of insertion, shorter learning curve, shorter operative time, quicker patient recovery and good success rates in curing stress incontinence. Available data showed an objective success rate of 72.3–77.3% at 24 months after mid-urethral sling surgery, and patient satisfaction was 86.3–88.1%. Regarding complications, mesh exposure was reported in 2.7–4.4%, voiding dysfunction requiring surgery in up to 3%, urinary tract infections (UTIs) in 10.7–17.1% and neurological symptoms in 5.4–9.7%.3 A total of 42% of patients experienced at least one adverse event and 12% had at least one serious adverse event.4 After 5 years of mid-urethral tape surgery treatment, success was 51.3% with retropubic tapes, satisfaction rates were 83%, 1.7% developed mesh exposure and 3.7% required surgical retreatment of SUI.5 After increasing concern about complications with mesh surgery, a large retrospective study of over 92,000 women in England was conducted during an 8 year period, and a complication rate in the region of 10% was quoted.6 However, there is lack of data on longer-term and meaningful outcomes to patients with overall complication rates. There was also no surgical experience in removing these meshes from the human body, as it was assumed that they would remain in the body forever.
We are currently seeing the results of the lack of information on long-term outcomes and complications, with a strong patient-led movement against synthetic mesh, based on the potentially devastating effects of retropubic, transobturator and mini-slings, amongst other types of mesh. The reported rate of complications for mesh-related procedures for SUI is less than for prolapsed organ surgery.7
The most common complications for tapes/slings reported are mesh exposure (extrusion/erosion) and pain, followed by urgency urinary incontinence (UUI), voiding dysfunction, recurrent UTI and SUI.8
The percentage of women requiring an intervention for mesh complications is between 2.3% and 6.1%.6,9 After 1 and 9 years of mid-urethral sling placement, an estimated 1.4% and 3.3%, respectively, had undergone removal.10
The aim of this review is to look at current best practice in the work-up and treatment of tape/sling-mesh-related complications for SUI in women.
Synthetic mesh is defined as a net-like material, which can be mono or multifilament and made from various types of polymers. The preferred type used for female synthetic slings is a polypropylene, monofilament with macropores mesh (type 1) since it causes less foreign-body response and improves tissue incorporation.11
Mesh can serve four main purposes:
(i) mesh inserted as slings for SUI in women, and includes retropubic slings, transobturator and single-incision mini-slings;
(ii) mesh inserted vaginally for anterior, vault and posterior compartment prolapse;
(iii) mesh inserted abdominally for uterine/cervical or vault prolapse: sacrocolpopexy and sacrohysteropexy; and
(iv) mesh inserted for rectal prolapse: rectopexy.
This article will concentrate only on synthetic mid-urethral slings. The first mesh-related complications were reported as early as a couple of years after the insertion of these devices.12
Diagnostic pathway for urinary incontinence mesh-related complications
There are no guidelines available as to which investigations must be performed in the various broad categories of mesh complications. History, examination and questionnaires are non-invasive, easy tools to help decide the next steps in diagnosis (Figure 1). We will address the complications and link them to the diagnostic tests. Complications in general have three main causes, and may occur on their own or in combination. These include mesh material factors, patient factors and surgeon factors.
Figure 1.
Diagnostic pathway.
CT, computed tomography; MRI, magnetic resonance imaging; PVR, post-void residual; TL, translabial; TV, transvaginal; US, ultrasound; UTIs, urinary tract infections; VUDS, videourodynamics.
History
A complete medical history is the first key to a comprehensive and assertive diagnosis of mesh-related complications. A list of comorbidities is essential to understand the probable cause of the problems. It is known that older age, diabetes mellitus, smoking, immunosuppression, prior pelvic radiation, and previous vaginal surgery may increase the risk of mesh problems.7,13,14 These factors also need to be considered prior to any surgery. Adequate glucose control, smoking cessation and weight loss can be beneficial for future management. Multidisciplinary evaluation is always encouraged, especially if there are other symptoms related to mesh, like bowel problems or musculoskeletal issues, that may need to be addressed in parallel. Hormonal status should be determined, particularly for further treatment options. Any other comorbidities such as autoimmune disorders and fibromyalgia need to be documented.
A thorough investigation of the indications, intra-operative and post-operative complications of mesh insertion, as well as the type of mesh used, and, ideally, operation notes, are fundamental for subsequent surgical planning.
If previous attempts to remove mesh have taken place, the surgical details, pathology reports (including length of mesh removed), any pictures of the mesh removed taken and outcomes should be stated.
Current symptom interrogation usually includes pain symptoms, mobility issues, vaginal discharge or bleeding, urinary symptoms with particular focus on continence, storage and voiding symptoms or other relevant infections and sexual dysfunction, including hispareunia (pain or injury to the partner).
Examination
A proper, detailed vaginal examination with a speculum will reveal mesh exposure sites, trigger points for pain, vaginal scarring, new or recurrent associated prolapse, probable fistula or infection signs and vaginal discharge. A pelvic examination can also show tender areas, signs of urine retention and previous surgical scars. In cases were examination is too painful or sub-optimal, it will need to be postponed and completed under anaesthesia.
Quality of life questionnaires
It is helpful to use validated questionnaires for objective assessment and registration of pelvic floor symptoms, sexual function, pain and quality of life.15,16 Some examples of these are the following: FSFI,17 ICIQ (LUTSqol, FLUTS, FLUTSsex, Vaginal symptoms, Bowel symptoms),18 EQ-5D,19 EQ-VAS, and the McGill Pain Questionnaire.20 It is important that a set of questionnaires is agreed upon and used regularly as that will also help assess outcomes. Unfortunately, there are currently no mesh-specific outcome questionnaires and these need to be developed urgently.
Urine dipstick
Testing urine is part of the usual work-up for patients with lower urinary tract symptoms, and therefore should be done routinely in mesh complications. Haematuria should always be subject to the usual diagnostic pathways and studied accordingly. In context of previous mesh insertion, blood and leukocytes are not uncommon in case of mesh extrusion into the urinary tract. Recurrent urinary infections because of extrusion or voiding dysfunction related to mesh can also lead to abnormal urine results.
Urethrocystoscopy
In patients with haematuria, recurrent UTIs and voiding dysfunction, direct visualisation of urethra and bladder is the preferred tool for diagnosis of extrusion into the lower urinary tract and associated complications, such as stone formation. It is important to record the exact site of exposure, colour and length of mesh in urethra/bladder. Scarring tissue or assessment of healthy, vascularised structures can help in planning future surgery.
Endoscopic evaluation is also useful in diagnosis of urinary fistula, giving information on size, location and associated complications.
If the patient has haematuria, it favours the possibility of disruption or inflammation of the urothelium. It is of the utmost importance to rule out more serious conditions causing the haematuria, such as urothelial cancer.
In case of recurrent UTIs, the usual pathway for their evaluation must be followed, taking into account that extrusion is one of the most common complications of mesh placement that can cause recurrent UTIs.
Flow rate and post void residual
Measurement of the residual urine and flow rate are important in those who have voiding dysfunction or lower urinary tract symptoms.
Cystography/cystourethrogram
In case of suspicion of a urinary fistula, and computed tomography (CT) urogram is not available or contraindicated, a cystography or cystourethrogram could be useful.
CT scan of pelvis and abdomen/CT urogram
A CT scan of pelvis and abdomen/CT urogram is useful as part of the haematuria and UTI work up. If a urinary fistula is suspected, a CT urogram is the gold standard. Other causes of pain can be assessed, as well as complications involving bowel. If there is infection or abscesses associated with mesh, this test can help define its extent.
Video-urodynamics
If a urodynamic study was performed before the mesh placement, video-urodynamics (VUDS) would be useful in planning management and counselling the patient.
After mesh insertion, when faced with complications, urodynamic studies are useful if a concomitant procedure is planned for recurrent incontinence. This gives more information regarding bladder support, outlet obstruction, ureteric reflux, post-void residual, bladder shape and size, bladder function during voiding, and can visualise urethral diverticula and fistula. If there are no lower urinary tract symptoms (LUTS) with a normal flow rate and post-void residual, then VUDS is not indicated.
VUDS is particularly relevant in women with urinary incontinence to help determine if it is a case of urgency, stress or mixed UI. In cases of high post-void residual urine, pressure/flow studies will help differentiate between detrusor underactivity and bladder outlet obstruction, and the choice of future surgery may be affected by this.
Translabial/transvaginal ultrasound
Ultrasound for mesh evaluation can be transperineal (translabial), transabdominal or transvaginal/introital.21 When performed by an experienced operator, it is a useful non-radiation test to assess the presence and position of mesh in the vagina and local complications like stones or exposure into the vagina and extrusion into the urinary tract, due to the high echogenicity of polypropylene mesh. The typical appearance is that of a honeycomb structure (Figure 2), although with time, as fibrosis and scarring take place, it might lose this common arrangement.22 Ultrasound gives information regarding mesh relation to the urethra and vaginal wall,15 which could help determine the type of mesh placed in case of unknown tape insertion surgery details. Transobturator tapes can be identified because the arms of the tape go lateral, in a ‘seagull’ shape, whilst retropubic transvaginal tapes gives a U-shaped image, with the arms travelling towards the pubic symphysis.22 Some radiologists advocate that the arms of the mesh are difficult to identify because of a longer distance from the transducer,23 and magnetic resonance imaging (MRI) could surpass ultrasound in this scenario.
Figure 2.
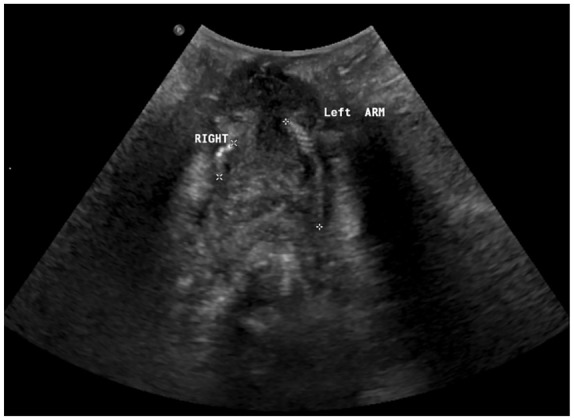
Right and left arms of a TVT mesh identified with ultrasound.
TVT, tension-free vaginal tape.
Magnetic resonance imaging
Mesh components are hypointense on T2-weighted MRI images.24 Some of the intensity may come from scar tissue around the mesh.23 Precise description of location, relation to other structures, extension and thickening of mesh characterise a good report. Identifying mesh in the retropubic and paraurethral region is better with MRI than ultrasound in some cases. Expert radiologists with knowledge in mesh complications are able to interpret these. Information about complications from mesh, like further investigation of pain, infection, osteomyelitis, sacrum or other organ involvement, can be diagnosed with MRI.
It is important to note that the investigations described in the preceding sections (summarised in Figure 2) help mainly to diagnose some of the complications and exclude others but may have no impact on surgical technique and removal of the mesh.
Mesh complications classification and standardisation
Revision surgery and complications from mesh have been reported only recently. This impairs evidence-based decision-making because there is not enough information on mesh complications and management, and even less on follow up after mesh revision surgery. There is also a lack of reporting of long-term outcomes of mesh removal surgery, limiting informed decision making for both clinicians and patients.
The lack of standardisation in terminology and classification system of mesh complications makes it difficult to compare and analyse available information and reach a consensus or provide recommendations. The International Urogynecological Association (IUGA)/International Continence Society (ICS) classification comprehensively describes the category of complication, time of diagnosis and the site involved in a thorough but complex manner25; therefore its actual clinical utility is not ideal.26 Surgical terminology, such as partial, complete or total removal of mesh, is not clearly defined and consensus must be pursued and standardised. Recently the American Urogynecologic Society (AUGS) and the IUGA developed a joint position statement with some standardisation of nomenclature. This document defines mesh revision as no removal of mesh or small edge removal, such that there is no change in the structural integrity of the device; they also divide vaginal excision as partial or complete, and, where segments outside of the vagina are removed, they define it as extravaginal mesh excision; lastly, total mesh excision consists of extirpation of 100% of the implant.27 These can also be confusing terms as complete vaginal excision is still a partial excision and certainly patient groups in the United Kingdom (UK) have highlighted this confusion, as acknowledged by an independent review on mesh complications being conducted.28 Although not mentioned in the AUGS-IUGA Joint Position Statement,27 we believe that measurement, recording of the length and photos of the mesh removed should be mandatory to have a clear history and evidence of mesh removal surgeries performed, which can aid if more than one surgery is required and is useful for legal purposes too. There also need to be registries and databases set up to help track these complications.
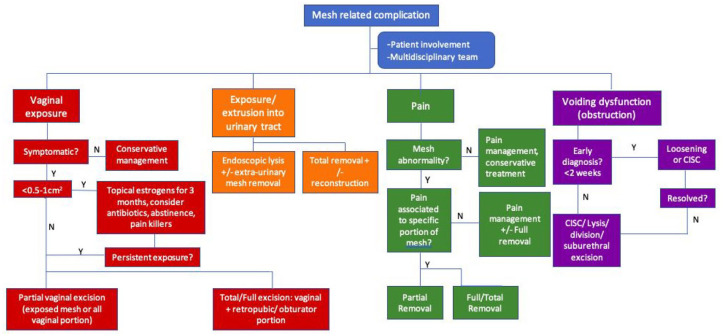
Management of mesh-related complications
Treatment has to be tailored to the patient’s symptoms, background and expectations (Figure 3). Management comprises involvement of the patient at every step of the decision making, a multidisciplinary team including physiotherapists, pain specialists and psychologists, and the surgical team (urology, gynaecology, colorectal, orthopaedics). Therefore, referral to specialised centres with multidisciplinary teams with expertise in mesh complications should be considered. It is generally accepted that mesh should be removed only if the patient has bothersome symptoms. It is, however, sometimes difficult to associate some of the symptoms to the mesh and, equally, some symptoms, especially pain, may not go away, or may go away and come back in the future or get worse after mesh removal. All risks have to be discussed with the patient and options given. Neurological and vaginal pain (dyspareunia) are also difficult to treat.
Figure 3.
Management algorithm.
CISC, clean intermittent self-catheterisation.
Patient involvement
Often, patients with mesh complications are quite challenging since their symptoms can be catastrophic with a great impact on their health and quality of life. Frequently, they have been seen and treated by various professionals, with partial or no improvement in symptoms and this carries emotional consequences such as disappointment, lack of trust in healthcare providers, anger, desperation or depression in addition to the physical problems that the mesh is causing.
It is crucial that the patient is involved at every step of the pathway. Options of treatment, with a thorough discussion and complete understanding of the possible risks and outcomes, including the impact in presenting symptoms and long-term consequences should take place. The possibility of needing more than one procedure, unsuccessful removal of mesh, recurrence of symptoms that led to mesh placement, no change or worsening of pain, need for major surgery and other organ damage should be clear. A detailed patient information leaflet would be very useful.
Multidisciplinary team
The urologist and/or urogynaecologist are usually the main professionals involved in treating the urinary incontinence of mesh patients, but an integral approach depending on the type of complication should be considered.
Pain is amongst the most common symptoms. Pain specialists and physiotherapists have a role to play in improving this complaint.15
Clinical psychologists and psychiatrists may help to treat the emotional distress related to mesh complications. Depression, anxiety and social isolation are common findings in this group of women.
Other surgeons might be involved, depending on the type of mesh and complications found. In our centre it is routine to include an orthopaedic surgeon during trans-obturator tape (TOT) removals as they are familiar with the anatomical region affected during TOT insertion. Similarly, in case of damage to bowel, colorectal surgeons are part of the decision making and surgical approach along with the urologist.
Conservative treatment
In the case of small vaginal exposure (less than 0.5–1 cm2), topical oestrogens and antibiotics along with intercourse abstinence may be considered before considering surgery.7,10 There is some evidence of a higher risk of treatment failure compared with surgery as an initial step,29 and up to 59.3% of women managed initially conservatively will eventually need surgical treatment.30 The 2019 National Institute for Health and Care Excellence (NICE) guidelines in the UK suggest reassessment after 3 months of conservative treatment. Asymptomatic patients can be managed by surveillance and topical treatment.7,31,32
If there is pain associated with exposure, painkillers including non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol, moving upwards in the ladder to opioids and/or neuroleptics and muscle relaxants such as amitriptyiline, gabapentin or pregabalin is advised. Physiotherapy and nerve block are other options available.
Referral to a clinical psychologist and the pain team may be helpful to help manage the patient in a holistic manner.
Mesh revision/excision surgery (removal)
Surgical options for mesh complications comprise covering of the exposed part if very small, division of the tape if causing voiding difficulty, partial or complete excision in one stage or two stages, and open or laparoscopic/robotic approaches.
Patients and surgeon must be aware that, in all surgical cases, complete excision of mesh might not be possible. The rate of worsening or failure from surgery for pre-existing symptoms is 10–15%.16 It is, however, extremely important to be honest and transparent with patients about what surgical approach will be taken, and whether a partial or full excision will be attempted. The majority of patients we encounter in our practice are keen on full excisions in one stage.
We use the following terminology for mesh excision (removal):
(1) Covering of the mesh with vaginal tissue: this does not involve removal or division of the mesh and only involves dissection of the vaginal tissue and using that to cover the exposed mesh.
(2) Division of the mesh: this involves cutting the mesh without removing any part of it, and is usually used in those with voiding dysfunction.
(3) Partial excision of the mesh: this involves removing only part of the mesh. The site of removal will have to be specified (vaginal, abdominal, retropubic, subdermal, transobturator) and length of mesh removed.
(4) Full excision of the mesh: removing all the mesh from one end to the other. For retropubic TVTs this involves removing the vaginal portion, the retropubic portion and the portion above the rectus sheath and under the skin. For TOTs, this would involve removing the mesh from both groins and will need a para-labial incision on each side to remove the whole mesh as well as a vaginal incision. Range of lengths removed for TOTs is 15–32 cm, and for retropubic tapes is 20–36 cm depending on body mass index.
(5) Completion removal/excision of the mesh: this is where they had a previous partial removal and the rest of the mesh needed to be removed fully.
Vaginal complications
Exposure of mesh into the vagina can cause discharge, spotting, dyspareunia, hispareunia, infection and pain. If infection is suspected, it must be assessed and treated.
If exposure is symptomatic, surgical options include: partial resection of the vaginal portion, which involves just the exposed mesh with a tension-free closure of the vaginal wall,33 removal of the vaginal portion or total removal of the mesh (vaginal and obturator/retropubic and suprapubic portions). Removal of all the vaginal portion has a higher risk of recurrence of SUI than removing part of the vaginal component, with partial removal having a higher risk of exposure in the future but full removal of the mesh encompasses risks including urinary tract injury.15 In all the previous procedures, tissue interposition (Martius flap or rectus fascia/tensor fascia lata graft) may be required in cases where the mesh may be adherent to the urethral wall resulting in urethral injury when removing it.
By definition, exposed mesh is infected and our surgical preference is to perform a full removal of the whole mesh; however, several factors have to be taken into consideration including the presence of pain, patient comorbidities and patient wishes.
Extrusion into the urinary tract
Complications and symptoms include haematuria, recurrent UTIs, stone formation, fistula, urgency and voiding difficulty. It is important to inform the patient that urinary symptoms might not improve, and new ones might appear. Likelihood of developing SUI is about 13–56%,34 with around 36% requiring another procedure for SUI in studies not limited to extrusion as only criteria for mesh excision.35 The only study reporting data exclusively for mesh excision found 7–59% SUI after surgery but follow up is for less than 1 year.36 There is a risk of developing a urinary fistula between the urethra and the vagina or bladder and vagina.
Mesh in the urinary tract, in general, must always be removed as it is a nidus for infection and stone formation. The least radical option is endoscopic lysis of the tape with laser or bipolar resection, which might allow the mesh to retract and facilitate a vaginal approach.37 Mesh excision, at the same time or as a second stage, will prevent further extrusions. Complete excision of mesh with urinary reconstruction can be done either through a vaginal, abdominal or minimally invasive approach (laparoscopic/robotic).
Depending on the site of exposure and patient’s preference, removal can be performed as a one stage or two stage procedure, usually involving vaginal and abdominal approaches. Our preference is to perform a full removal in one stage with an abdominal and vaginal incision for retropubic tapes and vaginal and groin incisions for transobturator tapes with the obvious advantages that a one stage procedure involves only one anaesthetic, one hospital admission and one recovery period as well as ensuring that the full mesh is removed.
Treatment for recurrent SUI, with an autologous fascial sling or colposuspension (in the absence of intrinsic sphincter deficiency) can be considered at the same time of removal if there was SUI prior to mesh removal or as a possible second procedure if SUI recurs after mesh removal.
To the best of the authors’ knowledge, there are no studies comparing abdominal open versus laparoscopic approach for mesh excision. Laparoscopy has a better visualisation of the retropubic space and the potential advantage of faster recovery and smaller incisions, although some studies have linked laparoscopic approach to a higher SUI recurrence. Robotic mesh removals have been reported, but no recommendations can be made so far with the available data. Laparoscopic procedures also do not always ensure that a full mesh removal has been performed, especially of the part above the rectus sheath in retropubic tapes and the transobturator part in TOTs.
Pain
Pain after mesh placement can be referred to the pelvic region, groin, legs and/or vagina. It can be caused by scarring, infection, exposure, direct nerve or muscle damage or without a clear reason.7 If no mesh abnormality is found, NICE guidelines suggest non-surgical treatment initially and if no improvement is achieved, multi-disciplinary (MDT) advice must be sought before any surgical treatment is decided on.15
A thorough vaginal examination, looking for trigger points with mesh palpation helps localise the pain, and reassures the clinician and the patient of an association between the mesh and pain. It can also guide further treatment in terms of deciding if partial or complete removal is necessary. Trigger point injections with local anaesthetic, with or without corticosteroids, as a trial of treatment to see if symptoms are relieved can be useful and can also help establish an association with mesh.38
Partial mesh excision may be considered if pain has a clear cause and is localised to a specific component of the sling. Complete excision can be especially challenging in cases of TOT since groin dissection is not an area familiar for urologists or urogynaecologists and complications can be serious. It is desirable to operate in conjunction with an orthopaedic or plastic surgeon. However, it is our opinion that if pain due to the mesh is the main complication, a total mesh removal is advised. Partial removals make future removals of any remnant mesh, if the pain persists, very difficult due to retraction of the mesh.
Reported outcomes for mesh excision for pain have a high variability. Pain may partially improve, completely disappear or get worse after partial removal of mesh. Recently, Dray et al. published persistence of pain in 42.3% of patients undergoing revision surgery for pain related to mesh, and de novo pain in 6.3% in a tertiary care hospital for sling revision.39
Voiding dysfunction
If bladder outlet obstruction or voiding dysfunction from a tight sling is diagnosed early (up to 2–3 weeks) after surgery, tape loosening is appropriate. After 2–3 weeks,40,41 because of fibrosis and scarring, lysis/division is the preferred procedure, with risk of recurrence of SUI in up to 63% of patients.41 If sling release is done 24 months after initial surgery, the likelihood of needing SUI surgery for recurrence is less than if done within 3 months of sling placement.42 Some studies show that temporary self-catheterisation is another option, with a possibility close to 50% of resolution of incomplete bladder emptying instead of sling lysis.41
If retention is the only complication from sling insertion, lysis or sub-urethral mesh division or excision are enough, with high resolution rates after revision surgery. Recurrence of stress incontinence after surgery for obstruction is around 14–42%.35
Stress incontinence surgery (concomitant or staged)
If mesh placed for stress incontinence is removed, there could be recurrence of leakage on effort. It can be argued that some support may remain even after mesh is taken out, avoiding recurrence due to scarring. In cases of recurrent SUI prior to mesh removal, some surgeons prefer to do a two-staged procedure, removing mesh first and reassessing symptoms to further decide on the need of a second anti-incontinence surgery while others prefer to do a concomitant surgery.
If recurrent stress incontinence is a problem, VUDS before surgery may help guide the decision. Firstly, to prove that it is in fact SUI and to look for concomitant urgency urinary incontinence. Secondly, it will give information regarding the most appropriate option for anti-incontinence surgery, differentiating intrinsic sphincter deficiency and urethral hypermobility, or maybe even an underactive detrusor during voiding and the potential risks of treating incontinence. There is currently no evidence to favour staged versus concomitant surgery for recurrent SUI. The main advantages for concomitant surgery is that it involves one anaesthetic, one incision and one recovery. The disadvantage is there may be a higher risk of urinary retention or if there was pain associated with the mesh prior to removal and the pain persists then it will not be clear as to the cause of the pain. The decision on which route to take will have to be individualised and agreed upon between the patient and surgeon.
Minimally invasive surgery
Laparoscopic, mainly transvesical, partial removals of mesh eroding into the bladder have been published.43,44 Some robotic case reports can be found in the literature, with positive results, particularly for intravesical exposure. There is not enough experience yet to support these approaches, other than the theoretical advantages described for other pelvic surgeries. Some surgeons advocate removal of mesh using the laparoscopic route45; however, there has to be clear documentation of how much length of mesh has been removed, especially if a full removal is the intention. In our experience, laparoscopic surgery does not remove the mesh fully in retropubic tapes, especially the supra-fascial/subdermal portion of the mesh, and in the transobturator route it is often difficult to get the mesh out from within the muscle fibres.
Bowel complications
Bowel injury following TVT, TOT or mini-sling are rare, but if they occur a colorectal surgeon must be involved in the counselling, decision making and surgical management. The patient needs to be informed that bowel diversion, temporary or permanent, may be needed, or small bowel resection if the small bowel is involved. Such complications can be picked up on MRI or CT scan and patients may present with a colo or entero-cutaneous fistula.
Conclusion
There is still a lot to learn from mesh problems. The best way to deal with mesh complications in terms of diagnostic studies is yet to be determined as evidence grows, with appropriate registration, standardisation and classification of the problems related to them and the outcomes after treatment. Individualisation and patient involvement throughout the process are vital, making sure that the patient understands the risks of treatment and the real possibility of improvement in the presenting symptoms. Collaboration from experts in all fields related to mesh diagnosis and management leads to better health outcomes and enhances integral patient care.
Treatment can be tailored to the patient’s symptoms, risks and expectations. When surgical management is suitable, it is the author’s preference to pursue a complete removal of mesh as a one stage procedure, predicting that further surgeries for this purpose will be more difficult if done after an initial partial removal. It is of the utmost importance in this matter, that detailed information of any surgery attempts of removal are correctly registered and available for the future.
Acknowledgments
The authors thank Shoba Philip, Consultant Radiologist, Southmead Hospital, Bristol for providing information and advice.
Footnotes
Author contributions: Paulina Bueno was responsible for the design of the review, research of data and writing the draft and final version
Hashim Hashim was responsible for the design of the review, support and supervision in draft writing and writing of the final version.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Hashim Hashim is chair of the International Continence Society working group on mesh complications.
Ethical approval: This study did not require an ethical board approval because it did not contain human or animal trials.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Paulina Bueno Garcia Reyes  https://orcid.org/0000-0002-1303-6470
https://orcid.org/0000-0002-1303-6470
Hashim Hashim  https://orcid.org/0000-0003-2467-407X
https://orcid.org/0000-0003-2467-407X
Contributor Information
Paulina Bueno Garcia Reyes, Bristol Urological Institute, Southmead Hospital, Bristol, UK.
Hashim Hashim, Bristol Urological Institute, Southmead Hospital, Bristol BS10 5NB, UK.
References
- 1. Irwin DE, Kopp ZS, Agatep B, et al. Tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 2011; 108: 1132–1139. [DOI] [PubMed] [Google Scholar]
- 2. Jarvis GJ. Surgery for genuine stress incontinence. Br J Obstet Gynaecol 1994; 101: 371–374. [DOI] [PubMed] [Google Scholar]
- 3. Albo ME, Litman HJ, Richter HE, et al. Treatment success of retropubic and transobturator mid urethral slings at 24 months. J Urol 2012; 188: 2281–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brubaker L, Norton PA, Albo ME, et al. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol 2011; 205: 498.e1–498.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal follow-up after retropubic and transobturator midurethral slings the urinary incontinence treatment network. J Urol 2015; 193: 203–210.25158274 [Google Scholar]
- 6. Keltie K, Elneil S, Monga A, et al. Complications following vaginal mesh procedures for stress urinary incontinence: an 8 year study of 92,246 women. Sci Rep 2017; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacDonald S, Terlecki R, Costantini E, et al. Complications of transvaginal mesh for pelvic organ prolapse and stress urinary incontinence: tips for prevention, recognition, and management. Eur Urol Focus 2016; 2: 260–267. [DOI] [PubMed] [Google Scholar]
- 8. Lee D, Dillon B, Lemack G, et al. Transvaginal mesh kits–how ‘serious’ are the complications and are they reversible? Urology 2013; 81: 43–49. [DOI] [PubMed] [Google Scholar]
- 9. Welk B, Al-Hothi H, Winick-Ng J. Removal or revision of vaginal mesh used for the treatment of stress urinary incontinence. JAMA Surg 2015; 150: 1167. [DOI] [PubMed] [Google Scholar]
- 10. Gurol-Urganci I, Geary RS, Mamza JB, et al. Long-term rate of mesh sling removal following midurethral mesh sling insertion among women with stress urinary incontinence. JAMA 2018; 320: 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gomelsky A, Dmochowski RR. Biocompatibility assessment of synthetic sling materials for female stress urinary incontinence. J Urol 2007; 178: 1171–1181. [DOI] [PubMed] [Google Scholar]
- 12. Ulmsten U, Henriksson L, Johnson P, et al. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J 1996; 7: 81–86. [DOI] [PubMed] [Google Scholar]
- 13. Kokanali MK, Doǧanay M, Aksakal O, et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur J Obstet Gynecol Reprod Biol 2014; 177: 146–150. [DOI] [PubMed] [Google Scholar]
- 14. Chen HY, Yeh LS, Chang WC, et al. Analysis of risk factors associated with surgical failure of inside-out transobturator vaginal tape for treating urodynamic stress incontinence. Int Urogynecol J 2007; 18: 443–447. [DOI] [PubMed] [Google Scholar]
- 15. NICE guidance - urinary incontinence and pelvic organ prolapse in women: management. BJU Int 2019; 123: 777–803. [DOI] [PubMed] [Google Scholar]
- 16. Lee D, Zimmern PE. An update on research and outcomes in surgical management of vaginal mesh complications. Expert Rev Med Devices 2019; 16: 569–580. [DOI] [PubMed] [Google Scholar]
- 17. Rosen R, Brown C, Heiman J, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000; 26: 191–208. [DOI] [PubMed] [Google Scholar]
- 18. Abrams P, Avery K, Gardener N, et al. The international consultation on incontinence modular questionnaire: www.iciq.net. J Urol 2006; 175: 1063–1066. [DOI] [PubMed] [Google Scholar]
- 19. Brooks R, De Charro F. EuroQol: the current state of play. Health Policy 1996; 37: 53–72. [DOI] [PubMed] [Google Scholar]
- 20. Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain 1975; 1: 277–299. [DOI] [PubMed] [Google Scholar]
- 21. Chan L, Tse V. Pelvic floor ultrasound in the diagnosis of sling complications. World J Urol 2018; 36: 753–759. [DOI] [PubMed] [Google Scholar]
- 22. Taithongchai A, Sultan AH, Wieczorek PA, et al. Clinical application of 2D and 3D pelvic floor ultrasound of mid-urethral slings and vaginal wall mesh. Int Urogynecol J 2019; 30: 1401–1411. [DOI] [PubMed] [Google Scholar]
- 23. Khatri G, Carmel ME, Bailey AA, et al. Postoperative imaging after surgical repair for pelvic floor dysfunction. RadioGraphics 2016; 36: 1233–1256. [DOI] [PubMed] [Google Scholar]
- 24. Ram R, Oliphant SS, Barr SA, et al. Imaging of pelvic floor reconstruction. Semin Ultrasound CT MR 2017; 38: 200–212. [DOI] [PubMed] [Google Scholar]
- 25. Haylen BT, de Ridder D, Freeman RM, et al. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. Epub ahead of print 25 November 2009. DOI: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 26. Riaz N, Wolden SL, Gelblum DY, et al. Clinical application of IUGA/ICS classification system for mesh erosion. Neurourol Urodyn 2016; 118: 6072–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joint position statement on the management of mesh-related complications for the FPMRS specialist. Female Pelvic Med Reconstr Surg 2020; 26: 219–232. [DOI] [PubMed] [Google Scholar]
- 28. Cumberlege BJ. Our concern over partial mesh removal. The Independent Medicines and Medical Devices Safety Review; 2019. [Google Scholar]
- 29. Barski D, Deng DY. Management of mesh complications after SUI and POP repair: review and analysis of the current literature. Biomed Res Int 2015; 2015: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abbott S, Unger CA, Evans JM, et al. Evaluation and management of complications from synthetic mesh after pelvic reconstructive surgery: a multicenter study. Am J Obstet Gynecol 2014; 210: 163.e1–163.e8. [DOI] [PubMed] [Google Scholar]
- 31. Bergersen A, Hinkel C, Funk J, et al. Management of vaginal mesh exposure: a systematic review. Arab J Urol 2019; 17: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolff GF, Winters JC, Krlin RM. Mesh excision: is total mesh excision necessary? Curr Urol Rep 2016; 17: 1–7. [DOI] [PubMed] [Google Scholar]
- 33. Zoorob D, Karram M. Management of mesh complications and vaginal constriction. a urogynecology perspective. Urol Clin North Am 2012; 39: 413–418. [DOI] [PubMed] [Google Scholar]
- 34. Shaw J, Wohlrab K, Rardin C. Recurrence of stress urinary incontinence after midurethral sling revision. Female Pelvic Med Reconstr Surg 2017; 23: 184–187. [DOI] [PubMed] [Google Scholar]
- 35. Ramart P, Ackerman AL, Cohen SA, et al. The risk of recurrent urinary incontinence requiring surgery after suburethral sling removal for mesh complications. Urology 2017; 106: 203–209. [DOI] [PubMed] [Google Scholar]
- 36. Jambusaria LH, Heft J, Reynolds WS, et al. Incontinence rates after midurethral sling revision for vaginal exposure or pain. Am J Obstet Gynecol 2016; 215: 764.e1–764.e5. [DOI] [PubMed] [Google Scholar]
- 37. Hengel AR, Carlson KV, Baverstock RJ. Prevention, diagnosis, and management of midurethral mesh sling complications. Can Urol Assoc J 2017; 11: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duckett J, Bodner-Adler B, Rachaneni S, et al. Management of complications arising from the use of mesh for stress urinary incontinence—International urogynecology association research and development committee opinion. Int Urogynecol J 2019; 30: 1413–1417. [DOI] [PubMed] [Google Scholar]
- 39. Dray E, Crosby E, Grable A, et al. A retrospective analysis of surgical outcomes and risk factors for persistent postoperative symptoms following synthetic mid urethral sling revision. J Urol 2019; 202: 339–346. [DOI] [PubMed] [Google Scholar]
- 40. Molden S, Bracken J, Nguyen A, et al. A retrospective multicenter study on outcomes after midurethral polypropylene sling revision for voiding dysfunction. Female Pelvic Med Reconstr Surg 2010; 16: 340–344. [DOI] [PubMed] [Google Scholar]
- 41. Brennand EA, Tang S, Birch C, et al. Early voiding dysfunction after midurethral sling surgery: comparison of two management approaches. Int Urogynecol J 2017; 28: 1515–1526. [DOI] [PubMed] [Google Scholar]
- 42. Abraham N, Makovey I, King A, et al. The effect of time to release of an obstructing synthetic mid-urethral sling on repeat surgery for stress urinary incontinence. Neurourol Urodyn 2015; 36: 349–353. [DOI] [PubMed] [Google Scholar]
- 43. Yoshizawa T, Yamaguchi K, Obinata D, et al. Laparoscopic transvesical removal of erosive mesh after transobturator tape procedure. Int J Urol 2011; 18: 861–863. [DOI] [PubMed] [Google Scholar]
- 44. Kim JH, Doo SW, Yang WJ, et al. Laparoscopic transvesical excision and reconstruction in the management of mid-urethral tape mesh erosion and stones around the bladder neck: initial experiences. BJU Int 2012; 110: E1009–E1013. [DOI] [PubMed] [Google Scholar]
- 45. Greenwell T, Cutner A. The anatomy and an illustrated description of a technique for combined laparoscopic and vaginal total removal of an obturator mid urethral tape. Transl Androl Urol 2018; 7: 978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]