Abstract
Aims:
This study aimed to (a) assess the effectiveness and safety of apatinib as a subsequent treatment for patients with sorafenib-resistant hepatocellular carcinoma (HCC), and (b) identify the clinical factors influencing their treatment outcomes.
Methods:
The electronic medical records of consecutive patients with newly diagnosed advanced HCC treated with first-line sorafenib from 2015 to 2017 were retrospectively reviewed. Patients who were confirmed to have primary resistance to sorafenib were enrolled in this study. The outcomes of patients treated with apatinib were compared with those of patients who received supportive care. The primary endpoint was overall survival (OS).
Results:
A total of 92 patients with sorafenib-resistant advanced HCC (84 men and 8 women; mean age, 51.9 years) were included. All patients had an etiology of hepatitis B. The median OS in the overall cohort was 5.0 months [95% confidence interval (CI): 3.9, 6.0]. Of 92 patients, 58 (63.0%) were treated with apatinib, and 34 (37.0%) received supportive care. Apatinib treatment was associated with longer survival times than supportive care for patients with sorafenib-resistant advanced HCC (median OS: 7.0 versus 4.0 months, p < 0.001). The results of the multivariate analysis demonstrated that liver tumor load [hazard ratio (HR): 3.653, 95% CI: 2.047, 5.965, p < 0.001] and extrahepatic spread (HR: 0.303, 95% CI: 0.231, 0.778, p = 0.003) were independent predictors of OS after apatinib treatment.
Conclusion:
This study showed that subsequent apatinib treatment may improve survival outcomes compared with supportive care for patients with sorafenib-resistant, advanced hepatitis B virus (HBV)-related HCC, especially for patients who have a lower liver tumor load and extrahepatic spread.
Keywords: antiangiogenesis, apatinib, hepatocellular carcinoma, resistant, sorafenib, survival benefits
Introduction
Hepatocellular carcinoma (HCC) is a major health problem worldwide.1 Approximately half of all HCC patients are diagnosed at an advanced stage and have a dismal prognosis (median survival time, 3–6 months).2,3 After the SHARP and ORIENTAL trials demonstrated that sorafenib significantly improved the overall survival (OS) rate, sorafenib was recommended as the first-line standard of care for advanced HCC.4–6 However, we found that the disease control rate was only 35.3–43%,4,5 and that is was especially low for patients with hepatitis B virus (HBV)-related HCC.7 In other words, more than 50% of patients with advanced HCC have no response to primary sorafenib treatment. Determining how patients with sorafenib-resistant HCC should be treated is urgent because the median survival time of these patients is less than 3 months.8
In the National Comprehensive Cancer Network (NCCN) Guidelines Version 2.2019 Hepatobiliary Cancers, regorafenib, cabozantinib, and ramucirumab are recommended as second-line systemic therapies for HCC patients who have disease progression while taking sorafenib; this is because phase III clinical trials have shown that these drugs provide survival benefits to patients with HCC who previously received sorafenib.9–13 However, we observed that the median duration of sorafenib treatment in those trials ranged from 4.1 months to 7.2 months.10–13 In other words, sorafenib was initially effective for the majority of the included patients who had later developed resistance to sorafenib. Previous studies have shown that the mechanisms of acquired resistance and primary resistance may be different, and that the efficacy of the drug may also be different.14 Therefore, whether these drugs benefit HCC patients who have primary resistance to sorafenib remains unclear.
Apatinib, an oral tyrosine kinase inhibitor agent, selectively inhibits vascular endothelial growth factor receptor (VEGFR) 2, and its binding affinity is 10 times that of sorafenib.15,16 Apatinib has been widely applied as an optional treatment for HCC patients in China, and several observational studies have shown that apatinib treatment is safe and effective for HCC patients.17–21 However, subsequent apatinib treatment for the specific subgroup of patients with sorafenib-resistant HCC has rarely been studied. Although our previous pilot study and several case reports showed that apatinib is a promising treatment for patients with sorafenib-resistant HCC,22–24 these studies were only single-arm studies or case reports and did not compare other treatments, nor did they identify which subgroup of patients would benefit most from apatinib treatment. Therefore, we conducted a comparative study for patients with sorafenib-resistant HCC who were either treated with apatinib or supportive care to evaluate whether apatinib treatment offers survival benefits. We also sought to identify clinical factors that were correlated with treatment outcomes.
Materials and methods
Patient selection
From January 2015 to December 2017, the electronic medical records of consecutive patients with newly diagnosed advanced HCC treated with first-line sorafenib were retrospectively analyzed. The inclusion criteria were as follows: (a) Patients were 18–75 years old; (b) All patients were pathologically or radiologically [contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI)] diagnosed with advanced HCC according to the American Association for the Study of Liver Diseases criteria2; (c) All patients had sorafenib-resistant HCC [sorafenib-resistant HCC was defined as the target tumor showing two successive progressions on imaging assessed by modified Response Evaluation Criteria in Solid Tumor (mRECIST) during the sorafenib treatment]; (d) Patients tolerated sorafenib treatment (at least 400 mg every day); (e) Patients had an Eastern Cooperative Oncology Group (ECOG) score of <2; and (f) Patients had a Child-Pugh score of <8. Before the initiation of apatinib, all patients were informed that apatinib was an alternative treatment because sorafenib was ineffective and because there were no universally recommended second-line treatments for patients who had sorafenib-resistant HCC during the study period. Moreover, all patients were informed of the approximate cost, anticipated outcomes, and potential side effects. Generally, the final decisions were made by the patients.
Due to the lack of a recommended second-line treatment for HCC during the study period, the treatment strategies included apatinib and supportive care. Apatinib treatment was performed if the patient accepted apatinib treatment. If the patient did not receive apatinib treatment, supportive care was performed. The exclusion criteria were as follows: (a) Patients who had received any other systemic chemotherapy, targeted therapy, or immune therapy; (b) Patients who discontinued sorafenib due to unacceptable adverse events (AEs); (c) patients who began apatinib treatment >4 weeks after the diagnosis of sorafenib resistance; (d) Patients who had a duration of apatinib treatment of <4 weeks; (e) Patients who had previous malignant tumors; or (f) Patients who had missing data.
All patients provided written informed consent, and the protocol was approved by the ethics committees of the institution.
Treatment protocol
The treatment protocol was previously described.22,25 The initial dosage of sorafenib was 400 mg given twice daily. Doses were adjusted based on intolerable AEs, according to version 3.0 of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE). If a patient was diagnosed with sorafenib-resistant HCC, apatinib treatment was recommended (250 mg per tablet, Jiangsu Hengrui Medicine, Lianyungang China). The initial dosage of oral apatinib was 500 mg daily, which was reduced to 250 mg per day in cases of unacceptable AEs. The apatinib treatment was discontinued if further dose reduction was required. Treatment continued until disease progression, clinical progression, death, unacceptable side effects, or withdrawal of consent by the patient.
Assessment and follow up
The follow up, including contrast-enhanced scans of the liver and chest, tests of liver function, measurements of alpha-fetoprotein levels, and physical examination was performed every month for the first 3 months after initiation of treatment, and every 2 months thereafter. The tumor response was assessed radiologically based on the mRECIST criteria.26 The objective response rate (ORR) was the sum of the complete response (CR) and partial response (PR), while the disease control rate (DCR) was the sum of the CR, PR, and stable disease (SD) rate. Apatinib administration could be continued beyond disease progression if the physician judged that the patient would benefit from continued administration. OS was defined as the time from the initiation of sorafenib until death or the last follow up. The AE grade was recorded based on CTCAE 3.0.
Statistical analysis
All statistical analyses were performed using SPSS software (SPSS version 16.0, SPSS, Chicago, IL, USA). For the baseline characteristics, categorical variables are described as frequencies and percentages, and continuous variables are expressed as the means ± standard deviation. The χ2 test was used to compare categorical variables, and t tests were used to compare continuous variables. The Kaplan–Meier method was used to estimate the survival curve between the two groups and compared by the log-rank test. Univariate analyses were performed with the log-rank test. Variables with p < 0.1 in the univariate analysis were entered into the multivariate analysis. A multivariate Cox proportional hazards model was used to identify risk factors correlated with survival. All statistical tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Study population
From January 2015 to December 2017, a total of 203 consecutive patients with advanced HCC who were treated with first-line sorafenib were analyzed. A total of 118 patients (58.1%) were diagnosed with sorafenib-resistant HCC. A total of 23 patients were excluded from the study because they did not meet the inclusion criteria. In addition, three patients were excluded due to an apatinib duration of less than 4 weeks. Finally, 92 patients who had advanced sorafenib-resistant HCC were enrolled in this study; 58 of them received apatinib treatment, and the other 34 received supportive care (Figure 1). The baseline characteristics of the overall cohort are summarized in Table 1. The mean patient age (±standard deviation) was 51.9 ± 8.7 years. Most of the patients were male (91.3%). Hepatitis B virus (HBV) infection (100%) and cirrhosis (94.6%) were the most common disease backgrounds. All patients were classified as advanced stage according to the Barcelona Clinic Liver Cancer (BCLC) system, 78 (84.7%) patients presented with portal vein thrombosis (PVTT), and 29 (31.5%) patients presented with extrahepatic spread. There were no differences between the two groups with respect to patient age, liver function, or tumor characteristics.
Figure 1.
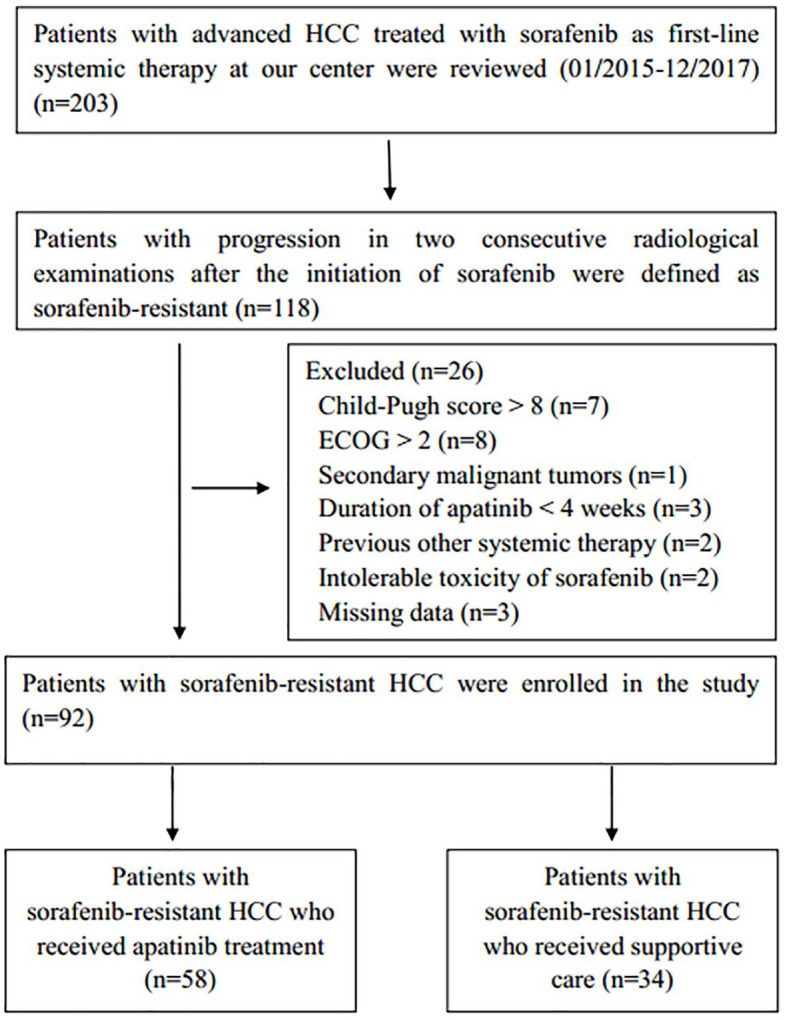
Flow diagram showing the patient selection process.
ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma.
Table 1.
Baseline characteristics of patients in both groups.
| Characteristics | Apatinib
group (n = 58) |
Supportive care group (n = 34) | p value |
|---|---|---|---|
| Age (years)* | 50.9 ± 8.7 | 53.7 ± 7.4 | 0.125 |
| Sex | 0.973 | ||
| Male | 53 (91.4) | 31 (91.2) | |
| Female | 5 (8.6) | 3 (8.8) | |
| Etiology | – | ||
| Hepatitis B | 58 (100) | 34 (100) | |
| Hepatitis C | 0 | 0 | |
| Other | 0 | 0 | |
| Cirrhosis | 0.648 | ||
| Present | 54 (93.1) | 33 (97.1) | |
| Absent | 4 (6.9) | 1 (2.9) | |
| Number of tumors | 0.169 | ||
| 1–5 | 9 (15.5) | 2 (5.9) | |
| >5 | 49 (84.5) | 32 (94.1) | |
| Liver tumor burden | 0.440 | ||
| <50% | 18 (31.0) | 8 (23.5) | |
| ⩾50% | 40 (69.0) | 26 (76.5) | |
| PVTT | 0.480 | ||
| Present | 48 (82.8) | 30 (88.2) | |
| Absent | 10 (17.2) | 4 (11.8) | |
| Extrahepatic spread | 0.206 | ||
| Present | 21 (36.2) | 8 (23.5) | |
| Absent | 37 (63.8) | 26 (76.5) | |
| ECOG | 0.666 | ||
| 0–1 | 55 (94.8) | 31 (91.2) | |
| 2 | 3 (5.2) | 3 (8.8) | |
| AFP (ng/ml) | 0.400 | ||
| <400 | 11 (19.0) | 9 (26.5) | |
| ⩾400 | 47 (81.0) | 25 (73.5) | |
| Child-Pugh class | 0.201 | ||
| A | 49 (84.5) | 25 (73.5) | |
| B | 9 (15.5) | 9 (26.5) | |
| Ascites | 0.068 | ||
| Present | 12 (22.4) | 13 (44.1) | |
| Absent | 46 (77.6) | 21 (55.9) | |
| ALT (U/l) | 49.5 ± 26.8 | 47.4 ± 25.7 | 0.721 |
| GGT (U/l) | 176.2 ± 102.7 | 194.1 ± 128.9 | 0.133 |
| Albumin (g/l) | 35.5 ± 4.0 | 34.1 ± 3.3 | 0.072 |
| Bilirubin (μmol/l) | 18.9 ± 6.4 | 18.4 ± 9.1 | 0.727 |
Data are presented as n (%).
Represents the mean ± standard deviation.
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; ECOG, Eastern Cooperative Oncology Group; GGT, γ-glutamyl transpeptidase; PVTT, portal vein tumor thrombosis.
Treatment outcome
The mean durations of sorafenib administration for the overall patients was 1.6 months (range 1–2.5 months). The mean durations of sorafenib administration for apatinib group and supportive care group were 1.5 months and 1.7 months, respectively. The median and mean durations of apatinib treatment were 5.2 months and 6.1 months (range, 1–32 months), respectively. In the apatinib group, follow-up imaging data during the second month of apatinib administration were available for 49 of 58 patients (84.5%); 13 patients (22.4%) had a PR, 19 (32.8%) had SD, and 17 (29.3%) had progressive disease (PD). Accordingly, the ORR was 22.4%, and the DCR was 55.2%. In the supportive care group, the treatment efficacy was not evaluated because there were no completely available follow-up images.
At the end of follow up (December 2018), 90 of the 92 (97.8%) patients had died. The median follow-up time of the cohort was 7.5 months (range, 3–37 months). The median OS of all patients was 5.0 months [95% confidence interval (CI): 3.9, 6.0]. The median OS time of the apatinib group was significantly longer than that of the supportive care group (7.0 months versus 4.0 months, p < 0.001) (Figure 2). The median and mean times to progression (TTPs) of sorafenib treatment were 1.0 months and 1.1 months, respectively. The median TTP of apatinib treatment was 3.0 months (95% CI: 2.5, 3.5).
Figure 2.
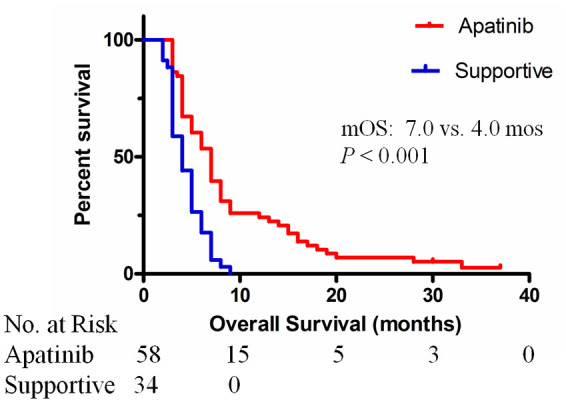
Kaplan–Meier curves showing the mOS of patients with sorafenib-resistant advanced HCC treated with apatinib or supportive care.
HCC, hepatocellular carcinoma; mOS, median overall survival. The median OS times were 7.0 months (95% CI: 5.8, 8.2) for the apatinib group and 4.0 months (95% CI: 2.9, 5.1) for the supportive care group (p < 0.001).
Predictive factors affecting survival
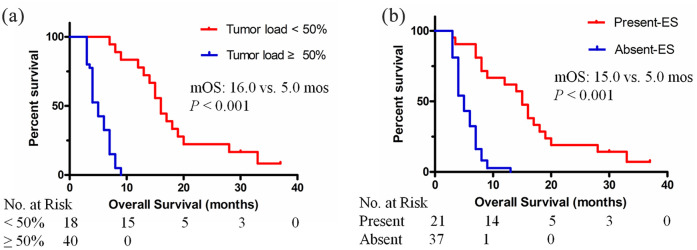
In the univariate analysis, treatment allocation, liver tumor load, number of tumors, PVTT, extrahepatic spread, Child-Pugh classification, ascites, and albumin were significantly associated with OS. In the multivariate Cox analysis, treatment allocation [hazard ratio (HR) = 2.837; 95% CI: 1.568, 5.132; p = 0.001] and liver tumor load (HR = 3.364; 95% CI: 2.147, 6.144; p < 0.001) were identified as independent prognostic factors associated with OS (Table 2). In the apatinib group, multivariate COX analysis showed that liver tumor load (HR = 3.653; 95% CI: 2.047, 5.965; p < 0.001) and extrahepatic spread (HR = 0.303; 95% CI: 0.231, 0.778; p = 0.003) were significant predictive factors for OS after apatinib treatment (details in Table 3). The median OS was 16.0 months (95% CI: 13.9, 18.1) for patients with a liver tumor load <50% and 5.0 months (95% CI: 4.3, 5.7) for patients with a liver tumor load ⩾50% (p < 0.001). The median OS was 15.0 months (95% CI: 12.8, 17.2) for patients with extrahepatic spread and 5.0 months (95% CI: 4.2, 5.8) for patients without extrahepatic spread (p < 0.001) (Figure 3).
Table 2.
Univariate and multivariate analysis of prognostic factors in total patients.
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Median OS, months (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Treatment | <0.001 | 0.001 | ||
| Apatinib | 7.0 (5.8–8.2) | Ref | ||
| supportive care | 4.0 (2.9–5.1) | 2.837 (1.568–5.132) | ||
| Age | 0.409 | – | – | |
| <52 | 5.0 (3.9–6.1) | |||
| ⩾52 | 5.0 (3.6–6.4) | |||
| Sex | 0.536 | – | – | |
| Male | 5.0 (3.9–6.1) | |||
| Female | 5.0 (1.3–8.7) | |||
| Cirrhosis | 0.708 | – | – | |
| Absent | 5.0 (3.9–6.1) | |||
| Present | 6.0 (1.7–10.3) | |||
| Liver tumor load | <0.001 | <0.001 | ||
| <50% | 13.0 (7.0–18.9) | Ref | ||
| ⩾50% | 4.0 (3.7–4.3) | 3.364 (2.147–6.144) | ||
| Number of tumors | 0.004 | 0.470 | ||
| >5 | 5.0 (4.1–5.9) | Ref | ||
| 1–5 | 13.0 (10.1–15.9) | 0.689 (0.251–1.893) | ||
| PVTT | <0.001 | 0.410 | ||
| Absent | 8.0 (4.3–11.7) | Ref | ||
| Present | 5.0 (4.5–5.5) | 1.500 (0.572–3.933) | ||
| Extrahepatic spread | <0.001 | 0.543 | ||
| Present | 9.0 (2.4–15.6) | Ref | ||
| Absent | 4.0 (3.3–4.7) | 0.808 (0.406–1.608) | ||
| ECOG | 0.807 | – | – | |
| 0–1 | 5.0 (3.7–6.3) | |||
| 2 | 5.0 (2.7–7.3) | |||
| Child-Pugh class | <0.001 | 0.323 | ||
| A | 7.0 (6.0–7.9) | Ref | ||
| B | 3.5 (3.1–3.9) | 1.505 (0.669–3.385) | ||
| Ascites | 0.001 | 0.284 | ||
| Absent | 6.0 (5.0–6.9) | Ref | ||
| Present | 4.0 (3.3–4.7) | 1.518 (0.707–3.260) | ||
| Albumin (g/l) | 0.024 | 0.737 | ||
| <35 | 4.0 (3.3–4.7) | Ref | ||
| ⩾35 | 6.0 (4.8–7.2) | 0.913 (0.537–1.552) | ||
| AFP level | 0.322 | – | – | |
| <400 | 7.0 (6.3–7.7) | |||
| ⩾400 | 5.0 (4.5–5.5) | |||
| GGT (U/l) | 0.608 | – | – | |
| >100 | 5.0 (3.8–6.2) | |||
| ⩽100 | 6.0 (4.8–7.2) | |||
| ALT (U/l) | 0.626 | – | – | |
| ⩽40 | 5.0 (3.8–6.2) | |||
| >40 | 6.0 (4.6–7.4) | |||
| Bilirubin (μmol/l) | 0.629 | – | – | |
| ⩽20 | 5.0 (4.2–5.8) | |||
| >20 | 6.0 (4.6–7.4) | |||
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GGT, γ-glutamyl transpeptidase; OS, overall survival; PVTT, portal vein tumor thrombosis.
Table 3.
Univariate and multivariate analysis of prognostic factors in the apatinib group.
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Median OS, months (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Liver tumor load | <0.001 | <0.001 | ||
| <50% | 16.0 (13.9–18.1) | Ref | ||
| ⩾50% | 5.0 (4.3–5.7) | 3.653 (2.047–5.965) | ||
| Number of tumors | 0.029 | 0.087 | ||
| >5 | 6.0 (4.8–7.2) | Ref | ||
| 1–5 | 13.0 (10.1–15.9) | 0.326 (0.090–1.175) | ||
| PVTT | <0.001 | 0.130 | ||
| Absent | 12.0 (3.1–16.9) | Ref | ||
| Present | 6.0 (4.3–7.7) | 2.935 (0.728–11.835) | ||
| Extrahepatic spread | <0.001 | 0.003 | ||
| Present | 15.0 (12.8–17.2) | Ref | ||
| Absent | 5.0 (4.2–5.8) | 0.303 (0.231–0.778) | ||
| Child-Pugh | 0.001 | 0.088 | ||
| A | 7.0 (6.1–7.9) | Ref | ||
| B | 3.5 (2.0–5.0) | 2.392 (0.879–6.508) | ||
| Ascites | 0.002 | 0.091 | ||
| Absent | 7.0 (6.0–8.0) | Ref | ||
| Present | 4.0 (3.4–4.6) | 2.208 (0.882–5.525) | ||
CI, confidence interval; OS, overall survival; PVTT, portal vein tumor thrombosis.
Figure 3.
Kaplan–Meier curves showing the OS of patients with sorafenib-resistant advanced HCC treated with apatinib, stratified into subgroups. (A) The median OS was 16.0 months (95% CI: 13.9, 18.1) for patients with a liver tumor load <50% and 5.0 months (95% CI: 4.3, 5.7) for patients with a liver tumor load ⩾50% (p < 0.001). (B) The median OS was 15.0 months (95% CI: 12.8, 17.2) for patients with extrahepatic spread and 5.0 months (95% CI: 4.2, 5.8) for patients without extrahepatic spread (p < 0.001).
ES, extrahepatic spread; HCC, hepatocellular carcinoma; mOS, median overall survival.
Adverse events related to apatinib
Adverse events related to apatinib are listed in Table 4. No patient experienced fatal AEs. A total of 16 patients (27.6%) had temporary reductions in their apatinib doses due to associated AEs. Two patients (3.4%) permanently discontinued apatinib treatment due to intolerable AEs, including dizziness, and dysphagia. The most common side effects related to apatinib were alopecia, weight loss, hypertension, hand-foot skin reaction (HFSR), fatigue, and diarrhea; 15 (25.9%) patients experienced 18 grade 3 or higher AEs, such as HFSR, hypertension, proteinuria, diarrhea, and dizziness.
Table 4.
Adverse events associated with apatinib in patients with sorafenib-resistant advanced HCC.
| Adverse event | All grades. n (%) | Grade 1–2. n (%) | Grade 3–4. n (%) |
|---|---|---|---|
| Weight loss | 45 (77.6) | 45 (77.6) | 0 |
| HFSR | 42 (72.4) | 39 (67.2) | 3 (5.2) |
| Hypertension | 35 (60.3) | 33 (56.9) | 3 (5.2) |
| Fatigue | 31 (53.4) | 31 (53.4) | 0 |
| Alopecia | 30 (51.7) | 30 (51.7) | 0 |
| Diarrhea | 27 (46.6) | 25 (43.1) | 2 (3.4) |
| Anorexia | 21 (36.2) | 19 (32.8) | 2 (3.4) |
| Proteinuria | 16 (27.6) | 15 (25.9) | 2 (3.4) |
| Pharyngolaryngeal pain | 6 (10.3) | 5 (8.6) | 1 (1.7) |
| Hoarseness | 7 (12.1) | 7 (12.1) | 0 |
| Oral mucositis | 4 (9.3) | 3 (5.1) | 1 (1.7) |
| Headache/dizziness | 5 (8.5) | 3 (5.1) | 2 (3.4) |
| Stomach ache | 5 (8.5) | 4 (6.8) | 1 (1.7) |
| Vomiting | 4 (6.8) | 4 (6.8) | 0 |
| Dysphagia | 3 (5.1) | 2 (3.4) | 1 (1.7) |
HCC, hepatocellular carcinoma; HFSR, hand-foot skin reaction.
Discussion
At present, the NCCN Guidelines on Hepatobiliary Cancers 2019 recommend regorafenib, cabozantinib, and ramucirumab as second-line systemic therapies for HCC patients who previously received sorafenib treatment.9 Nevertheless, the median TTP after sorafenib in these trials ranged from 4.1 to 7.2 months.11–13 In contrast, the median TTP after sorafenib treatment in the present study was only 1.0 month. Therefore, the survival benefits of these treatments for patients with sorafenib-resistant HCC are still unclear.
The present study has two important findings. The first is that the median survival time of the apatinib group was significantly longer than that of the supportive care group (7.0 versus 4.0 months, p < 0.001), which indicated that the subsequent apatinib treatment improved the survival outcomes of patients with an initially poor prognosis. This is the first study to compare the outcomes for patients with sorafenib-resistant HCC who either received apatinib or supportive care. Meantime, the ORR of our patients with sorafenib-resistant HCC who received apatinib treatment was 22.4%. The ORR in the present study was relatively higher than that of regorafenib for advanced HCC.10 The ORR was also higher than those reports in patients with sorafenib-refractory HCC.27–29 The most likely explanation for the improvement of survival outcomes by apatinib treatment among patients with sorafenib-resistant HCC may be the antitumor angiogenic actions of apatinib that occur via its selective inhibition of VEGFR-2, with a binding affinity that is 10 times higher than that of sorafenib.15,16 In contrast, because its anti-VEGFR2 ability is one-tenth that of apatinib, sorafenib is inefficient against sorafenib-resistant HCC. In our study, the median OS was 7.0 months in the apatinib treatment group, which was comparable with that of the study (OS: 7.2 months) for patients with sorafenib-refractory HCC who were treated with capecitabine combined with peginterferon α-2a.29 Interestingly, although the ORR of apatinib was relatively higher than that of regorafenib, the OS was significantly shorter than that of regorafenib. The most likely explanations may be that (a) the sample size in our study was significantly smaller than that of RESORCE trial, and the difference of ORR between the two studies may not directly reflected by the numerical value; (b) the imaging tumor response induced by the two drugs may be different. Apatinib, selectively inhibiting VEGFR2, may induce tumor shrinkage (especially for the extrahepatic lesions).23–25 Meanwhile, regorafenib, a multi-tyrosine kinases inhibitor, like sorafenib, may induce tumor necrosis, and most of the patients presented with SD, rarely presenting with PR10; (c) the cohort patients in RESORCE trial had acquired resistance to sorafenib; thus, those patients may have had less aggressive tumors and a longer survival time. We found that in the RESORCE trial, the median and mean duration of sorafenib were 7.8 months and 11.7 months, respectively. The median OS from the start of sorafenib and regorafenib in the regorafenib group and placebo group were 26.0 and 10.6 months and 19.2 and 7.8 months, respectively.10,11 In contrast, in the present study the median OS from the start of sorafenib in apatinib group and supportive group were 7.0 months and 4.0 months, respectively. The median and mean duration of sorafenib were 1.2 months and 1.6 months, respectively. Obviously, the cohort of patients in the RESORCE trial had acquired resistance to sorafenib, which may have had less aggressive tumors and better survival (the OS of patients in placebo group was 19.2 months). In contrast, our patients had primary resistance to sorafenib, namely, sorafenib-resistant HCC, who may have had more aggressive tumors (the OS of patients in supportive group was 4 months). In addition, another report showing the median OS of patients who did not respond to primary treatment of sorafenib was 87 days supported our results.8
The second important finding in the present study is that we identified liver tumor load and extrahepatic spread to be critical predictive factors influencing the outcomes of apatinib treatment. As presented in Figure 3, the median OS times of the patients in the subgroup with a lower liver tumor load and extrahepatic spread were 16.0 months and 15.0 months, respectively. Liver tumor load is a well-known risk factor for patients with HCC. There are reports showing that the final cause of death of most HCC patients is liver function failure resulting from the progression of intrahepatic tumors.30 Other studies have indicated that tumor load is an independent prognostic factor for HCC patients, as confirmed by our results.22,31 Although extrahepatic spread is a known predictive factor for a poor prognosis of HCC patients, it was found to be a favorable predictive factor for patients with sorafenib-resistant HCC after apatinib treatment. The most likely explanations are as follows: (a) first our enrolled advanced HCC patients included either PVTT or extrahepatic spread. In other words, PVTT was a worse factor than extrahepatic spread for patients with advanced HCC treated with apatinib, which is accordant with the fact that PVTT is a robust risk factor for patients with HCC31; (b) all enrolled patients were diagnosed with simultaneous HCC and extrahepatic spread, which may represent a specific group of HCC and have a specific biological nature. To our knowledge, the biological mechanism in the specific group of patients is not clear.32 In turn, based on our results, apatinib, inhibiting VEGFR2 10 times more than sorafenib activity, is effective while sorafenib is ineffective for the specific group of patients. Therefore, we propose a hypothesis that the VEGFR2 signaling pathway may play a key role in hepatocarcinogenesis in patients with HCC and extrahepatic spread. Of course, the mechanism of antitumor angiogenesis is singularly complex, and this hypothesis requires further confirmation in experimental studies. However, several observational studies demonstrating that apatinib treatment provides significant survival benefits to HCC patients with extrahepatic spread support this result.23–25,33
The side effects associated with apatinib have also been a priority in the management of cancer. Similarly, apatinib may induce many side effects on other targeted agents. The most frequent side effects were weight loss, alopecia, HFSR, fatigue, hypertension, and diarrhea, which are easily manageable and can be relieved with a dose reduction or the suspension of apatinib treatment. Although no patients experienced fatal AEs in the present study, the incidence rate of grade 3 or higher AEs reached 25.9%. Therefore, the side effects related to apatinib need to be closely monitored. Interestingly, we observed that patients who respond to apatinib can tolerate apatinib treatment well.
This study has several limitations. First, it is a retrospective study with a small sample size. Second, the data came from a single center. Nevertheless, the center had the largest population of liver cancer patients and had considerable experience in the treatment of liver cancers in South China.34 Third, all included patients had HBV-related HCC, which may be mainly because HBV is the major cause of hepatitis in China. If possible, other causes of HCC, such as hepatitis C virus infection, fatty liver, and alcohol abuse, should be further researched. Fourth, the apatinib treatment decision generally made by the patients may have potential selection bias despite the matched characteristics of the two groups. Although lenvatinib and immunotherapy are emerging and promising treatments, these drugs were not available during the present study periods. Nevertheless, the optional treatment using apatinib may be valuable for improving the survival of patients with sorafenib-resistant HCC who reach the endpoint in this study. Thus, a prospective randomized controlled trial is needed to test the efficacy of apatinib in the specific group of patients with sorafenib-resistant HCC.
In conclusion, this study suggests that subsequent apatinib treatment, compared with supportive care, improves the survival outcomes of patients with sorafenib-resistant advanced HCC. Apatinib treatment may serve as an alternative therapy for patients with advanced sorafenib-resistant HCC, especially for patients who have a lower liver tumor load and extrahepatic spread.
Footnotes
Author contributions: Conception/design: Yingqiang Zhang, Jiaping Li, Yong Chen; Provison of the study material: Yingqiang Zhang, Jiaping Li, Yong Chen; Collection of assembly of data: Guihua Huang, Hongfei Miao, Ze Song, Xiaoying Zhang; Wenzhe Fan, Yu Wang; Data analysis and interpretation: Yingqiang Zhang, Guihua Huang; Manuscript writing or revising: Yingqiang Zhang, Guihua Huang; Final approval of manuscript: All authors.
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Consent to publication: Obtained.
Ethics approval and consent to participate: The study protocol was approved by the Ethics Committees of the Seventh Affiliated Hospital, Sun Yat-sen University (No. 2018SYSUSH-002). Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yingqiang Zhang  https://orcid.org/0000-0003-0945-7405
https://orcid.org/0000-0003-0945-7405
Contributor Information
Yingqiang Zhang, Department of Radiology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China.
Guihua Huang, Digestive Medicine Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China.
Hongfei Miao, Division of Vascular and Interventional Radiology, Department of General Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Ze Song, Department of Oncology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China.
Xiaoying Zhang, Health Management Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China.
Wenzhe Fan, Department of Interventional Oncology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Yu Wang, Department of Interventional Oncology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Jiaping Li, Department of Interventional Oncology, The First Affiliated Hospital, Sun Yat-sen University, 58 Zhongshan Road, Guangzhou, 510080, P.R. China.
Yong Chen, Division of Vascular and Interventional Radiology, Department of General Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou, 510515, P.R. China.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943. [DOI] [PubMed] [Google Scholar]
- 4. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 5. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 6. Kane RC, Farrell AT, Madabushi R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009; 14: 95–100. [DOI] [PubMed] [Google Scholar]
- 7. Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017; 67: 999–1008. [DOI] [PubMed] [Google Scholar]
- 8. Cho JY, Paik YH, Lim HY, et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int 2013; 33: 950–957. [DOI] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network. NCCN guildelines version 2.2019: hepatobiliary cancers. 2019. [DOI] [PubMed] [Google Scholar]
- 10. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 11. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regarafenib for HCC: additional analyses from the phase 3 RESORCE trial. J Hepatol 2018; 69: 353–358. [DOI] [PubMed] [Google Scholar]
- 12. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased a-fetoprotein concentrations (REACH-2): a ranomiszed, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 282–296. [DOI] [PubMed] [Google Scholar]
- 13. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018; 379: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yue J, Lv D, Wang C, et al. Epigenetic silencing of miR-483-3p promotes acquired gefitinib resistance and EMT in EGFR-mutant NSCLC by targeting integrin β3. Oncogene 2018; 37: 4300–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011; 102: 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang C, Qin S. Apatinib targets both tumor and endothelial cells in hepatocellular carcinoma. Cancer Med 2018; 7: 4570–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan W, Yuan G, Fan H, et al. Apatinib combined with transarterial chemoembolization in patients with hepatocellular carcinoma and portal vein tumor thrombus: a multicenter retrospective study. Clin Ther 2019; 41: 1463–1476. [DOI] [PubMed] [Google Scholar]
- 18. Liu Z, Chen J, Fang Y, et al. The efficacy and safety of apatinib treatment for patients with unresectable or relapsed liver cancer: a retrospective study. J Cancer 2018; 9: 2773–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen S, Yu W, Zhang K, et al. Comparison of the efficacy and safety of transarterial chemoembolization with and without apatinib for the treatment of BCLC stage C hepatocellular carcinoma. BMC Cancer 2018; 18: 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther 2017; 18: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Guo Q, Xu R, et al. Efficacy and safety of sorafenib versus apatinib in the treatment of intermediate and advanced hepatocellular carcinoma: a comparative retrospective study. Onco Targets Ther 2018; 11: 3407–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Fan W, Wang Y, et al. Apatinib for patients with sorafenib-refractory advanced hepatitis B virus related hepatocellular carcinoma: results of a pilot study. Cancer Control 2019; 26: 1073274819872216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu H, Ma X, Zhao Y, et al. The excellent antitumor effect of apatinib alone as second-line therapy in a patient with sorafenib-refractory hepatocellular carcinoma a case report. Medicine (Baltimore) 2018; 97: e11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu H, Zhao Y, Wang X. The radiosensitive effect of apatinib for hepatocellualr carcinoma patient with big paraspinal metastasis a case report. Medicine (Baltimore) 2018; 97: e9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Fan W, Wang Y, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist 2015; 20: 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 52–60. [DOI] [PubMed] [Google Scholar]
- 27. Poggi G, Montagna B, Melchiorre F, et al. Hapatic intra-arterial cetuximab in combination with 5-fluorouracil and cisplatin as salvage treatment for sorafenib-refractory hepatocellular carcinoma. Anticancer Res 2011; 31: 3927–3933. [PubMed] [Google Scholar]
- 28. Goldstein R, Yu D, Gillmore R, et al. Oxaliplatin/5-fluorouracil in advanced hepatocellular carcinoma: case report and single-center retrospective review. Future Oncol 2014; 10: 2007–2014. [DOI] [PubMed] [Google Scholar]
- 29. Ogasawara S, Chiba T, Ooka Y, et al. A phase I/II trial of capecitabine combined with peginterferon α-2a in patients with sorafenib-refractory advanced hepatocellular carcinoma. Invest New Drugs 2014; 32: 762–768. [DOI] [PubMed] [Google Scholar]
- 30. Yoo DJ, Kim KM, Jin YJ, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol 2011; 26: 145–154. [DOI] [PubMed] [Google Scholar]
- 31. Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009; 29: 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarocchi M, Polvani S, Marroncini G, et al. Molecular mechanism of hepatitis B virus-induced hepatocarcinogenesis. World J Gastroenterol 2014; 20: 11630–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Du X, Chen D, Lin Z, et al. Efficacy of apatinib for advanced hepatocellular carcinoma with lung metastasis: a retrospective, multicenter study. Presented at 12th Annual Conference of the International Liver Cancer Association, 14-16 September 2018, London. [ePoster] [Google Scholar]
- 34. Zhang Y, Huang G, Wang Y, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist 2016; 21: 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]