Abstract
Background
Schizophrenia is associated with high health care resource utilization and treatment costs.
Objective
This study compared treatment patterns, health care resource utilization, and medical costs before and after a switch from oral antipsychotic drug (risperidone or paliperidone [RIS/PALI]) therapy to the long-acting injectable once-monthly paliperidone palmitate (PP1M) in patients with schizophrenia.
Methods
Data for adult patients (aged ≥18 years) with at least 1 diagnosis of schizophrenia who initiated treatment with oral RIS/PALI ≥6 months before switching and had continuous health plan enrollment during the study period before and after the switch were extracted from the Veterans Health Administration database. Treatment patterns, health care resource utilization, and costs were compared between the period 6 or 12 months before and after switching directly from oral RIS/PALI to PP1M.
Results
The analysis included 676 and 493 patients in the 6-month and 12-month cohorts, respectively. Adherence to oral RIS/PALI during the 12 months preswitch was 11.0% and 22.1% as measured by proportion of days covered and medication possession ratio ≥80%, respectively. During the 12 months postswitch, adherence to PP1M was 27.0% and 35.9%, respectively. Among patients treated with oral RIS/PALI, from 12 months pre- to 12 months post-PP1M switch, fewer all-cause inpatient stays (2.2 vs 1.1, respectively; P < 0.05) and a shorter mean length of inpatient stay (28.1 and 14.0 days, respectively; P < 0.05) were observed. This pattern was similar for both the number of mental health– and schizophrenia-related inpatient stays and length of stay. Compared with 12 months pre-PP1M switch, significantly higher mean numbers of all-cause outpatient visits and pharmacy visits were observed at 12 months postswitch. In line with health care resource utilization findings, at 12 months pre- versus 12 months post-PP1M switch we observed decreases in all-cause inpatient stay costs ($41,886 vs $20,489; P < 0.05) and increases in outpatient visit costs ($22,005 vs $29,069; P < 0.05). Findings for the 6-month cohort followed a similar pattern.
Conclusions
Post-PP1M switch, a decrease in total medical costs fully offset an increase in pharmacy costs, resulting in similar total costs. The findings suggest potential economic benefits of switching patients with schizophrenia from oral RIS/PALI to PP1M in the Veterans Health Administration.
Keywords: Health care resource utilization, Long-acting injectable antipsychotic drugs, Once-monthly paliperidone palmitate, Oral antipsychotic drugs, Real world, Schizophrenia, Switch
Introduction
Schizophrenia is a serious, chronic illness characterized by hallucinations, delusions, negative symptoms, and cognitive issues such as disorganized thinking.1 Approximately 2.4 million people in the United States (∼1.1% of the population) live with schizophrenia,2 which is ranked among the top-25 leading causes of disability worldwide.3 Among US veterans, the prevalence of schizophrenia was reported to be as high as 11%.4 Patients with schizophrenia experience diminished capacity for learning, working, self-care, and interpersonal relationships.5 Furthermore, the economic burden of managing schizophrenia in the United States is estimated to be $155.7 billion, with indirect costs such as caregiving considerably greater than the direct costs.6
The most common first-line treatment for schizophrenia is an oral antipsychotic agent (AP). However, approximately one-third of patients with schizophrenia are considered poorly adherent to oral AP treatment,7 resulting in unnecessary medication or dosage changes and the administration of additional pharmacologic treatments over time.7 Of 1.2 million people who reported having schizophrenia in the United States, 71% were found to be nonadherent to antipsychotic medications.8 Patients who are nonadherent to oral AP therapy are more likely to require hospitalization and emergency care and may be incorrectly labeled as treatment resistant in cases where the physician or clinician is unaware of their nonadherence.7 Accordingly, nonadherence to AP medication increases the burden of treatment of patients with schizophrenia on health care systems and is associated with high treatment costs.9
A review of the evidence suggests that long-acting injectable (LAI) therapies offer the potential to increase patient adherence because patients do not need to remember to take medication daily, and their physicians are assured of adherence based on the injection interval.10 Moreover, a number of studies suggest that LAI treatment decreases health care resource utilization (HRU) and related costs compared with oral APs,7,10, 11, 12 attributed to reduced inpatient admissions compared with treatment with oral APs.14 Once-monthly paliperidone palmitate (PP1M) is indicated for acute and maintenance therapy for patients with schizophrenia. Studies have shown PP1M to have several advantages over oral AP therapies. In patients with prior unsuccessful oral AP treatment, PP1M improved outcomes across symptom severity, subjective well-being, medication satisfaction, illness-related disorders of activity and participation, and patient functioning.15 It has also been demonstrated that PP1M improves outcomes in patients with schizophrenia and delays the time to treatment failure compared with 7 of the most commonly prescribed oral APs (including risperidone [RIS] and paliperidone [PALI]) in a real-world setting.16 However, there are limited real-world data available on patients who have switched from oral RIS/PALI to PP1M. Therefore, the objective of the present cohort analysis of the Veterans Health Administration (VHA) database was to compare treatment adherence, HRU, and overall costs during the 6 and 12 months before and after switching from oral RIS/PALI to PP1M in patients with schizophrenia.
Patients and Methods
Study design and patient selection
A retrospective cohort study with a pre–post analytical design was conducted using data from the VHA, the largest integrated health care system in the United States. In 2014, the US Department of Veterans Affairs provided medical services to nearly 6 million veterans and to more than 700,000 nonveterans (eg, for active duty military and reserve personnel and spouses, via the Civilian Health and Medical Program of the Department of Veterans Affairs, reimbursable services for affiliates, humanitarian care, and occupational immunizations such as hepatitis A and B and flu vaccinations for employees).17 Medical SAS (Statistical Analysis System) datasets spanned from January 1, 2014, to March 31, 2018. The datasets, extracted from the National Patient Care Database, are maintained by the VHA Office of Information at the Austin Information Technology Center (central repository for VHA data). The stability of VHA data sources allows for analysis of the continuity of patient care over multiple years. No identifiable patient information or medical records were disclosed for these analyses, except for those in compliance with applicable law. Institutional review board approval to conduct this study was not required, because the core study did not involve the collection, use, or transmittal of individuals’ identifiable data.
The study population included adult patients (aged ≥18 years) who had a diagnosis of schizophrenia according to the International Classification of Diseases, 9th/10th Revision, Clinical Modification codes: 295.XX (excluding 295.7 schizoaffective disorder) and F20.XX, F21 during the study period. Patients were excluded if they had received any prescription for PP1M during the preswitch period. Patients were required to have switched from oral RIS/PALI to PP1M based on criteria that the last antipsychotic drug before switching to PP1M was oral RIS/PALI. Patients had to have at least 1 claim for oral RIS/PALI or PP1M. The first dispensing of PP1M was defined as the index date.
Six-month cohort
Eligible patients had initiated treatment with oral RIS/PALI during the identification period (July 1, 2014, to September 30, 2017) and switched directly to PP1M. The first dispensing of PP1M was defined as the index date. Patients were required to have continuous health plan enrollment for 6 months before and after the index date.
Twelve-month cohort
Eligible patients had initiated treatment with oral RIS/PALI during the identification period (January 1, 2015, to March 31, 2017) and switched directly to PP1M. The first dispensing of PP1M was defined as the index date. Patients were required to have continuous health plan enrollment for 12 months before and after the switch.
Study measures
Treatment patterns were assessed for both 6 and 12 months pre- and post-PP1M switch, including the use of, and adherence to, any oral or LAI APs, and concomitant medications (including antidepressants, anxiolytics, and mood stabilizers) were recorded. Adherence was estimated by proportion of days covered (PDC), defined as the sum of nonoverlapping days of supply divided by a fixed period (ie, 6 or 12 months), and medication possession ratio (MPR) (defined as the sum of the days of supply during exposure divided by the duration of exposure to therapy). Adherence to therapy was defined as PDC or MPR ≥80%, which was considered an acceptable threshold that suggested very few days without treatment and, thus, reasonably continuous medication use.
All-cause, mental health (MH)– and schizophrenia-related HRU and costs were assessed. Medical claims were considered MH-related if there was an MH disorder (see Supplemental Appendix 1 in the online version at doi:XXXXXXXXXX) and/or schizophrenia diagnosis (International Classification of Diseases, 9th/10th Revision, Clinical Modification code: 295.XX [excluding 295.7 schizoaffective disorder]) in any position (primary or secondary) on the claim. Pharmacy claims were considered MH-related if they included a claim for an AP (see Supplemental Appendices 2 and 3 in the online version at doi:XXXXXXXXXX) and/or other MH-related medication (see Supplemental Appendix 4 in the online version at doi:XXXXXXXXXX).
Statistical analysis
To compare the 6-month and 12-month pre- and post-PP1M switch outcomes, including treatment adherence, HRU, and costs, P values were calculated using the McNemar test for categorical variables and the Wilcoxon signed-rank test for continuous variables to account for the paired nature of the data. Significant differences between pre- and post-PP1M switch values were identified using P values < 0.05. No adjustment for multiplicity was made. All the analyses were conducted using SAS statistical software (version 9.3, SAS Institute, Cary, North Carolina).
Results
Baseline demographic and clinical characteristics
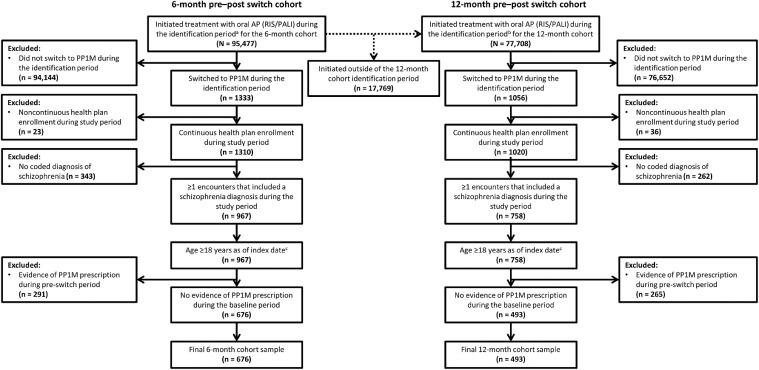
Two cohorts were defined for this analysis: a 6-month pre–post PP1M switch cohort and a 12-month pre–post PP1M switch cohort. In the 6-month cohort, a total of 676 patients were treated with oral RIS/PALI preswitch to PP1M and remained enrolled in their health plan for at least 6 months after switching (Figure 1). In the 12-month cohort, 493 patients were treated with oral RIS/PALI before switching directly to PP1M and remained enrolled in their health plan for at least 12 months after the switch (Figure 1).
Figure 1.
Selection criteria for patients enrolled for 6 and 12 months pre- and 6 and 12 months postswitch to once-monthly paliperidone palmitate (PP1M). AP = antipsychotic; PALI = paliperidone; RIS = risperidone. *The identification period for the 6-month cohort was defined as July 1, 2014, to September 30, 2017. †The identification period for the 12-month cohort was defined as January 1, 2015, to March 31, 2017. ‡The index period was defined as the first dispensing of PP1M.
Baseline demographic and clinical characteristics in the 6-month and 12-month cohorts were similar and are shown in Table 1. The mean (SD) age was 51.6 (13.7) years and 52.0 (13.7) years in the 6-month and 12-month cohorts, respectively. Patients in both groups were predominantly male, 90.8% and 91.3% in the 6-month and 12-month cohorts, respectively. The proportions of white patients were 45.9% and 42.8% and of black patients were 46.7% and 41.8% in the 6-month and 12-month cohorts, respectively.
Table 1.
Demographic and baseline clinical characteristics of patients who initiated treatment with risperidone or paliperidone 6 months or 12 months pre- and postswitch to once-monthly paliperidone palmitate.
| Characteristic* | 6-mo cohort (n = 676) | 12-mo cohort (n = 493) |
|---|---|---|
| Age (y) | 51.6 (13.7) | 52.0 (13.7) |
| Male sex | 614 (90.8) | 450 (91.3) |
| Race | ||
| White | 310 (45.9) | 230 (46.7) |
| Black | 289 (42.8) | 206 (41.8) |
| Other/unknown | 77 (11.4) | 57 (11.6) |
| Comorbid conditions | ||
| Quan-Charlson Comorbidity Index score | 0.74 (1.33) | 0.97 (1.50) |
| Mental health–related comorbidities | ||
| Post-traumatic stress disorder | 149 (22.0) | 124 (25.2) |
| Personality disorder | 53 (7.8) | 61 (12.4) |
| Anxiety | 121 (17.9) | 130 (26.4) |
| Suicide attempt or intentional injuries | 48 (7.1) | 43 (8.7) |
| Tobacco use | 266 (39.4) | 236 (47.9) |
| Bipolar disorder | 119 (17.6) | 109 (22.1) |
| Any depression disorder | 263 (38.9) | 240 (48.7) |
| Substance abuse | 311 (46.0) | 260 (52.7) |
| Mental health–related comorbidities† | 390 (57.7) | 319 (64.7) |
| Nonmental health–related comorbidities | ||
| Obesity | 110 (16.3) | 112 (22.7) |
| Diabetes mellitus | 138 (20.4) | 115 (23.3) |
| CVD – hyperlipidemia | 223 (33.0) | 210 (42.6) |
| CVD – hypertension | 275 (40.7) | 225 (45.6) |
| CVD – coronary artery disease | 25 (3.7) | 25 (5.1) |
| Hepatitis C infection | 23 (3.4) | 26 (5.3) |
| Chronic obstructive pulmonary disease | 76 (11.2) | 76 (15.4) |
CVD = cardiovascular disease.
Values for age and comorbid conditions are presented as mean (SD); values for sex, race, mental health–related comorbidities, and nonmental health–related comorbidities are presented as n (%).
Except for tobacco use and substance abuse.
Most patients had at least 1 diagnosis of a comorbidity in both the 6-month and 12-month pre–post PP1M cohorts, with a Quan Charlson Comorbidity Index score of 0.74 and 0.97, respectively. Substance abuse, tobacco use, and any depression disorder were the most common MH-related comorbidities in both cohorts, while the most common non–MH related comorbidities were cardiovascular disease, diabetes mellitus, and obesity for both cohorts (Table 1).
Treatment patterns
Six months pre- and post-PP1M
The mean (SD) PDC and MPR for patients receiving oral RIS/PALI was 0.4 (0.3) and 0.5 (0.3), respectively, at 6 months before the switch to PP1M. The mean (SD) PDC and MPR decreased to 0.2 (0.3) and 0.3 (0.4), respectively, at 6 months after the switch to PP1M. The reduction in mean PDC and MPR was significant at 6 months after the switch to PP1M (P < 0.0001). Adherence to oral RIS/PALI in the 6 months before the switch to PP1M, defined as proportion of patients achieving ≥80% PDC or MPR, was 16.4% and 25.7%, respectively. During the 6 months after the switch to PP1M, adherence to PP1M was 39.5% and 49.6%, as measured by PDC and MPR ≥80%, respectively (Table 2).
Table 2.
Comparison of treatment patterns 6 months pre- and postswitch to once-monthly paliperidone palmitate (PP1M) among patients who initiated treatment with risperidone or paliperidone (RIS/PALI).
| Treatment pattern | 6 months pre-PP1M switch (n = 676) | 6 months post-PP1M switch (n = 676) |
|---|---|---|
| AP use | ||
| Any oral APs | 676 (100.0)* | 405 (59.9)* |
| Atypical oral APs | 676 (100.0)* | 395 (58.4)* |
| Any LAI APs | 162 (24.0)* | 676 (100.0)* |
| Atypical LAI APs | 124 (18.3)* | 676 (100.0)* |
| Antidepressants | 419 (62.0)* | 405 (59.9)* |
| Anxiolytics | 316 (46.8)* | 305 (45.1)* |
| Mood stabilizers | 326 (48.2)* | 299 (44.2)*† |
| PDC‡ | ||
| Any agent | 0.6 (0.3)§ | 0.8 (0.3)†§ |
| ≥80% | 221 (32.7)* | 430 (63.6)*† |
| RIS/PALI | 0.4 (0.3)§ | 0.2 (0.3)†§ |
| ≥80% | 111 (16.4)* | 45 (6.7)*† |
| PP1M | – | 0.6 (0.3)§ |
| ≥80% | – | 267 (39.5)* |
| MPR|| | ||
| Any agent | 0.7 (0.3)§ | 0.9 (0.2)†§ |
| ≥80% | 328 (48.5)* | 539 (79.7)*† |
| RIS/PALI | 0.5 (0.3)§ | 0.3 (0.4)†§ |
| ≥80% | 174 (25.7)* | 117 (17.3)*† |
| PP1M | – | 0.7 (0.4)§ |
| ≥80% | – | 335 (49.6)* |
AP = antipsychotic; LAI = long-acting injectable; MPR = medication possession ratio; PDC = proportion of days covered.
Value is presented as n (%).
P < 0.05.
Defined as the sum of nonoverlapping days of supply divided by a fixed period (ie, 6 or 12 months) and the MPR. Adherence to therapy was defined as PDC ≥80%.
Value is presented as mean (SD).
Defined as the sum of the days of supply during exposure divided by the duration of exposure to therapy. Adherence to therapy was defined as MPR ≥80%.
Twelve months pre- and post-PP1M
The mean (SD) PDC and MPR for patients receiving oral RIS/PALI was 0.4 (0.3) and 0.4 (0.4), respectively, at 12 months before the switch to PP1M. The mean (SD) PDC and MPR decreased to 0.2 (0.3) and 0.2 (0.3), respectively, at 12 months after the switch to PP1M. The reduction in mean PDC and MPR was significant at 12 months after the switch to PP1M (P < 0.05). Adherence to oral RIS/PALI in the 12 months before the switch was 11.0% and 22.1%, measured by PDC and MPR ≥80%, respectively. During the 12 months after the switch, adherence to PP1M was 27.0% and 35.9%, respectively (Table 3).
Table 3.
Comparison of treatment patterns 12 months before and after switch to once-monthly paliperidone palmitate (PP1M) among patients treated with risperidone or paliperidone (RIS/PALI).
| Treatment patterns | 12 months pre-PP1M switch (n = 493) | 12 months post-PP1M switch (n = 493) |
|---|---|---|
| AP use | ||
| Any oral APs | 493 (100.0)* | 342 (69.4)* |
| Atypical oral APs | 493 (100.0)* | 334 (67.8)* |
| Any LAI APs | 125 (25.4)* | 493 (100.0)* |
| Atypical LAI APs | 93 (18.9)* | 493 (100.0)* |
| Antidepressants | 343 (69.6)* | 328 (66.5)* |
| Anxiolytics | 270 (54.8)* | 266 (54.0)* |
| Mood stabilizers | 261 (52.9)* | 246 (49.9)* |
| PDC† | ||
| Any agent | 0.6 (0.3)‡ | 0.7 (0.3)‡§ |
| ≥80% | 135 (27.4)* | 265 (53.8)*§ |
| RIS/PALI | 0.4 (0.3)‡ | 0.2 (0.3)‡§ |
| ≥80% | 54 (11.0)* | 22 (4.5)*§ |
| PP1M | – | 0.5 (0.3)‡ |
| ≥80% | – | 133 (27.0)* |
| MPR|| | ||
| Any agent | 0.7 (0.3)‡ | 0.9 (0.3)‡§ |
| ≥80% | 230 (46.7)* | 366 (74.2)*§ |
| RIS/PALI | 0.4 (0.4)‡ | 0.2 (0.3)‡§ |
| ≥80% | 109 (22.1)* | 64 (13.0)*§ |
| PP1M | – | 0.6 (0.4)‡ |
| ≥80% | – | 177 (35.9)* |
AP = antipsychotic; LAI = long-acting injectable; MPR = medication possession ratio; PDC = proportion of days covered.
Value is presented as n (%).
Defined as the sum of nonoverlapping days of supply divided by a fixed period (ie, 6 or 12 months) and medication possession ratio. Adherence to therapy was defined as PDC ≥80%.
Value is mean (SD).
P < 0.05.
Defined as the sum of the days of supply during exposure divided by the duration of exposure to therapy. Adherence to therapy was defined as MPR ≥80%.
HRU
Six months pre- and post-PP1M
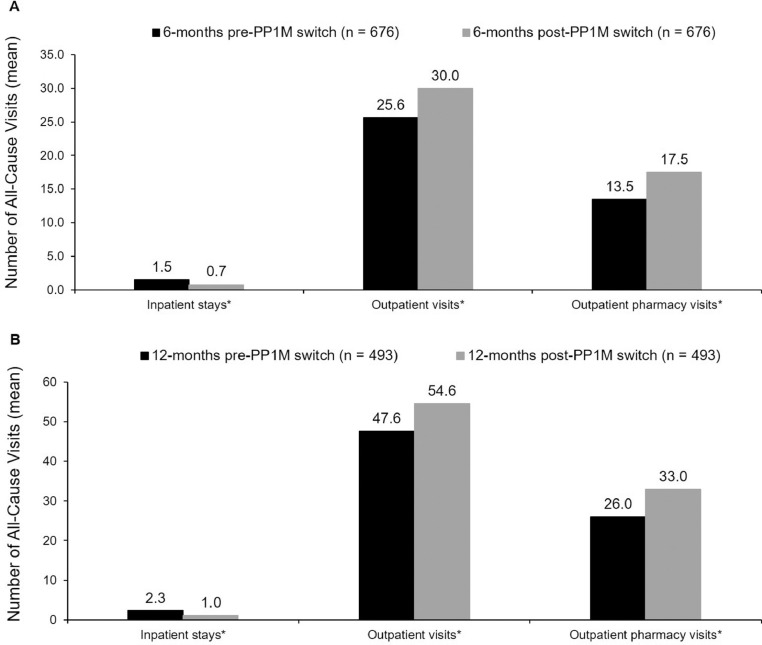
A significant reduction was seen in hospital inpatient use from 6 months pre- to 6 months post-PP1M switch, with reductions in the average number of all-cause inpatient stays (1.5 vs 0.7; P < 0.05) (Figure 2A) and in all-cause inpatient length of stay (18.2 vs 8.0 days; P < 0.05). Similar trends were seen for both the number of MH-related (1.1 vs 0.5; P < 0.05) and schizophrenia-related (0.5 vs 0.2; P < 0.05) inpatient stays, and for the MH-related (17.2 vs 7.8 days; P < 0.05) and schizophrenia-related inpatient length of stay (8.7 vs 3.5 days; P < 0.05). From 6 months pre- to 6 months post-PP1M switch, increases were observed in the total number of all-cause outpatient visits (25.6 vs 30.0; P < 0.05) and the number of all-cause outpatient pharmacy visits (13.5 vs 17.5; P < 0.05) (Figure 2A).
Figure 2.
Comparison of all-cause health care resource utilization during the 6 months (A) and 12 months (B) pre- and post-once-monthly paliperidone palmitate (PP1M) switch among patients who initiated treatment with oral risperidone or paliperidone. *P < 0.05.
Twelve months pre- and post-PP1M
Similarly, in the 12-month cohort, the number of all-cause inpatient stays was significantly reduced from 12 months pre- to 12 months post-PP1M switch (2.3 vs 1.0; P < 0.05) (Figure 2B), with a shorter length of stay (28.1 vs 14.0 days; P < 0.05) post-PP1M switch. This pattern was also observed for both the number of MH-related (1.5 vs 0.8; P < 0.05) and schizophrenia-related (0.6 vs 0.3; P < 0.05) inpatient stays, and for MH-related (27.1 vs 13.8 days; P < 0.05) and schizophrenia-related (13.2 vs 5.7 days; P < 0.05) inpatient length of stay. Comparing 12 months pre- to 12 months post-PP1M switch, increases were seen in the total number of all-cause outpatient visits (47.6 vs 54.6; P < 0.05) and number of all-cause outpatient pharmacy visits (26.0 vs 33.0; P < 0.05) (Figure 2B).
Health care costs
Six months pre- and post-PP1M
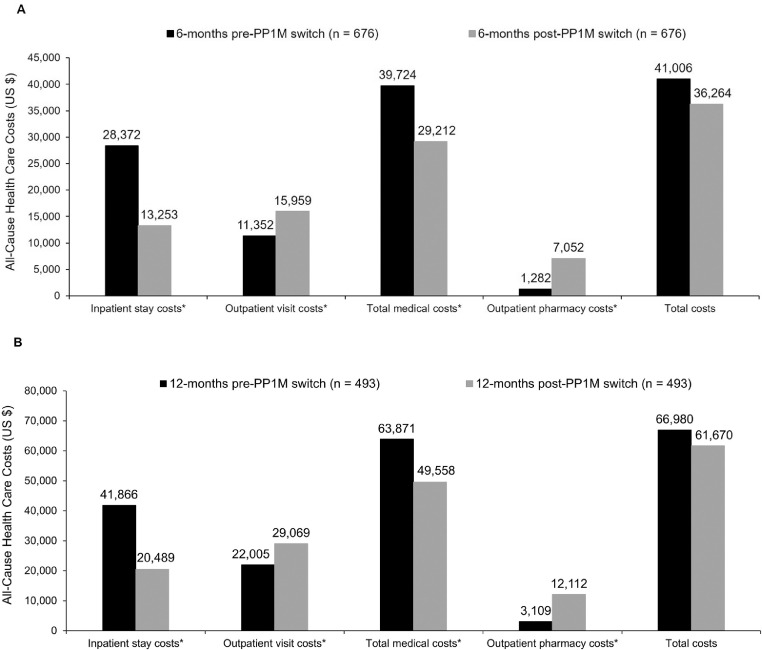
Compared with pre-PP1M switch, all-cause inpatient stay costs decreased markedly post-PP1M switch ($28,372 vs $13,253; P < 0.05); however, there was a rise in outpatient visit costs ($11,352 vs $15,959; P < 0.05) (Figure 3A). Total medical costs (inpatient plus outpatient costs combined) were lower postswitch (decreasing from $39,724 to $29,212; P < 0.05), but the increase in outpatient pharmacy costs ($1282 vs $7052; P < 0.05) resulted in total costs (inpatient, outpatient, and pharmacy costs) showing no significant change ($41,006 vs $36,264; P = 0.1617) (Figure 3A). MH-related costs and schizophrenia-related costs showed a similar pattern, with a slight decrease in MH-related total costs ($38,612 vs $33,596; P = 0.0842), and a modest increase in schizophrenia-related total costs ($19,309 vs $20,214; P < 0.05) being observed from 6 months pre- to 6 months post-PP1M switch.
Figure 3.
Comparison of all-cause health care costs during the 6 months (A) and 12 months (B) pre- and post-switch to once-monthly paliperidone palmitate (PP1M) dosing among patients who initiated treatment with oral risperidone or paliperidone. Total costs = outpatient, inpatient, and pharmacy costs; total medical costs = outpatient and inpatient costs. *P < 0.05.
Twelve months pre- and post-PP1M
Overall, the 12-month cohort showed similar results to the 6-month cohort, with pre- versus post-PP1M switch showing decreases in all-cause inpatient stay costs ($41,886 vs $20,489; P < 0.05) and increases in outpatient visit costs ($22,005 vs $29,069; P < 0.05) (Figure 3B). Total medical costs were lower after the switch (decreasing from $63,871 to $49,558; P < 0.05), but outpatient pharmacy costs increased from 12 months pre- to 12 months post-PP1M switch ($3109 vs $12,112; P < 0.05). All-cause total costs (inpatient, outpatient, and pharmacy costs) decreased from $66,980 to $61,670 (P = 0.8638) between 12 months pre- and post-PP1M switch (Figure 3B). The total MH-related costs and the total schizophrenia-related costs decreased from $61,978 to $55,786 (P = 0.7640) and increased from $30,108 to $31,904 (P < 0.05), respectively, between 12 months pre- and post-PP1M switch.
Discussion
The present study of the VHA database found that, among patients switching from oral RIS/PALI therapy to PP1M, those with schizophrenia had higher adherence, with lower HRU and no increase in all-cause total costs. The current analysis provides evidence that patients treated with LAI APs experienced improved adherence and significantly lower total medical costs compared with oral AP therapy, which is associated with relatively low levels of treatment adherence,18, 19, 20 high HRU,21,22 and high costs.12,23 The design of the current study allows a direct comparison of how costs changed in the same patients before and after switching from an oral AP to an LAI with a similar mechanism of action.
Oral APs have remained the first-line pharmacologic treatment option for patients with schizophrenia, with LAI APs typically reserved for patients believed to be nonadherent to oral treatment.10 However, the real-world effectiveness of LAIs has been demonstrated after their first hospital admission compared with oral APs for patients with schizophrenia.24 Furthermore, evidence from retrospective, cohort studies demonstrate that LAI treatment decreases HRU (inpatient stays) and related costs compared with oral APs.7,11, 12, 13 The lower medical costs when switching patients to PP1M observed in the current study are similar to findings reported in an analysis of costs among recently diagnosed patients with schizophrenia treated with oral APs or LAIs. In a real-world observational study, treatment with LAIs was associated with fewer inpatient admissions and fewer days spent in the hospital, and therefore significantly lower monthly inpatient costs ($4007 and $8769 for the LAI and oral AP cohorts, respectively).25 Patients treated with LAIs had higher medication costs compared with patients treated with oral APs, but both cohorts had similar total medical costs.25 US veterans with schizophrenia are reported to occupy more hospital beds at any given time than veterans with any other illness.26 A retrospective, longitudinal study conducted among US veterans revealed that the average annual all-cause total health care costs among US veterans with schizophrenia was $78,589 and $82,895 for patients treated with PP1M and oral APs, respectively.27 Furthermore, a subanalysis from the present study, conducted in patients with at least 1 prior hospitalization, reported that switching from oral RIS/PALI to PP1M may significantly improve HRU and provide potential cost savings in VHA database members with schizophrenia.28
Adherence for patients with schizophrenia is crucial and is a well-recognized challenge with oral APs that may lead to relapses and hospitalization (or rehospitalization), and hence increased medical-related costs.11,29, 30, 31 Patients treated with LAIs were less likely to be rehospitalized compared with patients treated with oral APs after a 2-year follow-up.24 The current retrospective study, conducted using data from the VHA database, shows that patients switching from oral RIS/PALI to PP1M have higher rates of adherence to PP1M than to oral RIS/PALI as measured by PDC and MPR, and physicians can be assured that patients are covered for the duration of the injection instead of needing to rely on patients to take their oral medication every day. In this retrospective study involving patients from the VHA database, increased adherence was associated with better control of schizophrenia, and, after the switch to PP1M, patients had fewer all-cause, MH-related, and schizophrenia-related inpatient stays. A higher number of outpatient visits was seen in the current study, but this is consistent with additional provider visits for administration of PP1M. Similar results have been seen in other studies of LAIs.14,25 With the administration of LAIs, such as PP1M, patients may have improved adherence owing to a sense of responsibility about keeping outpatient appointments, and without the burden of remembering daily oral medications. The findings from this VHA population suggest that these factors may contribute to enhanced management of schizophrenia in clinical practice.
The cost of acquiring LAIs may be a potential barrier to their use, and indeed in the current study the cost of outpatient pharmacy visits was higher after the switch to PP1M. However, although the acquisition cost of LAIs is higher, LAIs are associated with savings in inpatient costs and HRU.14 A retrospective claims database study reported that schizophrenia-related hospital costs during the 12-month period post-LAI treatment initiation decreased by an average of $5981 compared with the 12 months preceding treatment, whereas patients treated with oral APs saw an average increase in costs of $758.13 Furthermore, long-term LAI treatment (≥180 days) is associated with fewer hospitalizations and fewer days spent in the hospital compared with short-term LAI treatment (30–79 days), suggesting that the economic benefits associated with LAI treatment are increased over time.7 In the current study, patients had fewer inpatient stays, a decreased length of hospital stay, and lower inpatient and total medical costs, adding to the growing body of evidence that LAIs offer clinically meaningful gains in symptomatic and functional improvement in patients with schizophrenia.32
Limitations
Data from the current study must be interpreted with caution because retrospective administrative claims database studies are subject to inherent limitations, such as coding errors or diagnoses entered for administrative processing purposes rather than for clinical completeness. Furthermore, certain information (such as clinical and disease-specific parameters, including response to prior pharmacotherapy) is not readily available in claims data, and this information could influence study outcomes. For example, adherence is evaluated based on the presence of a claim for a filled prescription for an oral AP, which does not indicate that the medication was in fact taken or that is was taken as prescribed. Additional limitations include the minimal follow-up period of the study and a lack of patient characteristic assessment immediately pre- and postinitiation of treatment with PP1M. Specifically, the baseline period of 12 months may not have captured the first use of oral RIS/PALI for the subset of patients who have been using oral RIS/PALI for a longer duration. Furthermore, 23% of patients were being treated with an LAI before treatment switch and the reasons for switching were not documented. Likewise, the study did not account for changes in patient characteristics pre- and post-PP1M switch, which ideally should have been examined alongside assessing changes in outcomes.
Moreover, the current study used broad inclusion criteria and studied cohorts based on the available time of enrollment only. Subgroup analyses may identify patient groups with a greater or lesser benefit from switching from an oral AP to an LAI. Therefore, findings may not be generalizable to the overall US population, because the study utilized data from patients obtaining health care through the VHA system. Additionally, the current study sample also consisted of a high proportion of men with at least 1 prior hospitalization and who were aged 55 years and older and therefore may have had different comorbidities compared with the general population.
Conclusions
In this study, after the switch to PP1M from oral RIS/PALI alone, patients with schizophrenia demonstrated better adherence to antipsychotic medication and lower HRU. After PP1M switch, a decrease in total medical costs fully offset an increase in pharmacy costs, resulting in similar total costs. Specifically, all-cause inpatient hospitalizations and the number of days spent in the hospital were significantly decreased after the switch. Additionally, inpatient costs and total medical costs were significantly lower, with a significant reduction in all-cause medical costs fully offsetting the incremental all-cause pharmacy costs in both the 6- and 12-month post-PP1M initiation cohorts. Further research is necessary to determine whether similar findings would be observed in patients with at least 1 prior hospitalization.
Declaration of Competing Interest
Charmi Patel and Antoine El Khoury are employees of Janssen Pharmaceuticals and shareholders in its parent company, Johnson & Johnson. Ahong Huang, Li Wang, and Richa Bashyal are employees of STATinMED Research. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
This research was funded by Janssen Scientific Affairs, LLC. The authors thank ApotheCom (Yardley, Pennsylvania) for medical writing and editorial services, which were funded by Janssen Scientific Affairs. Ahong Huang, Li Wang, and Richa Bashyal were involved in the data analysis. All authors were involved in writing the manuscript and approved the final article.
References
- 1.Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P T. 2014;39:638-645. [PMC free article] [PubMed]
- 2.National Alliance on Mental Illness Schizophrenia Fact Sheet. 2017 . Available at: https://www.nami.org/Learn-More/Mental-Health-By-the-Numbers. Accessed May 13, 2019. [Google Scholar]
- 3.Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–373. doi: 10.2147/NDT.S96649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little JT. Prevalence of mental health disorders in geriatric U.S. military veterans. Am J Geriatr Psychiatry. 2018;26:546–547. doi: 10.1016/j.jagp.2018.01.204. [DOI] [PubMed] [Google Scholar]
- 5.Lafeuille MH, Gravel J, Lefebvre P. Patterns of relapse and associated cost burden in schizophrenia patients receiving atypical antipsychotics. J Med Econ. 2013;16:1290–1299. doi: 10.3111/13696998.2013.841705. [DOI] [PubMed] [Google Scholar]
- 6.Cloutier M, Aigbogun MS, Guerin A. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77:764–771. doi: 10.4088/JCP.15m10278. [DOI] [PubMed] [Google Scholar]
- 7.Correll CU, Citrome L, Haddad PM. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77:1–24. doi: 10.4088/JCP.15032su1. [DOI] [PubMed] [Google Scholar]
- 8.Desai R, Nayak R. Effects of medication nonadherence and comorbidity on health resource utilization in schizophrenia. J Manag Care Spec Pharm. 2019;25:37–46. doi: 10.18553/jmcp.2019.25.1.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dilla T, Ciudad A, Alvarez M. Systematic review of the economic aspects of nonadherence to antipsychotic medication in patients with schizophrenia. Patient Prefer Adherence. 2013;7:275–284. doi: 10.2147/PPA.S41609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biagi E, Capuzzi E, Colmegna F. Long-acting injectable antipsychotics in schizophrenia: literature review and practical perspective, with a focus on aripiprazole once-monthly. Adv Ther. 2017;34:1036–1048. doi: 10.1007/s12325-017-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafeuille MH, Tandon N, Tiggelaar S. Economic impact in medicaid beneficiaries with schizophrenia and cardiometabolic comorbidities treated with once-monthly paliperidone palmitate vs. oral atypical antipsychotics. Drugs Real World Outcomes. 2018;5:81–90. doi: 10.1007/s40801-018-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafeuille MH, Grittner AM, Fortier J. Comparison of rehospitalization rates and associated costs among patients with schizophrenia receiving paliperidone palmitate or oral antipsychotics. Am J Health Syst Pharm. 2015;72:378–389. doi: 10.2146/ajhp140219. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Wong B, Offord S, Mirski D. Healthcare cost reductions associated with the use of LAI formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Res. 2013;40:355–366. doi: 10.1007/s11414-013-9329-z. [DOI] [PubMed] [Google Scholar]
- 14.Young-Xu Y, Duh MS, Muser E. Impact of paliperidone palmitate versus oral atypical antipsychotics on health care resource use and costs in veterans with schizophrenia. J Clin Psychiatry. 2016;77:e1332–e1341. doi: 10.4088/JCP.16m10745. [DOI] [PubMed] [Google Scholar]
- 15.Hargarter L, Cherubin P, Bergmans P. Intramuscular long-acting paliperidone palmitate in acute patients with schizophrenia unsuccessfully treated with oral antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:1–7. doi: 10.1016/j.pnpbp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Alphs L, Benson C, Cheshire-Kinney K, Lindenmayer JP, Mao L, Rodriguez SC. Real-world outcomes of -paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: A randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76:554–561. doi: 10.4088/JCP.14m09584. [DOI] [PubMed] [Google Scholar]
- 17.Bagalman E.The number of veterans that use VA health care services: a fact sheet. 2014. https://fas.org/sgp/crs/misc/R43579.pdf. Accessed March 19, 2019.
- 18.Anderson JP, Icten Z, Alas V, Benson C, Joshi K. Comparison and predictors of treatment adherence and remission among patients with schizophrenia treated with paliperidone palmitate or atypical oral antipsychotics in community behavioral health organizations. BMC Psychiatry. 2017;17:346. doi: 10.1186/s12888-017-1507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21:754–768. doi: 10.18553/jmcp.2015.21.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilon D, Muser E, Lefebvre P, Kamstra R, Emond B, Joshi K. Adherence, healthcare resource utilization and Medicaid spending associated with once-monthly paliperidone palmitate versus oral atypical antipsychotic treatment among adults recently diagnosed with schizophrenia. BMC Psychiatry. 2017;17:207. doi: 10.1186/s12888-017-1358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSarkissian M, Lefebvre P, Joshi K. Health care resource utilization and costs associated with transitioning to 3-month paliperidone palmitate among US Veterans. Clin Ther. 2018;40:1496–1508. doi: 10.1016/j.clinthera.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 22.MacEwan JP, Kamat SA, Duffy RA. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatric Serv. 2016;67:1183–1188. doi: 10.1176/appi.ps.201500455. [DOI] [PubMed] [Google Scholar]
- 23.Pilon D, Tandon N, Lafeuille MH. Treatment patterns, health care resource utilization, and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther. 2017;39:1972–1985. doi: 10.1016/j.clinthera.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. doi: 10.1176/appi.ajp.2011.10081224. [DOI] [PubMed] [Google Scholar]
- 25.Shah A, Xie L, Kariburyo F, Zhang Q, Gore M. Treatment patterns, healthcare resource utilization and costs among schizophrenia patients treated with long-acting injectable versus oral antipsychotics. Adv Ther. 2018;35:1994–2014. doi: 10.1007/s12325-018-0786-x. [DOI] [PubMed] [Google Scholar]
- 26.Marder SR.Effective treatment for schizophrenia. United States Department of Veterans Affairs. https://www.hsrd.research.va.gov/publications/internal/pm_v7_n1.pdf. Accessed May 8, 2019. 2002.
- 27.Lefebvre P, Muser E, Joshi K. Impact of paliperidone palmitate versus oral atypical antipsychotics on health care resource use and costs in veterans with schizophrenia and comorbid substance abuse. Clin Ther. 2017;39:1380–1395. doi: 10.1016/j.clinthera.2017.05.356. e1384. [DOI] [PubMed] [Google Scholar]
- 28.El Khoury AC, Pilon D, Morrison L, Shak N, Llaneza A, Kim E, Lefebvre P. Projecting the long-term economic impact of once-monthly paliperidone palmitate versus oral atypical antipsychotics in Medicaid patients with schizophrenia. J Manag Care Spec Pharm. 2020;26:176–185. doi: 10.18553/jmcp.2020.26.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Amos TB, Gutkin SW, Lodowski N, Giegerich E, Joshi K. A systematic literature review of the clinical and health economic burden of schizophrenia in privately insured patients in the United States. Clinicoecon Outcomes Res. 2018;10:309–320. doi: 10.2147/CEOR.S156308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ascher-Svanum H, Zhu B, Faries DE. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2. doi: 10.1186/1471-244X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higashi K, Medic G, Littlewood KJ, Diez T, Granstrom O, De HM. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3:200–218. doi: 10.1177/2045125312474019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montemagni C, Frieri T, Rocca P. Second-generation long-acting injectable antipsychotics in schizophrenia: patient functioning and quality of life. Neuropsychiatr Dis Treat. 2016;12:917–929. doi: 10.2147/NDT.S88632. [DOI] [PMC free article] [PubMed] [Google Scholar]