This post hoc analysis of a randomized clinical trial examines the volumetric change of intraretinal fluid and subretinal fluid in patients with diabetic macular edema during anti–vascular endothelial growth factor treatment using deep learning algorithms.
Key Points
Question
Is it possible to automatically quantify intraretinal and subretinal fluid in eyes with diabetic macular edema using a deep learning algorithm in large optical coherence tomographic data sets and evaluate the associations of anti–vascular endothelial growth factor treatment with intraretinal and subretinal fluid volumetric changes?
Findings
In this post hoc analysis of a randomized clinical trial in which intraretinal and subretinal fluid was quantified using a fully automated algorithm, aflibercept and ranibizumab were associated with a greater reduction of intraretinal fluid than was bevacizumab. No difference among anti–vascular endothelial growth factor agents was observed regarding reduction of subretinal fluid.
Meaning
Automated quantification of intraretinal and subretinal fluid may be an objective approach to assess the effect of treatment for diabetic macular edema.
Abstract
Importance
Large amounts of optical coherence tomographic (OCT) data of diabetic macular edema (DME) are acquired, but many morphologic features have yet to be identified and quantified.
Objective
To examine the volumetric change of intraretinal fluid (IRF) and subretinal fluid (SRF) in DME during anti–vascular endothelial growth factor treatment using deep learning algorithms.
Design, Setting, and Participants
This post hoc analysis of a randomized clinical trial, the Diabetic Retinopathy Clinical Research Network (protocol T), assessed 6945 spectral-domain OCT volume scans of 570 eyes from 570 study participants with DME. The original trial was performed from August 21, 2012, to October 18, 2018. This analysis was performed from December 7, 2017, to January 15, 2020.
Interventions
Participants were treated according to a predefined, standardized protocol with aflibercept, ranibizumab, or bevacizumab with or without deferred laser.
Main Outcomes and Measures
The association of treatment with IRF and SRF volumes and best-corrected visual acuity (BCVA) during 12 months using deep learning algorithms.
Results
Among the 570 study participants (302 [53%] male; 369 [65%] white; mean [SD] age, 43.4 [12.6] years), the mean fluid volumes in the central 3 mm were 448.6 nL (95% CI, 412.3-485.0 nL) of IRF and 36.9 nL (95% CI, 27.0-46.7 nL) of SRF at baseline and 161.2 nL (95% CI, 135.1-187.4 nL) of IRF and 4.4 nL (95% CI, 1.7-7.1 nL) of SRF at 12 months. The presence of SRF at baseline was associated with a worse baseline BCVA Early Treatment Diabetic Retinopathy Study (ETDRS) score of 63.2 (95% CI, 60.2-66.1) (approximate Snellen equivalent of 20/63 [95% CI, 20/50-20/63]) in eyes with SRF vs 66.9 (95% CI, 65.7-68.1) (approximate Snellen equivalent, 20/50 [95% CI, 20/40-20/50]) without SRF (P < .001) and a greater gain in ETDRS score (0.5; 95% CI, 0.3-0.8) every 4 weeks during follow-up in eyes with SRF at baseline vs 0.4 (95% CI, 0.3-0.5) in eyes without SRF at baseline (P = .02) when adjusted for baseline BCVA. Aflibercept was associated with greater reduction of IRF volume compared with bevacizumab after the first injection (difference, 79.8 nL; 95% CI, 5.3-162.5 nL; P < .001) and every 4 weeks thereafter (difference, 10.4 nL; 95% CI, 0.7-20.0 nL; P = .004). Ranibizumab was associated with a greater reduction of IRF after the first injection compared with bevacizumab (difference, 75.2 nL; 95% CI, 1.4-154.7 nL; P < .001).
Conclusions and Relevance
Automated segmentation of fluid in DME revealed that the presence of SRF was associated with lower baseline BCVA but with good response to anti–vascular endothelial growth factor therapy. These automated spectral-domain OCT analyses may be used clinically to assess anatomical change during therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT01627249.
Introduction
The pathomechanisms of diabetic macular edema (DME), a common and visually detrimental complication of diabetic retinopathy (DR), are still not completely understood. Some of the most important signaling molecules involved in the process of DR, such as vascular endothelial growth factor (VEGF), interleukin 6, interleukin 10, tumor necrosis factor α, osteopontin, monocyte chemotactic protein 1, or intercellular adhesion molecule 1, have been identified.1,2,3,4 However, the sequence of events that ultimately leads to the accumulation of intraretinal fluid (IRF) and subretinal fluid (SRF) remains to be elucidated.
Anti-VEGF therapy has proved to be effective in the treatment of DME, and multiple randomized clinical trials have found functional gains and superiority compared with laser treatment in eyes with DME.5,6,7,8,9,10,11,12 The Diabetic Retinopathy Clinical Research Network, now called the DRCR Retina Network, conducted a multicenter randomized clinical trial (protocol T) to compare the efficacy of the 3 most commonly used anti-VEGF agents: ranibizumab, bevacizumab, and aflibercept.13 The study also provided data on the prognostic value of baseline visual acuity and central retinal subfield thickness for functional gains during therapy.14 Furthermore, the study produced large amounts of imaging data, providing valuable information about retinal anatomical changes during standardized therapy. However, many morphologic features have yet to be evaluated.
Artificial intelligence (AI) has recently become available to a larger community of researchers worldwide owing to improvements in computer hardware and software and represents a breakthrough in big data management. Deep learning algorithms are a novel AI-based approach toward automated image analysis and allow evaluation of large amounts of data, such as spectral-domain optical coherence tomography (SD-OCT) volume scans.15 Because IRF and SRF are associated with DME, automated and objective quantification is essential not only for a more profound understanding of the disease but also for objective evaluation of treatment efficacy (ie, resolution of IRF and SRF). The purpose of this study was to automatically identify and quantify SRF and IRF during 12 months and their association with best-corrected visual acuity (BCVA) using SD-OCT data from the DRCR.net protocol T trial.
Methods
Patient Population, Study Inclusion, and Treatment
We performed a post hoc analysis of a multicenter randomized clinical trial (DRCR.net protocol T trial [NCT01627249]) and included 570 study eyes of 570 patients. Of the 660 eyes included in the original study, 90 eyes were not included in this analysis because either time-domain OCT imaging had been performed (n = 60) or because of low image quality of SD-OCT images at baseline (n = 30). The original study was performed from August 21, 2012, to October 18, 2018. This analysis was performed from December 7, 2017, to January 15, 2020. The trial adhered to the Declaration of Helsinki,16 and all participants provided written informed consent before inclusion; data were deidentified. A detailed description of the methods for DRCR.net protocol T and statistical analysis plan has been published elsewhere.13 Anonymized SD-OCT volume images and clinical information assessed every 4 weeks for 1 year were included in the analysis. Main outcome measures, inclusion and exclusion criteria, and study examinations have been reported previously.13 Thus, only procedures relevant for the analysis of this study are described. Approval for this post hoc analysis was obtained from the ethics committee at the Medical University of Vienna.
Image Processing
The preprocessing steps contained automatic alignment and registration of the SD-OCT scans to obtain anatomical correspondence of all included volumes and an automatic segmentation of fluid compartments in the scans. An intrapatient registration was performed in all follow-up SD-OCT scans using vessel structures followed by interpatient registration at the full cohort level by centering the scans around the fovea as described previously.17 We validated the correctness of registration in all cases by overlaying the en face projections of follow-up scans and by validating the correct placement of fovea position. A deep learning convolutional neural network approach was applied, which classifies voxels as background, IRF, or SRF, as reported previously.18 Accuracy and reproducibility of the algorithm have been described previously, and the fluid quantification during anti-VEGF therapy was applied in a large population of patients with neovascular age-related macular degeneration.19 The IRF was defined as round, nonreflective to minimally reflective spaces (cysts) within the neuroretina, and SRF was defined as areas of nonreflective space between the posterior boundary of the neuroretina and the retinal pigment epithelium. Diffuse retinal thickening was not taken into account. IRF and SRF volumes were computed for the central fovea (circle with a 1-mm diameter) and for the parafovea (ring between 1 and 3 mm surrounding the fovea). The IRF in the fovea, SRF in the fovea, IRF in the parafovea, and SRF in the parafovea were measured. By applying the preprocessing steps on the entire longitudinal data set, individual series of fluid compartments receiving treatment compared with the entire cohort were obtained.
Statistical Analysis
On the basis of the previously reported significant effect of baseline BCVA in the treatment group comparison at 12 months, we chose to perform separate analyses for eyes with better (Early Treatment Diabetic Retinopathy Study [ETDRS] score ≥69; equivalent to Snellen ≥20/40) and worse (ETDRS score <69; equivalent to ≤20/50) BCVA at baseline in addition to the analysis of all study eyes.13 To assess changes in BCVA and fluid volume over time, we used the framework of linear repeated-measures mixed-effects models.20 These models allow an estimation of mean effects while accounting for the correlation between the repeated measurements of each patient. Considering that change over time was not linear, we used a piecewise linear model with the split point at the first follow-up visit, resulting in separate slopes for the first visit and the follow-up visits. This approach allowed us to capture the rapid changes in BCVA and fluid that occurred after the first anti-VEGF treatment more accurately than in a simple linear model.
Fixed effects contain the intercept and the 2 slope covariates. Depending on the research question, additional covariates were added and 3 models were calculated: (1) continuous variables of IRF in the fovea, SRF in the fovea, IRF in the parafovea, and SRF in the parafovea to determine the effect of fluid on vision, (2) the categorical variable of SRF at baseline (yes or no) to assess treatment response for the 2 groups, and (3) anti-VEGF agents (bevacizumab, ranibizumab, or aflibercept) to contrast the effect of agents on BCVA and fluid reduction. These 3 models were chosen to analyze in detail the most important and clinically relevant surrogate parameters for function (ie, ETDRS letter score) and retinal morphologic features (IRF and SRF) as well as the effects of different anti-VEGF agents.
In the random-effects structure, we included the intercept and both slopes, accounting for patient-specific BCVA and fluid volumes at the first visit (random intercept) and their change during treatment (random slopes). The mixed-effects framework allows an interpretation of the fixed-effects covariates as population mean effects in a similar way as covariates in ordinary linear regression. All parameters were determined by restricted maximum likelihood. Residual plots were used to validate the models’ assumptions. Goodness of fit was measured using coefficient of determination (R2) for mixed-effects models21,22 that consist of marginal R2, considering variance explained by fixed effects only, and conditional R2, considering both fixed and random effects. The 95% CIs were obtained by bootstrap sampling with 500 simulations. Both t statistics and P values were calculated based on Satterthwaite approximations.23 A 2-sided P < .05 was considered statistically significant. All computation was performed with R software, version 3.4.2 (R Foundation for Statistical Computing) using lme4 package, version 1.1.18.1).24
Results
In this post hoc analysis, we included 6945 SD-OCT volume scans of 570 eyes of 570 patients with DME (302 [53%] male; mean [SD] age, 43.4 [12.6] years). Of the 570 participants, 369 (65%) were white, 93 (16%) were African American, 87 (15%) were Hispanic, 7 (1%) were Asian, 6 (1%) were of more than 1 race/ethnicity, 4 (<1%) were Pacific Islanders, 3 were of unknown race/ethnicity (<1%), and 1 (<1%) was American Native. A total of 92 follow-up scans (1.3%) were excluded because of failed preprocessing attributable to inferior image quality or acquisition errors. At baseline, all 570 eyes (100%) had IRF and 235 (41%) had SRF. A total of 86 of 190 (45%) eyes in the aflibercept group, 77 of 188 eyes (41%) in the ranibizumab group, and 72 of 192 (38%) eyes in the bevacizumab group had SRF at baseline. At month 12, a total of 452 of 514 eyes (88%) still had IRF, and 82 of 514 eyes (16%) had SRF. The mean fluid volumes in the central 3 mm were 448.6 nL (95% CI, 412.3-485.0 nL) of IRF and 36.9 nL (95% CI, 27.0-46.7 nL) of SRF at baseline and 161.2 nL (95% CI, 135.1-187.4 nL) of IRF and 4.4 nL (95% CI, 1.7-7.1 nL) of SRF at 12 months. Visual acuity and IRF and SRF volumes at baseline and month 12 are listed in Table 1.
Table 1. BCVA, IRF, and SRF in All Study Eyes and in the High and Low VA Groups.
| Variable | Mean (95% CI) | P valuec | |||
|---|---|---|---|---|---|
| Better and worse baseline VA (n = 570) | Better baseline VAa (n = 298) | Worse baseline VAb (n = 272) | |||
| Mean BCVA at baseline, ETDRS score [Snellen equivalent] | 65.3 [20/50] (66.2 to 64.4) | 73.3 [20/40] (74.1 to 72.5) | 56.9 [20/80] (58.9 to 54.9) | NA | |
| Mean BCVA at 1 y, ETDRS score [Snellen equivalent] | 77.2 [20/32] (78.1 to 76.2) | 82.3 [20/25] (83.5 to 81.1) | 73.7 [20/32] (76.6 to 70.8) | <.001 | |
| BCVA change | |||||
| First 4 wk | 6.0 (5.4 to 6.6) | 4.2 (3.4 to 5.0) | 8.5 (6.6 to 10.6) | <.001 | |
| Every 4 wk | 0.5 (0.4 to 0.5) | 0.3 (0.3 to 0.4) | 0.5 (0.4 to 0.7) | <.001 | |
| IRF | |||||
| Mean IRF at baseline, nL | 448.6 (412.3 to 485.0) | 355.4 (317.9 to 392.9) | 550.8 (488.5 to 613.0) | <.001 | |
| Central 1 mm | 111.7 (104.7 to 118.7) | 96.0 (87.9 to 104.0) | 129.0 (117.5 to 140.5) | NA | |
| Parafovea | 336.9 (306.2 to 367.6) | 259.5 (227.9 to 291.0) | 421.8 (369.2 to 474.4) | NA | |
| Mean IRF at 1 y, nL | 161.2 (135.1 to 187.4) | 130.7 (102.8 to 158.7) | 201.6 (152.4 to 250.8) | .05 | |
| Central 1 mm | 42.7 (37.0 to 48.4) | 38.3 (31.7 to 44.9) | 50.9 (40.4 to 61.3) | NA | |
| Parafovea | 118.5 (97.3 to 139.8) | 92.4 (69.6 to 115.2) | 150.7 (110.9 to 190.5) | NA | |
| IRF change, nL | |||||
| First 4 wk | −199.4 (−173.1 to −221.6) | −150.5 (−111.6 to −185.4) | −270.7 (−181.7 to −357.1) | <.001 | |
| Every 4 wk | −6.7 (−5.2 to −8.3) | −5.7 (−3.4 to −8.0) | −6.9 (−1.6 to −12.4) | .46 | |
| SRF | |||||
| Mean SRF at baseline, nL | 36.9 (27.0 to 46.7) | 20.3 (11.8 to 28.9) | 55.0 (36.9 to 73.1) | <.001 | |
| Central 1 mm | 13.5 (10.9 to 16.2) | 9.6 (6.8 to 12.5) | 17.8 (13.2 to 22.5) | NA | |
| Parafovea | 23.3 (15.7 to 31.0) | 10.7 (4.5 to 16.9) | 37.2 (22.8 to 51.6) | NA | |
| Mean SRF at 1 y, nL | 4.4 (1.7 to 7.1) | 2.1 (0.8 to 3.3) | 7.4 (1.4 to 13.5) | NA | |
| Central 1 mm | 1.8 (1.0 to 2.7) | 1.3 (0.4 to 2.2) | 2.5 (0.9 to 4.2) | NA | |
| Parafovea | 2.6 (0.5 to 4.6) | 0.8 (0.3 to 1.3) | 4.9 (0.2 to 9.6) | NA | |
| SRF change, nL | |||||
| First 4 wk | −23.7 (−17.3 to −30.1) | −13.3 (−3.8 to −23.6) | −37.9 (−13.7 to −62.9) | .001 | |
| Every 4 wk | −0.7 (−0.3 to −1.1) | −0.3 (−0.9 to 0.2) | −1.2 (−2.6 to 0.4) | .04 | |
Abbreviations: BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; IRF, intraretinal fluid; NA, not applicable; SRF, subretinal fluid; VA, visual acuity.
Better VA was defined as an ETDRS score of 69 or better (approximate Snellen equivalent 20/32 to 20/40) at baseline.
Worse VA was defined as an ETDRS score of 68 or worse (approximate Snellen equivalent 20/50 or worse) at baseline.
P values indicate whether differences in reported mean values between the high VA and low VA group were significant as obtained from the mixed-effects models.
Table 2 provides the association between change in BCVA and change in IRF and SRF based on the location (central 1 mm vs parafovea) during 1 year. Our random-effects model resulted in a marginal R2 of 0.146 and a conditional R2 of 0.885. For every 10-nL reduction of IRF and SRF in the central 1 mm, BCVA will improve by a mean of 0.15 (95% CI, 0.10-0.20) and 0.34 (95% CI, 0.18-0.52) in ETDRS letter score. For every 10-nL reduction of IRF and SRF in the parafovea, BCVA will improve by a mean of 0.04 (95% CI, 0.03-0.06) and will decrease by 0.03 (95% CI, 0.04-0.09) in ETDRS letter score. Thus, presence of fluid in the parafovea did not seem to have clinically relevant effects on BCVA.
Table 2. Change in ETDRS Scores per 10 nL of IRF and SRF on BCVA Averaged Over All Study Visits.
| Variable | Better and worse baseline VA (n = 570) | P valuea | Worse baseline VAb (n = 272) | P valuea | Better baseline VAc (n = 298) | P valuea |
|---|---|---|---|---|---|---|
| Central 1 mm, mean (95% CI) | ||||||
| IRF | −0.15 (−0.10 to −0.20) | <.001 | −0.09 (−0.01 to −0.16) | .03 | −0.18 (−0.12 to −0.23) | .001 |
| SRF | −0.34 (−0.18 to −0.52) | <.001 | −0.18 (0.06 to −0.41) | .14 | −0.40 (−0.19 to −0.60) | .001 |
| Parafoveal, mean (95% CI) | ||||||
| IRF | −0.04 (−0.03 to −0.06) | <.001 | −0.04 (−0.02 to −0.07) | <.001 | −0.01 (−0.03 to <0.01) | .15 |
| SRF | 0.03 (0.09 to −0.04) | .41 | <0.01 (0.08 to −0.07) | .95 | 0.01 (<0.01 to 0.22) | .02 |
| R2 (marginal to conditional) | 0.146 to 0.885 | NA | 0.152 to 0.879 | NA | 0.158 to 0.732 | NA |
Abbreviations: BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; IRF, intraretinal fluid; NA, not applicable; SRF, subretinal fluid; VA, visual acuity.
P values for each fluid covariate tested the null hypothesis that there was no correlation with BCVA (mixed-effects model).
Better VA was defined as an ETDRS score of 69 or better (approximate Snellen equivalent 20/32 to 20/40) at baseline.
Worse VA was defined as an ETDRS score of 68 or worse (approximate Snellen equivalent 20/50 or worse) at baseline.
All anti-VEGF agents had an effect on fluid volume (Table 3); however, differences were noted in the associations between different anti-VEGF agents and IRF reduction (eFigure in the Supplement). Aflibercept was associated with greater reduction of IRF volume compared with bevacizumab after the first injection (difference, 79.8 nL; 95% CI, 5.3-162.5 nL; P < .001) and every 4 weeks thereafter (difference, 10.4 nL; 95% CI, 0.7-20.0; P = .004). Ranibizumab was associated with a greater reduction of IRF after the first injection compared with bevacizumab (difference, 75.2 nL; 95% CI, 1.4-154.7; P < .001), with only a borderline difference thereafter (difference, 6.3 nL; 95% CI, 2.9-16.1; P = .07). No difference between aflibercept and ranibizumab was identified. Furthermore, no difference was found in the reduction of SRF among the anti-VEGF agents identified.
Table 3. Association of Aflibercept, Ranibizumab, and Bevacizumab With BCVA, IRF, and SRF.
| Variable | IRF | P valuea | SRF | P valuea |
|---|---|---|---|---|
| Better and worse baseline VA, mean (95% CI) (n = 570)b | ||||
| Fluid change first 4 wk, nL | ||||
| Aflibercept injection | −211.0 (−254.9 to −173.7) | .001 | −13.7 (−20.6 to −6.7) | .001 |
| Bevacizumab injection | −131.2 (−168.4 to −92.4) | .001 | −15.2 (−23.0 to −7.6) | .001 |
| Ranibizumab injection | −206.4 (−247.1 to −169.8) | .001 | −15.4 (−22.0 to −9.1) | .001 |
| Fluid change every 4 wk, nL | ||||
| Aflibercept injection | −24.9 (−29.7 to −19.7) | .001 | −1.2 (−2.2 to −0.3) | .009 |
| Bevacizumab injection | −14.5 (−19.0 to −9.7) | .001 | −0.3 (−1.2 to 0.6) | .55 |
| Ranibizumab injection | −20.8 (−25.8 to −16.1) | .001 | −0.7 (−1.7 to 0.2) | .12 |
| R2 (marginal to conditional) | 0.086 to 0.912 | NA | 0.015 to 0.903 | NA |
| Worse baseline VA, mean (95% CI) (n = 272) | ||||
| Fluid change first 4 wk, nL | ||||
| Aflibercept injection | −278.1 (−348.7 to −214.1) | .001 | −32.1 (−47.6 to −17.2) | .001 |
| Bevacizumab injection | −188.9 (−254.3 to −121.4) | .001 | −37.4 (−53.4 to −22.1) | .001 |
| Ranibizumab injection | −310.7 (−367.9 to −239.1) | .001 | −38.7 (−53.4 to −23.7) | .001 |
| Fluid change every 4 wk, nL | ||||
| Aflibercept injection | −27.5 (−37.5/ −18.1) | .001 | −3.0 (−4.7 to −1.0) | .002 |
| Bevacizumab injection | −14.6 (−22.8 to −5.5) | .002 | −1.4 (−3.1 to 0.5) | .14 |
| Ranibizumab injection | −19.2 (−28.5 to −9.4) | .001 | −1.8 (−3.6 to −0.0) | .06 |
| R2 (marginal to conditional) | 0.108 to 0.890 | NA | 0.069 to 0.849 | NA |
| Better baseline VA, mean (95% CI) (n = 298) | ||||
| Fluid change first 4 wk, nL | ||||
| Aflibercept injection | −197.0 (−236.5 to −153.8) | .001 | −16.4 (−24.3 to −8.5) | .001 |
| Bevacizumab injection | −103.0 (−142.5 to −60.4) | .001 | −11.8 (−20.2 to −3.5) | .005 |
| Ranibizumab injection | −153.1 (−193.3 to −108.5) | .001 | −11.8 (−20.7 to −4.0) | .006 |
| Fluid change every 4 wk, nL | ||||
| Aflibercept injection | −5.3 (−8.2 to −2.4) | .001 | −0.1 (−0.8 to 0.4) | .72 |
| Bevacizumab injection | −4.1 (−6.9 to −1.4) | .005 | −0.4 (−1.0 to 0.3) | .23 |
| Ranibizumab injection | −7.7 (−10.5 to −5.0) | .001 | −0.5 (−1.1 to 0.2) | .12 |
| R2 (marginal to conditional) | 0.100 to 0.891 | NA | 0.029 to 0.830 | NA |
Abbreviations: BCVA, best-corrected visual acuity; IRF, intraretinal fluid; NA, not applicable; SRF, subretinal fluid; VA, visual acuity.
P values indicate the significance of the correlation of anti–vascular endothelial growth factor therapy on IRF or SRF (mixed-effects model).
Better VA was defined as an ETDRS score of 69 or better (approximate Snellen equivalent 20/32 to 20/40) at baseline. Worse VA was defined as an ETDRS score of 68 or worse (approximate Snellen equivalent 20/50 or worse) at baseline.
The presence of SRF at baseline was associated with a worse baseline BCVA of 63.2 (95% CI, 66.1-60.2) ETDRS letter score (approximate Snellen equivalent of 20/63 [95% CI, 20/50-20/63]) in eyes with SRF vs 66.9 (95% CI, 65.7-68.1) ETDRS letter score (approximate Snellen equivalent of 20/50 [95% CI, 20/40-20/50]) without SRF (P < .001) and a greater gain in ETDRS letter score (0.5 [95% CI, 0.3-0.8] every 4 weeks in eyes with SRF vs 0.4 [95% CI, 0.3-0.5] in eyes without SRF at baseline; P = .02) when adjusted for baseline BCVA.
Eyes with SRF also had more IRF volume at baseline (difference, 95.3%) and a higher reduction of IRF after the first anti-VEGF injection (Table 4). At month 12, no difference was identified in BCVA or IRF volume between eyes with and without SRF at baseline (Table 4). At month 12, SRF volumes in eyes with persistent SRF were too small to compute a robust model.
Table 4. Study Eyes Divided Into Eyes With and Without SRF at Baseline.
| Variable | Mean (95%) | P valuea | |
|---|---|---|---|
| No SRF (n = 335) | SRF (n = 235) | ||
| Mean BCVA at baseline, ETDRS score [Snellen equivalent] | 66.9 [20/50] (65.7 to 68.1) | 63.2 [20/63] (60.2 to 66.1) | .001 |
| Mean BCVA at 1 y, ETDRS score [Snellen equivalent] | 77.8 [20/32] (76.5 to 79.1) | 78.7 [20/25] (75.3 to 81.9) | .35 |
| BCVA change | |||
| First 4 wk | 5.2 (4.4 to 5.9) | 7.9 (6.0 to 9.7) | .001 |
| Per 4 wk | 0.4 (0.3 to 0.5) | 0.5 (0.3 to 0.8) | .02 |
| R2 (marginal to conditional) | 0.102 to 0.880 | NA | NA |
| Mean IRF, nL | |||
| Baseline | 319.7 (270.8 to 361.2) | 624.7 (507.7 to 737.7) | .001 |
| 1 y | 110.0 (75.4 to 138.7) | 156.7 (71.0 to 238.7) | .07 |
| IRF change, nL | |||
| First 4 wk | −110.4 (−75.4 to −139.9) | −346.5 (−266.3 to −425.3) | .001 |
| Per 4 wk | −6.6 (−4.7 to −8.8) | −5.8 (−0.6 to −11.0) | .63 |
| R2 (marginal to conditional) | 0.124 to 0.893 | NA | NA |
Abbreviations: BCVA, best-corrected visual acuity; IRF, intraretinal fluid; NA, not applicable; SRF, subretinal fluid.
P values indicate differences in reported mean values between the better visual acuity and worse visual acuity groups at baseline, as obtained from the mixed-effects models.
Discussion
In this post hoc analysis of a randomized clinical trial, we used a deep learning algorithm to quantify IRF and SRF and analyzed the association of treatment with fluid volume and BCVA. Recently, Vogl et al25 used a longitudinal statistical model based on automated fluid quantification in SD-OCT data to analyze the association of IRF and SRF with visual acuity in central retinal vein occlusion and to predict the trajectory of BCVA during treatment. Inclusion of IRF and SRF improved the fit of the model and helped in prognosis of individual variations in visual acuity over time. Despite similarities of retinal morphologic findings on OCT, the pathophysiologic features of retinal edema in DME are different from those of central retinal vein occlusion, which prompted us to investigate the association of fluid parameters with visual acuity in DME.
The low marginal R2 of 0.146 of the random-effects model (Table 2) indicates that the fixed effects (mean intercept, mean slopes, and mean effect of fluid volumes) explain only a small fraction of the variance in the BCVA trajectories. A marginal R2 reflects the predictive value of only the baseline parameters without any additional information, such as the slope of BCVA over time. A large fraction of the variance in the BCVA trajectories was not directly affected by fluid changes but may have been associated with other factors. However, subsuming these unmeasured effects in the patient-specific random effects (intercept plus slopes) allowed us to obtain a general good model fit, as indicated by the conditional R2 of 0.885. The low marginal R2 and thus the limited explanatory value of the model agree with outcomes of previous studies26,27 that investigated the correlation between retinal structural changes, such as retinal thickening, IRF, or SRF, and BCVA. Despite sophisticated automated methods for precise quantification of retinal structural alteration, baseline BCVA remains the best predictor of longitudinal BCVA outcomes.28 Retinal structural findings on SD-OCT, particularly central retinal thickening, are relatively poor surrogates for retinal function but are important biomarkers for the need for anti-VEGF treatment.28
Fluid volumes decreased after initiation of treatment and continued to decrease for 12 months. We analyzed the association of treatment with fluid volumes and observed that aflibercept and ranibizumab were associated with faster reduction of IRF than was bevacizumab (Table 3). However, there was no difference in the association between the different anti-VEGF agents and SRF reduction. This finding may suggest that SRF and IRF in DME have different pathophysiologic backgrounds and are not merely fluid accumulations from the same origin. However, there may also be an anatomical explanation for this finding: SRF may not reach the subretinal pigment epithelium space, even though it is originating from the same source as IRF. Despite the limited association of IRF and SRF with visual function, the presence and amount of fluid may be used as an objective measure of response to therapy when automated fluid measurement has become accessible in clinical routine.19
The IRF was present in all eyes at baseline, whereas SRF was present in only a subset of eyes (41%) at baseline and at lower quantities than IRF (Table 1). The lower overall volume of SRF at baseline and during follow-up may explain the stronger association of SRF with BCVA compared with IRF (Table 2).
Of interest, although SRF had a negative association with BCVA at baseline, it indicated a good response to anti-VEGF treatment and higher BCVA gain per month of follow-up (Table 4). Although this finding seems counterintuitive at first glance, the presence of SRF is associated with good visual prognosis in a variety of retinal diseases, such as age-related macular degeneration, central retinal vein occlusion, or central serous chorioretinopathy, suggesting a protective association of SRF with photoreceptors independent of the underlying disease.29,30,31,32 The origin and prognostic value of SRF in DME is controversial.27,33,34,35,36,37,38,39 Deák et al40 used microperimetry to identify retinal morphologic changes associated with decreased retinal sensitivity and found that SRF and large outer nuclear layer cysts are the 2 morphologic features with the greatest negative association with retinal function. In our study, we found that SRF in the central 1 mm, even in small quantities, was associated with BCVA, whereas IRF was only associated with BCVA if present in larger volumes, in line with the findings described by Deák et al.40 Gerendas et al27 recently investigated the predictive value of central intraretinal cysts and SRF for visual acuity in a post hoc analysis of the RESTORE/RESTORE-extension studies (A 12 Month Core Study to Assess the Efficacy and Safety of Ranibizumab [Intravitreal Injections] in Patients With Visual Impairment Due to Diabetic Macular Edema and a 24 Month Open-label Extension Study). They found that the height of intraretinal cysts at baseline was a good predictor for functional and anatomical improvement. Furthermore, the presence of SRF indicated good response to ranibizumab therapy and a poor response to laser therapy, in line with the outcomes of our study.
Sophie et al38 investigated patient characteristics correlating with BCVA at month 24 in DME in a post hoc analysis of the RISE (A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus) and RIDE (A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus) trials. Submacular fluid at baseline was associated with excellent visual outcome in patients treated with ranibizumab in their study. One group37 suggested that the higher visual gain usually observed in DME with SRF may be associated with lower baseline BCVA. Another explanation may be that the presence of SRF interferes with photoreceptor alignment and thus with the wave-guiding abilities of photoreceptors, also known as the Stiles-Crawford effect, and, thus, leads to a decrease in BCVA but not irreversible structural damage to the retina if treated in a timely manner.41,42 In animal models of induced retinal detachment, photoreceptor outer segments degenerated during serous retinal detachment but had regrowth as early as 3 days after reattachment.43,44,45 This restoration of functional anatomy may be associated with the early visual gain in eyes with SRF (Figure).
Figure. Trajectory of Best-Corrected Visual Acuity (BCVA) in Eyes With and Without Subretinal Fluid (SRF) at Baseline .
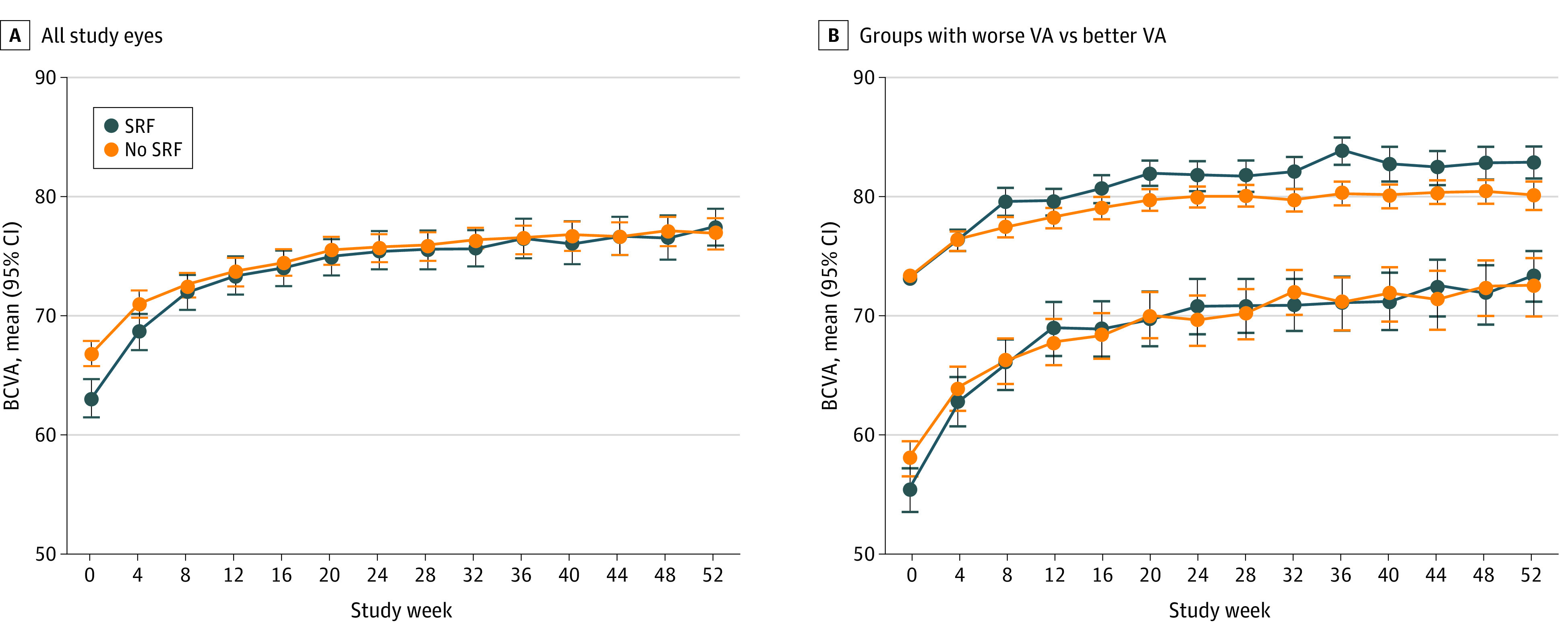
A, BCVA scores for all eyes. B, Eyes divided into better visual acuity (VA) (a baseline Early Treatment Diabetic Retinopathy Study [ETDRS] score of ≥69 [approximate Snellen equivalent of 20/32-20/40]) and worse VA (baseline ETDRS score of <69 [approximate Snellen equivalent of ≤20/50]) groups. Error bars indicate the 95% CIs of the mean value estimated by bootstrap analysis.
In our study IRF had a negative association with visual function if present in large quantities. We inferred that retinal structural damage occurred as a consequence of retinal deformation and stretching of cellular elements secondary to the presence of large cystoid spaces.
With use of AI, an automated and objective profiling of the efficacy of different substances was possible by automated fluid quantification. Despite the limited correlation with visual function found in our study, the presence and amount of IRF and SRF served as measures of individual treatment response. Thus, automated fluid segmentation could support the clinical applicability of individualized medicine.
Strengths and Limitations
Strengths of the study include a large number of study participants, a standardized study protocol, and an objective and automated segmentation algorithm. This study also has limitations, including the retrospective study design and missing data for some visits. Furthermore, we evaluated IRF and SRF but did not account for other morphologic factors, such as disorganization of retinal inner layers, the integrity of photoreceptor layers, or the presence of intraretinal hyperreflective foci or septae between intraretinal cysts. Adding more retinal morphologic markers to the statistical model may improve the identification of clinically relevant factors associated with treatment response. Furthermore, the follow-up period was limited to 12 months.
Conclusions
With use of an automated segmentation algorithm, even minimal amounts of IRF and SRF could be segmented in patients with DME and a difference in IRF reduction was found among anti-VEGF substances. The presence of SRF was associated with low baseline BCVA but good response to anti-VEGF therapy. Artificial intelligence may be used to automatically measure fluid volumes in a reliable way and thus may facilitate a comparison of the differential efficacy of anti-VEGF agents.
eFigure. Trajectory of Intraretinal and Subretinal Fluid Volumes
References
- 1.Felfeli T, Juncal VR, Hillier RJ, et al. . Aqueous humor cytokines and long-term response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2019;206:176-183. doi: 10.1016/j.ajo.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 2.Hillier RJ, Ojaimi E, Wong DT, et al. . Aqueous humor cytokine levels and anatomic response to intravitreal ranibizumab in diabetic macular edema. JAMA Ophthalmol. 2018;136(4):382-388. doi: 10.1001/jamaophthalmol.2018.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cacciamani A, Esposito G, Scarinci F, et al. . Inflammatory mediators in the vitreal reflux of patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2019;257(1):187-197. doi: 10.1007/s00417-018-4169-4 [DOI] [PubMed] [Google Scholar]
- 4.Lim SW, Bandala-Sanchez E, Kolic M, et al. . The influence of intravitreal ranibizumab on inflammation-associated cytokine concentrations in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59(13):5382-5390. doi: 10.1167/iovs.17-23325 [DOI] [PubMed] [Google Scholar]
- 5.Bressler SB, Glassman AR, Almukhtar T, et al. ; Diabetic Retinopathy Clinical Research Network . Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57-68. doi: 10.1016/j.ajo.2015.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown DM, Nguyen QD, Marcus DM, et al. ; RIDE and RISE Research Group . Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013-2022. doi: 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 7.Elman MJ, Qin H, Aiello LP, et al. ; Diabetic Retinopathy Clinical Research Network . Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312-2318. doi: 10.1016/j.ophtha.2012.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do DV, Nguyen QD, Boyer D, et al. ; da Vinci Study Group . One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658-1665. doi: 10.1016/j.ophtha.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 9.Elman MJ, Ayala A, Bressler NM, et al. ; Diabetic Retinopathy Clinical Research Network . Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375-381. doi: 10.1016/j.ophtha.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heier JS, Korobelnik JF, Brown DM, et al. . Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123(11):2376-2385. doi: 10.1016/j.ophtha.2016.07.032 [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi T, Li X, Koh A, et al. ; REVEAL Study Group . The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology. 2015;122(7):1402-1415. doi: 10.1016/j.ophtha.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Erfurth U, Lang GE, Holz FG, et al. ; RESTORE Extension Study Group . Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121(5):1045-1053. doi: 10.1016/j.ophtha.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 13.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells JA, Glassman AR, Jampol LM, et al. ; Diabetic Retinopathy Clinical Research Network . Association of baseline visual acuity and retinal thickness with 1-year efficacy of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. JAMA Ophthalmol. 2016;134(2):127-134. doi: 10.1001/jamaophthalmol.2015.4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1-29. doi: 10.1016/j.preteyeres.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Vogl WD, Waldstein SM, Gerendas BS, Schmidt-Erfurth U, Langs G. Predicting macular edema recurrence from spatio-temporal signatures in optical coherence tomography images. IEEE Trans Med Imaging. 2017;36(9):1773-1783. doi: 10.1109/TMI.2017.2700213 [DOI] [PubMed] [Google Scholar]
- 18.Schlegl T, Waldstein SM, Bogunovic H, et al. . Fully automated detection and quantification of macular fluid in OCT using deep learning. Ophthalmology. 2018;125(4):549-558. doi: 10.1016/j.ophtha.2017.10.031 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Erfurth U, Vogl WD, Jampol LM, Bogunović H. Application of automated quantification of fluid volumes to anti-VEGF therapy of neovascular age-related macular degeneration. Ophthalmology. 2020;S0161-6420(20)30268-2. doi: 10.1016/j.ophtha.2020.03.010 [DOI] [PubMed] [Google Scholar]
- 20.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963-974. doi: 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- 21.Johnson PC. Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol Evol. 2014;5(9):944-946. doi: 10.1111/2041-210X.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133-142. doi: 10.1111/j.2041-210x.2012.00261.x [DOI] [Google Scholar]
- 23.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics. 1946;2(6):110-114. doi: 10.2307/3002019 [DOI] [PubMed] [Google Scholar]
- 24.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 25.Vogl WD, Waldstein SM, Gerendas BS, Schlegl T, Langs G, Schmidt-Erfurth U. Analyzing and predicting visual acuity outcomes of anti-VEGF therapy by a longitudinal mixed effects model of imaging and clinical data. Invest Ophthalmol Vis Sci. 2017;58(10):4173-4181. doi: 10.1167/iovs.17-21878 [DOI] [PubMed] [Google Scholar]
- 26.Gerendas BS, Bogunovic H, Sadeghipour A, et al. . Computational image analysis for prognosis determination in DME. Vision Res. 2017;139:204-210. doi: 10.1016/j.visres.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Gerendas BS, Prager S, Deak G, et al. . Predictive imaging biomarkers relevant for functional and anatomical outcomes during ranibizumab therapy of diabetic macular oedema. Br J Ophthalmol. 2018;102(2):195-203. doi: 10.1136/bjophthalmol-2017-310483 [DOI] [PubMed] [Google Scholar]
- 28.Deák GG, Schmidt-Erfurth UM, Jampol LM. Correlation of central retinal thickness and visual acuity in diabetic macular edema. JAMA Ophthalmol. 2018;136(11):1215-1216. doi: 10.1001/jamaophthalmol.2018.3848 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd Clark W, Liu M, Kitchens J, Wang PW, Haskova Z. Baseline characteristics associated with early visual acuity gains after ranibizumab treatment for retinal vein occlusion. BMC Ophthalmol. 2019;19(1):11. doi: 10.1186/s12886-018-1012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein ML, Van Buskirk EM, Friedman E, Gragoudas E, Chandra S. Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol. 1974;91(4):247-250. doi: 10.1001/archopht.1974.03900060257001 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1-24. doi: 10.1016/j.preteyeres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 32.Philip AM, Podkowinski D, Pablik E, et al. . Presence of subretinal fluid at baseline preserves from photoreceptor alterations in diabetic macular edema and cystoid macular edema due to central retinal vein occlusion. Invest Ophthalmol Vis Sci. 2016;57(12):4164. [Google Scholar]
- 33.Vujosevic S, Torresin T, Berton M, Bini S, Convento E, Midena E. Diabetic macular edema with and without subfoveal neuroretinal detachment: two different morphologic and functional entities. Am J Ophthalmol. 2017;181:149-155. doi: 10.1016/j.ajo.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 34.Seo KH, Yu SY, Kim M, Kwak HW. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina. 2016;36(3):588-595. doi: 10.1097/IAE.0000000000000770 [DOI] [PubMed] [Google Scholar]
- 35.Zur D, Iglicki M, Busch C, Invernizzi A, Mariussi M, Loewenstein A; International Retina Group . OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology. 2018;125(2):267-275. doi: 10.1016/j.ophtha.2017.08.031 [DOI] [PubMed] [Google Scholar]
- 36.Iglicki M, Lavaque A, Ozimek M, et al. . Biomarkers and predictors for functional and anatomic outcomes for small gauge pars plana vitrectomy and peeling of the internal limiting membrane in naïve diabetic macular edema: the VITAL study. PLoS One. 2018;13(7):e0200365. doi: 10.1371/journal.pone.0200365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giocanti-Aurégan A, Hrarat L, Qu LM, et al. . Functional and anatomical outcomes in patients with serous retinal detachment in diabetic macular edema treated with ranibizumab. Invest Ophthalmol Vis Sci. 2017;58(2):797-800. doi: 10.1167/iovs.16-20855 [DOI] [PubMed] [Google Scholar]
- 38.Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology. 2015;122(7):1395-1401. doi: 10.1016/j.ophtha.2015.02.036 [DOI] [PubMed] [Google Scholar]
- 39.Gaucher D, Sebah C, Erginay A, et al. . Optical coherence tomography features during the evolution of serous retinal detachment in patients with diabetic macular edema. Am J Ophthalmol. 2008;145(2):289-296. doi: 10.1016/j.ajo.2007.09.029 [DOI] [PubMed] [Google Scholar]
- 40.Deák GG, Bolz M, Ritter M, Prager S, Benesch T, Schmidt-Erfurth U; Diabetic Retinopathy Research Group Vienna . A systematic correlation between morphology and functional alterations in diabetic macular edema. Invest Ophthalmol Vis Sci. 2010;51(12):6710-6714. doi: 10.1167/iovs.09-5064 [DOI] [PubMed] [Google Scholar]
- 41.Stiles WS, Crawford BH. The luminous efficiency of rays entering the eye pupil at different points. Proc R Soc Lond. 1933;112(778):428-450. [Google Scholar]
- 42.Enoch JM. Vertebrate receptor optics and orientation. Doc Ophthalmol. 1980;48(2):373-388. doi: 10.1007/BF00141466 [DOI] [PubMed] [Google Scholar]
- 43.Guérin CJ, Anderson DH, Fariss RN, Fisher SK. Retinal reattachment of the primate macula: photoreceptor recovery after short-term detachment. Invest Ophthalmol Vis Sci. 1989;30(8):1708-1725. [PubMed] [Google Scholar]
- 44.Anderson DH, Guérin CJ, Erickson PA, Stern WH, Fisher SK. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27(2):168-183. [PubMed] [Google Scholar]
- 45.Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. Retinal detachment in the cat: the pigment epithelial-photoreceptor interface. Invest Ophthalmol Vis Sci. 1983;24(7):906-926. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Trajectory of Intraretinal and Subretinal Fluid Volumes


