This randomized clinical trial evaluates the association of pathologic complete response with event-free survival and distant recurrence–free survival in subpopulations of women with high-risk operable breast cancer treated with standard therapy or one of several novel agents.
Key Points
Question
Is there an association between pathologic complete response (pCR) and survival end points in neoadjuvant treatment of early breast cancer with various novel therapeutics?
Findings
In this follow-up analysis of a randomized clinical trial of 950 patients with breast cancer, a strong individual-level association was found between pCR and event-free survival and distant recurrence–free survival. Excellent outcomes were associated with pCR for all standard subtypes of breast cancer, including pCR owing to experimental regimens.
Meaning
The findings of this study suggest that pCR may be a robust prognostic biomarker for excellent long-term outcomes at the individual patient level for patients with early, high-risk breast cancer.
Abstract
Importance
Pathologic complete response (pCR) is a known prognostic biomarker for long-term outcomes. The I-SPY2 trial evaluated if the strength of this clinical association persists in the context of a phase 2 neoadjuvant platform trial.
Objective
To evaluate the association of pCR with event-free survival (EFS) and pCR with distant recurrence–free survival (DRFS) in subpopulations of women with high-risk operable breast cancer treated with standard therapy or one of several novel agents.
Design, Setting, and Participants
Multicenter platform trial of women with operable clinical stage 2 or 3 breast cancer with no prior surgery or systemic therapy for breast cancer; primary tumors were 2.5 cm or larger. Women with tumors that were ERBB2 negative/hormone receptor (HR) positive with low 70-gene assay score were excluded. Participants were adaptively randomized to one of several different investigational regimens or control therapy within molecular subtypes from March 2010 through 2016. The analysis included participants with follow-up data available as of February 26, 2019.
Interventions
Standard-of-care neoadjuvant therapy consisting of taxane treatment with or without (as control) one of several investigational agents or combinations followed by doxorubicin and cyclophosphamide.
Main Outcomes and Measures
Pathologic complete response and 3-year EFS and DRFS.
Results
Of the 950 participants (median [range] age, 49 [23-77] years), 330 (34.7%) achieved pCR. Three-year EFS and DRFS for patients who achieved pCR were both 95%. Hazard ratios for pCR vs non-pCR were 0.19 for EFS (95% CI, 0.12-0.31) and 0.21 for DRFS (95% CI, 0.13-0.34) and were similar across molecular subtypes, varying from 0.14 to 0.18 for EFS and 0.10 to 0.20 for DRFS.
Conclusions and Relevance
The 3-year outcomes from the I-SPY2 trial show that, regardless of subtype and/or treatment regimen, including 9 novel therapeutic combinations, achieving pCR after neoadjuvant therapy implies approximately an 80% reduction in recurrence rate. The goal of the I-SPY2 trial is to rapidly identify investigational therapies that may improve pCR when validated in a phase 3 confirmatory trial. Whether pCR is a validated surrogate in the sense that a therapy that improves pCR rate can be assumed to also improve long-term outcome requires further study.
Trial Registration
ClinicalTrials.gov Identifier: NCT01042379
Introduction
Neoadjuvant therapy and adjuvant systemic therapy are equally effective in improving breast cancer survival. However, neoadjuvant therapy improves rates of breast-conserving surgery owing to tumor downstaging1,2 and allows assessment of response. The Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) working group evaluated the latter by conducting a pooled patient-level meta-analysis of nearly 12 000 patients from 12 randomized trials, largely with various regimens of standard chemotherapy, to assess the relationship between pathologic complete response (pCR) rates, event-free survival (EFS), and overall survival.3 Overall, pCR was found to have long-term benefit for patients (EFS hazard ratio, 0.48), with the strongest association observed for more aggressive breast cancer subtypes, such as triple-negative breast cancer (hormone receptor [HR]–negative, ERBB2 [formerly HER2]–negative disease) (EFS hazard ratio, 0.24), and ERBB2-positive disease (EFS hazard ratio, 0.39). Based on these data, the US Food and Drug Administration and the European Medicines Agency issued guidance for the use of pCR as a primary end point in support of accelerated drug approval.4,5 Given the number of promising new agents, this provided the opportunity for new trial designs to rapidly identify drugs with the greatest activity.
I-SPY2 is a neoadjuvant, adaptively randomized, multicenter phase 2 platform trial evaluating investigational therapies in combination with standard-of-care chemotherapy for high-risk breast cancer,6,7,8 with pCR as the primary end point. The trial’s goals are to match investigational therapies with responsive subtypes. Using a multiarm design and a master protocol, I-SPY2 has continuously enrolled patients since 2010 and has completed the evaluation of 15 investigational therapies. Here, we report the relationship between pCR status and 3-year outcomes (EFS and DRFS) for the first 950 patients randomized across 10 therapies (including control).
Methods
Study Design
I-SPY2 (NCT01042379) is a multicenter platform trial (protocol in Supplement 1; eFigure 1 in Supplement 2)7,8,9 that used adaptive randomization across 8 subtypes defined by HR expression, ERBB2 status, and genomic risk of recurrence per the 70-gene assay (MammaPrint, Agendia) as previously described.9,10 Twenty percent of patients in each of the 8 subtypes were randomized to control therapy.
The primary end point of I-SPY2 was pCR, defined as the absence of invasive disease in breast and axillary nodes (ypT0/is, ypN0) at time of surgery. Patients who progressed, withdrew consent, or received nonprotocol therapy before surgery are adjudicated as non-pCR. All patients were observed for long-term outcome, reporting at least annually. Secondary end points include residual cancer burden,11 EFS, and DRFS.
The I-SPY2 trial receives institutional review board approval for the master protocol for each site. As new drugs are added to the trial, each site receives approval for the amendment, and amendment approval is required for continued enrollment on the trial. All participating sites received local institutional review board approval. The I-SPY2 Data and Safety Monitoring Board met monthly to review patient safety and study progress.
Participants
Eligible patients were 18 years and older and have clinical stage 2 or 3 breast cancer with no prior surgery or systemic therapy for breast cancer. Primary tumors must be 2.5 cm or greater as measured by imaging or physical examination with patient Eastern Cooperative Oncology Group performance status 0 or 1.12 Tumors that are ERBB2 negative/HR positive with low 70-gene assay score were excluded, as there is no benefit of cytotoxic chemotherapy in this subpopulation.10 All patients provided written informed consent prior to screening for the trial, and a second treatment consent was obtained after randomization to open-label treatment (before treatment was initiated).
Treatment Arms
Control therapy was 12 weekly cycles of paclitaxel (80 mg/m2) followed by 4 cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2). Control therapy for ERBB2-positive tumors also included weekly trastuzumab (4 mg/kg loading dose followed by 2 mg/kg during weeks 2-12) in combination with paclitaxel; pertuzumab treatment was an experimental arm. When pertuzumab received accelerated approval in September 2013, randomization to paclitaxel and trastuzumab was stopped.13
From trial initiation in March 2010 until November 2016, I-SPY2 completed evaluation of 10 experimental agents or combinations: neratinib,7 veliparib plus carboplatin,8 trebananib (AMG386),14 ganitumab,15 MK2206,16 pertuzumab,17 trastuzumab emtansine plus pertuzumab,18 ganetespib,19 pexidartinib (PLX3397),20 and pembrolizumab.21 Each arm included paclitaxel except for the trastuzumab emtansine plus pertuzumab arm. Pexidartinib was discontinued for safety concerns,20 having accrued only 9 patients, and was therefore excluded from this analysis. Six of the 9 experimental therapies “graduated” as described. The current analysis includes participants randomized to any of these 10 therapies (including control) who had follow-up information available as of February 26, 2019.
Pathology
Locations of lesions were marked with clips before therapy to ensure identification of the tumor bed at surgery. Pathologic assessment followed the residual cancer burden method, where residual cancer burden of 0 is pCR.11 This is consistent with the standardized procedures of the College of American Pathologists as described in section IV.E of the US Food and Drug Administration guidance.4,22
Statistical Analysis
Participants were grouped according to pCR status. Baseline characteristics were compared using a χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables. Patients’ EFS was assessed as time from treatment consent to any locoregional or distant recurrence or death from any cause; DRFS was time to distant recurrence or death from any cause. Patients without events were censored at last follow-up.
Cox proportional hazard modeling, with significance assessment using the score (log rank) test, was used to estimate the hazard ratio for pCR vs non-pCR and its 95% CI. All P values are 2-sided, and significance level is P < .05. Kaplan-Meier estimates of 3-year EFS and DRFS for the 2 groups are presented. Analyses were performed over the entire population and within HR/ERBB2 subtypes. Bayesian modeling of EFS, stratified for subtype, was used to estimate the hazard ratios for pCR vs non-pCR within individual treatment arms (see eMethods in Supplement 2). Statistical analyses were performed using Intel Fortran Compiler, version 19.0.2 (Intel Corporation).
Results
Patients
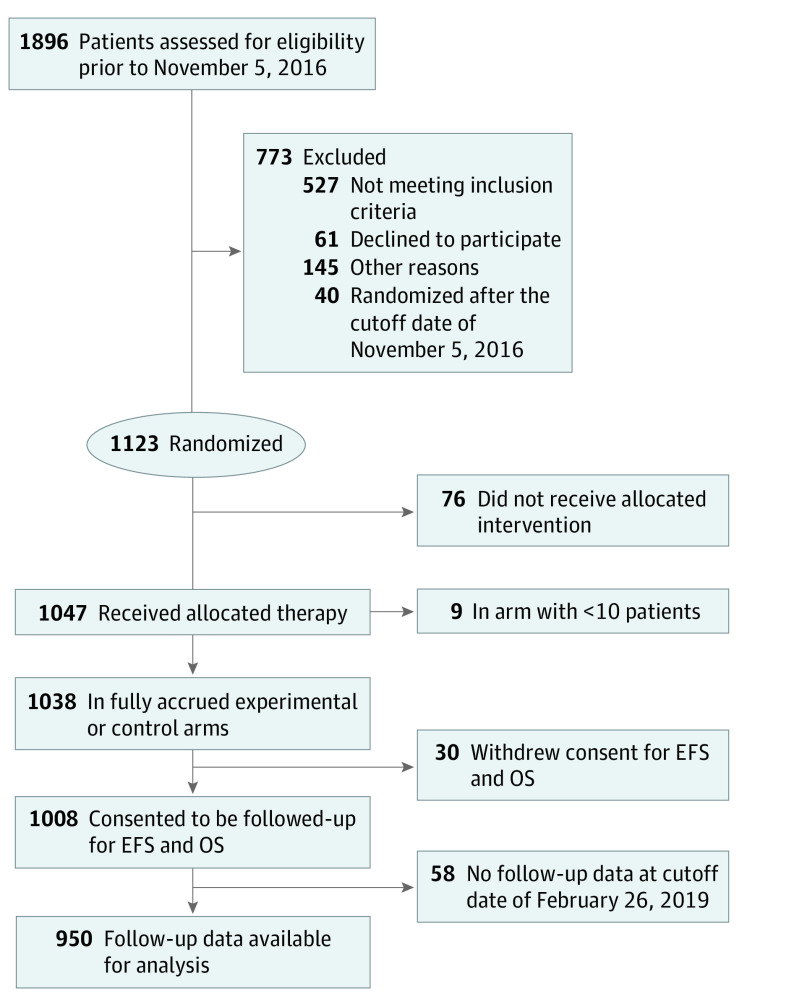
From March 3, 2010, to November 5, 2016, a total of 1896 patients were screened for eligibility for the trial, and 1123 were randomized; 773 patients did not proceed to randomization for various reasons (Figure 1). A total of 1038 patients received treatment on 1 of 9 arms in this analysis. Of these, 950 had follow-up data available prior to the data cutoff date of February 26, 2019. No statistical differences were observed in age or race among patients who achieved pCR (median [range] age, 49 [25-73] years) vs those who did not (median [range] age, 49 [23-77] years) (eTable 1 in Supplement 2), nor were there significant differences between the 2 groups in nodal status, time from treatment consent to surgery, or length of follow-up.
Figure 1. CONSORT Diagram.
EFS indicates event-free survival; OS, overall survival.
Rates of pCR
Of the 950 patients, 330 (34.7%) achieved pCR. Rates of pCR (Table) were lowest for HR-positive, ERBB2-negative tumors (17.4% [63 of 361]), increasing approximately additively for ERBB2 positivity and HR negativity, up to 68% (61 of 90) for HR-negative, ERBB2-positive tumors. For patients receiving control therapy, pCR rates were 19.3% for the entire population (39 of 202), varying by subtype; 15% (14 of 92) for HR-positive, ERBB2-negative tumors; 21% (17 of 80) in HR-negative, ERBB2-negative tumors; 17% (3 of 18) for HR-positive, ERBB2-positive tumors; and 42% (5 of 12) in HR-negative, ERBB2-positive tumors. Rates of pCR were higher for those in the investigational arms compared with those in the control arms.
Table. Pathologic Complete Response (pCR) Rates and Hazard Ratios of Event-Free Survival (EFS) and Distant Recurrence–Free Survival (DRFS) for pCR vs Not pCR by Molecular Subtypea.
| Subtype | No. | pCR rate, % (95% CIb) | Hazard ratio (95% CI) | |
|---|---|---|---|---|
| EFS | DRFS | |||
| HR+ ERBB2− | 361 | 17 (14-22) | 0.14 (0.03-0.55) | 0.16 (0.04-0.64) |
| HR+ ERBB2+ | 173 | 40 (33-48) | 0.15 (0.03-0.63) | 0.10 (0.01-0.77) |
| HR− ERBB2− | 326 | 42 (36-47) | 0.18 (0.09-0.34) | 0.20 (0.10-0.40) |
| HR− ERBB2+ | 90 | 68 (57-77) | 0.14 (0.05-0.41) | 0.18 (0.06-0.53) |
| All | 950 | 35 (32-38) | 0.19 (0.12-0.31) | 0.21 (0.13-0.34) |
Abbreviation: HR, hormone receptor.
The observation that every hazard ratio for molecular subtype is smaller than the overall hazard ratio is not a typographic error. It is an example of the Simpson paradox, which was observed as well in the I-SPY1 trial.23 This observation demonstrates the importance of considering molecular subtype when evaluating the association of pCR with EFS and DRFS.
Based on binomial exact (Clopper-Pearson) CI method.
Association of pCR With EFS and DRFS
There were 1265 woman-years of follow-up for the 330 patients who achieved pCR, with 19 events reported (0.0150/y), and 2125 woman-years of follow-up for the 620 patients not achieving pCR, with 169 events reported (0.0795/y). Three-year EFS was 95% for patients achieving pCR compared with 78% for non-pCR, with a hazard ratio of 0.19 (95% CI, 0.12-0.31; Figure 2A and Table). Similarly, 3-year DRFS was 95% for those with pCR vs 81% for those without, with a hazard ratio of 0.21 (95% CI, 0.13-0.34; Figure 2B). Patients who achieved pCR showed a 3-year EFS of 93% to 97% regardless of subtype (Figure 2C). There were differences by subtype for those not achieving pCR, with 3-year EFS ranging from 57% for HR-negative, ERBB2-positive tumors to 89% for HR-positive, ERBB2-positive tumors (Figure 2D).
Figure 2. Kaplan-Meier Survival Curves.
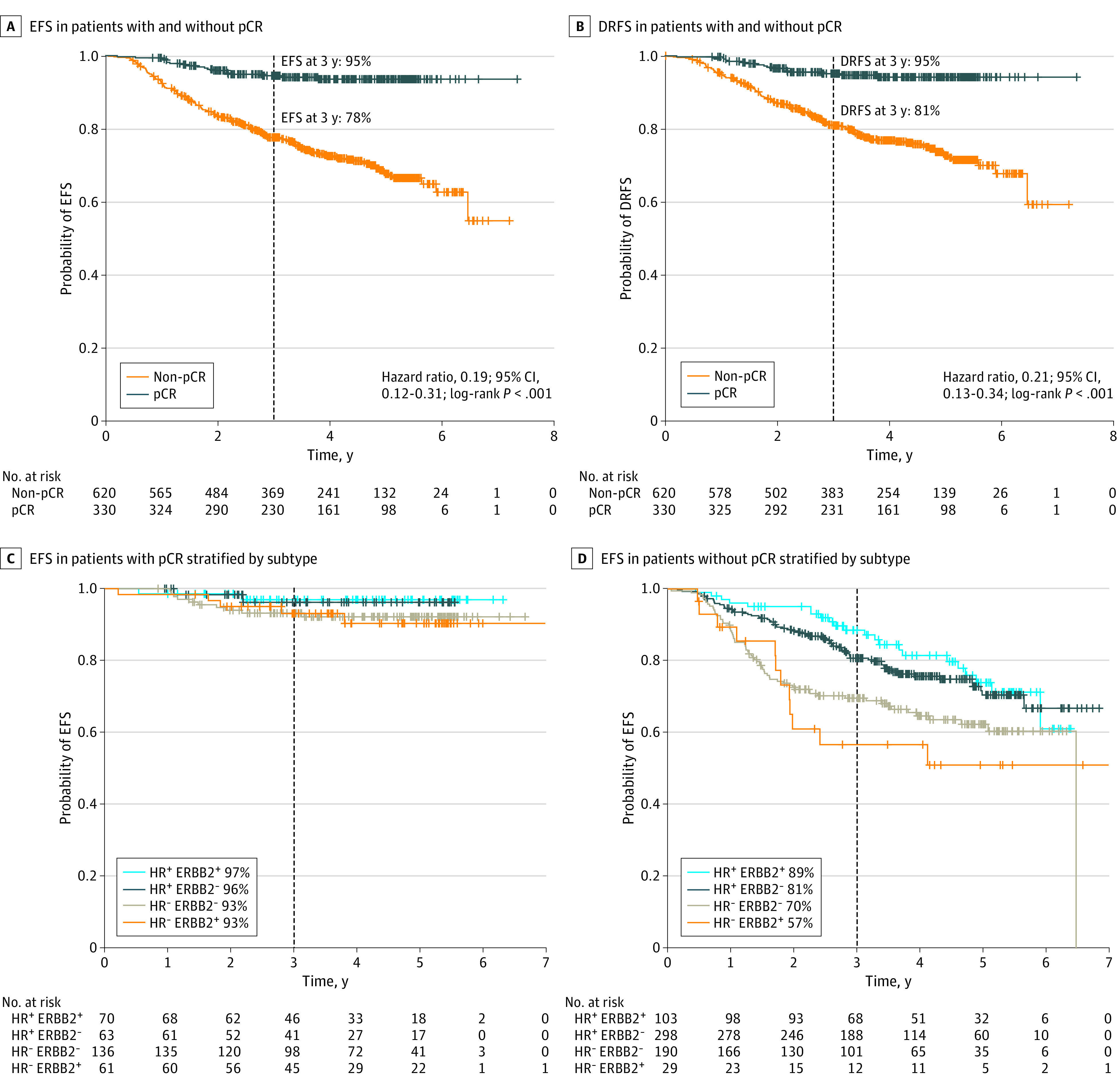
Kaplan-Meier survival curves for event-free survival (EFS) (A), and distant recurrence–free survival (DRFS) (B), in populations achieving pathologic complete response (pCR) at surgery (gray) and those with residual disease at time of surgery (orange). Kaplan-Meier survival curves for EFS among patients achieving pCR (C) and those who did not (D), stratified by hormone receptor (HR) and ERBB2 subtype (HR+ ERBB2+: light blue; HR+ ERBB2−: dark blue; HR− ERBB2−: gray; HR− ERBB2+: orange).
Hazard ratios for EFS were consistent across all subtypes of breast cancer, ranging from 0.14 (95% CI, 0.03-0.55) in HR-positive, ERBB2-negative tumors to 0.18 (95% CI, 0.05-0.41) in HR-positive, ERBB2-positive tumors regardless of the treatment arm. Hazard ratios for DRFS varied similarly. Kaplan-Meier plots of EFS and DRFS by subtype are provided in eFigures 2 and 3 in Supplement 2. Bayesian modeled subtype-adjusted EFS hazard ratios by pCR vs non-pCR within individual arms are provided in eFigure 4 in Supplement 2, with median (range) estimates ranging from 0.05 (0.003-0.24) to 0.45 (0.14-1.10).
Postsurgical Therapy
All patients were recommended to receive appropriate postsurgical therapy for their disease subtype, administered per the discretion of the treating physician. Of the 838 patients for whom data were available, a higher proportion of non-pCR patients received additional adjuvant therapies when compared with those who achieved pCR (eTable 2 in Supplement 2). Thirty-six of 540 patients (6.7%) not achieving pCR received additional adjuvant chemotherapy compared with only 2 of 298 (0.7%) in the pCR group. More postneoadjuvant therapy was given during the later years of the trial (eFigure 5 in Supplement 2). For example, in patients with triple-negative breast cancer who did not achieve pCR, only 2 of 56 (4%) received additional systemic therapy from 2010 to 2012, increasing to 5 of 68 (7%) during 2013 to 2014 and to 11 of 43 (25%) during 2015 to 2016.
Discussion
Since the start of enrollment in 2010, the rate of pCR varied across the molecular subtypes of breast cancer in the I-SPY2 trial. Regardless of subtype or treatment regimen, a strong and consistent association between individual pCR and EFS/DRFS with an overall hazard ratio of 0.19 (95% CI, 0.12-0.31) with median follow-up of 3.8 years was observed. After 3 years of follow-up, a clear separation of the curves suggests that these results will persist over time, but this awaits further analysis and follow-up.
Our results are consistent with the findings of the CTNeoBC (Collaborative Trials in Neoadjuvant Breast Cancer) pooled analysis. We did observe smaller EFS hazard ratios with a somewhat shorter follow-up period, a difference that in part may be explained by differences in patient populations. The I-SPY2 trial does not enroll patients with tumors smaller than 2.5 cm or HR-positive, ERBB2-negative, 70-gene–assay (MammaPrint) low-risk tumors. Only a small number of patients with HR-negative or ERBB2-positive (n = 17) genomic low-risk tumors were enrolled. The I-SPY2 trial focused on a group with high risk of early recurrence, as demonstrated in 2 validation series.24,25 The MINDACT (Microarray in Node-negative Disease May Avoid Chemotherapy) trial, which evaluated the 70-gene assay to predict benefit of adjuvant therapy, demonstrated that women with clinically high-risk but genomic low-risk tumors did not have risk for early recurrence, nor did they benefit from chemotherapy.10 Inclusion of HR-positive/70-gene–assay low-risk patients as in CTNeoBC could therefore dilute the effect of pCR on EFS, leading to a higher hazard ratio.
Whether pCR is a validated surrogate in the sense that a therapy that improves pCR rate can be assumed to also improve long-term outcome requires further study. The I-SPY2 trial is not powered or intended to evaluate whether an increase in pCR rate translates to an improvement in EFS for individual experimental therapies relative to control. However, small improvements in pCR, as seen with the use of bevacizumab in the ARTemis trial,26 would not rise to the level of a graduation threshold in the I-SPY trial, which is designed to identify agents with a clinically important effect of the rates of pCR. Instead, I-SPY2 is designed to identify large pCR improvements and provide the rationale for larger confirmatory trials in appropriate subsets of patients, as has been validated in the KEYNOTE-522 trial27 and the BrighTNess trial.28 These showed similar pCR rates for chemotherapy with investigational agents (pembrolizumab and veliparib/carboplatin, respectively) as reported here, with an early indication of improved EFS. In I-SPY2, pCR rates were similar or higher for all of the experimental agents compared with the control regimen of paclitaxel plus doxorubicin and cyclophosphamide. Further confirmatory trials, along the lines of KEYNOTE-522, can use I-SPY2 results to develop new standard-of-care regimens.
We and many others have now shown that, for individuals, pCR achieved after neoadjuvant therapy is associated with excellent EFS and DRFS. Neoadjuvant therapy allows early assessment of a clinically relevant response end point (pCR) for patients, identifying individuals who do well on their initial therapy alone. As such, an immediate estimate of benefit is available that cannot be determined when adjuvant chemotherapy is given after surgery. Given the number of promising therapies for breast cancer and the need to determine efficacy for specific subgroups of patients, neoadjuvant therapy provides an opportunity to understand a new therapy’s mechanistic antitumor effect and correlate with antitumor activity measured by imaging and pathologic response. The contribution of the I-SPY program is that the association between pCR and EFS appears to hold true for individuals who received novel therapeutic combinations.16 Follow-up will continue in I-SPY2 to assess the longer-term association with pCR and overall survival.
In a patient-level meta-analysis by Spring et al29 that included more than 27 000 women treated with the neoadjuvant approach, again we see the very strong correlation of pCR with EFS. Importantly, for the almost 8000 women who had additional adjuvant therapy following neoadjuvant therapy, no benefit of additional therapy was observed in patients who achieved pCR. While it is not yet known if additional systemic therapy can be discontinued after pCR in all subtypes, especially in HR-positive, ERBB2-negative and HR-negative, ERBB2-positive tumors, trials to test this hypothesis, such as the COMPASS trial (NCT04266249) and the DeCrescendo trial (in planning through the Breast International Group), are under way. These data provide support for the de-escalation of therapy with the possibility to avoid unnecessary toxic effects.
Continuing the same regimen after non-pCR may not improve long-term outcomes. The KATHERINE trial enrolled patients with residual disease after neoadjuvant ERBB2-based therapy.30 Use of ado-trastuzumab emtansine, instead of continued trastuzumab, resulted in improved 3-year invasive and distant disease-free survival with a hazard ratio of 0.50. This argues strongly that lack of pCR requires a change in therapy, as continuing the same regimen given in the neoadjuvant setting was inferior. The CREATE-X trial,31 which randomized patients to capecitabine vs no additional therapy, furthers the argument that the poor prognostic implications of non-pCR can be addressed by adding additional new therapy in triple-negative breast cancer.
Limitations
The goal of the I-SPY2 trial was to rapidly identify active agents and combinations to increase the chance of achieving pCR. The association of pCR with EFS and DRFS is based on a 3-year follow-up; longer follow-up will yield additional information about the strength of this association for pCR. The association between pCR and overall survival will also require additional long-term follow-up. Furthermore, larger confirmatory neoadjuvant trials will be able to generate sufficient data to establish whether pCR can be considered a validated surrogate of EFS.
Conclusions
The strength of the I-SPY2 study is that it shows that pCR, regardless of high-risk subtype and type of treatment and across 9 investigational targeted biologics, is associated with a much better outcome for individuals who achieve pCR. The goal of the I-SPY2 trial was to rapidly identify active agents and combinations to increase the chance of achieving pCR. Larger confirmatory neoadjuvant trials will be able to generate sufficient data to establish whether pCR can be considered a validated surrogate of EFS. Perhaps more importantly, these data should drive and inspire us to think about how to maximize the chance that each individual can achieve pCR. These data provide a clear experimental rationale for serial adjustments to systemic therapy prior to surgery with therapies directed at specific subtypes.
Trial Protocol and Summary of Protocol Amendments
eMethods.
eFigure 1. I-SPY2 study schema, illustrating multiple experimental arms compared with a common control, adaptive randomization and schedule of assessments.
eFigure 2. Kaplan-Meier survival curves for Event-free Survival for each molecular subtype.
eFigure 3. Kaplan-Meier survival curves Distant Relapse-free Survival for each molecular subtype.
eFigure 4. Forest plot showing Bayesian modeled EFS hazard ratios by pCR vs non-pCR for each therapy, adjusting for molecular subtype.
eFigure 5. Trends in use of adjuvant therapy in patients with residual disease following neoadjuvant treatment and surgery since I-SPY2 opened in 2010. over time in I-SPY2.
eTable 1. Baseline characteristics of participants in the analysis set who achieved pCR prior to surgery and those with residual disease at surgery.
eTable 2. Use of adjuvant therapy in the I-SPY2 population, by subtype and pCR result.
Data Sharing Statement
References
- 1.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672-2685. doi: 10.1200/JCO.1998.16.8.2672 [DOI] [PubMed] [Google Scholar]
- 2.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24(13):2019-2027. doi: 10.1200/JCO.2005.04.1665 [DOI] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration Guidance for industry: pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. Accessed November 26, 2017. https://www.fda.gov/media/83507/downloadhttps://www.fda.gov/downloads/drugs/guidances/ucm305501.pdf
- 5.European Medicines Agency The role of pathological complete response as an endpoint in neoadjuvant breast cancer studies (EMA/CHMP/151853/2014). Accessed March 25, 2018. https://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/04/WC500165781.pdf
- 6.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97-100. doi: 10.1038/clpt.2009.68 [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Liu MC, Yee D, et al. ; I-SPY 2 Investigators . Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11-22. doi: 10.1056/NEJMoa1513750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rugo HS, Olopade OI, DeMichele A, et al. ; I-SPY 2 Investigators . Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23-34. doi: 10.1056/NEJMoa1513749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999-2009. doi: 10.1056/NEJMoa021967 [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F, van’t Veer LJ, Bogaerts J, et al. ; MINDACT Investigators . 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717-729. doi: 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 11.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414-4422. doi: 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 13.Esserman LJ, DeMichele A. Accelerated approval for pertuzumab in the neoadjuvant setting: winds of change? Clin Cancer Res. 2014;20(14):3632-3636. doi: 10.1158/1078-0432.CCR-13-3131 [DOI] [PubMed] [Google Scholar]
- 14.Albain KS, Leyland-Jones B, Symmans F, et al. Abstract P1-14-03: the evaluation of trebananib plus standard neoadjuvant therapy in high-risk breast cancer: results from the I-SPY 2 TRIAL. Cancer Res. 2016;76(4)(suppl). doi: 10.1158/1538-7445.SABCS15-P1-14-03 [DOI] [Google Scholar]
- 15.Yee D, Paoloni M, van’t Veer L, et al. The evaluation of ganitumab/metformin plus standard neoadjuvant therapy in high-risk breast cancer: Results from the I-SPY 2 trial (Abstract P6-11-04). Cancer Res. 2017;77(4)(suppl). doi: 10.1158/1538-7445.SABCS16-P6-11-04 [DOI] [Google Scholar]
- 16.Chien AJ, Tripathy D, Albain KS, et al. ; I-SPY 2 Consortium . MK-2206 and standard neoadjuvant chemotherapy improves response in patients with human epidermal growth factor receptor 2–positive and/or hormone receptor–negative breast cancers in the I-SPY 2 trial. J Clin Oncol. 2020;38(10):1059-1069. doi: 10.1200/JCO.19.01027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buxton M, DeMichele AM, Chia S, et al. Efficacy of pertuzumab/trastuzumab/paclitaxel over standard trastuzumab/paclitaxel therapy for HER2+ breast cancer: results from the neoadjuvant I-SPY 2 TRIAL (Abstract CT106). Cancer Res. 2016;76(14)(suppl). doi: 10.1158/1538-7445.AM2016-CT106 [DOI] [Google Scholar]
- 18.DeMichele AM, Moulder S, Buxton M, et al. Efficacy of T-DM1+pertuzumab over standard therapy for HER2+ breast cancer: Results from the neoadjuvant I-SPY 2 TRIAL (Abstract CT042). Cancer Res. 2016;76(14)(suppl). doi: 10.1158/1538-7445.AM2016-CT042 [DOI] [Google Scholar]
- 19.Forero A, Yee D, Buxton MB, et al. Efficacy of Hsp90 inhibitor ganetespib plus standard neoadjuvant therapy in high-risk breast cancer: results from the I-SPY 2 trial (Abstract P6-11-02). Cancer Res. 2017;77(4)(suppl). doi: 10.1158/1538-7445.SABCS16-P6-11-02 [DOI] [Google Scholar]
- 20.Piawah S, Hyland C, Umetsu SE, Esserman LJ, Rugo HS, Chien AJ. A case report of vanishing bile duct syndrome after exposure to pexidartinib (PLX3397) and paclitaxel. NPJ Breast Cancer. 2019;5(1):17. doi: 10.1038/s41523-019-0112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 Trial. JAMA Oncol. 2020;6(5):676-684. doi: 10.1001/jamaoncol.2019.6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.College of American Pathologists Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Accessed March 25, 2018. https://documents.cap.org/protocols/cp-breast-invasive-18protocol-4100.pdf
- 23.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 trial—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242-3249. doi: 10.1200/JCO.2011.39.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van ’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530-536. doi: 10.1038/415530a [DOI] [PubMed] [Google Scholar]
- 25.Buyse M, Loi S, van’t Veer L, et al. ; TRANSBIG Consortium . Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98(17):1183-1192. doi: 10.1093/jnci/djj329 [DOI] [PubMed] [Google Scholar]
- 26.Earl HM, Hiller L, Dunn JA, et al. ; ARTemis Investigators Group . Disease-free and overall survival at 3.5 years for neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin and cyclophosphamide, for women with HER2 negative early breast cancer: ARTemis trial. Ann Oncol. 2017;28(8):1817-1824. doi: 10.1093/annonc/mdx173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid P, Cortés J, Dent R, et al. KEYNOTE-522: phase 3 study of pembrolizumab + chemotherapy vs placebo + chemotherapy as neoadjuvant treatment, followed by pembrolizumab vs placebo as adjuvant treatment for early-stage high-risk triple-negative breast cancer. Ann Oncol. 2019;30(suppl 5). doi: 10.1093/annonc/mdz394 [DOI] [PubMed] [Google Scholar]
- 28.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497-509. doi: 10.1016/S1470-2045(18)30111-6 [DOI] [PubMed] [Google Scholar]
- 29.Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838-2848. doi: 10.1158/1078-0432.CCR-19-3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Minckwitz G, Huang CS, Mano MS, et al. ; KATHERINE Investigators . Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 31.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Summary of Protocol Amendments
eMethods.
eFigure 1. I-SPY2 study schema, illustrating multiple experimental arms compared with a common control, adaptive randomization and schedule of assessments.
eFigure 2. Kaplan-Meier survival curves for Event-free Survival for each molecular subtype.
eFigure 3. Kaplan-Meier survival curves Distant Relapse-free Survival for each molecular subtype.
eFigure 4. Forest plot showing Bayesian modeled EFS hazard ratios by pCR vs non-pCR for each therapy, adjusting for molecular subtype.
eFigure 5. Trends in use of adjuvant therapy in patients with residual disease following neoadjuvant treatment and surgery since I-SPY2 opened in 2010. over time in I-SPY2.
eTable 1. Baseline characteristics of participants in the analysis set who achieved pCR prior to surgery and those with residual disease at surgery.
eTable 2. Use of adjuvant therapy in the I-SPY2 population, by subtype and pCR result.
Data Sharing Statement