Abstract
The macro‐ and microstructural characteristics of sleep electroencephalography have been associated with several aspects of executive functioning. However, only a few studies have addressed the association of sleep characteristics with the learning involved in the acquisition of executive functions, and no study has investigated this for planning and problem‐solving skills in the developing brain of children. The present study examined whether children's sleep stages and microstructural sleep characteristics are associated with performance improvement over repeated assessments of the Tower of Hanoi task, which requires integrated planning and problem‐solving skills. Thirty children (11 boys, mean age 10.7 years, SD = 0.8) performed computerized parallel versions of the Tower of Hanoi three times across 2 days, including a night with polysomnographically assessed sleep. Pearson correlations were used to evaluate the associations of Tower of Hanoi solution time improvements across repeated assessments with sleep stages (% of total sleep time), slow‐wave activity, and fast and slow spindle features. The results indicated a stronger performance improvement across wake in children with more Stage N2 sleep and less slow‐wave sleep. Stronger improvements across sleep were present in children in whom slow spindles were more dense, and in children in whom fast spindles were less dense, of shorter duration and had less power. The findings indicate that specific sleep electroencephalography signatures reflect the ability of the developing brain to acquire and improve on integrated planning and problem‐solving skills.
Keywords: children, executive functioning, slow‐wave sleep, spindles, Tower of Hanoi
1. INTRODUCTION
The importance of sleep for learning and cognitive performance is well recognized. In the last decades, several studies have found better executive functioning (EF) in children and adolescents with sleep of longer duration and better quality (Anderson, Storfer‐Isser, Taylor, Rosen, & Redline, 2009; Kuula et al., 2015; Sadeh, Gruber, & Raviv, 2002, 2003). EF encompasses a set of cognitive abilities, such as inhibition, working memory and cognitive flexibility, which require prefrontal cortex involvement and are needed for goal‐directed behaviour (Diamond, 2013). Integrated, these abilities enable complex or higher‐order EF, including problem‐solving and planning (Diamond, 2013; Miyake et al., 2000; Nigg, 2017). EF develops throughout childhood into late adolescence, and is linked to maturational changes of the prefrontal cortex and connected (sub)cortical structures (Casey, Tottenham, Liston, & Durston, 2005; Diamond, 2013; Kharitonova, Martin, Gabrieli, & Sheridan, 2013; Satterthwaite et al., 2013). Moreover, training interventions targeting EF in children and adolescents showed that executive functions, particularly working memory and cognitive flexibility, can be improved with practice (Jaeggi, Buschkuehl, Jonides, & Shah, [Link]; Karbach & Unger, 2014).
Aside from maturation‐ and practice‐related improvements, sleep is also known to support EF (Kuriyama, Mishima, Suzuki, Aritake, & Uchiyama, 2008; Zinke, Noack, & Born, 2018). A more extensively studied role of sleep in cognition concerns the consolidation of procedural memories of perceptual and motor skills in adults (e.g., Diekelmann & Born, 2010; Rasch & Born, 2013; Walker, Brakefield, Morgan, Hobson, & Stickgold, 2002) and in children (Fischer, Wilhelm, & Born, 2007; Wilhelm, Diekelmann, & Born, 2008). However, little is known about the contribution of sleep‐related processes to the consolidation or enhancement of cognitive procedures related to problem‐solving and planning skills.
Ashworth, Hill, Karmiloff‐Smith, and Dimitriou (2014) showed in children aged 6–12 years that performance on the Tower of Hanoi (TOH), a task that primarily involves executive functions such as planning and problem‐solving, improved only across a period that included sleep but not across a similar period of wakefulness only. This finding suggests that sleep supports the learning of planning and problem‐solving; however, the involvement of specific sleep stages and microstructural sleep characteristics was not addressed. Studies have begun to identify associations between microstructural sleep characteristics and EF or cognitive ability in children. For example, Chatburn et al. (2013) found negative associations between measures of working memory, planning ability and spindle frequency in children aged 4–12 years. In other studies, sleep spindle activity (i.e. frequency) during non‐rapid eye movement (NREM) sleep was related to general cognitive ability and working memory in school‐aged children (Geiger et al., 2011; Gruber et al., 2013; Hoedlmoser et al., 2014). Other spindle characteristics, such as spindle amplitude, duration and density, were not associated with general cognitive ability (Gruber et al., 2013). Combined, these studies do not provide an unequivocal view on the role of sleep characteristics on EF in children, and none of these studies addressed learning of planning and problem‐solving in children.
In general, findings in adults suggest that consolidation of procedural memories, which are involved in the mastery of TOH performance, particularly benefit from rapid eye movement (REM) sleep, although Stage 2 sleep (spindles) and slow‐wave activity (SWA) seem to be involved as well (Ackermann & Rasch, 2014; Born, Rasch, & Gais, 2006; Diekelmann & Born, 2010; Fogel & Smith, 2006; Peters, Smith, & Smith, 2007).
Recently, Nielsen et al. (2015) investigated whether sleep characteristics were associated with planning and problem‐solving skills in adults. They found a stronger overnight performance improvement (completion time) on the TOH in participants with more Stage 2 sleep, less slow‐wave sleep (SWS) and a higher sleep spindle density in both Stage 2 and SWS. Another study in adults investigated how the pre‐sleep acquisition of the TOH task affected subsequent sleep (Fogel, Ray, Binnie, & Owen, 2015). As compared with the baseline night, the densities of fast sleep spindles during Stage 2 sleep and SWS increased after the first task completion. Moreover, subjects with a stronger increase in spindle density showed more overnight improvement in speed and accuracy. A study on the effect of an intensive working memory training on subsequent sleep in children and adolescents aged 10–16 years showed increased SWA, which was positively associated with overnight increments in working memory performance (Pugin et al., 2015).
So far, studies on the role of sleep in planning and problem‐solving skills in children are scarce. Therefore, the present study aimed to investigate the associations of sleep stages and a wide range of microstructural sleep characteristics (fast and slow sleep spindle features, SWA) with improvement in EF performance across 12‐hr periods that included primarily sleep or wakefulness only. Based on previous findings in adults (Fogel et al., 2015; Nielsen et al., 2015), we hypothesized that overnight performance improvements on the TOH are associated with increased Stage 2 sleep and higher spindle density. We complemented the investigation by exploring associations of sleep stages, spindles and SWA for performance improvements across wakefulness.
2. METHODS
2.1. Participants
Participants were recruited through a competition, aimed to increase awareness of an interest in scientific research, among primary schools in the Netherlands. Children from the winning school (grades 5 and 6) were invited, and 32 children participated in the current study. The data of two children previously diagnosed with Pervasive Developmental Disorder—Not Otherwise Specified were excluded from analysis. The final sample consisted of 30 healthy children (11 boys, 19 girls) aged 9.5–12.8 years (M = 10.7, SD = 0.8 years). The study was approved by the medical ethics committee of the VU University Medical Center, Amsterdam, the Netherlands, and written informed consent was obtained for all participants. The same sample has been previously investigated by Astill et al. (2014).
2.2. Task
Planning and problem‐solving skills were assessed with a computerized version of the TOH, which is equivalent to the classic wooden version (Mataix‐Cols & Bartrés‐Faz, 2002; Simon, 1975). Participants were instructed to rearrange four disks of different colour and size across three pegs in order to attain a goal configuration with the fewest possible number of moves. There were three rules: (a) move only one disk at a time; (b) do not place a larger disk on top of a smaller disk; and (c) disks should be placed on one of the pegs displayed. We used a four‐disk version of the TOH that consisted of 14 trials covering seven grades of difficulty (i.e. the minimum number of moves necessary to solve the problem). The first two trials were intended to practice, and could be solved in one and two moves, respectively. The remaining 12 trials increased in difficulty every two trials, starting at two‐move solutions and ending at seven‐move solutions. The number of required moves was announced on the screen at the start of each trial. Then the problem was shown with the goal configuration on top of the screen and the TOH initial position below. During self‐paced problem‐solving, feedback on the remaining number of moves was provided on the screen. Extra moves were not allowed. Errors (i.e. making an unnecessary or wrong move) were followed by a screen (2 s) that informed the children that they had made a mistake, after which they had to start afresh. Upon completion of the trial, participants were shown a confirmation, after which they continued with the next trial. There was no time limit and trials were continued until completion. Measures recorded for each trial were the number of errors and the total time required to solve the problem (minus 2 s per error, for the feedback screen displayed after each error). To measure TOH performance, we calculated the average solution time for the six trials that require five, six or seven moves because only the more difficult trials address planning and problem‐solving, which was the focus of our investigation.
2.3. Procedure
The experimental design covered four consecutive weekdays during which the TOH was assessed five times. A scheme of the assessments can be found in Astill et al. (2014; Figure 1). The first and final sessions of the TOH were performed in the morning at school, whereas the three middle sessions (morning session at 09:30 hours, evening session at 21:45 hours, second morning session at 09:30 hours) were administered in the Science Museum “Nemo” (Amsterdam, the Netherlands). After each session, the children were asked to indicate their subjective level of tiredness on a 10 cm visual analogue scale (VAS) ranging from “Not tired at all” to “Very tired”. The analyses only included data obtained during the three middle sessions, which were assessed under controlled conditions in the Science Museum. The interval between the a.m. session and the p.m. session on the first day at the museum contained only wakefulness. During daytime, all children participated in the same activity programme that focused on educating about the brain and sleep in a playful way. The interval between the p.m. session on the first day at the museum and the a.m. session on the second day contained a period of sleep during which polysomnography (PSG) recordings were obtained. Children slept in a sleep‐lab that was built for the occasion of this study in the museum. Each child slept in a comfortable bed in a private space created by room dividers. During sleep, children wore in‐ear headphones for acoustic stimulation intervention, which showed no effect on any sleep variable (Astill et al., 2014). Every three children were supervised by one sleep technician. The nights before and after the PSG recording were regular non‐monitored nights at home. Regularity of bedtime in the week before the study was usually so (5–7 times per week) for 77% of the children and sometimes (2–4 times per week) for the remaining 23% as indicated by parents. In order to minimize learning effects, four parallel versions of the TOH task were created by adapting the start and goal configurations. Each child received a different version every next session, and these version sequences differed between children in a balanced way.
Figure 1.
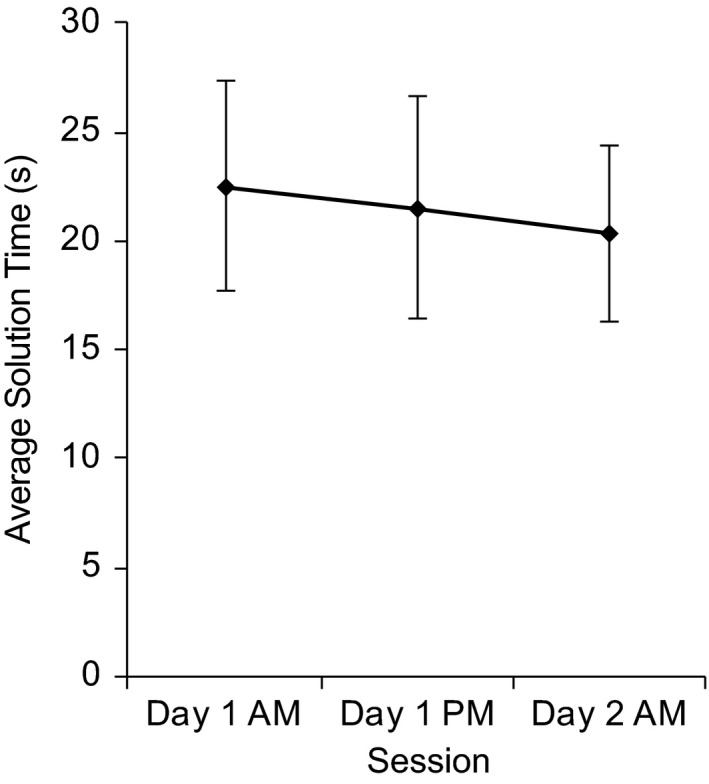
Average solution time for difficult trials (i.e. five‐, six‐ or seven‐move solutions) across the three assessments of the Tower of Hanoi (TOH). Error bars represent 1 SD
2.4. Polysomnography
Polysomnography was recorded with eight electrodes: for electroencephalography (EEG) two electrodes were positioned on frontopolar (Fpz) and central (Cz) positions according to the 10–20 system; for electrooculography two electrodes were placed diagonally, one 1 cm above and the other 1 cm below the outer corner of the eyes; for electromyography two electrodes were attached submentally (chin muscles); a ground electrode was positioned on the forehead and a reference electrode on the left mastoid (A1). Signals were recorded with the Embla A10 system (Flaga hf, Reykjavik, Iceland). The Embla A10 system initially samples the data at 2000 Hz, and subsequently down‐samples it digitally to 200 Hz. Furthermore, it applies high‐pass filtering (transition band below 1 Hz, –3 dB at 0.3 Hz) and a 50 Hz notch filter (1 Hz bandwidth). The montage of electrodes was validated against the AASM C4‐A1 montage (Van Sweden, Kemp, Kamphuisen, & Van Der Velde, 1990), and previously applied successfully to obtain sleep variables, including spindle parameters, in adults with and without sleep disorders (Fronczek et al., 2008; Raymann & Van Someren, 2008) and in children (Astill et al., 2014; Piantoni et al., 2013).
2.5. Quantitative analysis of sleep macrostructure, SWA and sleep spindles
Polysomnography was scored according to standard criteria (Iber, Ancoli‐Israel, Chesson, & Quan, 2007). To quantify sleep macrostructure the following measures were derived: time in bed, total sleep time (TST), sleep onset latency, latency to first REM period, wake after sleep onset, sleep efficiency (TST relative to time in bed), and the percentage of sleep stages N1, N2, N3 and REM relative to TST. SWA was calculated as average power in the range of 1–4.5 Hz in Stage N2 and Stage N3 across the entire night, separately for the Fpz and Cz channel.
Sleep spindles in Stage N2 and Stage N3 during the entire night were automatically quantified as previously described in detail in Astill et al. (2014). The automated spindle detection method is universal, i.e. not specific to a montage, and has successfully been applied to the sleep EEG of children assessed with 128 channels (Lustenberger, Wehrle, Tushaus, Achermann, & Huber, 2015) and with the current montage (Astill et al., 2014; Piantoni et al., 2013). Spindle features quantified were duration, maximal amplitude, duration × maximal amplitude, power and density (the number of spindles per 30 s epoch of sleep). These were separately calculated using both Fpz and Cz channels for slow (frequency < 12 Hz) and fast (frequency ≥ 12 Hz) spindles. The 12 Hz cut‐off was determined in a data‐driven way using the frequency distributions of typically slower spindles at Fpz and typically faster spindles at Cz, as previously reported by Astill et al. (2014).
2.6. Statistical analyses
Before we averaged the solution time for the six most difficult trials per session, we inspected the data for outliers per grade of difficulty. In total 11 outliers (> 3 SD from average) were excluded: five‐move solutions (n = 2 trials); six‐move solutions (n = 6); and seven‐move solutions (n = 3). After quantifying TOH performance as the average solution time (s) for each session, performance change scores were calculated by subtracting average solution times of day 1 p.m. session from day 1 a.m. session (ΔTOH across wake), and day 2 a.m. session from day 1 p.m. session (ΔTOH across sleep). Positive change scores indicate TOH performance improvement, i.e. a shortening of solution times across sessions. Descriptive statistics and histograms were used to verify normality of all variables. A normal distribution was confirmed for the average solution time on all three sessions, macrostructural characteristics of sleep, SWA and sleep spindle characteristics. Visual inspection of boxplots and scatterplots suggested one outlier that was excluded from analyses investigating ΔTOH across sleep, after having confirmed that the value crossed the usual threshold of 1.5 times the inter‐quartile range above the third quartile.
A one‐way repeated‐measures ANOVA was performed to investigate whether there was an overall learning effect on the TOH across the three sessions (day 1 a.m. session, day 1 p.m. session, day 2 a.m. session). Pearson correlation coefficients were calculated to quantify associations of sleep characteristics with TOH performance improvement across intervals of wake and sleep. Pearson's correlation coefficients (r) indicate effect sizes, which can be considered, respectively, small .10 ≤ r < .30; moderate .30 ≤ r < .50; or large r ≥ .50 (Cohen, 1988). Initial planning and problem skill levels might influence the TOH improvement across wake and across sleep. Therefore, observed associations were reanalysed with the initial TOH average solution times as a covariate (i.e. day 1 a.m. session and day 1 p.m. session, respectively) using multiple regression analyses to obtain part correlations. Part (or semi‐partial) correlations quantify the correlation between two variables after removing the effect of the covariate only from the independent variable. In a similar way, analyses were adjusted for the increase in tiredness during the day (ΔVAS day 1 p.m.–a.m.). All analyses were conducted with SPSS (Statistical Package for Social Sciences) Version 23.0. An alpha level of .05 (two‐sided) was used to indicate statistical significance.
3. RESULTS
In two children, one of the three TOH sessions was missing because of malfunctioning equipment. The EEG variables based on the Cz channel (SWA Cz and spindle features) of two children were omitted from the analyses due to a consistently noisy signal. No Stage N1 sleep was detected in two children. Pair‐wise deletion was applied to these missing data in the Pearson correlation analyses. Sleep characteristics are presented in Table 1.
Table 1.
Sleep characteristics (N = 30)
| M | SD | |
|---|---|---|
| Macrostructure characteristics | ||
| Time in bed (min) | 464.68 | 9.66 |
| TST (min) | 431.97 | 25.31 |
| Sleep onset latency (min) | 19.27 | 12.80 |
| First REM latency (min) | 94.32 | 37.89 |
| Sleep efficiency | 92.95 | 4.84 |
| Wake after sleep onset (min) | 9.43 | 11.51 |
| Stage N1 (% TST)a | 2.60 | 1.84 |
| Stage N2 (% TST) | 38.97 | 7.24 |
| Stage N3 (% TST) | 33.96 | 5.78 |
| Stage R (% TST) | 23.70 | 4.50 |
| SWA Fpz (μV2) | 194.90 | 78.10 |
| SWA Cz (μV2)a | 349.65 | 117.75 |
| Slow sleep spindle characteristics a | ||
| Duration (s) | 1.11 | 0.12 |
| Amplitude (μV) | 22.45 | 3.33 |
| Duration*Amplitude (μVs) | 25.65 | 5.30 |
| Power (μV2 Hz−1) | 7.40 | 3.14 |
| Frequency (Hz) | 11.24 | 0.17 |
| Density (# per 30 s epoch) | 1.15 | 0.37 |
| Fast sleep spindle characteristics a | ||
| Duration (s) | 1.12 | 0.12 |
| Amplitude (μV) | 22.89 | 3.35 |
| Duration*Amplitude (μVs) | 26.96 | 5.61 |
| Power (μV2 Hz−1) | 8.51 | 3.58 |
| Frequency (Hz) | 12.85 | 0.23 |
| Density (# per 30 s epoch) | 1.25 | 0.42 |
REM, rapid eye movement; Stage N1, NREM 1; Stage N2, NREM2; Stage N3, SWS or NREM3; Stage R, REM; SWA, slow‐wave activity (average power in the range of 1–4.5 Hz in Stage N2 and Stage N3 across the entire night); TST, total sleep time.
N = 28.
3.1. TOH performance and tiredness
No significant overall learning effect was found, because the one‐way repeated‐measures ANOVA showed that the small improvement in TOH solution time over the three assessments (Figure 1) did not reach significance at the group level due to high between‐subject variability (day 1 a.m. session: M = 22.51, SD = 4.83; day 1 p.m. session: M = 21.53, SD = 5.13; day 2 a.m. session: M = 20.28, SD = 4.02; F 2,54 = 2.54, p = .088, n = 28).
As expected, children were more tired in the evening (M = 5.7, SD = 2.4) than in the prior morning (M = 2.1, SD = 2.2; t 27 = –5.93, p < .001), but not very tired given the average answer in the mid‐range of the VAS. No residual tiredness was detected on the morning of day 2 (M = 2.8, SD = 2.3) as compared with the first morning (t 27 = –1.11, p = .275). The increase in tiredness during day 1 at the museum (ΔVAS day 1 p.m.–a.m.) showed no significant associations with Stage N2 (p = .701), Stage N3 (p = .313), SWA (Fpz, p = .560; Cz, p = .721), performance on the TOH at the p.m. session (p = .091), and neither with TOH performance improvement across wake (p = .971) nor across sleep (p = .323). These results suggest that differences in tiredness do not explain differences in sleep characteristics or TOH performance.
3.2. TOH performance improvement across wake
3.2.1. Sleep stages and SWA
A stronger TOH improvement (faster average solution time) across wake was seen in children with more Stage N2 and/or less Stage N3 sleep (Figure 2a,b; Table 2). Stage N2 sleep and N3 were negatively correlated (r = –.62, p < .001). To control for initial TOH performance differences, part correlations were performed. The association of TOH improvement with Stage N2 sleep was reduced to a trend (r part = .32, p = .071), while the effect for N3 remained significant (r part = –.40, p = .021). Adjusting the observed associations by including the increase in tiredness during the day (ΔVAS day 1 p.m.–a.m.) as a covariate yielded similar results: TOH improvement across wake remained significant with Stage N2 sleep (r part = .40, p = .045) and Stage N3 sleep (r part = –.43, p = .027). Other sleep stages did not show significant associations with TOH performance improvement across wakefulness, and neither did SWA (Table 2).
Figure 2.
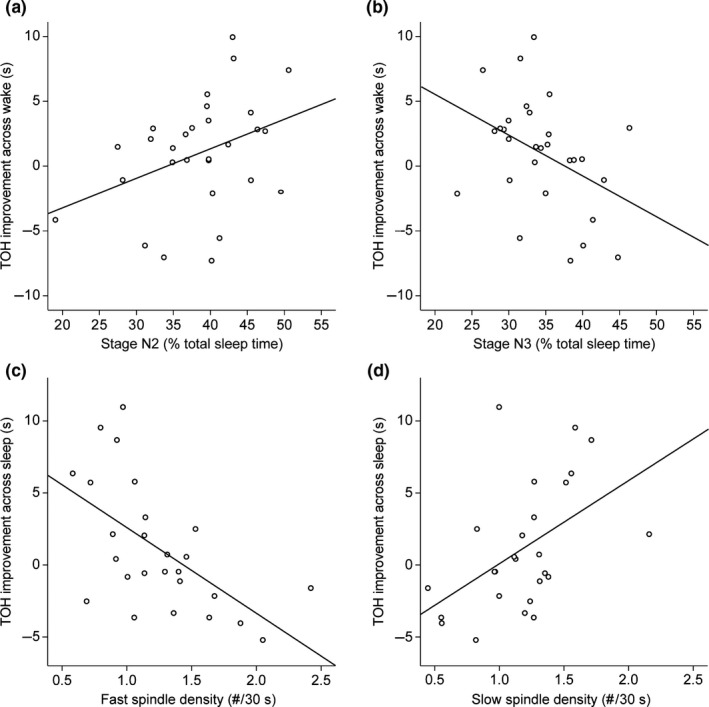
(a and b) The association of Tower of Hanoi (TOH) performance improvement (i.e. faster average solution time) across wake with (a) Stage N2 (% total sleep time [TST]), r = .37, p = .048; (b) Stage N3 (% TST), r = –.40, p = .030. A stronger improvement is associated with more Stage N2 and less Stage N3. (c and d) The association of TOH performance improvement across sleep with (c) Fast spindle density (# per 30 s epoch), r = –.59, p = .002; (d) slow spindle density (# per 30 s epoch), r = .49, p = .010. A stronger improvement is associated with a lower density of fast spindles and a higher density of slow spindles
Table 2.
Pearson correlation coefficients of sleep stages and SWA with improvement in TOH performance (i.e. decline in average solution time) on difficult trials. Significant correlations are shown in bold font.
| Sleep stages | Improvement in TOH across wake (n = 29) | Improvement in TOH across sleep (n = 28) | ||
|---|---|---|---|---|
| r | p | r | p | |
| Wake after sleep onset (% TST) | –.10 | .626 | –.03 | .892 |
| Stage N1 (% TST)a | –.04 | .841 | –.17 | .417 |
| Stage N2 (% TST) | .37 | .048 | –.31 | .112 |
| Stage N3 (% TST) | –.40 | .030 | .30 | .121 |
| Stage R (% TST) | .03 | .887 | .14 | .476 |
| SWA F pz (μV2) | –.07 | .738 | .14 | .468 |
| SWA C z (μV2)b | –.12 | .554 | .15 | .462 |
Stage N1, NREM 1; Stage N2, NREM2; Stage N3, SWS or NREM3; Stage R, REM; SWA, slow‐wave activity (average power in the range of 1–4.5 Hz in Stage N2 and Stage N3 across the entire night); TOH, Tower of Hanoi; TST, total sleep time.
N = 28.
Data Cz channel missing for two participants.
3.2.2. Sleep spindles
The change in TOH solution time across wakefulness was not significantly associated with any sleep spindle characteristic (Table 3).
Table 3.
Pearson correlation coefficients of sleep spindle characteristics with improvement in TOH performance (i.e. decline in average solution time) on difficult trials. Significant correlations are shown in bold font.
| Sleep spindle characteristics | Improvement in TOH across wake (n = 27) | Improvement in TOH across sleep (n = 26) | ||
|---|---|---|---|---|
| r | p | r | p | |
| Fast | ||||
| Duration (ms) | .03 | .894 | –.40 | .043 |
| Amplitude (μV) | .28 | .164 | –.19 | .358 |
| Duration*Amplitude (μVs) | .26 | .188 | –.37 | .060 |
| Power (μV2 Hz−1) | .30 | .127 | –.41 | .039 |
| Density (# per 30 s epoch) | .05 | .793 | –.59 | .002 |
| Slow | ||||
| Duration (ms) | .06 | .757 | .09 | .677 |
| Amplitude (μV) | .24 | .221 | –.13 | .533 |
| Duration*Amplitude (μVs) | .21 | .285 | –.05 | .814 |
| Power (μV2 Hz−1) | .19 | .339 | –.09 | .676 |
| Density (# per 30 s epoch) | –.07 | .728 | .49 | .010 |
TOH, Tower of Hanoi.
3.3. TOH performance improvement across sleep
3.3.1. Sleep stages and SWA
No significant associations were found between TOH performance changes across sleep and the sleep stages or SWA (Table 2).
3.3.2. Sleep spindles
A stronger TOH improvement (faster average solution time) across sleep was seen in children whose fast spindles had a lower density, shorter duration and less power, and/or whose slow spindles had a higher density (Figure 2c,d; Table 3). Fast and slow spindle densities were negatively correlated (r = –.76, p < .001). Although initial planning and problem skill levels might influence the TOH improvement across sleep, part correlations showed that the associations of overnight TOH improvement with fast spindle density (r part = –.47, p = .008) as well as with slow spindle density (r part = 0.42, p = .020) remain significant. The other fast spindle features, duration and power, were no longer related with overnight TOH improvement after controlling for initial performance. Similar results were obtained in regression models that were adjusted for changes in tiredness. The associations of TOH improvement across sleep with both fast spindle density (r part = –.58, p = .003) and slow spindle density (r part = .44, p = .033) remained significant. After adjusting for the increase in tiredness across the day, the other fast spindle features, duration and power, were no longer related with overnight TOH improvement.
4. DISCUSSION
The present study addressed whether individual differences in sleep stages and microstructural sleep characteristics of children aged between 9 and 13 years are associated with performance improvement over repeated assessments of the TOH task, which requires integrated planning and problem‐solving skills.
The results showed that stronger performance increments on the TOH across wakefulness were found in children with more Stage N2 and less Stage N3 sleep (both moderate effects). Developmental studies show that the amount of SWS is attenuated from age 9 to 16 years in favour of more Stage 2 sleep (Jenni & Carskadon, [Link]; Tarokh & Carskadon, 2010), and that these changes in EEG across age may reflect the decline in synaptic connectivity among neurons during the transition towards adulthood. For the current study, this suggests that improving planning and problem‐solving skills across wakefulness is the result of more efficient neural processing, i.e. more advanced brain maturation, of children with more Stage 2 sleep and less SWS. Our results are in line with recent suggestions that Stage 2 sleep and SWS are involved in the acquisition and refinement of planning and problem‐solving skills on the TOH in adults (Fogel et al., 2015). TOH performance improvement across sleep was not associated with Stage 2 sleep and SWS. It is not unlikely that the effect of more efficient neural processing might surface only across wakefulness, when the brain has to cope with more environmental demands and with external interference (Rasch & Born, 2013). We did not find associations between TOH improvement across wakefulness with electrophysiological features of Stage 2 sleep and SWS, for example sleep spindles or SWA. This is in contrast with previous work (Buchmann et al., 2011), suggesting that SWA is related to decreases in grey matter during development, and with studies suggesting that SWA reflects cortical plasticity induced by prior learning and use of neural networks involved in EF and motor skills (Pugin et al., 2015; Wehrle & Latal, 2017; Wilhelm et al., 2014).
The findings regarding TOH performance improvements across sleep showed that stronger improvements in planning and problem‐solving skills were present in children in whom fast spindles were less dense, of shorter duration and had less power, and in whom slow spindles were more dense. These associations of sleep spindle features with TOH performance increments (moderate to large effects) suggest that the neural processes during NREM sleep contribute to the consolidation of planning and problem‐solving skills. Our results correspond to previous findings in children, in which enhanced overnight motor skills (Astill et al., 2014) were related to higher slow spindle density. Moreover, our negative associations for spindle characteristics are in line with studies in children that found relations between spindle frequency and EF or general cognitive ability (Chatburn et al., 2013; Geiger et al., 2011; Gruber et al., 2013). While Gruber et al. (2013) found results only for spindle frequency, we also showed associations with spindle power, duration and density.
So far, studies in children did not focus on mastery of planning and problem‐solving skills. In adults, overnight TOH performance improvement was associated with higher spindle density (Nielsen et al., 2015), specifically for fast spindles (Fogel et al., 2015). In contrast, the current study in children showed TOH performance improvement to be associated with a higher density of slow spindles and a lower density of fast spindles. This discrepancy suggests interesting developmental differences between childhood and adult spindle properties, such as amount and topographical distribution of fast and slow sleep spindles (Clawson, Durkin, & Aton, 2016; D'atri, Novelli, Ferrara, Bruni, & De Gennaro, 2018; Scholle, Zwacka, & Scholle, 2007; Tarokh & Carskadon, 2010). Sleep spindle features may be indicative of maturity of the neuronal networks involved in EF.
Mastery of the TOH could involve a declarative memory component (Winter, Broman, Rose, & Reber, 2001; Xu & Corkin, 2001). Spindles and slow waves have shown to play important roles in declarative memory consolidation. The active system consolidation model (ACS; Born & Wilhelm, 2012) states that novel associations induced by daytime experiences are initially stored temporarily in the hippocampus. During subsequent sleep, neocortical slow oscillations drive a dialogue between the neocortex and hippocampus that, together with thalamocortical spindles and sharp‐wave ripples, ensues reactivation of the hippocampal memory representations and their redistribution to the neocortex for long‐term storage (Born & Wilhelm, 2012). The ACS model concerns declarative memory consolidation with a key role for the hippocampus. However, hippocampal involvement in the TOH is likely to be minimal. A functional magnetic resonance imaging study in healthy adults failed to find significant hippocampal involvement during TOH performance. Instead, activation was confined to a fronto‐parietal system (Fincham, Carter, Van Veen, Stenger, & Anderson, 2002). It thus remains unclear whether the ACS model applies to mastery of the TOH task. Mastery of the TOH may also involve a procedural learning component. Whereas overnight improvement on procedural tasks has initially been proposed to involve REM sleep, several studies suggest an involvement of slow waves and spindles as well (for reviews, see Ulrich, 2016; Walker & Stickgold, 2004). The specific role of spindles and other EEG components in the mastery of planning and problem‐solving skills needs further attention in future research.
We found only associations with NREM sleep stages and related electrophysiological features, while our findings revealed no association between TOH performance improvement and REM sleep (percentage relative to TST). These results are in line with the results of Nielsen et al. (2015) and Smith et al. (), where actual time spent in REM was not related to TOH improvement in adults. One explanation might be that TOH performance strongly taps into declarative memory because of the explicit remembrance of solution strategies (Winter et al., 2001). It is known that declarative memory depends more on NREM than on REM sleep (Rasch & Born, 2013), and therefore may explain our negative findings regarding REM sleep.
Of note, it remains unknown whether our findings are specific to the acquisition of planning and problem‐solving skills, or that they apply to a more general cognitive factor. At least three arguments can be raised against the involvement of general intelligence. First, several studies in children failed to find significant associations of general intelligence with TOH and other executive function task performances (Bull, Espy, & Senn, 2004). Second, different associations with sleep were found for a procedural learning task performed in the same sample of children as reported here (Astill et al., 2014). Third, a general factor is unlikely given the finding that individuals differ significantly with respect to the tasks on which performance worsens most with sleep loss (Van Dongen, Baynard, Maislin, & Dinges, 2004).
The results of the current study must be interpreted taking into account some limitations. First, the study design contained no adaption night, and the intervals of sleep and wake were not counter‐balanced across children. A more optimal design was not possible due to the unique set‐up of a playful educational event including a night in the museum. The design limitation precluded a fully balanced comparison of overnight consolidation effects with the effects after a similar period of wake. A second limitation is that the sleep duration of the children was restricted, because the p.m. assessment was on average 1 hr and 3 min (SD = 22 min) later than the habitual bedtimes as reported by parents. However, the distribution of sleep stage durations is comparable to what is known for children (Jenni & Carskadon, [Link]), and sleep quality indicated by sleep efficiency was good. Even if the analyses on TOH performance improvement were co‐varied by the increase in tiredness during the day, the majority of the observed associations (i.e. with Stage N2, Stage N3, slow and fast spindle density) remained significant, except for fast spindle duration and power, which were no longer related with overnight TOH improvement. For future research it is recommended to perform sleep recordings at home adhering to the children's habitual sleep schedule. A third limitation is that it is not trivial to investigate accuracy or speed accuracy trade‐off using a computerized version of the TOH. In the current version, the children had to start over with the problem if they made an error. This discourages random clicking but results in quite non‐linear changes in accuracy, depending on when the errors are made. An alternative implementation could be problematic for computerized assessment as well: if an error would only result in a notification without further consequence, fast random clicking would be encouraged. It is worth mentioning that previous research found a speed accuracy trade‐off on the TOH only for children aged 8–9 years and not for 11–12 year olds (Schiff & Vakil, 2015). Finally, a much larger and diverse sample would be needed to address differences and interactions related to sex, pubertal status and sleep disturbances.
In sum, the importance of sleep for learning and EF is well studied. Nevertheless, findings regarding the associated sleep processes are inconsistent and are scarce for higher‐order EF, such as planning and problem‐solving skills in children. Therefore, we included sleep stages and various microstructural sleep characteristics, such as fast and slow sleep spindles, and SWA, to examine their association with improvement in EF performance across sleep and wakefulness. The findings indicated that specific sleep EEG signatures, especially sleep spindles, reflect the ability of the developing brain to acquire integrated planning and problem‐solving skills.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors have contributed to this manuscript by carrying out one or more of the following activities: study design, data collection, data analysis, interpretation of results, preparation of the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the Netherlands Organization of Scientific Research (NWO), The Hague: National Initiative Brain & Cognition Research Program “Innovative Learning Materials and Methods” under Grant 056‐32‐013; and VICI Innovation Grant 453‐07‐001. Data were obtained during “The Great Sleep Experiment” event, organized and sponsored by Netherlands Organization for Scientific Research (NWO) and the Netherlands Institute for Neuroscience. The event was also sponsored by Beter Bed, Medcare, IBM, Amstel Botel, Cambridge Neurotechnology, J&J Pharmaceutical Research and Development, Nederlandse Vereniging voor Slaap‐Waak Onderzoek, Onderzoeksschool Neurowetenschappen Amsterdam, Philips, SEIN Zwolle, Gelre ziekenhuizen Zutphen, OLVG Amsterdam, Sint Lucas Andreas Ziekenhuis Amsterdam, Spaarne Ziekenhuis Hoofddorp, and Zaans Medisch Centrum Zaandam. The authors want to thank all participating children and their teachers, and all 22 volunteering lab technicians and the research staff who made “The Great Sleep Experiment” possible. Finally, the authors would like to thank Prof. R. Huber for his kind permission to use and adapt his automated spindle detection scripts, Prof. M. Massimini for the slow‐wave script, and Dr R.G. Astill, Dr G. Piantoni and F. van Schalkwijk MSc for their assistance in preparing the data.
Vermeulen MCM, Van der Heijden KB, Swaab H, Van Someren EJW. Sleep spindle characteristics and sleep architecture are associated with learning of executive functions in school‐age children. J Sleep Res. 2019;28:e12779 10.1111/jsr.12779
REFERENCES
- Ackermann, S. , & Rasch, B. (2014). Differential effects of non‐REM and REM sleep on memory consolidation? Current Neurology and Neuroscience Reports, 14, 430 10.1007/s11910-013-0430-8 [DOI] [PubMed] [Google Scholar]
- Anderson, B. , Storfer‐Isser, A. , Taylor, H. G. , Rosen, C. L. , & Redline, S. (2009). Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics, 123, e701–e707. 10.1542/peds.2008-1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth, A. , Hill, C. M. , Karmiloff‐Smith, A. , & Dimitriou, D. (2014). Sleep enhances memory consolidation in children. Journal of Sleep Research, 23, 302–308. [DOI] [PubMed] [Google Scholar]
- Astill, R. G. , Piantoni, G. , Raymann, R. J. , Vis, J. C. , Coppens, J. E. , Walker, M. P. , … Van Someren, E. J. (2014). Sleep spindle and slow wave frequency reflect motor skill performance in primary school‐age children. Frontiers in Human Neuroscience, 8, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born, J. , Rasch, B. , & Gais, S. (2006). Sleep to remember. Neuroscientist, 12, 410–424. 10.1177/1073858406292647 [DOI] [PubMed] [Google Scholar]
- Born, J. , & Wilhelm, I. (2012). System consolidation of memory during sleep. Psychological Research, 76, 192–203. 10.1007/s00426-011-0335-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann, A. , Ringli, M. , Kurth, S. , Schaerer, M. , Geiger, A. , Jenni, O. G. , … Huber, R. (2011). EEG sleep slow‐wave activity as a mirror of cortical maturation. Cerebral Cortex, 21, 607–615. 10.1093/cercor/bhq129 [DOI] [PubMed] [Google Scholar]
- Bull, R. , Espy, K. A. , & Senn, T. E. (2004). A comparison of performance on the Towers of London and Hanoi in young children. Journal of Child Psychology and Psychiatry, 45, 743–754. 10.1111/j.1469-7610.2004.00268.x [DOI] [PubMed] [Google Scholar]
- Casey, B. J. , Tottenham, N. , Liston, C. , & Durston, S. (2005). Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences, 9, 104–110. 10.1016/j.tics.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Chatburn, A. , Coussens, S. , Lushington, K. , Kennedy, D. , Baumert, M. , & Kohler, M. (2013). Sleep spindle activity and cognitive performance in healthy children. Sleep, 36, 237–243. 10.5665/sleep.2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson, B. C. , Durkin, J. , & Aton, S. J. (2016). Form and function of sleep spindles across the lifespan. Neural Plasticity, 2016, 6,936,381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- D'atri, A. , Novelli, L. , Ferrara, M. , Bruni, O. , & De Gennaro, L. Different maturational changes of fast and slow sleep spindles in the first four years of life. Sleep Medicine, 2018, 42, 73–82. 10.1016/j.sleep.2017.11.1138 [DOI] [PubMed] [Google Scholar]
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann, S. , & Born, J. (2010). The memory function of sleep. Nature Reviews Neuroscience, 11, 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Fincham, J. M. , Carter, C. S. , Van Veen, V. , Stenger, V. A. , & Anderson, J. R. Neural mechanisms of planning: A computational analysis using event‐related fMRI. Proceedings of the National Academy of Science USA, 2002, 99, 3346–3351. 10.1073/pnas.052703399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, S. , Wilhelm, I. , & Born, J. (2007). Developmental differences in sleep's role for implicit off‐line learning: Comparing children with adults. Journal of Cognitive Neuroscience, 19, 214–227. 10.1162/jocn.2007.19.2.214 [DOI] [PubMed] [Google Scholar]
- Fogel, S. M. , Ray, L. B. , Binnie, L. , & Owen, A. M. (2015). How to become an expert: A new perspective on the role of sleep in the mastery of procedural skills. Neurobiology of Learning and Memory, 125, 236–248. 10.1016/j.nlm.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Fogel, S. M. , & Smith, C. T. (2006). Learning‐dependent changes in sleep spindles and Stage 2 sleep. Journal of Sleep Research, 15, 250–255. 10.1111/j.1365-2869.2006.00522.x [DOI] [PubMed] [Google Scholar]
- Fronczek, R. , Raymann, R. J. , Overeem, S.et al. (2008). Manipulation of skin temperature improves nocturnal sleep in narcolepsy. Journal of Neurology, Neurosurgery and Psychiatry, 79, 1354–1357. 10.1136/jnnp.2008.143610 [DOI] [PubMed] [Google Scholar]
- Geiger, A. , Huber, R. , Kurth, S. , Ringli, M. , Jenni, O. G. , & Achermann, P. (2011). The sleep EEG as a marker of intellectual ability in school age children. Sleep, 34, 181–189. 10.1093/sleep/34.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, R. , Wise, M. S. , Frenette, S. . (2013). The association between sleep spindles and IQ in healthy school‐age children. International Journal of Psychophysiology, 89, 229–240. 10.1016/j.ijpsycho.2013.03.018 [DOI] [PubMed] [Google Scholar]
- Hoedlmoser, K. , Heib, D. P. J. , Roell, J.et al. (2014). Slow sleep spindle activity, declarative memory, and general cognitive abilities in children. Sleep, 37, 1501–U1313. 10.5665/sleep.4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber, C. , Ancoli‐Israel, S. , Chesson, A. L. Jr , & Quan, S. F. (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology, and technical specifications. Westchester, IL: American Academy of Sleep Medicine. [Google Scholar]
- Jaeggi, S. M. , Buschkuehl, M. , Jonides, J. , & Shah, P.(2011). Short‐ and long‐term benefits of cognitive training. Proceedings of the National Academy of Science USA, 108, 10,081–10,086. 10.1073/pnas.1103228108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni, O. G. , & Carskadon, M. A.(2005). Normal human sleep at different ages: Infants to adolescents In Sleep Research Society, SRS basics of sleep guide (pp. 11–19). Westchester, IL: Sleep Research Society. [Google Scholar]
- Karbach, J. , & Unger, K. (2014). Executive control training from middle childhood to adolescence. Frontiers in Psychology, 5, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonova, M. , Martin, R. E. , Gabrieli, J. D. , & Sheridan, M. A. (2013). Cortical gray‐matter thinning is associated with age‐related improvements on executive function tasks. Developmental Cognitive Neuroscience, 6, 61–71. 10.1016/j.dcn.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama, K. , Mishima, K. , Suzuki, H. , Aritake, S. , & Uchiyama, M. (2008). Sleep accelerates the improvement in working memory performance. Journal of Neuroscience, 28, 10,145–10,150. 10.1523/jneurosci.2039-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuula, L. , Pesonen, A. K. , Martikainen, S.et al. (2015). Poor sleep and neurocognitive function in early adolescence. Sleep Medicine, 16, 1207–1212. 10.1016/j.sleep.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Lustenberger, C. , Wehrle, F. , Tushaus, L. , Achermann, P. , & Huber, R. (2015). The multidimensional aspects of sleep spindles and their relationship to word‐pair memory consolidation. Sleep, 38, 1093–1103. 10.5665/sleep.4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix‐Cols, D. , & Bartrés‐Faz, D. (2002). Is the use of the wooden and computerized versions of the Tower of Hanoi puzzle equivalent? Applied Neuropsychology, 9, 117–120. 10.1207/s15324826an0902_8 [DOI] [PubMed] [Google Scholar]
- Miyake, A. , Friedman, N. P. , Emerson, M. J. , Witzki, A. H. , Howerter, A. , & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Nielsen, T. , O'Reilly, C. , Carr, M. , Dumel, G. , Godin, I. , Solomonova, E. , … Paquette, T. (2015). Overnight improvements in two REM sleep‐sensitive tasks are associated with both REM and NREM sleep changes, sleep spindle features, and awakenings for dream recall. Neurobiology of Learning and Memory, 122, 88–97. 10.1016/j.nlm.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Nigg, J. T. (2017). Annual Research Review: On the relations among self‐regulation, self‐control, executive functioning, effortful control, cognitive control, impulsivity, risk‐taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58, 361–383. 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, K. R. , Smith, V. , & Smith, C. T. (2007). Changes in sleep architecture following motor learning depend on initial skill level. Journal of Cognitive Neuroscience, 19, 817–829. 10.1162/jocn.2007.19.5.817 [DOI] [PubMed] [Google Scholar]
- Piantoni, G. , Astill, R. G. , Raymann, R. J. , Vis, J. C. , Coppens, J. E. , & Van Someren, E. J. (2013). Modulation of gamma and spindle‐range power by slow oscillations in scalp sleep EEG of children. International Journal of Psychophysiology, 89, 252–258. 10.1016/j.ijpsycho.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Pugin, F. , Metz, A. J. , Wolf, M. , Achermann, P. , Jenni, O. G. , & Huber, R. (2015). Local increase of sleep slow wave activity after three weeks of working memory training in children and adolescents. Sleep, 38, 607–614. 10.5665/sleep.4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch, B. , & Born, J. (2013). About sleep's role in memory. Physiological Reviews, 93, 681–766. 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymann, R. J. , & Van Someren, E. J. (2008). Diminished capability to recognize the optimal temperature for sleep initiation may contribute to poor sleep in elderly people. Sleep, 31, 1301–1309. [PMC free article] [PubMed] [Google Scholar]
- Sadeh, A. , Gruber, R. , & Raviv, A. (2002). Sleep, neurobehavioral functioning, and behavior problems in school‐age children. Child Development, 73, 405–417. 10.1111/1467-8624.00414 [DOI] [PubMed] [Google Scholar]
- Sadeh, A. , Gruber, R. , & Raviv, A. (2003). The effects of sleep restriction and extension on school‐age children: What a difference an hour makes. Child Development, 74, 444–455. 10.1111/1467-8624.7402008 [DOI] [PubMed] [Google Scholar]
- Satterthwaite, T. D. , Wolf, D. H. , Erus, G. . (2013). Functional maturation of the executive system during adolescence. Journal of Neuroscience, 33, 16,249–16,261. 10.1523/jneurosci.2345-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff, R. , & Vakil, E. (2015). Age differences in cognitive skill learning, retention and transfer: The case of the Tower of Hanoi Puzzle. Learning and Individual Differences, 39, 164–171. 10.1016/j.lindif.2015.03.010 [DOI] [Google Scholar]
- Scholle, S. , Zwacka, G. , & Scholle, H. C. (2007). Sleep spindle evolution from infancy to adolescence. Clinical Neurophysiology, 118, 1525–1531. 10.1016/j.clinph.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Simon, H. A. (1975). The functional equivalence of problem solving skills. Cognitive Psychology, 7, 268–288. 10.1016/0010-0285(75)90012-2 [DOI] [Google Scholar]
- Smith, C. T. , Nixon, M. R. & Nader, R. S. (2004). Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learning Memory, 11, 714-719. 10.1101/lm.74904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh, L. , & Carskadon, M. A. (2010). Developmental changes in the human sleep EEG during early adolescence. Sleep, 33, 801–809. 10.1093/sleep/33.6.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, D. (2016). Sleep spindles as facilitators of memory formation and learning. Neural Plasticity, 2016, 1,796,715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen, H. P. A. , Baynard, M. D. , Maislin, G. , & Dinges, D. F. (2004). Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait‐like differential vulnerability. Sleep, 27, 423. [PubMed] [Google Scholar]
- Van Sweden, B. , Kemp, B. , Kamphuisen, H. A. , & Van Der Velde, E. A. (1990). Alternative electrode placement in (automatic) sleep scoring (Fpz‐Cz/Pz‐Oz versus C4‐A1). Sleep, 13, 279–283. 10.1093/sleep/13.3.279 [DOI] [PubMed] [Google Scholar]
- Walker, M. P. , Brakefield, T. , Morgan, A. , Hobson, J. A. , & Stickgold, R. (2002). Practice with sleep makes perfect: Sleep‐dependent motor skill learning. Neuron, 35, 205–211. 10.1016/s0896-6273(02)00746-8 [DOI] [PubMed] [Google Scholar]
- Walker, M. P. , & Stickgold, R. (2004). Sleep‐dependent learning and memory consolidation. Neuron, 44, 121–133. 10.1016/j.neuron.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Wehrle, F. M. , & Latal, B. (2017). Sleep EEG maps the functional neuroanatomy of executive processes in adolescents born very preterm. Cortex, 86, 11–21. 10.1016/j.cortex.2016.10.011 [DOI] [PubMed] [Google Scholar]
- Wilhelm, I. , Diekelmann, S. , & Born, J. (2008). Sleep in children improves memory performance on declarative but not procedural tasks. Learning & Memory, 15, 373–377. 10.1101/lm.803708 [DOI] [PubMed] [Google Scholar]
- Wilhelm, I. , Kurth, S. , Ringli, M. , Mouthon, A. L. , Buchmann, A. , Geiger, A. , … Huber, R. (2014). Sleep slow‐wave activity reveals developmental changes in experience‐dependent plasticity. Journal of Neuroscience, 34, 12,568–12,575. 10.1523/jneurosci.0962-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, W. E. , Broman, M. , Rose, A. L. , & Reber, A. S. (2001). The assessment of cognitive procedural learning in amnesia: Why the tower of Hanoi has fallen down. Brain and Cognition, 45, 79–96. 10.1006/brcg.2000.1257 [DOI] [PubMed] [Google Scholar]
- Xu, Y. , & Corkin, S. H. M. (2001). Revisits the Tower of Hanoi Puzzle. Neuropsychology, 15, 69–79. 10.1037/0894-4105.15.1.69 [DOI] [PubMed] [Google Scholar]
- Zinke, K. , Noack, H. , & Born, J. (2018). Sleep augments training‐induced improvement in working memory in children and adults. Neurobiology of Learning and Memory, 147, 46–53. 10.1016/j.nlm.2017.11.009 [DOI] [PubMed] [Google Scholar]


