Summary
Narcolepsy type 1 is characterised by an increase in body weight after disease onset, frequently leading to obesity. It was suggested that this weight gain may be counteracted by treatment with sodium oxybate. We here provide longitudinal body mass index data of patients with narcolepsy type 1 after starting treatment with sodium oxybate, compared with patients in whom treatment with modafinil was initiated. Eighty‐one individuals with narcolepsy type 1 fulfilled the entry criteria for this retrospective study: 59 had newly started treatment with sodium oxybate and 22 had newly started modafinil. Gender‐specific differences between both treatment groups were compared using Student's t tests and mixed effect modeling. Patients using sodium oxybate lost weight, with a mean body mass index decrease of 2.56 kg/m2 between the first and last measurement (women; p = .001) and 0.84 kg/m2 (men; p = .006). Patients using modafinil, however, gained weight, with a mean body mass index increase of 0.57 kg/m2 (women; p = .033) and 0.67 kg/m2 (men; p = .122). Medication (p = .006) and baseline body mass index (p = .032) were predictors for body mass index decrease. In conclusion, treatment with sodium oxybate is associated with a body mass index reduction in narcolepsy type 1, whereas modafinil treatment is not. This effect is most pronounced in those who already have a higher baseline body mass index.
Keywords: weight loss, central nervous system stimulants, hydroxybutyrates, hypersomnia, dyssomnias
1. INTRODUCTION
Narcolepsy type 1 is a chronic neurological disorder characterised by excessive daytime sleepiness, cataplexy, sleep paralysis, hypnagogic hallucinations and disturbed nocturnal sleep (Black, Yamanaka, & Kilduff, 2017). In addition to the classical symptoms, other symptoms have been reported. These include autonomic abnormalities and obesity (Fronczek et al., 2008). Narcolepsy is caused by a loss of hypothalamic hypocretin (orexin)‐producing neurons (Nishino et al., 2001). Hypocretin neurons project throughout the central nervous system to areas known to be important in the control of sleep–wakefulness, but also to areas important in neuroendocrine homeostasis, autonomic regulation and the control of feeding (Willie, Chemelli, Sinton, & Yanagisawa, 2001).
From as early as the 1930s it has been reported that obesity is more prevalent in narcolepsy patients than in healthy controls (Daniels, 1934; Wang et al., 2016). Abdominal fat deposition and waist circumference were found to be significantly increased in narcolepsy patients (Kok et al., 2003), who also have a higher prevalence of the metabolic syndrome compared with idiopathic hypersomnia patients (Poli et al., 2009). Onset of narcolepsy type 1 was also associated with rapid weight gain in children (Ponziani et al., 2016). The cause of the observed obesity in narcolepsy has not been elucidated. It is probably not secondary to inactivity or to medication use (Black et al., 2017). Studies of eating habits showed conflicting results regarding caloric intake, the prevalence of eating disorders in narcolepsy (Fortuyn et al., 2008) and basal metabolic rate. A recent study in children with narcolepsy type 1 showed a lower basal metabolic rate (BMR) closely after disease onset, which returned to normal levels in the following months (Wang et al., 2016). Therefore, it is hypothesised that narcolepsy induces a change in the individual body mass index (BMI) set point (Dahmen, Tonn, Messroghli, Ghezel‐Ahmadi, & Engel, 2009), but the exact mechanism causing this hypothesised change in narcolepsy patients remains unclear. Management of BMI in narcolepsy is important, as a higher BMI seems to be a risk factor for diseases, such as type 2 diabetes and cardiovascular disease (Kok et al., 2003), and can lead to psychosocial and work‐related problems (Narbro et al., 1996; World Health Organization, 2000).
Recent observations suggest that paediatric and adult narcolepsy patients lose weight when using sodium oxybate (SXB; Boscolo‐Berto et al., 2012; Ponziani et al., 2016). Weight loss in narcolepsy patients with (mean loss of 5.1 kg) and without cataplexy (mean loss of 2 kg) treated with SXB has been reported (Husain, Ristanovic, & Bogan, 2009), while another study showed a 5.2‐kg weight loss after 3 months of SXB treatment (Donjacour et al., 2014). This has not yet been confirmed and long‐term follow‐up data are not available. We here provide longitudinal BMI data of narcolepsy type 1 patients after starting treatment with SXB, compared with patients in whom treatment with modafinil was initiated. Our hypothesis is that BMI decreases upon introduction of SXB, whereas use of modafinil will not affect BMI.
2. METHODS
2.1. Subjects
Medical records of consecutive individuals diagnosed with narcolepsy type 1, attending the outpatient clinic of the Sleep‐Wake Center Stichting Epilepsie Instellingen Nederland (SEIN) and the outpatient clinic of the Leiden University Medical Center between 2009 and 2017, were reviewed retrospectively. All individuals included (n = 81) fulfilled the criteria for narcolepsy type 1 as formulated in the third edition of the International Classification of Sleep Disorders (ICSD‐3, 2014). Only those who initiated treatment with either SXB or modafinil and used it for at least 3 months were included. Those who had already used one of the medications were excluded. Patients were also excluded if there were no measurements recorded, if they suffered from another disorder that is associated with obesity (e.g. hypothyroid disease) or if they were under the age of 18 years. The follow‐up period ended prematurely if the SXB or modafinil treatment was discontinued, or when additional medication was started. Relevant co‐medication use (antidepressants, methylphenidate or dexamphetamine) was extracted from the patient records. The decision to prescribe either modafinil or SXB was a clinical decision based on the presence and severity of the various narcolepsy symptoms. Weight at treatment initiation did not play a role in this decision.
2.2. Study design
This is a retrospective follow‐up study in which the above‐described individuals were followed up to 31 July 2017. Weight, height and medication, assessed at the beginning of each visit to the outpatient clinic, were extracted from the records. BMI was calculated by dividing the weight (kg) by the squared length (m2) for each visit.
2.3. Statistical analysis
Differences at baseline in participant characteristics between the SXB and modafinil groups were calculated with the Mann–Whitney U test (age), chi‐squared test (gender and co‐medication) and Student's t test (baseline BMI and treatment duration). To estimate if the BMI of the study cohort was significantly higher than that of the general Dutch population, data from Statistics Netherlands (Centraal Bureau voor de Statistiek, 2016) were used.
To assess whether statistically significant BMI changes occurred during follow‐up, a paired sample Student′s t test was performed to compare BMI at baseline with BMI at the last visit for four separate groups (based on gender and treatment). Because this comparison is still subject to factors influencing BMI and there was a single outcome parameter, a linear mixed model with a random slope and a random intercept for each individual was fitted. The outcome was the difference in BMI with respect to baseline. As fixed effects, we added medication, gender, baseline BMI and follow‐up duration, and also the interactions of medication, gender and baseline BMI with follow‐up duration. Normality of the fitted mixed model was subsequently assessed using scatter plots and quantile‐quantile plots of the model's residuals. Correlations between medication dose and BMI change for individual patients were assessed using Spearman's rank‐order correlation tests; p‐values below .05 were deemed significant. Bonferroni corrections were executed when needed. All analyses were conducted using the IBM SPSS Statistics 23 software package.
3. RESULTS
3.1. Patient characteristics
Fifty‐nine individuals started treatment with SXB and 22 with modafinil (Table 1). Follow‐up frequency and duration varied considerably among patients (range, 0.14–6.94 years). There were no significant differences found in age and baseline BMI between groups. More men were in the SXB group. Mean duration of treatment for the SXB group was longer than that for the modafinil group.
Table 1.
Characteristics of narcolepsy type 1 patients
| SXB | Modafinil | p‐value | |
|---|---|---|---|
| N | 59 | 22 | |
| Age (years) | 34.5 ± 13.4 | 39.0 ± 19.7 | .332 |
| Male (%) | 38 (64%) | 6 (27%) | .005 |
| Baseline BMI (kg/m2) | 28.6 ± 4.3 | 26.5 ± 5.5 | .070 |
| Follow‐up (years) | 2.0 ± 1.7 | 1.2 ± 1.2 | .054a |
| Male | 1.9 ± 1.5 | 0.9 ± 0.7 | .152a |
| Female | 2.2 ± 1.9 | 1.3 ± 1.4 | .134a |
| HLA‐DQB1*06:02 + | 56/57 | 18/19 | .408 |
| Hypocretin < 110 pg/ml | 32/35 | 6/7 | .638 |
Data indicate mean ± standard deviation.
BMI, body mass index; SXB, sodium oxybate.
p‐values result from Student's t tests for the difference between treatment groups.
To adjust for multiple testing, p‐values <.05/4 were considered to be significant.
3.2. Co‐medication
There were no significant differences between the percentages of patients in the SXB and the modafinil groups who received no additional medication (p = .209) and patients who used antidepressants (p = .094). The number of patients using methylphenidate or dexamphetamine was higher in the SXB group (p = .007; Table 2).
Table 2.
Co‐medication of narcolepsy type 1 patients
| SXB (N = 59) | Modafinil (N = 22) | p‐value | |
|---|---|---|---|
| None | 23 (39.0) | 12 (54.5) | .209 |
| Antidepressant | 12 (20.3) | 8 (36.4) | .167 |
| Methylphenidate/dexamphetamine | 24 (40.7) | 2 (9.1) | .007 |
Data indicate number of patients (%).
Antidepressants (N SXB‐group/N modafinil‐group): clomipramine (7/6), venlafaxine (3/0), imipramine (2/1), citalopram (0/1). Median treatment 0.96 years (range 0.25–19 years).
p‐values result from Student's t tests for the difference between treatment groups.
SXB, sodium oxybate.
3.3. BMI in narcolepsy type 1 patients is higher than mean BMI in Dutch people above 18 years old
The mean BMI of the patient cohort differed significantly from that of a representative sample of Dutch people aged over 18 years. In men, the mean BMI of our study population was significantly higher than the mean BMI of Dutch men aged over 18 years (mean ± standard error of the mean [SEM] 27.9 ± 0.58 kg/m2 versus 25.3 kg/m2; p < .001). Likewise, women in our patient groups had a significantly higher BMI than the average for Dutch women aged over 18 years (mean ± SEM 28.2 ± 0.93 kg/m2 versus 25.8 kg/m2; p < .001).
3.4. BMI decreases in patient groups between first and last measurements
Four separate groups were constructed based on treatment and gender. Those treated with SXB lost weight during follow‐up (−1.58 kg/m2, standard deviation [SD] 2.12 kg/m2; p < .001; Figures 1 and 2). Women using SXB lost weight, with a mean BMI decrease of 2.56 kg/m2 (SD 2.20 kg/m2; p = .001), which corresponds to an average weight loss of 7.1 kg; men had a mean BMI decrease of 0.84 kg/m2 (SD 1.71 kg/m2; p = .006), which corresponds to 2.8 kg. Patients using modafinil, however, gained weight (0.60 kg/m2, SD 0.91 kg/m2; p = .005). A mean BMI increase of 0.57 kg/m2 (SD 0.96 kg/m2; 1.6 kg) was found in women (p = .033), whereas a mean BMI increase of 0.67 (SD 0.88 kg/m2; 2.3 kg) was observed in men (p = .122). After adjustment for multiple testing, the BMI increase in women using modafinil failed to reach statistical significance.
Figure 1.
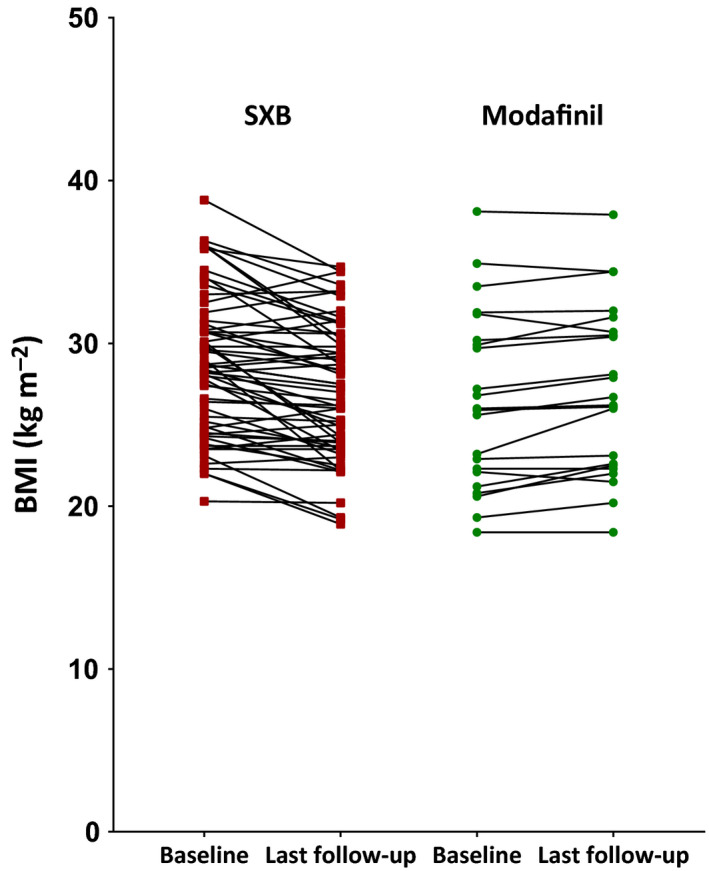
Body mass index (BMI) values at baseline and at last follow‐up of patients in whom treatment with sodium oxybate (SXB; red, N = 59) was newly started and patients in whom treatment with modafinil (green, N = 22) was newly started. Each data point represents one patient at either baseline or follow‐up. Individual values per patient are linked
Figure 2.
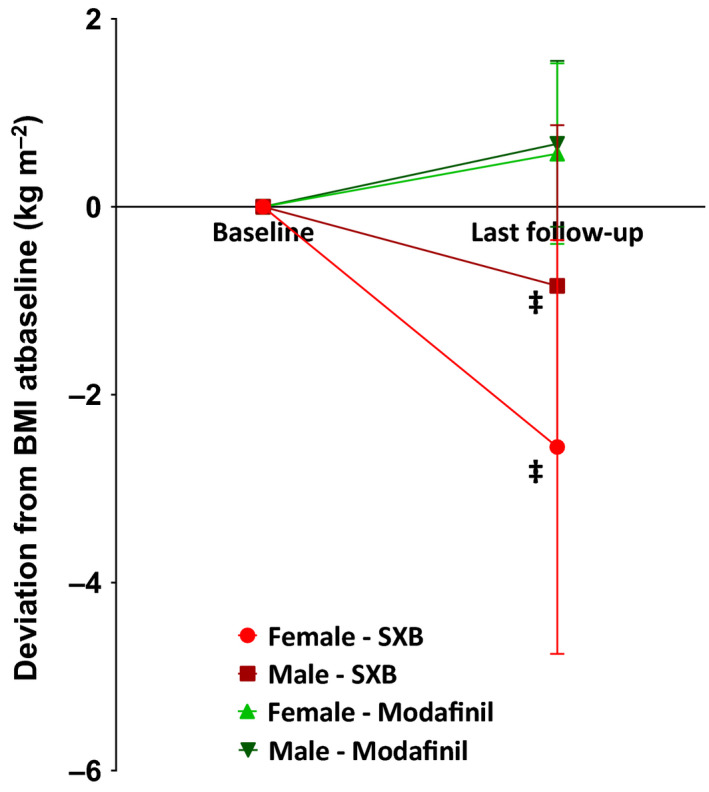
Patients treated with sodium oxybate (SXB) showed a decrease in body mass index (BMI) during follow‐up, whereas patients treated with modafinil did not. p‐values are derived from Student's paired t tests comparing normalised BMI values at baseline and at last follow‐up. Mean follow‐up for SXB and modafinil was 2.0 ± 1.7 versus 1.2 ± 1.2 (p = .054). *p < .05, †p < .01, ‡p < .001
3.5. BMI at baseline and medication type influence BMI difference over time
Patients using SXB showed a decrease in BMI that was larger than the BMI deviation of modafinil users (F(1,69.396) = 8.180, p = .006). A higher baseline BMI was found to predict a more pronounced BMI decrease (F(1,26.040) = 5.137, p = .032). The effect gender had on BMI deviations was found not to be significant (F(1,72.923) = 3.464, p = .067). An overview of these and all other main and interaction effects can be found in Table 3. Mean values of BMI differences from baseline for four groups based on gender and medication type are depicted in Figure 3.
Table 3.
Main effects and interaction effects of the fitted mixed model; narcolepsy type 1 patients using both modafinil and sodium oxybate excluded
| Parameter | Estimate (95% CI) | F‐ and p‐values |
|---|---|---|
| Gender | Female: −0.751 (−1.56; 0.05) | F(1,72.923) = 3.464, p = .067 |
| Study drug | SXB: −1.198 (−2.03; −0.36) | F(1,69.396) = 8.180, p = .006 |
| Baseline BMI | −0.017 (−0.10; 0.06) | F(1,71.713) = 0.177, p = .675 |
| Follow‐up (years) | −0.154 (−0.54; 0.23) | F(1,40.695) = 0.690, p = .411 |
| Gender*follow‐up | Female: −0.333 (−0.96; 0.30) | F(1,27.231) = 1.178, p = .287 |
| Study drug*follow‐up | SXB: −0.911 (−1.62; −0.20) | F(1,34.531) = 6.784, p = .013 |
| Baseline BMI*follow‐up | −0.067 (−0.13; −0.01) | F(1,26.040) = 5.137, p = .032 |
BMI, body mass index.
Figure 3.
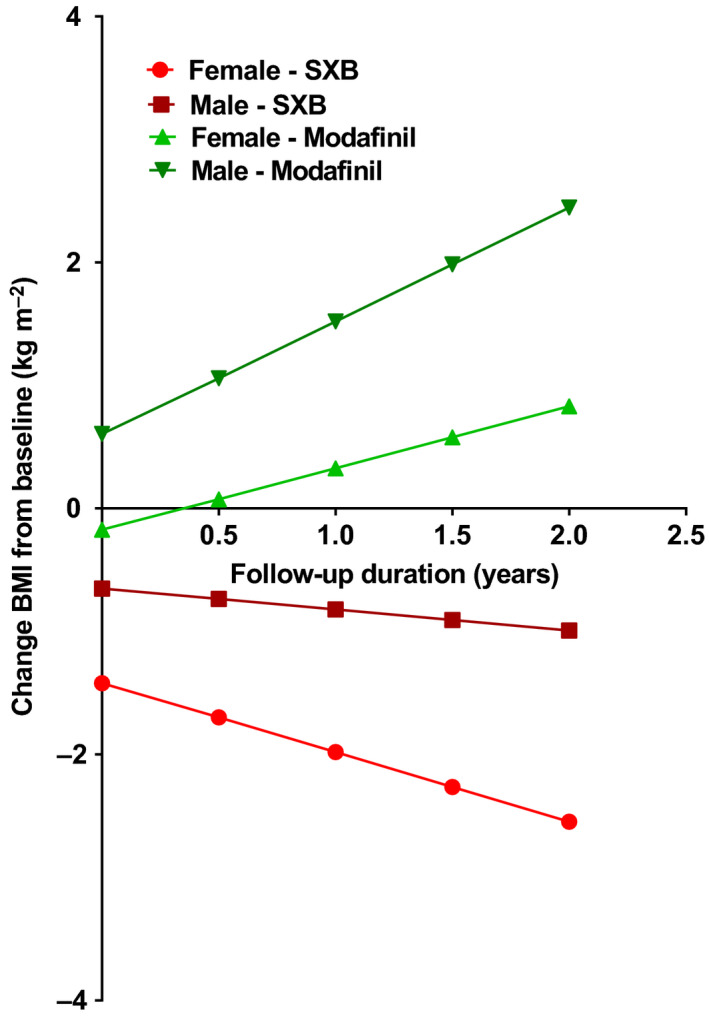
Mixed‐model estimations of the averaged subject in four groups based on gender and medication type. Body mass index (BMI) at baseline is set at 28.0 kg/m2, the grand mean of our study population. Values for different groups at follow‐up duration = 0 depicts the average baseline BMI value of all patients in that particular group. SXB, sodium oxybate
3.6. BMI change in patients is not correlated with either modafinil or SXB dose
A higher dose of either modafinil or SXB was not correlated with BMI change in our patient cohort. Spearman's rho was .188 in modafinil‐treated patients (p = .402) and .153 in SXB‐treated patients (p = .294).
3.7. BMI decrease in SXB group is not explained by methylphenidate or dexamphetamine use
Given the fact that stimulant use is significantly higher in the patient group in which SXB treatment was initiated, a mixed model was fitted on the dataset in which patients using methylphenidate or dexamphetamine were excluded (n = 24 in the SXB group, n = 2 in the modafinil group; data not shown). Also in this fitted model there was a significant main effect of SXB on BMI (F(1,40.073) = 16.003, p < .001). Additionally, we fitted a mixed model in which dexamphetamine or methylphenidate use was added both as a main effect and as an interaction effect with time (Table S1). The main effect of dexamphetamine or methylphenidate use was significant (F(1,78.787) = 10.215, p = .002). However, there still remained a highly significant main effect of SXB on BMI (F(1,66.799) = 14.916, p < .001).
4. DISCUSSION
Our findings (i) confirm that SXB reduces BMI in narcolepsy type 1 patients and (ii) suggest that this is a long‐lasting effect. Even when accounting for baseline BMI, gender, treatment duration and the interactions between them, the mixed model we fitted statistically significantly demonstrates a BMI decrease in the SXB group, in contrast to a BMI increase in the modafinil group. The BMI decrease in the SXB group is seen in both male and female patients but is more pronounced in women. Patients using modafinil gain rather than lose weight. Another interesting finding is that a higher BMI at baseline predicts a more pronounced decrease in BMI during medication use over time. This suggests that BMI decrease constitutes an additional beneficial effect of SXB for narcolepsy type 1 patients, especially for those with a higher BMI at baseline. A correlation between medication dose and BMI change is not found in our cohort.
Our findings are in line with a previous report (Husain et al., 2009) on weight loss amongst patients treated with SXB. Patients with narcolepsy type 1 and type 2 were assessed, yet without a control group, and data on age, gender or BMI of the smaller cohort were lacking.
Narcolepsy patients have a higher prevalence of obesity than the general population. Indeed, the mean baseline BMI of our cohort was significantly higher than that of a representative sample of Dutch people aged older than 18 years.
The exact mechanism by which SXB leads to weight loss is unclear, although several theories exist. It is known (Donjacour et al., 2011) that SXB leads to a consistent increase in nocturnal growth hormone (GH) secretion and that SXB strengthens the temporal relation between GH secretion and slow‐wave sleep. GH is a potent lipolytic agent and a GH deficiency decreases lean body mass while increasing fat mass. It was suggested that SXB could lead to an increase in lipolysis by restoring GH secretion. This hypothesis was tested in a study showing that SXB stimulates lipolysis in narcolepsy type 1 (Donjacour et al., 2014). Participants in this study lost on average 5.2 kg in 3 months of SXB treatment, which supports our results (Husain et al., 2009). If weight loss is mediated through this pathway, gender differences in fat metabolism (Williams, 2004) could account for the different trends in BMI change between men and women in our study.
Another hypothesis is that a BMI decrease results from the effect of SXB on ghrelin and leptin secretion. A comparison between narcolepsy type 1 patients and healthy controls (Donjacour et al., 2013) did not show any differences in ghrelin and leptin secretion after 3 months of SXB treatment. It was speculated there that the weight loss may also be due to a decrease of food intake and an increase of physical activity, leading to a negative energy balance secondary to the sleep‐promoting effects of SXB.
In addition to these hypotheses, we propose that the fact that patients who start using SXB are required to cease using alcohol might play an additional role in BMI loss in this patient group. Even though the exact relation between alcohol consumption and weight gain is complex, it can be said that alcohol consumption leads to weight gain (Suter, 2005).
The effects of SXB on BMI were compared with modafinil, a commonly prescribed therapy for narcolepsy. The decision to treat an individual with either SXB or modafinil is not always clear cut. SXB is more often prescribed when cataplexy and disturbed nocturnal sleep are the most incapacitating symptoms (Bosch, Quednow, Seifritz, & Wetter, 2012; Boscolo‐Berto et al., 2012), whereas modafinil is prescribed in those suffering most from excessive daytime sleepiness (Guilleminault & Cao, 2011). If cataplexy had an effect on weight, it could therefore lead to a selection bias in our study. There are no data on the relation between cataplexy and BMI. We have also no reason to expect other confounding parameters to be present than the ones we accounted for in our analysis. Earlier studies on the effect of modafinil in narcolepsy type 1 showed no significant BMI changes in those treated with it (Moldofsky, Broughton, & Hill, 2000; US Modafinil in Narcolepsy Multicenter Study Group, 1998). These studies had a shorter follow‐up time compared with our study.
Our study has a few limitations. Firstly, due to the retrospective nature, we were not able to randomise patients and we were dependent on the data that was present in the medical records for each individual. The retrospective design led to incomplete data about symptom severity at treatment initiation and made correlation between treatment effects on BMI and treatment effects on other symptoms impossible. We were, however, able to review the medical records of all patients with narcolepsy type 1 who had treatment started at the outpatient clinic, thereby reducing the risk of selection bias. Our cohort is also larger than those presented in earlier studies on weight loss and SXB (Donjacour et al., 2013; Husain et al., 2009), and we only included narcolepsy type 1 patients as diagnosed based on ICSD‐3 guidelines.
Co‐medication was a concern in our study. The use of methylphenidate or dexamphetamine was found to be significantly different between groups. Methylphenidate, a stimulant often used in narcolepsy, is known to decrease appetite and dietary fat intake in healthy subjects (Goldfield, Lorello, & Doucet, 2007). In narcolepsy type 1 no such effect has been observed (Kok et al., 2003). It remains unclear whether these findings also lead to a decrease in BMI. Given that in our study all patients using these two medications were on a stable dose for at least 3 months before initiating treatment with SXB or modafinil, it was not likely that this influenced our results in a significant way. We assessed the effect of these stimulants in two ways. Removing individuals using methylphenidate or dexamphetamine from the model still shows a highly significant effect of SXB on BMI. In addition, adding use of dexamphetamine or methylphenidate to the model shows that it leads to a BMI reduction, but does not interfere with the BMI decrease that is demonstrated in the SXB group. Besides stimulants, a non‐significant difference in antidepressant use was found between both groups. Results on the effect on BMI of the most frequently used antidepressant clomipramine are contradictory: two groups have reported an increase in appetite and weight gain in patients using clomipramine (Maina, Albert, Salvi, & Bogetto, 2004; Paige et al., 2015). However, this effect was only shown in patients without narcolepsy and for a higher dose than normally prescribed in narcolepsy type 1. Another group found no BMI changes in narcolepsy patients using clomipramine who were already on a stable dose (Kok et al., 2003).
5. CONCLUSION
We confirm that treatment with SXB is associated with a decrease in BMI in narcolepsy type 1 patients, whereas modafinil treatment is not. For the majority of narcolepsy type 1 patients, BMI decrease therefore constitutes an additional beneficial effect of SXB. The weight loss is more pronounced in those with a higher BMI at baseline. Possible weight loss could therefore be another reason to opt for treatment with SXB in narcolepsy type 1, especially in those with a higher BMI. Due to the retrospective nature of this study further prospective and longitudinal studies are needed to confirm our results and further characterise BMI change dynamics.
CONFLICTS OF INTERESTS
GJL has served as a paid member of the UCB advisory board and received lecture fees and conference travel support from UCB Pharma. GJL also carried out consultancy for UCB Pharma and provided expert testimony for UCB Pharma, Jazz Pharmaceuticals and Bioprojet. CED received lecture fees from UCB Pharma. The other authors report no conflict of interests.
AUTHOR CONTRIBUTIONS
Study design: MSS, CED, WPvdM, EWvZ, RF and GJL. Data entry: MSS, IMS, CED and RF. Data analysis: MSS, IMS, CED, WPvdM, EWvZ and RF. Writing and reviewing the manuscript: MSS, IMS, CED, WPvdM, EWvZ, RF and GJL.
Supporting information
ACKNOWLEDGEMENTS
The authors thank P.P. Buijserd‐Amesz for her help in acquiring the data and J.W. Sander for critically reviewing this manuscript, specifically concerning the use of the English language.
Schinkelshoek MS, Smolders IM, Donjacour CE, et al. Decreased body mass index during treatment with sodium oxybate in narcolepsy type 1. J Sleep Res. 2019;28:e12684 10.1111/jsr.12684
REFERENCES
- Black, S. W. , Yamanaka, A. , & Kilduff, T. S. (2017). Challenges in the development of therapeutics for narcolepsy. Progress in Neurobiology, 152, 89–113. 10.1016/j.pneurobio.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, O. G. , Quednow, B. B. , Seifritz, E. , & Wetter, T. C. (2012). Reconsidering GHB: Orphan drug or new model antidepressant? Journal of Psychopharmacology, 26, 618–628. 10.1177/0269881111421975 [DOI] [PubMed] [Google Scholar]
- Boscolo‐Berto, R. , Viel, G. , Montagnese, S. , Raduazzo, D. I. , Ferrara, S. D. , & Dauvilliers, Y. (2012). Narcolepsy and effectiveness of gamma‐hydroxybutyrate (GHB): A systematic review and meta‐analysis of randomized controlled trials. Sleep Medicine Reviews, 16, 431–443. 10.1016/j.smrv.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Centraal Bureau voor de Statistiek . (2016). Lengte en gewicht van personen, ondergewicht en overgewicht; vanaf 1981. http://statline.cbs.nl/Statweb/publication/?VW=T&DM=SLNL&PA=81565NED&D1=a&D2=a&D3=0-1,5&D4=0&D5=0,10,20,30,33-34&HD=170316-1454&HDR=T&STB=G1,G2,G3,G4. Accessed March 16, 2017.
- Dahmen, N. , Tonn, P. , Messroghli, L. , Ghezel‐Ahmadi, D. , & Engel, A. (2009). Basal metabolic rate in narcoleptic patients. Sleep, 32, 962–964. [PMC free article] [PubMed] [Google Scholar]
- Daniels, L. (1934). Narcolepsy. Medicine, 13, 1–122. 10.1097/00005792-193413010-00001 [DOI] [Google Scholar]
- Donjacour, C. E. H. M. , Aziz, N. A. , Overeem, S. , Kalsbeek, A. , Pijl, H. , & Lammers, G. J. (2014). Glucose and fat metabolism in narcolepsy and the effect of sodium oxybate: A hyperinsulinemic‐euglycemic clamp study. Sleep, 37, 795–801. 10.5665/sleep.3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donjacour, C. E. H. M. , Aziz, N. A. , Roelfsema, F. , Frölich, M. , Overeem, S. , Lammers, G. J. , & Pijl, H. (2011). Effect of sodium oxybate on growth hormone secretion in narcolepsy patients and healthy controls. American Journal of Physiology‐Endocrinology and Metabolism, 300, e1069–e1075. 10.1152/ajpendo.00623.2010 [DOI] [PubMed] [Google Scholar]
- Donjacour, C. E. H. M. , Pardi, D. , Aziz, N. A. , Frölich, M. , Roelfsema, F. , Overeem, S. , … Lammers, G. J. (2013). Plasma total ghrelin and leptin levels in human narcolepsy and matched healthy controls: Basal concentrations and response to sodium oxybate. Journal of Clinical Sleep Medicine, 9, 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuyn, H. A. , Swinkels, S. , Buitelaar, J. , Renier, W. O. , Furer, J. W. , Rijnders, C. A. , … Overeem, S. (2008). High prevalence of eating disorders in narcolepsy with cataplexy: A case‐control study. Sleep, 31, 335–341. 10.1093/sleep/31.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek, R. , Overeem, S. , Reijntjes, R. , Lammers, G. J. , van Dijk, J. G. , & Pijl, H. (2008). Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin‐deficient human narcolepsy. Journal of Clinical Sleep Medicine, 4, 248–254. [PMC free article] [PubMed] [Google Scholar]
- Goldfield, G. S. , Lorello, C. , & Doucet, E. (2007). Methylphenidate reduces energy intake and dietary fat intake in adults: A mechanism of reduced reinforcing value of food? American Journal of Clinical Nutrition, 86, 308–315. 10.1093/ajcn/86.2.308 [DOI] [PubMed] [Google Scholar]
- Guilleminault, C. , & Cao, M. T. (2011). Narcolepsy: Diagnosis and management In Kryger M. H., Roth T., & Dement W. C. (Eds.), Principles and practice of sleep medicine (pp. 957–968). St. Louis, MO: Elsevier Saunders; 10.1016/B978-1-4160-6645-3.00085-2 [DOI] [Google Scholar]
- Husain, A. M. , Ristanovic, R. K. , & Bogan, R. K. (2009). Weight loss in narcolepsy patients treated with sodium oxybate. Sleep Medicine, 10, 661–663. 10.1016/j.sleep.2008.05.012 [DOI] [PubMed] [Google Scholar]
- ICSD‐3 . (2014). International Classification of Sleep Disorders (3rd ed.). Darien, IL: American Academy of Sleep Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok, S. W. , Overeem, S. , Visscher, T. L. S. , Lammers, G. J. , Seidell, J. C. , Pijl, H. , & Meinders, A. E. (2003). Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obesity Research, 11, 1147–1154. 10.1038/oby.2003.156 [DOI] [PubMed] [Google Scholar]
- Maina, G. , Albert, U. , Salvi, V. , & Bogetto, F. (2004). Weight gain during long‐term treatment of obsessive‐compulsive disorder: A prospective comparison between serotonin reuptake inhibitors. Journal of Clinical Psychiatry, 65, 1365–1371. 10.4088/JCP.v65n1011 [DOI] [PubMed] [Google Scholar]
- Moldofsky, H. , Broughton, R. J. , & Hill, J. D. (2000). A randomized trial of the long‐term, continued efficacy and safety of modafinil in narcolepsy. Sleep Medicine, 1, 109–116. 10.1016/S1389-9457(99)00014-3 [DOI] [PubMed] [Google Scholar]
- Narbro, K. , Jonsson, E. , Larsson, B. , Waaler, H. , Wedel, H. , & Sjöström, L. (1996). Economic consequences of sick‐leave and early retirement in obese Swedish women. International Journal of Obesity and Related Metabolic Disorders, 20, 895–903. [PubMed] [Google Scholar]
- Nishino, S. , Ripley, B. , Overeem, S. , Nevsimalova, S. , Lammers, G. J. , Vankova, J. , … Mignot, E. (2001). Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Annals of Neurology, 50, 381–388. 10.1002/(ISSN)1531-8249 [DOI] [PubMed] [Google Scholar]
- Paige, E. , Korda, R. , Kemp‐Casey, A. , Rodgers, B. , Dobbins, T. , & Banks, E. (2015). A record linkage study of antidepressant medication use and weight change in Australian adults. Australian and New Zealand Journal of Psychiatry, 49, 1029–1039. 10.1177/0004867415607365 [DOI] [PubMed] [Google Scholar]
- Poli, F. , Plazzi, G. , Di Dalmazi, G. , Ribichini, D. , Vicennati, V. , Pizza, F. , … Pagotto, U (2009). Body mass index‐independent metabolic alterations in narcolepsy with cataplexy. Sleep, 32, 1491–1497. 10.1093/sleep/32.11.1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponziani, V. , Gennari, M. , Pizza, F. , Balsamo, A. , Bernardi, F. , & Plazzi, G. (2016). Growing up with type 1 narcolepsy: Its anthropometric and endocrine features. Journal of Clinical Sleep Medicine, 12, 1649–1657. 10.5664/jcsm.6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter, P. M. (2005). Is alcohol consumption a risk factor for weight gain and obesity? Critical Reviews in Clinical Laboratory Sciences, 42, 197–227. 10.1080/10408360590913542 [DOI] [PubMed] [Google Scholar]
- US Modafinil in Narcolepsy Multicenter Study Group . (1998). Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. Annals of Neurology, 43, 88–97. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Wu, H. , Stone, W. S. , Zhuang, J. , Qiu, L. , Xu, X. , … Zhao, Z. (2016). Body weight and basal metabolic rate in childhood narcolepsy: A longitudinal study. Sleep Medicine, 25, 139–144. 10.1016/j.sleep.2016.06.019 [DOI] [PubMed] [Google Scholar]
- Williams, C. M. (2004). Lipid metabolism in women. Proceedings of the Nutrition Society, 63, 153–160. 10.1079/PNS2003314 [DOI] [PubMed] [Google Scholar]
- Willie, J. T. , Chemelli, R. M. , Sinton, C. M. , & Yanagisawa, M. (2001). To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annual Review of Neuroscience, 24, 429–458. 10.1146/annurev.neuro.24.1.429 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2000). Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series, 894, 1–253. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


