Abstract
Morphine is the opioid most commonly used for neonatal pain management. In intravenous form, it is administered as continuous infusions and intermittent injections, mostly based on empirically established protocols. Inadequate pain control in neonates can cause long-term adverse consequences; however, providing appropriate individualized morphine dosing is particularly challenging due to the interplay of rapid natural physiological changes and multiple life-sustaining procedures in patients who cannot describe their symptoms. At most institutions, morphine dosing in neonates is largely carried out as an iterative process using a wide range of starting doses and then titrating to effect based on clinical response and side effects using pain scores and levels of sedation. Our background data show that neonates exhibit large variability in morphine clearance resulting in a wide range of exposures which are poorly predicted by dose alone. Here, we describe the development and implementation of an electronic health record (EHR)-integrated, model-informed decision support platform for the precision dosing of morphine in the management of neonatal pain. The platform supports pharmacokinetic model-informed dosing guidance and has functionality to incorporate real-time drug concentration information. The feedback is inserted directly into prescribers’ workflows so that they can make data-informed decisions. The expected outcomes are better clinical efficacy and safety with fewer side effects in the neonatal population.
Introduction
Every day in the United States, thousands of neonates receive morphine for neonatal pain management.(1) Background data analyzed at Cincinnati Children’s Hospital Medical Center and data from earlier studies show that infants who are placed on morphine treatment exhibit large variability in morphine pharmacokinetic (PK) and clearance resulting in a wide range of exposures (up to 30 fold) which is poorly predicted by the current weight based morphine dosing regimens.(2–4) Inadequate pain control in neonates can have long-term adverse consequences;(5) however, providing appropriate individualized morphine dosing is particularly challenging due to the interplay of rapid natural physiological change and multiple life-sustaining procedures in patients who cannot describe symptoms. Morphine is the opioid most commonly used for neonatal pain management. In intravenous form, it is administered in various combinations of continuous infusions and intermittent injections, mostly based on empirically established protocols.(6) Optimal morphine dosing would provide effective analgesia while minimizing adverse side effects like apnea, decreased gut motility, and drug dependence, but these have and have yet to be established for this population.
The PK of morphine have been described extensively in the neonatal population.(3, 4, 7–10) However, clear pharmacodynamic (PD) endpoints describing the morphine concentration-response relationship are not yet available.(11) The population PK studies identified size, age and maturation, mechanical ventilation, and organ dysfunction as major factors contributing to variability in PK. While several population models are available, few clinical practice guidelines have incorporated this information. At many institutions, guidelines use a wide range of starting doses and typically rely on subsequent titration to effect based on pain scores and levels of sedation since each infant exhibits widely variable individual responses to morphine and many other medications.(6) For neonates, clinicians rely on behavioral signs of pain using scoring systems, such as the Neonatal Pain, Agitation and Sedation Scale (NPASS), and physiological indicators, such as changes in heart rate, respiratory rate, and blood pressure. While taking these indicators into account, dose adjustment itself mostly relies on clinical experience and typically has a large “trial and error” component. We hypothesized that providing user-friendly decision support for the precision dosing of morphine will improve pain management and reduce the need for breakthrough or rescue doses while also reducing the risk of harm side effects and opiate dependence.
Here, we describe the development and implementation of an Electronic Health Record (EHR)-embedded decision support platform for individualized precision drug treatment in neonates using morphine. The goal of the visual dashboard was to provide clinicians with valuable dosing information at the time of medication ordering and support precision dosing of pain and sedation medications for each individual patient.
Methods
Prototype NeoRelief platform development
A prototype NeoRelief platform was developed using the PK engine of the Edsim++ software, an object oriented visual PK/PD modeling tool (Mediware, Prague, Czech Republic) running on the Microsoft .NET platform (.NET Standard) and written in C#. The Edsim software includes a large extendable library of advanced PK/PD objects that can be used for building complex models using application plug-ins. The application programming interface (API) of the platform was set up as a web service (ASP.NET Core WebAPI) and provides access for different applications through the internet and intranet (Supplemental Figure S1). This architecture follows the micro services paradigm (https://en.wikipedia.org/wiki/Microservices), where an individual hospital uses a number of services, each dedicated to a single drug (e.g. morphine, midazolam, acetaminophen).
Data elements to be automatically extracted from the EHR include patient clinical data (date of birth, gestational age in weeks, weight, sex, serum creatinine, and liver function); the medication history, including the type of intravenous administration and infusion duration (continuous infusion, intermittent bolus), dose, date and times, number of doses; and measured plasma morphine concentrations (when available). The PK models used to generate the predicted plasma concentration visualizations are generated by calling a well-documented RESTful (REpresentational State Transfer) API from the Edsim++ platform. This is a decoupled composite application designed and implemented with secure, extensible integration in mind using the current capabilities of the EHR and the Edsim package. To allow real time PK/PD evaluation, composite scores using the Neonatal Pain, Agitation, and Sedation Scale (NPASS) were used.(12) The NPASS represents a clinically-validated, consistent, age-appropriate assessment and documentation methodology for infant pain and sedation in the neonatal intensive care unit (NICU). In our NICU, scores are collected every 4 hours, providing a quantitative assessment of subjective descriptions that often drive patient therapy.
To provide broad functionality during development, six neonatal population PK morphine models from the literature were programmed in the prototype tool, including the reported empiric and mechanistic structural models with their respective covariate relationships (Tables S1 and S2; supplemental material[dummy]).(3, 4, 8, 13–15) To accommodate all reported possible covariates, the overall structure of the model was defined as follows:
Where P is the PK model parameter (CL or V), Pstd represents the mean population parameter for a 70 kg adult male, Fsize represents the size factor (using allometric scaling of weight), Fmat represents the maturation factor (using a sigmoid Emax model), Forg represents an organ function factor (in case of compromised renal and liver function), Fage represents the age factor, and Fsex represents sex differences (male or female). The NeoRelief service can be instructed to use a specific PK/PD model. If the “Auto” model is selected (default setting), either the Holford model (2012)(14) or the Holford_Meta (Bouwmeester extension)(13) model is used, depending on the presence or absence of M3G and M6G observations. The semi-mechanistic covariate model described by Holford et al. (2012) was selected as the default model, as it includes a scaler for size, a separate function for maturation, and a factor for when neonates receive mechanical ventilation. In this model, morphine clearance is defined as:
where CLi is the morphine clearance in the ith patient, CLstd is the population mean clearance, Fsize is the allometric scaling relationship, Fmat represents the maturation of morphine clearance expressed as a percentage of the adult clearance, and Fvent is a factor indicating lower morphine clearance when on mechanical ventilation (Table S2). The prototype NeoRelief platform also includes a selection option to automatically display concentration time profiles of the metabolites M3G and M6G. NeoRelief automatically selects the appropriate model based on the requested output: do not show metabolite concentration(14) or do show metabolites.(13) In case of morphine administration via the oral route, the default model uses standard first-order absorption of morphine-sulphate as its input.(16) For prediction of M3G and M6G after oral administration, a more complex equation was programmed to take into account the first-pass effect on metabolite formation.(17) In this model, the net bioavailability (Fbio) is split into the fraction absorbed from the gut (F) and the hepatic bioavailability Fhp = 1 − Eh. The net bioavailability is then calculated as Fbio = F × (1 − Eh), where Eh is hepatic extraction.
Bayesian estimator
A module for Bayesian estimation was included in the NeoRelief platform based on existing Edsim++ PK engine functionality. This curve-fitting capability allows individual PK parameter estimates using a Bayesian procedure.(18) Data used for the model-informed individual PK estimation included the medication history together with morphine concentration feedback information while taking into account NPASS data and patient status with respect to growth and maturation and treatment (e.g. mechanical ventilation) over the course of treatment.(19) For the generation of dose suggestions NeoRelief includes a history aware dosing feature. This functionality allows that a dose is not calculated as if were the first dose, but that any previous dosing events are taken into consideration. History aware dosing can deal with two scenarios: i) in case of exposures higher than the selected target. an extension for the interval is suggested while postponing the next dose; ii) in case of underdosing, an extra loading dose in combination with a new maintenance dose is suggested to rapidly achieve the desired target.
Evaluation of morphine population PK models
Evaluation of the morphine population PK models from the literature when integrated into the decision support software was performed by implementing published equations and covariate logic in Microsoft Excel (Version 2010, Microsoft Corporation, Redmond, Washington). Six different models where implemented and tested as part of the NeoRelief platform. The models were named as follows: Holford,(13, 14) Anand,(3) Knibbe,(8) Wang-1 and Wang-2,(4) and Knosgaard.(15) The Wang-1 model is an empirical model that scales parameters using a variable exponential value (k). In contrast to the Wang-2 model which uses one parameter set, this model uses two parameter sets to describe patients of all ages.(4) Model parameter details are summarized in the [dummy_similar]supplementary materials (Tables S1 and S2). Validation was performed using patient cases across different age cohorts as described by Krekels et al.(20) Age cohorts consisted of nine different patient scenarios: 32 week preterm neonate (1 day old, 0.5 kg), 32 week preterm neonate (2 weeks old, 1.0 kg), 38 week term neonate (1 day old, 3.5 kg), 40 week term neonate (2 weeks old, 4.0 kg), 3 month old infant born term (6.0 kg), 6 month old infant (7.5 kg), one year old infant (10 kg), 2 year old infant (13 kg), and a 3 year old infant (17 kg). Krekels et al. also reported the Anand and Knibbe model estimates, which were used to compare results predicted with the NeoRelief platform. For each model, a spreadsheet function was implemented for calculating parameter values (e.g. clearance) as a function of patient physiological parameters (e.g. age, term, weight and ventilation status). Next, the spreadsheet functions were used to calculate parameter estimates (morphine clearance, volumes of distribution, and distribution clearances) across age categories with defined representative patients. The outcomes using different published models were compared with those generated by the NeoRelief models (Edsim++). Models were accepted when an exact match of parameter estimates could be generated with the published data.
In addition, Bayesian estimation was tested using retrospective morphine concentration data from a well-characterized cohort of preterm neonates who enrolled in a prospective opportunistic PK study using a minimal-risk design with discarded blood samples in infants in the NICU receiving morphine as part of their care.(2) Eligible infants received intravenous morphine for at least 24 hours for analgesia, had no signs of liver or kidney abnormalities, and did not undergo therapeutic hypothermia or extracorporeal membrane oxygenation (ECMO). Clinical judgment determined the need for continuous morphine infusion and frequency of intermittent bolus doses of morphine. A typical starting infusion dose of morphine was 0.03–0.05 mg/kg/h with intermittent bolus doses as ‘needed’ (PRN, pro re nata) managed by the nursing staff per protocol. The results were compared with published data and used as part of the evaluation of the models available in the NeoRelief platform.(9, 14, 20)
Morphine and metabolites assay
Morphine concentrations in plasma were quantified using a validated liquid chromatography with tandem mass spectrometry assay using stable isotope labeled internal standards. Plasma samples were prepared for analysis using solid-phase extraction as recently described.(21) The lower limit of quantification was 2 ng/mL, and the assay was linear over the range of 1–200 ng/ml. The inter-assay accuracy (% bias) ranged from −2.6–13% across the calibration range with an overall inter-assay precision of <10% across the dynamic range. The major glucuronide metabolites of morphine, M3G and M6G were quantified within the same tandem mass spectrometry assay used for morphine but with monitoring of the MRM transition m/z 462 >286 for both compounds after chromatographic separation.(21)
Human factors engineering
After development of the prototype NeoRelief version, a human factors engineering analysis using user-centered design methodologies was performed to further engage neonatal clinicians (neonatologists, neonatal fellows, pharmacists, and nurse practitioners) in contributing to the design of the final prototype.(22) User-centered insights were used to develop a user-validated, lightweight, interactive data visualization tool incorporated directly into the EHR. The primary goal of this analysis was to better understand how a dosing decision support application can enhance the dosing decision process within the NICU. A secondary goal was to facilitate the education of the clinical team on the importance of optimal dosing and the impact of a drug’s PK/PD on the patient’s clinical response with changes in pain and sedation. The human factors research answered critical clinical questions through design, prototypes, and testing ideas with potential operators using a compressed timeframe in which ideas were generated and feedback was received from clinicians and the interdisciplinary team. The project included onsite observation and interviews with clinicians and nursing staff over a 2-month period. The interviews included a System Usability Scale (SUS)(23) evaluation, which focused current dosing process. Testing included ethnographic (observation and contextual inquiry) and usability testing (based on paper and interactive prototype versions) to build a lightweight, interactive data visualization prototype for roll out and prospective evaluation.(24)
Integration in the EHR and clinical workflow
EHR-integration was accomplished with security, data quality, and extensibility as requirements. We adopted a secure architecture from our onset of feature declaration through design and implementation to product review. The patient clinical data used to inform the model are supplied through integration capabilities offered in standard Epic webservice offerings. The authentication capability is achieved through the Epic FHIR (Fast Healthcare Interoperability Resource) webservices. The design of the application and the models specific to the requirements of the application rely on an integration API created during implementation that transforms the EHR-specific data to the application’s required data structures. The backend API calls the Edsim RESTful service using anonymized clinical information from the patient record, including morphine dosing, weight, and both chronological and corrected postmenstrual age, and the API responds with a detailed time-series model.
Results
Model evaluations
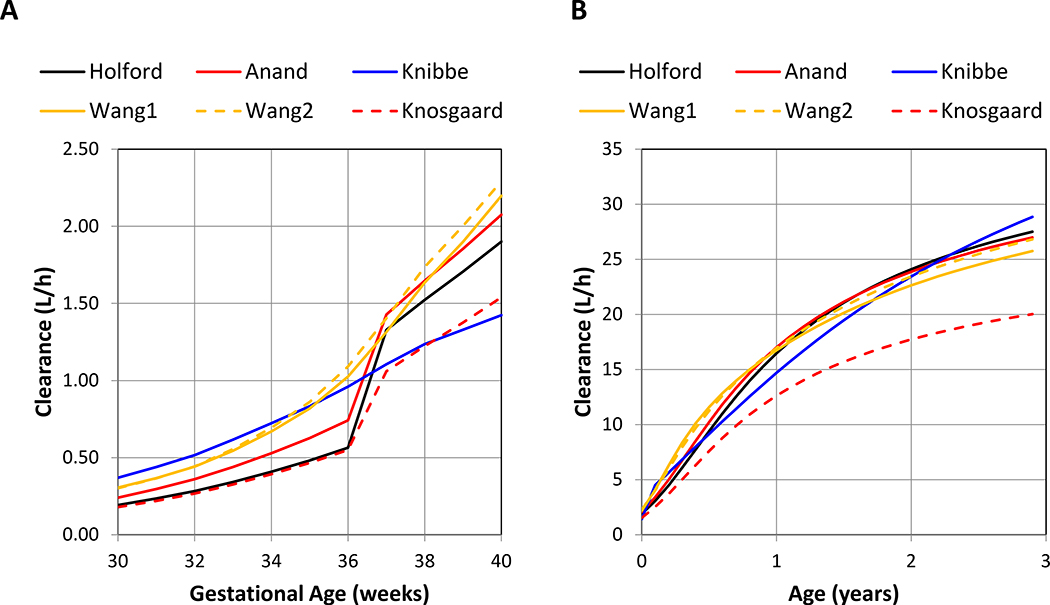
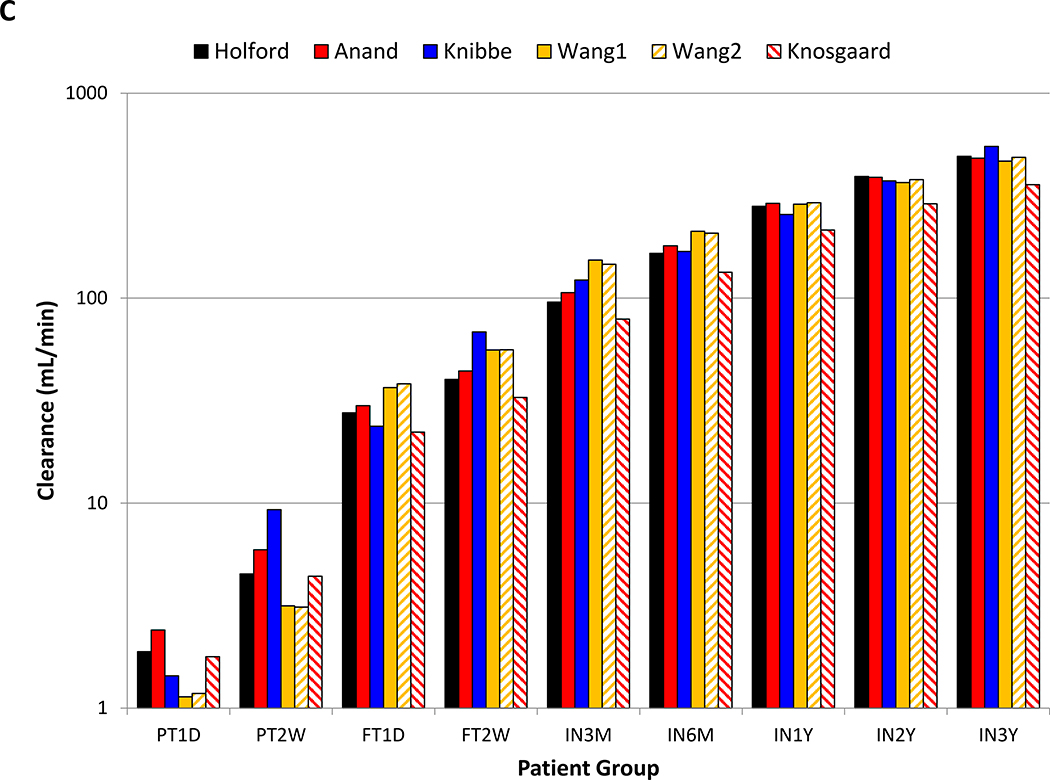
Six models where implemented and tested as part of the initial NeoRelief platform. Model parameters for each of the models are summarized in the supplementary materials (Tables S1 and S2) The worksheet functions and NeoRelief model output yielded identical results (Table S3) and passed validation. Figure 2 graphically presents how each model predicts the increase in morphine clearance as a function of gestational age in weeks (Figure 2A) and patient age in years (Figure 2B). Figure 2C compares numerical morphine clearance predictions of all models for the nine patient scenarios from preterm to 3 years of age. This provides the NeoRelief platform with the functionality to take maturation of morphine clearance into consideration as a function of neonatal growth and development over time.
Figure 2.
Summary of internal validation of the morphine population models.
Panels A, present the model predicted morphine clearance estimates (in L/h) as a function of gestational age from 30 to 40 weeks; Panel B, present the model predicted morphine clearance estimates (in L/h) as a function of age from zero to 3 years of age; Panel C, present the model predicted morphine clearance estimates (in mL/min) for each of the nine case examples: a 32 week preterm neonate (PT1D), a 32 week preterm neonates (PT2W), a 38 week term neonate (FT1D), a 40 week term neonate (FT2W), a 3 month old infant born term (IN3M), a 6 month old infant (IN6M), and a one year old infant (IN1Y), a 2 year old infant (IN2Y), and a 3 year old infant (IN3Y).
Patient weight was derived using gestational age (Panel A: data from https://www.momjunction.com/articles/baby-weight-gain-and-weight-chart_00362524/#gref) or the post-natal age (Panel B: standard growth curve).
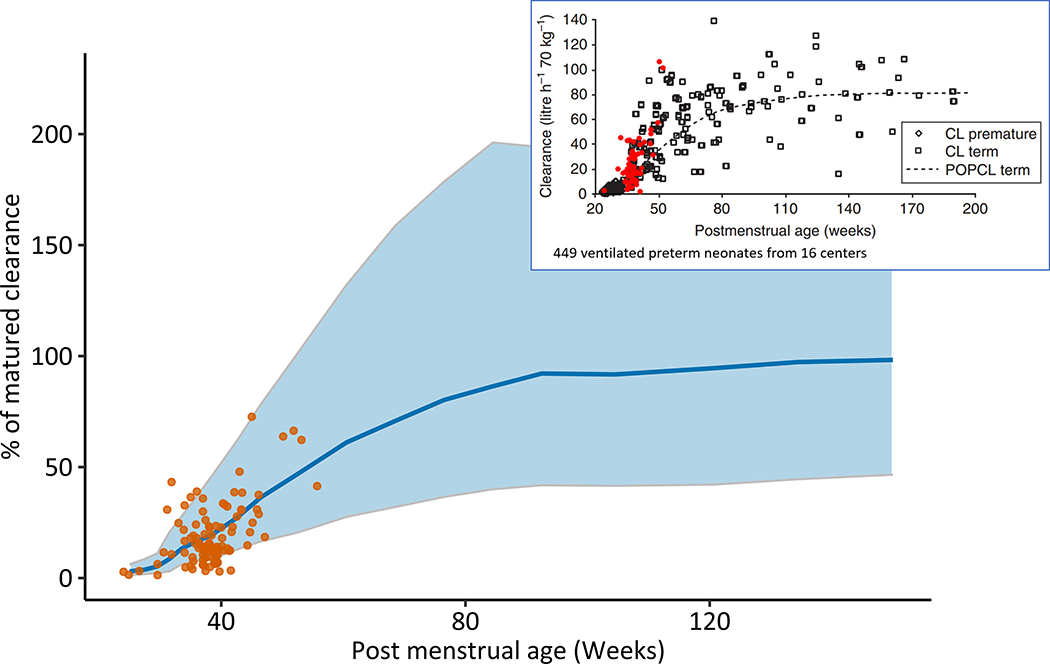
Figure 3 shows morphine clearance estimates generated with the NeoRelief platform using concentrations collected from 102 neonates.(2) Clearance was estimated with the default Holford model,(14) and the results were overlaid with a simulated morphine clearance distribution generated using the Anand model.(3) The insert also displays the reported morphine clearance data of Anand et al. (2008)(3) with our data, indicating that the morphine disposition in our neonates resembles that of the neonates participating in the NEOPAIN study.
Figure 3.
Morphine clearance estimates generated with the NeoRelief platform versus results from the NEOPAIN study.
Closed circles represent morphine clearance estimates in newborns treated in NICU at Cincinnati Children’s analyzed using NeoRelief and expressed as percentage of the mature morphine clearance (84.2 L/l per 70 kg)(3). The solid line and shaded areas represent the median and 5–95 percentiles, respectively of morphine clearance values simulated using the Anand model. The figure insert shows the original clearance estimate data of the NEOPAIN study with morphine clearance estimates (red symbols) for our neonatal cohort generated with NONMEM(2) and verified with the NeoRelief platform.
Software Prototype
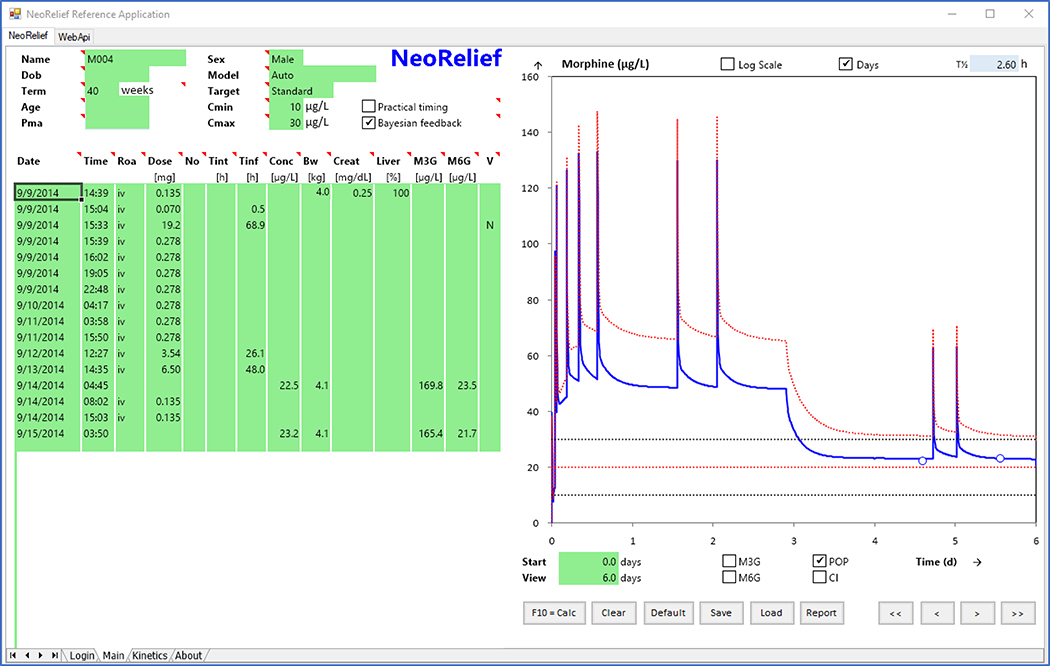
A graphical representation of the initial NeoRelief prototype (version 1.61) is shown in Figure 1. Separate fields are defined (in green) for input of patient demographic data, dosing information, and concentration results. There is a dropdown window for model selection of published models with ‘Auto’ representing the default model.(14) This version includes a dosing table that can be manually populated after which the model-predicted concentration time profile is generated (orange dotted line) via the calculation (F10/calc) button. Similar model predicted profiles for metabolites can be generated by selecting the M3G and M6G boxes. Once concentration results become available and by clicking the Bayesian feedback box, an individual concentration time profile is generated by selecting the calculation button (blue solid line). Measured morphine concentrations are represented by the open circles. Tentative concentration targets as suggested in the literature for postoperative neonates and infants(11, 19, 25) are represented by the horizontal dotted lines.
Figure 1.
NeoRelief software platform prototype. Depicted are the dosing table that can be populated with morphine doses by continuous infusion and intermittent bolus administration after which the model-predicted concentration time profile is generated (orange dotted line) using the calculation (F10/calc) button. Similar model predicted profiles for the metabolites can be generated by checking the M3G and M6G boxes. Once concentration results become available and by clicking the Bayesian feedback box an individual concentration time profile is generated after selecting the calculation button (blue solid line); with measured morphine concentrations are represented by the open circles. The dotted lines represent the potential target range of 10 to 30 ng/mL (mean 20 ng/mL in red) as suggested in the literature. Target plasma concentrations of morphine, which are needed to determine the infusion rate, have not been firmly established in the pediatric population, although concentrations around 20 ng/ml have been suggested for postoperative neonates and infants. (11, 19, 25)
Human Factors Engineering
Two human factors engineers conducted human factor engineering onsite at CCHMC Newborn Intensive Care Unit over one month. Guided by existing precision dosing application prototypes designed by physicians at CCHMC and in combination with insights from the interviews, a series of wireframe paper prototypes of a dosing support application were created and presented to clinicians for feedback. This process guided the development of a minimally interactive prototype for evaluation by the clinical team members. A total of 40 participants provided input to the prototype. All participants were self-selected clinicians including attending physicians, neonatal fellows, clinical pharmacists, and nurse practitioners who completed the System Usability Score survey(23), participated in face-to-face interviews, and provided evaluations of the wireframes and interactive prototypes. The prototype was iteratively revised in response to user-centered design feedback, resulting in progressively improving SUS results. Participants found the following aspects of the dosing decision to be important, and indicated that the use of a support application would improve their experience in these areas: a) History of morphine use and response; b) NPASS related to morphine dose; c) Understanding of morphine use related to a specific patient event; and d) Team collaboration.
Some functions of the software were found to be more useful for specific roles. For nurse practitioners, the word ‘concentration’ was considered vague and confusing, and the graph correlating dosing with PK profile predictions did not communicate any value to them and was ignored. On the other hand, clinical pharmacists found these graphs useful despite them not fitting their established mental model or current method of making dosing decisions for morphine. Presentation of the morphine infusion and bolus dose data combined with the NPASS was considered very helpful to all participants. All participants desired to see infusion and bolus doses in relation to NPASS values. Users felt a tool to visually present historical trends over days or weeks would benefit and support individual patient dosing decisions. While using plasma morphine concentrations and related model predicted profiles would require training in both the mental model and the tool, the result was felt to increase the confidence of the team in making dosing decisions.
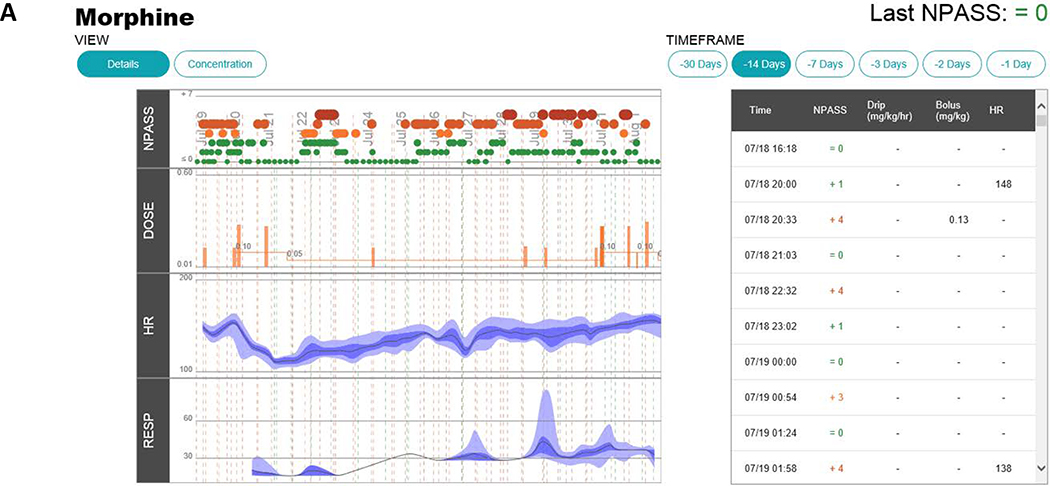
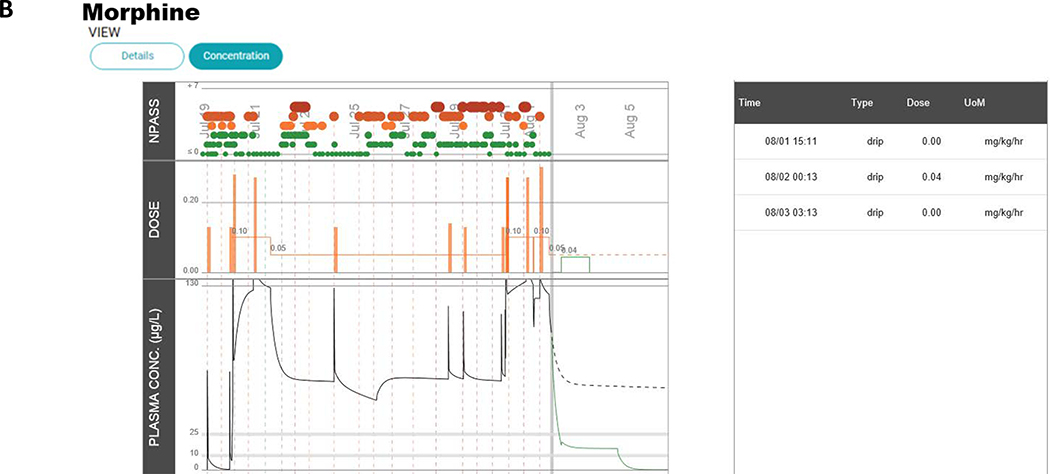
The current version of the morphine precision dosing decision support application is shown in Figure 4. As can be seen in comparison to the prototype in Figure 1, the application significantly changed as a result of the human factors engineering process in terms of presentation and what is reported to providers. To accommodate the different provider roles, conceptual models, and workflows, the application was divided into two complementary views. The landing page (Figure 4A; under the details tab) graphically presents NPASS, morphine dose (infusion and PRN doses), heart rate, and respiratory rate. NPASS scores and dosing events are also summarized chronologically in table format to be viewed over different selectable time frames. A second screen (Figure 4B; via the concentration tab) shows the NPASS scores, the dosing information, and the predicted serum morphine concentration time profile. In the final EHR embedded NeoRelief version there is no model selection option as this was found to be confusing and not valued by actual users in the NICU. The default model is the Holford model.(14)
Figure 4.
Screen shots of the NeoRelief application as displayed in the electronic health record.
Panel A; shows the default landing page (and activated via the “details tab” in the left-hand corner). This page graphically presents NPASS, morphine dose (infusion and PRN doses), heart rate, and breathing frequency. NPASS scores and dosing events are also summarized chronologically in table format to be viewed over different selectable time frames. Panel B; a second screen (activated via the “concentration tab”) summarizes the NPASS data, the doses administered (Infusion “drip” and “bolus” doses), and the model-based translation of the dosing regimen into a morphine concentration time profile (morphine plasma concentration in μg/L). Dosing events (date, time, type of dose, and dose unit) are also summarized in Table format. The symbol size and colors (green, yellow, orange, and red) represent the severity (red, orange, yellow, green) represent the severity of the NPASS: score 0–3 (green); 4–5 (shades of orange); 6–7 (shades of red). As all patient info, doses and time(s) of dosing, and concentration results in the EHR embedded platform are automatically being pulled from the EHR, the data entry panels of the prototype as shown in Figure 1 have disappeared.
Discussion
This project was designed to establish an EHR-integrated decision support for PK model-informed precision dosing of morphine as part of individualized neonatal pain management. Currently, most opiate and benzodiazepine dosing is performed without knowledge of predicted or actual drug concentrations in vivo. Our preliminary data showed a wide range of morphine exposures which are poorly predicted by dose. We used user-centered participatory design and human factors engineering to develop and implement a precision dosing dashboard into clinical workflow.
While multiple neonatal population PK models for morphine are available, few have been implemented as part of clinical practice or are available for use at the bedside. The NeoRelief platform provides this functionality in a user-centered and human factors engineering-based design, and is one of the first EHR embedded decision supports systems to translate morphine dose into exposure while simultaneously depicting markers of response and clinical events in the form of pains scores, heart rate, and respiratory rate. We envision that the prospective collection of these data will allow us to further delineate the therapeutic exposure targets that to date have not been well identified.(11) In an initial analysis of morphine concentrations, Bayesian predicted PK profiles, and pains score data-pairs were categorized as “on target” (low pain with target or below-target concentration), “warning” (high pain with target or above-target concentration) or “alert” (high pain with below-target concentration or “low” pain with above-target concentration).(26) A large percentage of neonates were found to be exposed to high morphine concentrations relative to their pain scores. The PK/PD analysis resulted in an informative heat map of exposure-response data to be used as a next step in the identification of specific PK/PD targets depending on indication and comorbidity. Lastly, such an integrated tool will allow for systematic data collection that as a large real world data set can be mined and analyzed for exposure response relationships using machine learning techniques.(27)
One of the most important findings from the human factors engineering evaluation was the fact that a system designed for expert use requires training. The NeoRelief decision support application in this respect is not different. Most notably, the initial proposed morphine concentration readout and projected PK profiles did not resonate with nurse practitioner’s mental models. Participants had difficulty using this information for dosing decisions. Surprisingly, usability tests showed that the information of time within the target concentration range also did not add value to the dosing decision process. However, throughout the design discussions with clinicians, there was broad consensus that the long-term benefits of the system would outweigh the time investment and burden of learning and training.
During the development phase, the NICU clinical pharmacist was the only individual who had access to the live tool. The platform is currently in production and prospectively being evaluated and modified through cooperative design methods with two neonatologists and a clinical pharmacist in the NICU to inform the refinement of the system and testing during the implementation stage. Of particular importance was the focus of incorporating the decision support tool into the EHR in a way that fits into providers’ existing clinical workflows. To be successful, the precision dosing clinical decision support dashboard must satisfy the “five rights” of clinical decision support, to supply the “right information” to the “right person” in the “right intervention format” through the “right channel” at the “right time in workflow.”(28) User-centered design, optimal quality assurance and safety testing techniques, user acceptance testing, and an evidence-based informatics implementation with an extreme sensitivity to impact on workflows will ensure a successful implementation and minimizes the risk of the intervention. The project team has implemented pre- and post-release technical checks and user surveys to assess for any unintended consequences. These included technical checks as quality assurance with regard to data quality and completeness by clinical champions and survey activities to adjust and align on clarity of presentation and usability. Users will be prescribers placing orders for morphine on NICU-based patients. As part of this process, rapid iterative development cycles to refine the individualized precision dosing tool are implemented. The first phase of the prospective evaluation is the use of the tool to better match morphine dose to predicted exposures without concentration feedback. This is to evaluate if we can avoid high morphine concentrations relative to pain scores as noted in the preliminary PK/PD evaluation.(26) A next phase in the evaluation will incorporate providing feedback in terms of real-time drug concentration information directly into prescribers’ workflows so that they can make data-informed decisions, thus allowing the tailoring of the dose to individual therapeutics targets and needs.
Our overarching vision is that integrating a real time, model-informed, clinically-individualized PK profile into prescribing clinicians’ existing workflows will improve safety and efficacy of morphine dosing for neonates. A visual dashboard will provide clinicians with valuable dosing information at the time of medication ordering to facilitate precision dosing of pain and sedation medications for each individual patient. This would improve pain management and have the potential of reducing the need for breakthrough or rescue doses while also reducing the risk of harm from side effects and opioid dependence. Some potential drawbacks during the early phase of evaluation could be delayed order writing, and a mismatch between model predicted and actual morphine exposure, given that real time concentration results will not be implemented until a later phase. The availability of morphine (and metabolites) concentration results would also be needed to evaluate the development of opiate tolerance and how this might be predicted by the tool. Planned next steps include incorporation of pharmacogenetic information (e.g. OCT1)(10, 29) and expansion of the platform to provide clinical decision support for other commonly used, high-risk medications. Using the same techniques as for incorporation of morphine PK models, we have developed PK models for midazolam(30–36) and acetaminophen,(37–39) which we plan to integrate into the precision dosing platform display, thereby augmenting the clinical picture informing analgesic and sedative medication dosing. Further extensions will incorporate additional pain medications such as fentanyl and remifentanyl(39), provide model-informed support for the precision treatment of neonatal abstinence syndrome (opiate withdrawal resulting from in-utero drug exposure)(40), and extend these PK/PD concepts to other areas such as antimicrobial or antiepileptic medication dosing.(19) The expected outcomes will be enhanced individualized and evidence-based pharmacotherapy, resulting in better clinical efficacy and safety with fewer side effects in the neonatal population.
Supplementary Material
Figure S1. NeoRelief application software architecture.
Table S1. Overview of morphine PK population models implemented in the NeoRelief application.
Data are summarized as: year of publication, number of compartments, model type (empiric, mechanistic), supplied output (M, M3G, M6G concentration prediction), covariates, PK variables (CL, Q, V), and inclusion of maturation model based on PMA and /or PNA.
Table S2. Modeling details. Describing the morphine population PK models and related equations as implemented in the prototype NeoRelief application.
Table S3. NeoRelief predictive performance results based on nine clinical case scenarios.
Study Highlights.
- What is the current knowledge on the topic?
- Pain management with intravenous morphine in neonates is challenging and mostly carried out as an iterative process using a wide range of starting doses followed by titrating to effect based on clinical response and side effects using pain scores and levels of sedation.
- What question did this study address?
- Can a user-friendly decision-support tool be developed and successfully integrated into the EHR? We hypothesized that providing user-friendly decision support for the precision dosing of morphine has the potential to improve pain management and reduce the need for breakthrough or rescue doses while also reducing the risk of harmful side effects and opioid dependence.
- What does this study add to our knowledge?
- We describe the development and implementation of an Electronic Health Record (EHR)-embedded decision support platform (NeoRelief) for individualized precision drug treatment in neonates using morphine. The NeoRelief application translate morphine dose into a PK profile and exposure while simultaneously depicting markers of response and clinical events in the form of pains scores, heart rate, and respiratory rate. This provide clinicians with actionable information at the time of medication ordering in support of individualized precision dosing of morphine in the neonatal intensive care unit.
- How might this change clinical pharmacology or translational science?
- The NeoRelief precision dosing decision application is designed to deliver the “five rights” of clinical decision support: the “right information” to the “right person” in the “right intervention format” through the “right channel” at the “right time in the workflow.” The expected outcomes are improved pain management and safety with fewer side effects in the neonatal population.
Acknowledgments
Funding
Funding for the development and implementation of the morphine decision support platform (NeoRelief) project came from the Gerber Foundation, the State of Ohio, Ohio Development Services Agency, Ohio Third Frontier, Grant Control No. TECG20170361, the Cincinnati Children’s Hospital Medical Center’s Innovation Fund, and in part by a T1 translational research award by the University of Cincinnati Center for Clinical and Translational Science and Training; National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425.
Joshua C. Euteneuer and Thomas J. Duggan were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number 5T32HD069054 (Cincinnati Training Program in Pediatric Clinical & Developmental Pharmacology; Vinks, PI).
Footnotes
Conflict of Interest
AAV, FM, DB, TJD, DEC, SK, TD, KDRS, JZ, JCE, TM, and KD declared no competing interests for this work. NP is president of Medimatics a company that provides consulting services on medical information systems located in Maastricht, the Netherlands. EK receives royalties for the licensing of acute kidney injury algorithms to VigiLanz Corporation. These algorithms are unrelated to the work presented here. PS and RK are employees of Pomiet, LLC a healthcare consulting company specializing in user-centered lean software development located in Cincinnati, OH. As an Associate Editor for Clinical Pharmacology & Therapeutics, Alexander A. Vinks was not involved in the review or decision process for this paper.
Supporting Information
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
References
- (1).Hall RW & Shbarou RM Drugs of choice for sedation and analgesia in the neonatal ICU. Clin Perinatol 36, 215–26, vii (2009). [DOI] [PubMed] [Google Scholar]
- (2).Euteneuer JC et al. Large Variability in Morphine Exposure during Standard of Care Dosing in Critically Ill Neonates. Clin Pharmacol Ther 1003, S45. [Google Scholar]
- (3).Anand KJ et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. British journal of anaesthesia 101, 680–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wang C et al. Developmental changes in morphine clearance across the entire paediatric age range are best described by a bodyweight-dependent exponent model. Clinical drug investigation 33, 523–34 (2013). [DOI] [PubMed] [Google Scholar]
- (5).FDA. General Anesthetic and Sedation Drugs: Drug Safety Communication - FDA Approves Label Changes for Use in Young Children. <https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm555631.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery> (2017).
- (6).Zeilmaker-Roest GA et al. An international survey of management of pain and sedation after paediatric cardiac surgery. BMJ Paediatrics Open 1, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bouwmeester NJ, Anderson BJ, Tibboel D & Holford NH Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. British journal of anaesthesia 92, 208–17 (2004). [DOI] [PubMed] [Google Scholar]
- (8).Knibbe CA et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clinical pharmacokinetics 48, 371–85 (2009). [DOI] [PubMed] [Google Scholar]
- (9).Krekels EH, Tibboel D, Danhof M & Knibbe CA Prediction of morphine clearance in the paediatric population : how accurate are the available pharmacokinetic models? Clinical pharmacokinetics 51, 695–709 (2012). [DOI] [PubMed] [Google Scholar]
- (10).Emoto C, Fukuda T, Johnson TN, Neuhoff S, Sadhasivam S & Vinks AA Characterization of Contributing Factors to Variability in Morphine Clearance Through PBPK Modeling Implemented With OCT1 Transporter. CPT Pharmacometrics Syst Pharmacol 6, 110–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Anderson BJ & van den Anker J Why is there no morphine concentration-response curve for acute pain? Paediatr Anaesth 24, 233–8 (2014). [DOI] [PubMed] [Google Scholar]
- (12).Hillman BA, Tabrizi MN, Gauda EB, Carson KA & Aucott SW The Neonatal Pain, Agitation and Sedation Scale and the bedside nurse’s assessment of neonates. J Perinatol 35, 128–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bouwmeester NJ, Anderson BJ, Tibboel D & Holford NHG Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. British journal of anaesthesia 92, 208–17 (2004). [DOI] [PubMed] [Google Scholar]
- (14).Holford NH, Ma SC & Anderson BJ Prediction of morphine dose in humans. Paediatr Anaesth 22, 209–22 (2012). [DOI] [PubMed] [Google Scholar]
- (15).Knosgaard KR, Foster DJ, Kreilgaard M, Sverrisdottir E, Upton RN & van den Anker JN Pharmacokinetic models of morphine and its metabolites in neonates:: Systematic comparisons of models from the literature, and development of a new meta-model. Eur J Pharm Sci 92, 117–30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Juul RV et al. A Pharmacokinetic-Pharmacodynamic Model of Morphine Exposure and Subsequent Morphine Consumption in Postoperative Pain. Pharm Res 33, 1093–103 (2016). [DOI] [PubMed] [Google Scholar]
- (17).Lotsch J, Weiss M, Ahne G, Kobal G & Geisslinger G Pharmacokinetic modeling of M6G formation after oral administration of morphine in healthy volunteers. Anesthesiology 90, 1026–38 (1999). [DOI] [PubMed] [Google Scholar]
- (18).Proost JH & Meijer DK MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 22, 155–63 (1992). [DOI] [PubMed] [Google Scholar]
- (19).Euteneuer JC, Kamatkar S, Fukuda T, Vinks AA & Akinbi HT Suggestions for Model-Informed Precision Dosing to Optimize Neonatal Drug Therapy. J Clin Pharmacol 59, 168–76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Krekels EH, van Hasselt JG, Tibboel D, Danhof M & Knibbe CA Systematic evaluation of the descriptive and predictive performance of paediatric morphine population models. Pharm Res 28, 797–811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wood M & Morris M (2007). Quantification of morphine, morphine-3-glucuronide and morphine-6-glucuronide in biological samples by LC/MS/MS. (Waters Corporation, UK Limited, Manchester, UK, 2007). [Google Scholar]
- (22).Beuscart-Zephir MC, Aarts J & Elkin P Human factors engineering for healthcare IT clinical applications. Int J Med Inform 79, 223–4 (2010). [DOI] [PubMed] [Google Scholar]
- (23).Brooke J SUS: A “quick and dirty” usability scale In: Usability Evaluation in Industry (eds. Jordan PW, Thomas B, Weerdmeester BA and McClelland AL) (Taylor and Francis, London, 1996). [Google Scholar]
- (24).Miller K et al. Interface, information, interaction: a narrative review of design and functional requirements for clinical decision support. J Am Med Inform Assoc 25, 585–92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Krekels EH et al. Evidence-based morphine dosing for postoperative neonates and infants. Clinical pharmacokinetics 53, 553–63 (2014). [DOI] [PubMed] [Google Scholar]
- (26).Duggan TJ et al. PK/PD Modeling: What Can We Learn About Morphine Treatment in the Neonate? In Pediatric Academic Societies Meeting. [Google Scholar]
- (27).Van Driest SL & Choi L Real-World Data for Pediatric Pharmacometrics: Can We Upcycle Clinical Data for Research Use? Clin Pharmacol Ther 106, 84–6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Osheroff J et al. Improving Outcomes with Clinical Decision Support: An Implementer’s Guide. 2nd Edition edn. (Co-published by HIMSS, the Scottsdale Institute, AMIA, AMDIS and SHM: 2012). [Google Scholar]
- (29).Hahn D, Emoto C, Euteneuer JC, Mizuno T, Vinks AA & Fukuda T Influence of OCT1 Ontogeny and Genetic Variation on Morphine Disposition in Critically Ill Neonates: Lessons From PBPK Modeling and Clinical Study. Clin Pharmacol Ther 105, 761–8 (2019). [DOI] [PubMed] [Google Scholar]
- (30).Ahsman MJ, Hanekamp M, Wildschut ED, Tibboel D & Mathot RA Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clinical pharmacokinetics 49, 407–19 (2010). [DOI] [PubMed] [Google Scholar]
- (31).Harte GJ, Gray PH, Lee TC, Steer PA & Charles BG Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. J Paediatr Child Health 33, 335–8 (1997). [DOI] [PubMed] [Google Scholar]
- (32).Burtin P et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther 56, 615–25 (1994). [DOI] [PubMed] [Google Scholar]
- (33).Brussee JM et al. Predicting CYP3A-mediated midazolam metabolism in critically ill neonates, infants, children and adults with inflammation and organ failure. Br J Clin Pharmacol 84, 358–68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ince I et al. Critical illness is a major determinant of midazolam clearance in children aged 1 month to 17 years. Ther Drug Monit 34, 381–9 (2012). [DOI] [PubMed] [Google Scholar]
- (35).Ince I et al. A novel maturation function for clearance of the cytochrome P450 3A substrate midazolam from preterm neonates to adults. Clinical pharmacokinetics 52, 555–65 (2013). [DOI] [PubMed] [Google Scholar]
- (36).Peeters MY et al. Pharmacokinetics and pharmacodynamics of midazolam and metabolites in nonventilated infants after craniofacial surgery. Anesthesiology 105, 1135–46 (2006). [DOI] [PubMed] [Google Scholar]
- (37).Wang C et al. Population pharmacokinetics of paracetamol across the human age-range from (pre)term neonates, infants, children to adults. J Clin Pharmacol 54, 619–29 (2014). [DOI] [PubMed] [Google Scholar]
- (38).Cook SF et al. Population Pharmacokinetics of Intravenous Paracetamol (Acetaminophen) in Preterm and Term Neonates: Model Development and External Evaluation. Clinical pharmacokinetics 55, 107–19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Baarslag MA et al. Paracetamol and morphine for infant and neonatal pain; still a long way to go? Expert Rev Clin Pharmacol 10, 111–26 (2017). [DOI] [PubMed] [Google Scholar]
- (40).Wiles JR et al. Pharmacokinetics of Oral Methadone in the Treatment of Neonatal Abstinence Syndrome: A Pilot Study. J Pediatr 167, 1214–20 e3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. NeoRelief application software architecture.
Table S1. Overview of morphine PK population models implemented in the NeoRelief application.
Data are summarized as: year of publication, number of compartments, model type (empiric, mechanistic), supplied output (M, M3G, M6G concentration prediction), covariates, PK variables (CL, Q, V), and inclusion of maturation model based on PMA and /or PNA.
Table S2. Modeling details. Describing the morphine population PK models and related equations as implemented in the prototype NeoRelief application.
Table S3. NeoRelief predictive performance results based on nine clinical case scenarios.