Abstract
The objective of this study was to investigate the current status and trends in antidepressant use and expenditure in China from 2013 to 2018. The study had a retrospective design based on prescribing data on antidepressant drugs, which was sourced from the Hospital Prescription Analysis Cooperative Project. The trends in hospital visits and corresponding expenditure on antidepressant drugs were examined. Subgroup analyses were carried out by sex, age, and drug class. A total of 1,795,230 outpatient prescriptions were collected from 79 hospitals in six major cities in China. Hospital visits with antidepressant prescriptions rose significantly from 244,626 in 2013 to 348,718 in 2018, reflecting a 42.6% increase (P < 0.05). The antidepressant expenditure also rose, increasing from 48.0 million Chinese yuan in 2013 to 64.8 million Chinese yuan in 2018. There were approximately 1.6 times more antidepressant prescriptions written for women than for men. The most frequent age category for antidepressant prescriptions was 45–64 years. The most commonly prescribed antidepressants were selective serotonin reuptake inhibitors (N06AB) and other antidepressants (N06AX), whereas tricyclic antidepressants (N06AA) accounted for only a small part of the total antidepressant prescriptions. Flupentixol/melitracen and escitalopram were the most frequently prescribed antidepressants. Antidepressant prescribing rates continue to increase in China, although the prescribing patterns have changed over the past few years. The wide use of expensive antidepressants and those with weak clinical evidence raises concerns regarding the rational use of antidepressants. This study provides a basis for future stewardship by the government and medical institutions.
Keywords: prescriptions, cost, selective serotonin reuptake inhibitor, tricyclic antidepressant, flupentixol/melitracen, escitalopram
Introduction
Antidepressant medications are currently prescribed for a wide range of conditions, and an increasing trend in antidepressant prescriptions have been reported in many countries, including the United States, Australia, and some European countries (1–5). There are a number of reasons for this increase, including growing public awareness of depression, changes in patients’ attitudes, the expansion of therapeutic indications, and the impact of commercial interests (6, 7). In clinical practice, antidepressants are prescribed for many conditions other than depression, such as anxiety, sleeping disorders, psychosis, and neuropathic pain (3, 8). This has led to concern that antidepressants are being overprescribed.
The antidepressants available on the market have increased in recent years, providing prescribers with a variety of options. The choice of an antidepressant is influenced by drug profiles, physician characteristics, patient characteristics, regulation and reimbursement policies, and other factors (9). Antidepressants can induce several severe adverse effects, including self-inflicted injury, myocardial infarction, and stroke (10–12). Discontinuing or decreasing use of antidepressants, especially selective serotonin reuptake inhibitors (SSRIs), may lead to the appearance of withdrawal symptoms (13–15). Furthermore, concerns have been raised regarding whether the prescription of antidepressants is always justified (3). It is important to understand national trends and patterns of antidepressant use.
With the exception of one study that was restricted to patients with schizophrenia, little is known about the profile of antidepressant use in China (16). Therefore, this study aimed to describe the time trends and patterns of antidepressant prescriptions for adults from 2013 to 2018, using a large dataset.
Methods
Ethics
This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University (Reference Number: 20191011-18). Informed consent was waived as part of the approval.
Study Setting and Population
This study was designed as a retrospective research based on prescription data. Antidepressant prescription data were obtained from the database of the Hospital Prescription Analysis Cooperative Project, which has been widely used in Chinese pharmacoepidemiology studies (17, 18). The database contained prescription information on sampling days of participating hospitals. There were 40 randomized sampling days per year, with 10 sampling days each quarter. For each prescription, the collected information included the prescription code, sex and age of the patient, prescription date, patient’s diagnosis, generic drug name, dose, and cost.
In the current study, outpatient prescriptions meeting the following criteria were extracted and included: (1) The prescriptions contained at least one antidepressant; (2) were prescribed for patients aged over 18 years; (3) were issued from 2013 to 2018; and (4) were issued in hospitals that participated in the program continuously during the study period and were located in Beijing, Tianjin, Shanghai, Guangzhou, Chengdu, or Hangzhou. No restrictions were imposed regarding indications because antidepressants are used to treat many conditions. However, prescriptions with incomplete information were excluded.
Drug Classes
Antidepressant drugs were classified into five categories according to the World Health Organization Anatomical Therapeutic Chemical Index: (1) non-selective monoamine reuptake inhibitors (tricyclic antidepressants, TCAs): amitriptyline, clomipramine, doxepin, maprotiline; (2) N06AB, SSRIs: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline; (3) N06AF, non-selective monoamine oxidase inhibitor; (4) N06AG, selective monoamine oxidase A inhibitor; and (5) N06AX, other antidepressants: agomelatine, bupropion, flupentixol/melitracen, reboxetine, neurostan, mirtazapine, mianserin, trazodone, duloxetine, milnacipran, and venlafaxine (4, 19).
Data Analysis
The main units of analysis in this study were hospital visits where antidepressants were prescribed and expenditure on antidepressants. The number of visits was defined as the number of prescriptions meeting the inclusion criteria. Expenditure was defined as the cost of antidepressants. The yearly number of visits and expenditure were calculated, and the trends were analyzed.
Subgroup analyses by age, sex, and drug class were conducted. Three age groups were created to determine whether the trends in antidepressant use were being driven by a particular age group. The three age groups were young adults (18–44 years), middle-aged adults (45–64 years), and older adults (65 years and older).
The data were processed using Microsoft Access software. The rank-sum test was used to assess the statistical significance of trends for visits and expenditure. The Cochran–Armitage trend test was applied to determine the statistical significance of prescribing trends in drugs and drug classes. R V.3.3.0 (http://www.R-project.org) software was used for the statistical analysis.
Results
Total Trends in Antidepressant Visits and Expenditure
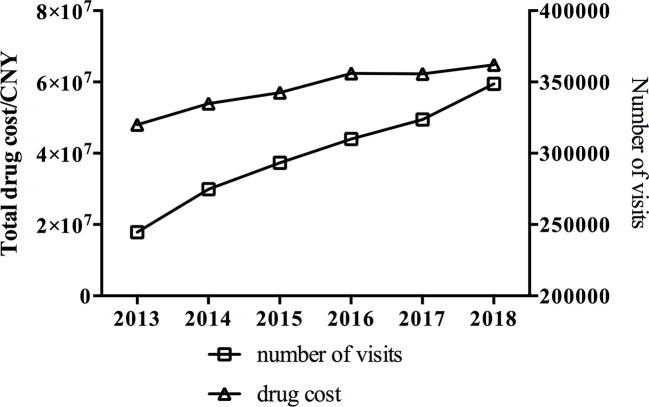
A total of 1,795,230 outpatient antidepressant prescriptions issued from 2013 to 2018 were reviewed in this study. These prescriptions were from 79 hospitals located in six major cities in China. All included hospitals were state-owned general hospitals. Of all the prescriptions, 42.1% were prescribed by psychiatrists and 33.0% were prescribed by neurological physicians. As Figure 1 indicates, both visits and expenditure increased over time (both P < 0.05). The prescribing of antidepressant medications increased substantially from 244,626 in 2013 to 348,717 in 2018, reflecting a 42.6% increase over the study period. A 34.9% increase in prescribing costs was found over the same period—from 48 million Chinese yuan in 2013 to 64.8 million Chinese yuan in 2018.
Figure 1.
Trends in visits and cost of antidepressants in 79 hospitals located in six major cities in China from 2013 to 2018.
Trends by Age and Sex
We stratified the analysis of the trends in the number of antidepressant visits for any indication by age group and sex, and the results are shown in Table 1 . The number of antidepressant visits for patients aged 18–44 years, 45–64 years, and 65 years and older all increased. Patients aged 45–64 years accounted for more than 40% of the visits. However, the percentage of older adult patients showed a small but significant increase over time (P < 0.05). Antidepressant drugs were more frequently prescribed to women, who accounted for 1.6 times more antidepressant visits than men (P < 0.05). However, the ratio of female-to-male users remained constant over the study period (P > 0.05).
Table 1.
Demographic characteristics of included patients, 2013-2018.
| Number of patients (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||||||||
| Age (years) | |||||||||||||
| 18-44 | 69382 | (28.36) | 76073 | (27.69) | 80713 | (27.52) | 84434 | (27.23) | 88209 | (27.25) | 95589 | (27.41) | |
| 45-64 | 107308 | (43.87) | 122116 | (44.45) | 129030 | (43.99) | 134851 | (43.49) | 139719 | (43.16) | 147865 | (42.40) | |
| 65 up | 67936 | (27.77) | 76550 | (27.86) | 83588 | (28.50) | 90792 | (29.28) | 95812 | (29.60) | 105263 | (30.19) | |
| Sex | |||||||||||||
| Male | 90616 | (37.04) | 101609 | (36.98) | 109247 | (37.24) | 116328 | (37.52) | 119893 | (37.03) | 127958 | (36.69) | |
| Female | 154010 | (62.96) | 173130 | (63.02) | 184084 | (62.76) | 193749 | (62.48) | 203847 | (62.97) | 220759 | (63.31) | |
Trends by Type of Drug
A total of 21 antidepressants in three drug classes were involved in this study. No instances of prescriptions for non-selective monoamine oxidase inhibitor (N06AF) or selective monoamine oxidase A inhibitor (N06AG) were observed. The antidepressant prescription visits and the percentage of the total prescriptions made up by each drug and drug class were examined, and the results are shown in Table 2 . The total expenditure on antidepressant medications and the percentage of this accounted for by each drug are summarized in Table 3 .
Table 2.
Antidepressant prescription visits by drug and drug class, 2013-2018.
| ATC code | Medicine | Number of visits (%) | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| N06AA | 14827(6.06) | 15634(5.69) | 14834(5.06) | 14606(4.71) | 13373(4.13) | 12790(3.67) | |
| Amitriptyline | 8694(3.55) | 9184(3.34) | 8891(3.03) | 8906(2.87) | 8658(2.67) | 8402(2.41) | |
| Clomipramine | 731(0.30) | 692(0.25) | 655(0.22) | 641(0.21) | 654(0.20) | 647(0.19) | |
| Doxepin | 5155(2.11) | 5533(2.01) | 5084(1.73) | 4867(1.57) | 3877(1.20) | 3554(1.02) | |
| Maprotiline | 247(0.10) | 225(0.08) | 204(0.07) | 192(0.06) | 184(0.06) | 187(0.05) | |
| N06AB | 126478(51.7) | 134902(49.1) | 145625(49.6) | 156543(50.5) | 161658(49.9) | 172718(49.5) | |
| Citalopram | 13637(5.57) | 15727(5.72) | 17707(6.04) | 17024(5.49) | 16216(5.01) | 14207(4.07) | |
| Escitalopram | 19850(8.11) | 28585(10.4) | 36162(12.3) | 42182(13.6) | 48379(14.9) | 55279(15.9) | |
| Fluoxetine | 13509(5.52) | 14731(5.36) | 12908(4.40) | 12878(4.15) | 11780(3.64) | 11942(3.42) | |
| Fluvoxamine | 4771(1.95) | 6701(2.44) | 7829(2.67) | 8275(2.67) | 8571(2.65) | 9849(2.82) | |
| Paroxetine | 42861(17.5) | 37254(13.6) | 36880(12.6) | 39487(12.7) | 39531(12.2) | 42447(12.2) | |
| Sertraline | 31850(13.0) | 31904(11.6) | 34139(11.6) | 36697(11.8) | 37181(11.5) | 38994(11.2) | |
| N06AX | 103321(42.2) | 124203(45.2) | 132872(45.3) | 138928(44.8) | 148709(45.9) | 163209(46.8) | |
| Agomelatine | 133(0.05) | 97(0.04) | 49(0.02) | 3(0.00) | 293(0.09) | 2255(0.65) | |
| Bupropion | 482(0.20) | 518(0.19) | 575(0.20) | 723(0.23) | 951(0.29) | 936(0.27) | |
| Duloxetine | 10503(4.29) | 14255(5.19) | 14302(4.88) | 17449(5.63) | 20420(6.31) | 23937(6.86) | |
| Flupentixol/melitracen | 48685(19.9) | 58622(21.3) | 63769(21.7) | 60977(19.7) | 63824(19.7) | 65617(18.8) | |
| Reboxetine | 13(0.01) | 9(0.00) | 0(0.00) | 0(0.00) | 0(0.00) | 0(0.00) | |
| Mianserin | 623(0.25) | 598(0.22) | 766(0.26) | 916(0.30) | 1093(0.34) | 1170(0.34) | |
| Mirtazapine | 15482(6.33) | 17780(6.47) | 17621(6.01) | 18592(6.00) | 19660(6.07) | 21489(6.16) | |
| Milnacipran | 0(0.00) | 0(0.00) | 0(0.00) | 8(0.00) | 135(0.04) | 210(0.06) | |
| Neurostan | 352(0.14) | 347(0.13) | 83(0.03) | 101(0.03) | 535(0.17) | 559(0.16) | |
| Trazodone | 8552(3.50) | 9977(3.63) | 10869(3.71) | 11950(3.85) | 13595(4.20) | 16295(4.67) | |
| Venlafaxine | 18496(7.56) | 22000(8.01) | 24838(8.47) | 28209(9.10) | 28203(8.71) | 30741(8.82) | |
Table 3.
Total expenditure on antidepressant medications dispensed from 2013 to 2018 by drug and drug class.
| ATC code | Medicine | Cost of Chinese yuan (%) | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| N06AX | 162184(0.34) | 150912(0.28) | 139197(0.24) | 134399(0.22) | 115441(0.19) | 144083(0.22) | |
| Amitriptyline | 81596(0.17) | 79001(0.15) | 75502(0.13) | 72435(0.12) | 52052(0.08) | 63106(0.10) | |
| Clomipramine | 39563(0.08) | 33029(0.06) | 27268(0.05) | 24916(0.04) | 19019(0.03) | 20180(0.03) | |
| Doxepin | 28889(0.06) | 28691(0.05) | 26473(0.05) | 27750(0.04) | 35418(0.06) | 52145(0.08) | |
| Maprotiline | 12137(0.03) | 10191(0.02) | 9954(0.02) | 9298(0.01) | 8952(0.01) | 8653(0.01) | |
| N06AB | 30366454(63.2) | 31897367(59.2) | 34035848(59.7) | 37415695(60.0) | 37273217(59.9) | 38231262(59.0) | |
| Citalopram | 3304822(6.88) | 3918401(7.27) | 4367593(7.66) | 4172154(6.69) | 3801416(6.11) | 3069586(4.74) | |
| Escitalopram | 6023948(12.5) | 8369067(15.5) | 10747229(18.9) | 13046681(20.9) | 14256349(22.9) | 15536616(24.0) | |
| Fluoxetine | 3659219(7.62) | 3946896(7.32) | 3272764(5.74) | 3477740(5.57) | 3042443(4.89) | 2957276(4.56) | |
| Fluvoxamine | 794085(1.65) | 1103158(2.05) | 1290382(2.26) | 1433769(2.30) | 1437000(2.31) | 1560367(2.41) | |
| Paroxetine | 10530022(21.9) | 8498899(15.8) | 7881856(13.8) | 8256006(13.2) | 7553850(12.1) | 7691849(11.9) | |
| Sertraline | 6054358(12.6) | 6060945(11.2) | 6476025(11.4) | 7029344(11.3) | 7182157(11.5) | 7415567(11.4) | |
| N06AX | 17494875(36.4) | 21874231(40.6) | 22823643(40.0) | 24842641(39.8) | 24864451(39.9) | 26414705(40.8) | |
| Agomelatine | 72644(0.15) | 60197(0.11) | 37379(0.07) | 1781(0.00) | 124678(0.20) | 687905(1.06) | |
| Bupropion | 123598(0.26) | 143183(0.27) | 161264(0.28) | 198178(0.32) | 244859(0.39) | 184247(0.28) | |
| Duloxetine | 4042906(8.42) | 5651351(10.5) | 5402316(9.5) | 6508445(10.4) | 7025245(11.3) | 7662359(11.8) | |
| Flupentixol/Melitracen | 4221026(8.79) | 5385611(9.99) | 5855594(10.3) | 5510436(8.8) | 5140978(8.3) | 4971748(7.7) | |
| Reboxetine | 1230(0.00) | 643(0.00) | 0(0.00) | 0(0.00) | 0(0.00) | 0(0.00) | |
| Mianserin | 100041(0.21) | 89928(0.17) | 112878(0.20) | 132846(0.21) | 155082(0.25) | 158740(0.25) | |
| Milnacipran | 0(0.00) | 0(0.00) | 0(0.00) | 3911(0.01) | 51236(0.08) | 74276(0.11) | |
| Mirtazapine | 3070120(6.39) | 3457236(6.41) | 3320667(5.83) | 3367417(5.40) | 3273662(5.26) | 3361283(5.19) | |
| Neurostan | 46357(0.10) | 50620(0.09) | 11840(0.02) | 15783(0.03) | 83083(0.13) | 91459(0.14) | |
| Trazodone | 714002(1.49) | 860164(1.60) | 971800(1.70) | 1068978(1.71) | 1107519(1.78) | 1307992(2.02) | |
| Venlafaxine | 5102949(10.6) | 6175297(11.5) | 6949906(12.2) | 8034867(12.9) | 7658109(12.3) | 7914696(12.2) | |
The most frequently prescribed antidepressant class was SSRIs, which remained in the leading position in terms of both the percentage of the total antidepressant prescription visits and the percentage of the expenditure during the study period. Although TCAs accounted for a small amount of both visits and costs, the percentages nevertheless decreased from 6.1% to 3.7% of antidepressant prescription visits and from 0.34% to 0.22% of the total cost from 2013 to 2018 (both P < 0.05). For the other antidepressant class (N06AX), a slight but continuous increase was seen in the percentage of all antidepressant prescription visits, which rose from 42.2% in 2013 to 46.8% in 2018 (P < 0.05). Over the same period, the expenditure on N06AX antidepressants increased from 36.4% to 40.8% of the total expenditure on antidepressants (P < 0.05).
The most frequently prescribed antidepressant was flupentixol/melitracen, followed by escitalopram, paroxetine, and sertraline. The visits where each of these four antidepressants was prescribed increased during the study period; however, escitalopram was the only antidepressant that showed an increase in terms of the percentage of antidepressant prescription visits where this drug was prescribed (all P < 0.05). The greatest antidepressant expenditure at the end of the study period was for escitalopram, followed by venlafaxine, paroxetine, duloxetine, and sertraline. The percentage of the total antidepressant expenditure accounted for by escitalopram increased dramatically from 12.5% in 2013 to 24.0% in 2018 (P < 0.05). In contrast, the percentage of the total expenditure accounted for paroxetine decreased from 21.9% in 2013 to 11.9% in 2018 (P < 0.05). Other antidepressants exhibited small and insignificant fluctuations.
Discussion
This study examined the trends in antidepressant use in China using a large anonymized database. Progressive increases from 2013 to 2018 in the number of hospital visits where antidepressants were prescribed and in the expenditure on antidepressants were revealed in the study. We also analyzed antidepressants by age, sex, and drug class.
The overall trend in antidepressant use was similar to the trends that have been observed in other countries (20–23). This may suggest an increase in the burden of depression, the diagnosis of depression, or the prescription of antidepressants for conditions other than depression. Less than half of the antidepressant prescriptions examined in this study were issued by a psychiatrist, indicating that special concern should be paid to the rational use of antidepressants, particularly for indications other than depression. Antidepressant prescription visits increased among all age groups over the study period, whereas antidepressant prescription is generally found to be more frequent for patients aged 45–64 years. The antidepressant prescription visits of patients aged over 65 years increased relatively rapidly, and the percentage of visits accounted for by patients in this age group also increased. More attention should be paid to older adult users of antidepressants. In this study, we found that approximately 1.6 times more antidepressant prescriptions were prescribed to women than to men. This is not surprising, given that the prevalence and incidence of depression, as well as other psychiatric disorders such as anxiety and bipolar disorder, have previously been found to be higher for women than for men (20, 24–26). There was no difference in the sex ratio for antidepressant visits across the study years, which indicates that the relative distribution of the burden of depression and related conditions between men and women has not changed.
Our analysis showed that SSRIs accounted for nearly half of the antidepressant visits in China during the study period. However, our findings for the use of SSRIs were nevertheless lower than previous reports of SSRI use in other countries. In many countries, SSRIs are the predominant antidepressants, making up 60%–70% of the market (2, 27, 28). The clinical guidelines for treating depression suggest SSRIs (mostly escitalopram, paroxetine, and sertraline) as the preferred pharmacological treatment in adults (29, 30); however, the antidepressant efficacy of SSRIs is not superior to that of TCAs (31). SSRIs are also used to treat anxiety disorders, but the efficacy of SSRIs in this application is not superior to that of benzodiazepines (32). The reported overall adverse event rates of SSRIs and TCAs are comparable, but SSRIs are reported to have a lower incidence rate of cardiovascular effects and higher rates of neurogenic adverse effects (33). The percentage of the total antidepressant expenditure accounted for by SSRIs over the six years of study was almost 60%, indicating that SSRIs are relatively expensive. Considering that commercial interests have a major impact on the prescription of antidepressants, attention should be paid to the wide use of high-cost antidepressants (34, 35).
The prescription of TCAs was low despite these drugs having been the cornerstone of antidepressant pharmacotherapy for a long time. A similar trend has been observed in many other countries, although TCA use is still greater than SSRI use in Germany and an increasing trend has been reported for TCA use in the Netherlands (1, 4). The disadvantages of TCAs in terms of their cardiovascular side effects, high overdose fatality, and relatively great possibility of drug–drug interactions are responsible for the declining trend in TCA use (36, 37). However, other antidepressants—particularly SSRIs—also have serious problems because of withdrawal (14, 15). The higher use of TCAs than SSRIs in Germany suggests the important role of TCAs (1). The low price of TCAs is not a disadvantage, but this is another important reason for the decline (7).
A total of 21 antidepressant drugs were reported in this study. Flupentixol/melitracen, a mixture of a type of TCA and a classical antipsychotic component reported to be associated with significant improvement in quality of life independent of the presence of anxiety or depression, held the lead position in the antidepressant market from 2013 to 2018 (38). This is why the use of drugs in the other antidepressant class (N06AX) was much higher in our findings than in previous work in other countries. Some studies have suggested the efficacy of flupentixol/melitracen in treating resistant depression, ulcerative colitis, and several other conditions (39, 40). However, there is very limited evidence to support the use of this combination, and its popularity appears to coincide with marketing efforts. Flupentixol/melitracen is produced by a Danish company and is not registered in Denmark, the United States, the United Kingdom, Japan, or India (41). It can be assumed that flupentixol/melitracen is rarely used in these countries, despite the lack of reports on this. Special concern for safety and rational use should be raised regarding the wide use of this antidepressant with weak clinical evidence (42).
Escitalopram, the S-enantiomer of racemic citalopram, became the most frequently prescribed SSRI and moved into second place in terms of antidepressant prescriptions overall at the end of this study. This situation is quite different compared with other countries. The most commonly used SSRI in the United States, the United Kingdom, Italy, and Sweden has been shown to be citalopram, and sertraline has been reported as the most frequently used SSRI in Iran (2, 23, 24, 27, 43). Escitalopram is currently approved in over 100 countries and has been shown to have a good efficacy and safety profile (44–46). Escitalopram’s price varies by country. Although escitalopram was approved with a high price in some countries, previous studies have reported that escitalopram has a cost–utility advantage over other antidepressants in the treatment of major depression (47, 48). Escitalopram is expensive in China, elevating the total expenditure on SSRIs ( Table 3 ). The pharmacoeconomic profile of the drug has not been evaluated for depression or other indications in China.
There are several limitations to this study. Our analysis was based on prescription data only; therefore, the appropriateness of the antidepressant therapy could not be evaluated, nor could the outcome of antidepressant therapy. Data on the indications for prescribing antidepressants were lacking, and this topic needs to be investigated in further studies. Finally, the prescription data were extracted from hospitals located in major cities in China, which might have induced bias.
Conclusion
In this study, trends in antidepressant use and expenditure in six major cities in China were evaluated using a large database. Antidepressant prescription visits and expenditure on antidepressants were found to have increased over the study period. Furthermore, the most frequently prescribed antidepressants in this study differed from those reported in other countries. The wide use of antidepressants with weak clinical evidence or high costs raises concerns regarding the rational use of antidepressants. Using data from 79 hospitals in China from 2013 to 2018, this study provides a basis for future stewardship by the government and medical institutions.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceptualization: ZY, LY. Data curation: ZY, JZ. Formal analysis: LY, JZ, YZ, ZY. Funding acquisition: JZ, ZY. Investigation: LY, JZ, YZ. Methodology: ZY; Resources: ZY. Validation: LY, YZ. Visualization: LY. Writing-original draft: ZY, JZ. Writing—review and editing: YZ, LY.
Funding
This work was funded by the Health Commission of Zhejiang Province and Traditional Chinese Medicine Administration of Zhejiang, China (2017KY089 and 2016ZQ027). The funders played no role in this research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would thank Hospital Prescription Analysis Corporation Program of `China for collecting and providing the data.
References
- 1. Abbing-Karahagopian V, Huerta C, Souverein PC, De Abajo F, Leufkens HGM, Slattery J, et al. Antidepressant prescribing in five European countries: application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur J Clin Pharmacol (2014) 70(7):849–57. 10.1007/s00228-014-1676-z [DOI] [PubMed] [Google Scholar]
- 2. Andersson Sundell K, Gissler M, Petzold M, Waern M. Antidepressant utilization patterns and mortality in Swedish men and women aged 20-34 years. Eur J Clin Pharmacol (2011) 67(2):169–78. 10.1007/s00228-010-0933-z [DOI] [PubMed] [Google Scholar]
- 3. Noordam R, Aarts N, Verhamme KM, Sturkenboom MCM, Stricker BH, Visser LE. Prescription and indication trends of antidepressant drugs in the Netherlands between 1996 and 2012: a dynamic population-based study. Eur J Clin Pharmacol (2015) 71(3):369–75. 10.1007/s00228-014-1803-x [DOI] [PubMed] [Google Scholar]
- 4. González-López MC, Rodríguez-López CM, Parrón-Carreño T, Luna JD, Del Pozo E. Trends in the dispensation of antidepressant drugs over the past decade (2000–2010) in Andalusia, Spain. Soc Psychiatry Psychiatr Epidemiol (2015) 50(5):705–12. 10.1007/s00127-014-0995-9 [DOI] [PubMed] [Google Scholar]
- 5. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. national health and nutrition examination survey. J Clin Psychiatry (2014) 75(2):169–77. 10.4088/JCP.13m08443 [DOI] [PubMed] [Google Scholar]
- 6. Mojtabai R. Americans’ atitudes toward psychiatric medications: 1998-2006. Psychiatr Serv (2009) 60(8):1015–23. 10.1176/ps.2009.60.8.1015 [DOI] [PubMed] [Google Scholar]
- 7. Fava GA. Long-term treatment with antidepressant drugs: the spectacular achievements of propaganda. Psychother Psychosom (2002) 71(3):127–32. 10.1159/000056279 [DOI] [PubMed] [Google Scholar]
- 8. Mojtabai R, Oflson M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Aff (2011) 30(8):1434–42. 10.1377/hlthaff.2010.1024 [DOI] [PubMed] [Google Scholar]
- 9. Forns J, Pottegård A, Reinders T, Poblador-Plou B, Morros R, Brandt L, et al. Antidepressant use in Denmark, Germany, Spain, and Sweden between 2009 and 2014: incidence and comorbidities of antidepressant initiators. J Affect Disord (2019) 249:242–52. 10.1016/j.jad.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 10. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ (2011) 343:d4551. 10.1136/bmj.d4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parihar HS, Yin H, Gooch JL, Allen S, John S, Xuan J. Trends in hospital admissions due to antidepressant-related adverse drug events from 2001 to 2011 in the U.S. BMC Health Serv Res (2017) 17(1):1–7. 10.1186/s12913-017-1993-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The safety, tolerability and risks associated with the ue of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom (2016) 85(5):270–88. 10.1159/000447034 [DOI] [PubMed] [Google Scholar]
- 13. Fava GA, Cosci F, Offidani E, Guidi J. Behavioral toxicity revisited: Iatrogenic comorbidity in psychiatric evaluation and treatment. J Clin Psychopharmacol (2016) 36(6):550–3. 10.1097/JCP.0000000000000570 [DOI] [PubMed] [Google Scholar]
- 14. Fava GA, Benasi G, Lucente M, Offidani E, Cosci F, Guidi J. Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychother Psychosom (2018) 87(4):195–203. 10.1159/000491524 [DOI] [PubMed] [Google Scholar]
- 15. Fava GA, Cosci F. Understanding and managing withdrawal syndromes after discontinuation of antidepressant drugs. J Clin Psychiatry (2019) 80(6):19com12794. 10.4088/JCP.19com12794 [DOI] [PubMed] [Google Scholar]
- 16. Li Q, Su YA, Xiang YT, Shu L, Yu X, Ungvari GS, et al. Adjunctive antidepressant use in schizophrenia in China: a national survey (2002–2012). Hum Psychopharmacol (2017) 32(1):1–5. 10.1002/hup.2571 [DOI] [PubMed] [Google Scholar]
- 17. Yu L, Chen X, Yu Z. Trends of antidementia drugs use in outpatients with Alzheimer’s disease in six major cities of China: 2012-2017. Int Clin Psychopharmacol (2019) 34(6):312–6. 10.1097/YIC.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 18. Yu L, Ding K, Luo L, Yu Z. Prescribing trends of glaucoma drugs in six major cities of China from 2013 to 2017. PloS One (2020) 15(1):e0227595. 10.1371/journal.pone.0227595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organisation (WHO) (2018). WHO Collaborating Center for Drug Statistics Methodology. ATC/DDD Index. Available at: https://www.whocc.no/atc_ddd_index/ [assessed July 11, 2020].
- 20. Mars B, Heron J, Kessler D, Davies NM, Martin RM, Thomas KH, et al. Influences on antidepressant prescribing trends in the UK: 1995–2011. Soc Psychiatry Psychiatr Epidemiol (2017) 52(2):193–200. 10.1007/s00127-016-1306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilkinson S, Mulder RT. Antidepressant prescribing in New Zealand between 2008 and 2015. N Z Med J (2018) 131(1485):52–9. [PubMed] [Google Scholar]
- 22. Rhee TG. Continuing versus new antidepressant use in older adults: US prescribing trends from 2006 to 2015. Eur Geriatr Med (2018) 9(4):551–5. 10.1007/s41999-018-0075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sultana J, Italiano D, Spina E, Cricelli C, Lapi F, Pecchioli S, et al. Changes in the prescribing pattern of antidepressant drugs in elderly patients: an Italian, nationwide, population-based study. Eur J Clin Pharmacol (2014) 70(4):469–78. 10.1007/s00228-013-1636-z [DOI] [PubMed] [Google Scholar]
- 24. Zhong W, Kremers HM, Yawn BP, Bobo WV, St. Sauver JL, Ebbert JO, et al. Time trends of antidepressant drug prescriptions in men versus women in a geographically defined US population. Arch Womens Ment Health (2014) 17(6):485–92. 10.1007/s00737-014-0450-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canuto A, Weber K, Baertschi M, Andreas S, Volkert J, Dehoust MC, et al. Anxiety disorders in old age: psychiatric comorbidities, quality of life, and prevalence according to age, gender, and country. Am J Geriatr Psychiatry (2018) 26(2):174–85. 10.1016/j.jagp.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 26. Ferrari AJ, Stockings E, Khoo JP, Erskine HE, Degenhardt L, Vos T, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord (2016) 18(5):440–50. 10.1111/bdi.12423 [DOI] [PubMed] [Google Scholar]
- 27. Soleymani F, Taheri F, Roughead E, Nikfar S, Abdollahi M. Pattern of antidepressant utilization and cost in Iran from 2006 to 2013 in comparison with other countries. J Epidemiol Glob Health (2018) 8(3-4):213–9. 10.2991/j.jegh.2018.06.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bauer M, Monz BU, Montejo AL, Quail D, Dantchev N, Demyttenaere K, et al. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur Psychiatry (2008) 23(1):66–73. 10.1016/j.eurpsy.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence NICE guideline. Depression in adults: recognition and management. (2009) Available at: https://www.nice.org.uk/guidance/cg90 [assessed July 11, 2020]. [PubMed]
- 30. Guidi J, Tomba E, Cosci F, Park SK, Fava GA. The role of staging in planning psychotherapeutic interventions in depression. J Clin Psychiatry (2017) 78(4):456–63. 10.4088/JCP.16r10736 [DOI] [PubMed] [Google Scholar]
- 31. Undurraga J, Baldessarini RJ. Direct comparison of tricyclic and serotonin-reuptake inhibitor antidepressants in randomized head-to-head trials in acute major depression: systematic review and meta-analysis. J Psychopharmacol (2017) 31(9):1184–9. 10.1177/0269881117711709 [DOI] [PubMed] [Google Scholar]
- 32. Gomez AF, Barthel AL, Hofmann SG. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: a meta-analytic review. Expert Opin Pharmacother (2018) 19(8):883–94. 10.1080/14656566.2018.1472767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grohmann R, Rüther E, Engel RR, Hippius H. Assessment of adverse drug reactions in psychiatric inpatients with the AMSP drug safety program: methods and first results for tricyclic antidepressants and SSRI. Pharmacopsychiatry (1999) 32(1):21–8. 10.1055/s-2007-979184 [DOI] [PubMed] [Google Scholar]
- 34. Fava GA. Rational use of antidepressant drugs. Psychother Psychosom (2014) 83(4):197–204. 10.1159/000362803 [DOI] [PubMed] [Google Scholar]
- 35. Fava GA, Rafanelli C. Iatrogenic factors in psychopathology. Psychother Psychosom (2019) 88(3):129–40. 10.1159/000500151 [DOI] [PubMed] [Google Scholar]
- 36. Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand (2007) 101(s403):17–25. 10.1111/j.1600-0447.2000.tb10944.x [DOI] [PubMed] [Google Scholar]
- 37. Spina E, Scordo MG. Clinically significant drug interactions with antidepressants in the elderly. Drugs Aging (2002) 19(4):299–320. 10.2165/00002512-200219040-00004 [DOI] [PubMed] [Google Scholar]
- 38. Hashash JG, Abdul-Baki H, Azar C, Elhajj II, El Zahabi L, Chaar HF, et al. Clinical trial: a randomized controlled cross-over study of flupenthixol + melitracen in functional dyspepsia. Aliment Pharmacol Ther (2008) 27(11):1148–55. 10.1111/j.1365-2036.2008.03677.x [DOI] [PubMed] [Google Scholar]
- 39. Zhou X, Zhao L, Yang P, Chen Y, Ruan XZ. The efficacy of flupentixol-melitracen in the adjuvant therapy of ulcerative colitis in the chinese population: a meta-analysis. Gastroenterol Res Pr (2019) 2019:3480732. 10.1155/2019/3480732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bares M, Novak T, Kopecek M, Stopkova P, Sos P. Is combined treatment more effective than switching to monotherapy in patients with resistant depression? A retrospective study. Neuroendocrinol Lett (2009) 30(6):723–8. [PubMed] [Google Scholar]
- 41. Bhaumik S. India’s health ministry bans pioglitazone, metamizole, and flupentixol-melitracen. BMJ (2013) 347:f4366. 10.1136/bmj.f4366 [DOI] [PubMed] [Google Scholar]
- 42. Solanki MS, Banwari G. Irrational fixed dose combinations of psychotropic drugs in India: cause of concern. Indian J Pharmacol (2016) 48(4):468–9. 10.4103/0253-7613.186192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mars B, Heron J, Gunnell D, Martin RM, Thomas KH, Kessler D. Prevalence and patterns of antidepressant switching amongst primary care patients in the UK. J Psychopharmacol (2017) 31(5):553–60. 10.1177/0269881117693748 [DOI] [PubMed] [Google Scholar]
- 44. Pastoor D, Gobburu J. Clinical pharmacology review of escitalopram for the treatment of depression. Expert Opin Drug Metab Toxicol (2014) 10(1):121–8. 10.1517/17425255.2014.863873 [DOI] [PubMed] [Google Scholar]
- 45. Si T, Wang G, Yang F, Fang Y, Fang M, Li J, et al. Efficacy and safety of escitalopram in treatment of severe depression in Chinese population. Metab Brain Dis (2017) 32(3):891–901. 10.1007/s11011-017-9992-5 [DOI] [PubMed] [Google Scholar]
- 46. Stein DJ, Khoo JP, Ahokas A, Jarema M, Van Ameringen M, Vavrusova L, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25-50 mg/day) versus escitalopram (10-20 mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol (2018) 28(8):970–9. 10.1016/j.euroneuro.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 47. Armstrong EP, Malone DC. Erder MH. A Markov cost-utility analysis of escitalopram and duloxetine for the treatment of major depressive disorder. Curr Med Res Opin (2008) 24(4):1115–21. 10.1185/030079908X273309 [DOI] [PubMed] [Google Scholar]
- 48. Wade AG, Fernández JL, François C, Hansen K, Danchenko N, Despiegel N. Escitalopram and duloxetine in major depressive disorder: a pharmacoeconomic comparison using UK cost data. Pharmacoeconomics (2008) 26(11):969–81. 10.2165/00019053-200826110-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.