Abstract
Objective
As noninvasive biological markers gain increasing popularity in pediatric research, it is critical to understand how study participants perceive these measures, especially among groups underrepresented in biobehavioral research, like children and people of color. The purpose of this study was to examine acceptability and feasibility of hair and salivary biomarker collection in an urban community sample of ethnically diverse children (age 4 to 10 years).
Methods
Ninety-seven mother–child dyads were recruited for a cross-sectional follow up study of the Minding the Baby® home visiting intervention. Children were Hispanic (63%), Black (34%), and multi-racial (3.1%). A conventional content analysis was conducted using two sources of data: (1) mothers’ responses to open-ended interview questions on their views and suggestions regarding biomarker collection, and (2) field notes recorded by investigators.
Results
Forty-four percent of mothers reported biomarker-related questions or concerns, including questions about the purpose of biomarker testing, and concerns about cosmetic issues, child discomfort, and future use of biomarker data. Mothers also offered positive feedback and advice for collection. Issues affecting feasibility included children’s hair length and style, refusal to participate, and behavioral or developmental issues.
Conclusions
Hair and salivary biomarker collection was largely acceptable and feasible in this sample. Strategies for promoting ethical and sensitive biomarker collection include respectful explanations and parental involvement, creating a comfortable and safe environment for the child, flexible collection strategies, and attention to development, cultural preferences and perspectives.
Keywords: Child health, Mothers, Parent–child relations, Health disparities
Introduction
Pediatric researchers are increasingly using biological markers (biomarkers) to examine the complex ways in which biology, behavior, and the environment interact to influence child health. Development of noninvasive biomarkers for use in pediatric populations, including sampling hair and saliva, has led to critical advancements in scientific knowledge such as better understanding the link between early childhood adversity and lifelong health (Gunnar and Donzella 2002; Johnson et al. 2013; Shonkoff et al. 2012). As use of these markers continues to expand, it is critical to examine their acceptability and feasibility in children and people of color, who are largely underrepresented in biobehavioral research (Kuhlman et al. 2019). While less invasive than collecting blood serum, children and their guardians may still be wary of hair and saliva collection, as this is not part of routine clinical care. Biological samples, like hair, may also hold important cultural meaning for families (Pergament 1999; Wright et al. 2018). Further, families of color may not trust research due to a history of unethical practices and exploitation in many marginalized communities (Corbie-Smith et al. 2002). Thus, identifying threats to acceptability and strategies for enhancing feasibility is critical for promoting sensitive and ethical approaches to hair and salivary biomarker collection.
Hair Collection
In pediatric research, hair is often used to measure environmental exposures, such as secondhand smoke via cotinine levels, or chronic stress via cortisol concentrations (Al-Delaimy 2002; Bates et al. 2017; Stalder et al. 2017). While hair collection is generally considered a simple and painless procedure, in a recent feasibility study with mothers and toddlers from low-income backgrounds and identifying as Black and White, only 94 out of 142 eligible families (66.2%) consented to hair collection (Bates et al. 2019). Of those who refused to participate, 43.5% reported concerns about disrupting hair appearance as their primary reason for refusal. The authors also noted that some data collectors “may not have felt comfortable approaching mothers for hair collection” (p.2), which may have affected participation. In a feasibility study of hair cortisol collection among adolescents of Non-Hispanic Black/African-American and White ancestry, 471 out of 516 eligible youth (91.3%) consented to participate (Ford et al. 2016). Among those who provided a reason for refusal, responses included “concerns over privacy or collection of biomaterial” (n = 6) and “did not want to have hair cut due to extensions or braids” (n = 3). Youth of Black/African-American ancestry were also less likely to participate due to insufficient hair or refusal when compared to their White peers.
Saliva Collection
Saliva is often collected as a less invasive alternative to blood serum to measure biomarkers of stress and inflammation, including cortisol and pro-inflammatory cytokines (Granger et al. 2007). In a recent review of salivary cortisol collection in children (N = 31 studies), six psychosocial influences on acceptability and feasibility were identified: (1) uncertainty and misconceptions, (2) cultural and ethnic values, (3) family rules and values, (4) difficulty following protocols and procedures, (5) burden of multiple samples, and (6) child refusal or resistance (Condon 2016). In a recent qualitative study of biobehavioral research acceptability among Hispanic adolescents, authors identified the following themes: (1) protection of human subjects is important to participants, (2) comfort with providing different types of biological data varies depending on different factors, (3) engagement in biobehavioral research should be grounded in a cultural lens, (4) providing bilingual research staff is essential, and (5) adolescents have various motivations for participating (Johnson et al. 2018). Given these wide-ranging influences on acceptability and feasibility, it is important to further investigate preferences and perspectives that may be specific to defined groups, including young children and people of various races and ethnicities.
Study Purpose
Past research demonstrates that families may have unique perspectives and needs that should be carefully considered in biobehavioral research studies (Bates et al. 2019; Condon 2016; Ford et al. 2016; Johnson et al. 2018). However, to our knowledge, acceptability among parents of early school-age children has not been studied, and while researchers often describe reasons for participant refusal (Bates et al. 2019; Bryson et al. 2019; Ford et al. 2016), questions or concerns among consenting individuals are often not addressed. Manuscripts focused on biomarker methodologies also rarely address feasibility related to specific age, socioeconomic, or cultural groups (Greff et al. 2018; Kudielka et al. 2012). In the current study, we aimed to address these gaps by examining acceptability and feasibility of hair and salivary biomarker collection among ethnically diverse children (age 4–10 years) who, along with their mothers, participated in a follow up study of Minding the Baby® (MTB), a home visiting program for urban, low-income, first time mothers (Sadler et al. 2013; Slade et al. 2019).
Methods
Participants were mother–child dyads who enrolled in the cross-sectional Early School Age follow-up study of the MTB randomized controlled trial (RCT). In the MTB RCT, mothers participated from the second trimester of pregnancy until the child was two years of age (Sadler et al. 2013; Slade et al. 2019). In the MTB Early School Age Follow-up study, intervention and control group mother–child dyads with children ages four to ten years were recruited. Inclusion criteria included residence within the state of Connecticut and maternal custody or regular contact with the child (see Londono Tobon et al., under review for complete study methods). We obtained Yale University Institutional Review Board approval prior to data collection. Mothers provided informed consent and children seven years and older provided assent.
Data Collection
We collected data at private locations, such as participants’ homes, the Yale School of Nursing Center for Biobehavioral Research, and private spaces within community libraries. Hair and saliva were collected from child participants as part of the follow up study protocol. To assess hair cortisol, we collected three centimeters of hair from the posterior vertex of the child’s scalp. To sample the hair, we used a blunt cut at the scalp to collect a bundle of approximately 20–30 strands. Holding the hair at the scalp end, we then cut the bundle to be three centimeters in length and weighed the bundle on a small digital scale. If necessary, we repeated this process until a minimum of 20 mg of hair was collected. Saliva samples were analyzed for c-reactive protein and a panel of inflammatory cytokines. For each child, we attempted to collect saliva using passive drool, as this is the preferred for analysis of salivary cytokines (Salimetrics® 2020). For children who were unable to provide saliva samples using the passive drool method, cotton swabs were offered as an alternative. One saliva sample was collected from each participant. Additional information about biomarker collection and storage protocols is described elsewhere (Condon et al. 2018).
At the time of biomarker collection, mothers were asked two open ended questions: (1) “What questions do you have about our collection of hair or saliva for this study?” And (2) “What advice do you have for myself or other researchers who are collecting hair and saliva samples in children? What can we do to improve the process?” The investigators recorded verbatim responses by hand during the research visit, and then later typed and stored responses on an electronic secure server. After the completion of each research visit, the investigators electronically recorded field notes, including any descriptive information related to hair and saliva collection. All participant responses and field notes were deidentified, except for the child’s age, gender, and race/ethnicity, and were imported into a Microsoft Excel file for data management and coding.
Data Analysis
We used univariate statistics to describe sample demographics. We used Chi-Square and Fisher’s exact test to compare whether hair or saliva collection differed between former intervention and control group families or between participants identifying as Black or Hispanic.
We used a conventional content analysis approach for analysis of open-ended questions and field notes (Hsieh and Shannon 2005). To assess acceptability of hair and salivary biomarker collection, we conducted a content analysis of mothers’ responses to the open-ended questions. To assess feasibility of our approach, we conducted a content analysis of investigators’ field notes. Information from these data sources was coded into 17 general codes and then sorted into categories. We made note of the child’s age, gender, and race/ethnicity for each relevant quote or field note to aid analysis and interpretation. Categories of coded notes and questions were reviewed and discussed by three investigators until consensus was reached. To enhance reliability, the first author kept a detailed audit trail of decisions and findings. Internal validity was addressed by reviewing the findings and implications with interdisciplinary members of the MTB team.
Results
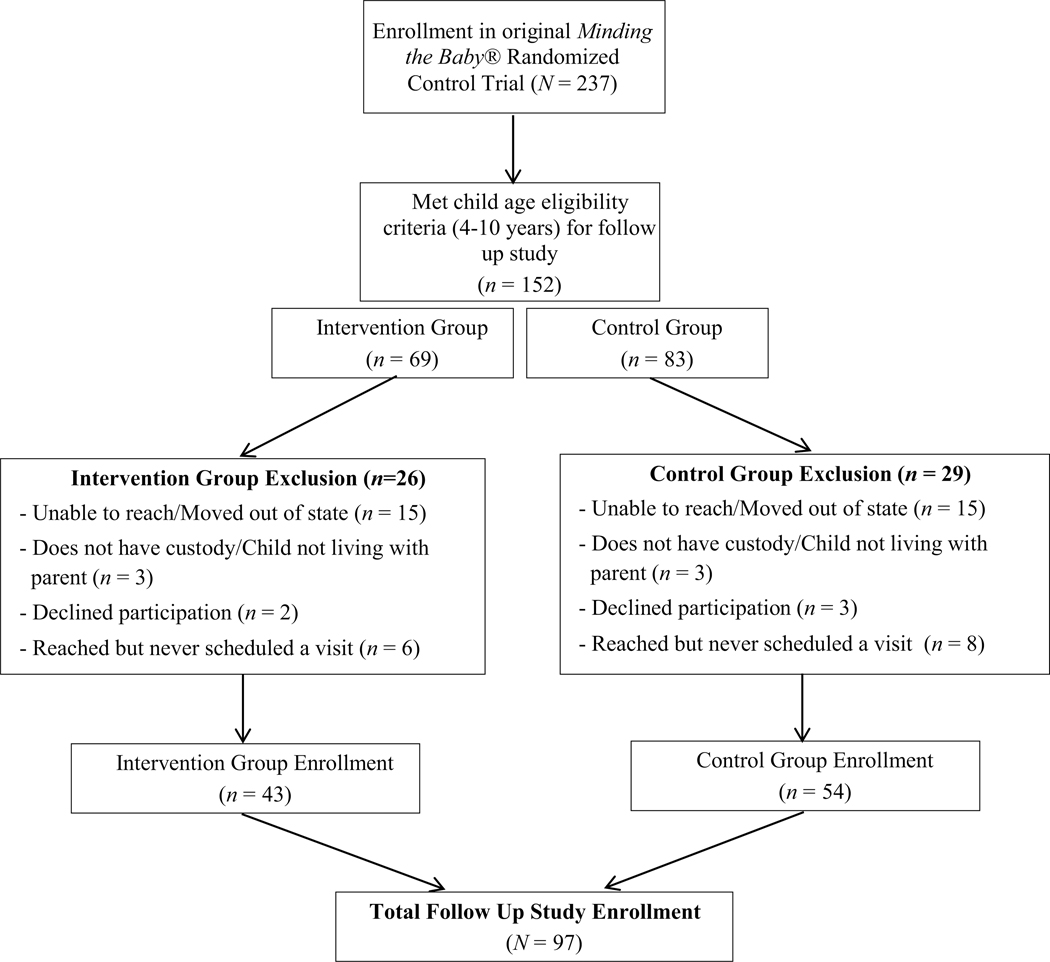
Ninety-seven mother–child dyads participated in the MTB Early School Age (4 to 10 years) follow up study. Families self-identified as Hispanic (62% mothers, 63% children), non-Hispanic Black (33% mothers, 34% children), and multi-racial (5.2% mothers, 3.1% children). Puerto Rican and Dominican were the most commonly reported Hispanic ethnic backgrounds. Forty-eight percent of children were girls and over 90% of mothers reported receiving some form of public assistance (see Appendices A and B for enrollment and sample descriptions).
We received valid laboratory results for 83% of hair samples and 94% of saliva samples (Table 1). The most common reason for missing hair values was short hair length, and for saliva values was insufficient saliva quantity per the laboratory report. Saliva collection did not significantly differ between children of Black and Hispanic ancestry, but differences in hair collection were noted. We successfully collected hair samples and received valid laboratory results for 90% of children of Hispanic ancestry but only 69% of children of Black ancestry (p = 0.01). This discrepancy was largely driven by short hairstyles in boys of Black/African ancestry, which prohibited hair collection.
Table 1.
Hair and saliva collection methods and reasons for missing data
| Total (N = 97) | Intervention (n = 43) | Control (n = 54) | P value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Hair collected and valid laboratory result received | ||||
| Yes | 80 (83) | 35 (81) | 45 (83) | 0.49a |
| No | 17 (18) | 8 (19) | 9 (17) | |
| Reasons for missing hair valuec | ||||
| Hair too short | 9 (53) | 3 (38) | 6 (67) | |
| Mother refused | 2 (12) | 1 (13) | 1 (11) | |
| Child refused | 2 (12) | 0 (0) | 2 (22) | |
| Unknown | 4 (24) | 4 (50) | 0 (0) | |
| Saliva collected and valid laboratory result received | ||||
| Yes | 91 (94) | 39 (91) | 52 (96) | 0.45b |
| No | 6 (6.2) | 4 (9.3) | 2 (3.7) | |
| Reasons for missing saliva valuec | ||||
| Mother refused | 1 (17) | 0 | 1 (50) | |
| Child refused | 1 (17) | 1 (25) | 0 | |
| Quantity not sufficient | 2 (33) | 1 (25) | 1 (50) | |
| Unknown | 2 (33) | 2 (50) | 0 | |
| Saliva collection method | ||||
| Passive drool | 80 (88) | 34 (87) | 46 (89) | 0.99b |
| Cotton swab | 11 (12) | 5 (13) | 6 (12) | |
Chi-square test
Fisher’s exact test
Group differences not tested due to small cell sizes
Acceptability
The majority (56%) of all mothers enrolled reported no questions or concerns in response to the open-ended questions (Table 2). Of those who did, mothers most commonly (n = 15) requested clarification about the biomarker testing. For example, many asked to review what would be measured in the hair and saliva, and whether they would receive results after analysis. Mothers (n = 11) also reported concerns about the cosmetic effects of hair collection, particularly mothers of girls with hair styled in braids or ponytails. Five mothers reported concerns about whether the procedures would be uncomfortable for the child. This included one mother who refused to allow hair and saliva collection from her child due to concerns about invasiveness, but did consent to participate in the other aspects of the study (boy, Black, age 9). Others (n = 3) expressed concern about what would be done with the biological samples or test results after study completion. One mother reported that she was personally comfortable with biomarker collection, but that the child’s grandmother was concerned that the findings might be reported to Child Protective Services (boy, Hispanic, age 6). Another reported concern that someone might “do bad things” or “curse” the hair, citing religious practices as the reason for her objection; this mother consented to saliva collection only (boy, Hispanic, age 4).
Table 2.
Summary of mothers’ responses to open-ended questions regarding acceptability of hair/saliva collection
| Category | Responses N (%) | Exemplary quotes/notes |
|---|---|---|
| No questions or concerns | 55 (56) | 28 (51.9%) control mothers and 27 (62.7%) intervention mothers reported no questions or concerns about hair or saliva biomarkers |
| Clarification regarding biomarker testing | 15 (15.5) | Mother asked for clarification regarding what would be measured with the biomarker tests |
| “What are the tests for? Will I get results?” | ||
| Mother wanted to know if she will be informed if stress levels are very high | ||
| Mother wondered about results and whether she will be contacted | ||
| Cosmetic concerns | 11 (11.3) | Mother concerned about braiding removal |
| Mother worried about how much hair will be cut; agreed to allow small amounts to be cut at a time and measured on a scale so that no extra hair taken | ||
| Mother concerned about taking hair out of ponytail because hair thick/curly | ||
| Positive comments | 9 (9.3) | “It’s something cool to learn about, it’s interesting” |
| Mother did saliva sampling herself as a child for a different study so she said she knows what’s involved and is not worried | ||
| “I don’t find it horrible, it’s just a few strands” | ||
| Mother has participated in past research studies with her son because she knows it can help other people | ||
| Advice for hair/saliva collection | 8 (8.2) | “Make sure you say it’s not going to hurt” |
| Mother asked us to be slow, soft, and gentle with hair collection because daughter has curly hair and sensitive scalp | ||
| Mother suggested cutting hair when her son is distracted because he may get upset | ||
| “He might want mom to help” | ||
| Concerns about child discomfort | 5 (5.1) | Mother concerned that cutting hair would hurt her child because her daughter’s scalp is sensitive |
| Mother concerned about hair being pulled and that it may be painful or uncomfortable for the child | ||
| Mother refused to participate in hair and saliva collection because she felt it was too invasive and did not think her husband would feel comfortable | ||
| Concerns about use of data | 3 (3.1) | Mother stated that child’s grandmother was concerned about type of testing that would be done and whether information would be provided to Child Protective Services |
| Mother stated she wants to make sure we’re not using it for anything else | ||
| Mother concerned that people might do bad things with hair and curse it |
Some mothers’ responses were coded under more than one theme; percentages represent number of responses that endorsed a specific theme, not individual participants
Mothers (n = 9) also offered positive feedback in response to the open ended questions. One mother reported that she was willing to participate because “she knows it can help other people” (boy, Hispanic, age 8). Others (n = 8) provided advice for hair and saliva collection, with many emphasizing the importance of making the child comfortable, being gentle, and providing reassurance (Table 2).
Feasibility
Hair Collection
Issues related to hair collection included hairstyle, hair length/texture, and child refusal (Table 3). Many children, particularly girls, had braided or other hairstyles that created challenges for obtaining a sample from the posterior vertex of the scalp. While some mothers gave permission to remove the braids or ponytails, others refused, especially if the hair was professionally styled, which is often expensive. For those participants, we collected stray hairs that had come loose; sometimes it was possible to obtain these strands from the posterior vertex scalp area, but other times the nape of the neck or other areas were used. This technique was acceptable to mothers, and also allowed us to collect enough for laboratory analyses. Another common issue with hair collection was short hair length or light texture/thin hair strands, which often made it difficult to obtain an adequate quantity for analysis. To address this, we started by cutting a small sample of hair and weighing it on a digital scale; if necessary, additional samples were cut until an adequate weight was reached. Two children (boys, ages 4 and 6 years) were distressed by the hair collection procedures and actively refused to participate, even after attempts to calm or distract the child; this was honored, and no hair collection was done. A third child (girl, Black, age 6) initially refused to participate, but was willing after her mother volunteered to have her hair cut first.
Table 3.
Hair collection issues and problem solving strategies
| Category | Exemplary field notes |
|---|---|
| Hair style (e.g. braids, ponytails) | Child’s hair in two braids, was able to take hair from scalp from pieces hanging out without removing braids. |
| Hair in braids, mother undid one of the braids in the back of the head for hair sample. | |
| Child’s hair in multiple ponytails, mother would prefer not to take them out. Collected hair from stray strands in between ponytails. | |
| Mother did not want to remove braids in posterior vertex area of scalp because she doesn’t know how to put them back in, asked to cut hair towards the nape of neck. | |
| Hair length/texture | Hair collected from scalp area slightly above posterior vertex due to longer hair length. |
| Mother states she avoided getting a haircut for child this week in preparation for sample collection, but hair was still very short; collected small amounts from 4 spots on head. | |
| Hair was very light; collected small amounts from 5–6 different locations until adequate weight reached on digital scale. | |
| Child refusal | Child very upset by the idea of someone cutting his hair; child continued to refuse after taking a break, so hair not collected. |
| Child was hesitant about hair collection until mother offered to have her hair collected first. | |
| Mother reported that child gets very upset during haircuts and requested that hair be cut while he was distracted; attempted collection while child was sitting on mother’s lap and looking at phone, but stopped when child became upset. |
Saliva Collection
Although parents were counseled to avoid feeding the child immediately prior to the research visit, a number of children were noted to be eating or drinking upon arrival. In these cases, saliva collection was moved to the end of the one-hour visit. For all children, mouths were rinsed thoroughly with water five to ten minutes prior to saliva collection. While most children were ultimately able to provide a saliva sample, eleven children were unsuccessful with the passive drool method and cotton swabs were used. These children tended to be younger (4 to 5 years), and while they appeared to understand the instructions, were developmentally unable to provide passive drool; rather, they were blowing into the collection tube or mimicking mouth movements only. A few children were unable to provide passive drool due to behavioral issues. These children, all four-year-old boys, were uncooperative even with the assistance of a parent (Table 4).
Table 4.
Saliva collection issues and problem solving strategies
| Issues | Exemplary field notes |
|---|---|
| Food/drink | Saliva red-tinged on first collection, and child reported eating red candy approximately 1 hour earlier. Sample discarded and child instructed to rinse mouth out again multiple times. Saliva still slightly pink-tinged, but improved, so sample used. |
| Child eating hamburger and drinking soda upon arrival, so saliva collection delayed until the end of the visit. | |
| Developmental issues | Child was cooperative but unable to produce any passive drool during attempts, kept mimicking noises and mouth movements only. Collected saliva by swab. |
| Child unable to provide saliva using passive drool method. After demo and multiple instructions, kept “blowing” into adapter with no saliva. Cotton swab was successful. | |
| Behavioral issues | Child kept biting on the swab and laughing; mother retrieved swab from child’s mouth. |
| Attempted saliva collection after 20 min of play. Used demo device; child tried but spit very small amounts and lost interest after multiple attempts. | |
| Collection strategies | |
| Help from parents | Child had difficulty with passive drool; was successful after coaching from father. |
| Child reluctant to complete saliva collection despite multiple attempts; child kept refusing whenever asked to try by investigator. Mother coached her through it and she was successful. | |
| Child refused to provide sample for investigator; mother asked for a second cotton swab and demonstrated on herself, showing the child it was “like a lollipop.” Child kept swab in his mouth while mother had hers in as well. He then allowed mom to hold his swab and move it around his mouth until enough saliva was collected. | |
| Demonstration and other strategies | Used demo to walk child through saliva collection. Child preferred to spit while I wasn’t looking. |
| Child had difficulty with passive drool despite multiple attempts and instructions by both researcher and mother. Had child drool into a clean cup and poured saliva into appropriate tube. | |
Strategies to aid saliva collection were also identified (Table 4). Assistance from parents was particularly effective, including when the parent provided instruction to the child or demonstrated the process by providing a sample herself. Demonstration by the investigators was also effective, and “demo” collection devices were brought to all research visits so that visual aids would be available when necessary. Other strategies included allowing privacy to provide the saliva sample, as some children, especially girls, were uncomfortable “spitting” in front of others.
Discussion
Results of this study demonstrate that hair and salivary biomarker collection is largely acceptable and feasible in an ethnically diverse urban community sample of mothers and early school age children. Findings of this study also reveal important considerations for pediatric researchers to ensure accurate, sensitive, and ethical approaches to biomarker collection, including educating and reassuring parents about collection procedures, creating a comfortable and safe environment for the child, and actively involving parents in the collection process. Participants’ cultural, religious, and social backgrounds should also be carefully considered prior to biomarker collection.
Communicating with Parents
While the majority of mothers in our study did not report any questions or concerns regarding biomarker collection, almost half did have follow up questions, even after providing informed consent. Thus, it is important for researchers to reiterate the reasons for collecting the samples, and clarify whether there are any remaining concerns about the procedures or use of data. If sufficient concerns are identified, families should be reminded that they have the opportunity to withdraw their consent at any time. It is also important to counsel parents on whether individualized results of biomarker testing will be received; this may be particularly challenging for biological markers without established pediatric reference ranges or meaningful clinical interpretations (Condon 2018). In these cases, it may be helpful to explain to parents that while individualized results will not be provided, the data will be used to help understand the phenomena of interest and may benefit others in the future (Kuhlman et al. 2019). Providing a summary of study findings to all participants is an additional ethical and feasible approach to communicating research results (Shalowitz and Miller 2005).
Effective communication with parents, so that they are able to explain the procedures to the child, may also help improve feasibility of hair and saliva collection. Especially for younger children, having a parent explain or demonstrate the process greatly improved children’s cooperation and successful collection. Anticipatory guidance, such as visually demonstrating how much hair would be cut from the scalp, was also helpful in many cases. Early, clear communication with parents may also aid adherence to protocol standards, such as not eating or scheduling a haircut or dental appointment immediately prior to the research visit.
Creating a Safe and Comfortable Environment
Biomarker collection procedures should allow children time to warm up, practice collection-related tasks as necessary, and take breaks as needed. The developmental age of the child should also be carefully considered prior to biomarker collection. In our study, we found that preschool-age children often had difficulty providing passive drool samples, but that children over age five were largely successful with this technique. Providing demonstrations, either by the investigator or parent, also proved to aid the collection process. Other strategies to aid collection may include developmentally appropriate games or pretend play, such as “playing hair stylist” for hair collection. We found use of age-appropriate language to also be helpful; for example, children were more responsive to the description “water in the mouth” than the terms “saliva” or “drool.” Some children were also embarrassed to provide saliva samples, or felt that “spitting” would get them in trouble, but these barriers were easily resolved with counseling.
Need for Flexibility
Creative strategies were often necessary to successfully collect hair and saliva samples. This included collection of stray hair strands adjacent to ponytails and braids, and use of cotton swabs as an alternative to passive drool when necessary for developmental or behavioral reasons. When alternative collection strategies were used, we conducted sensitivity analyses with the biomarker laboratory results. However, it should be noted that the posterior vertex of the scalp is thought to be the most reliable area for hair cortisol collection (Sauvé et al. 2007), and thus our results may still have been affected in undetected ways. Further, while passive drool is assumed to contain a mix of saliva from all salivary glands, cotton swabs may only collect saliva from one area of the mouth, depending on swab placement. Our combined use of cotton swabs and passive drool, while feasible, may have also affected our analysis in ways undetected by our sensitivity tests (Granger et al. 2007). To enhance reliability and guide sensitivity analyses in future studies, researchers should develop a system for documenting any deviations from the study protocol, especially in studies with multiple data collectors. Anticipatory guidance regarding potential challenges and alternative strategies may also improve biomarker collection efforts, and training videos may also help support sensitive and accurate collection methods (for an excellent example, see (Wright et al. 2018). Careful consideration of the study population prior to selecting biomarkers, including the developmental stages of participants, may help also avoid variations in collection methods.
Cultural Considerations
Cultural traditions and religious practices may influence acceptability of biomarker collection (Kuhlman et al. 2019). Community engaged research methods, including consultation with community members, may be necessary to identify and address concerns prior to data collection (O’Campo et al. 2016). Hair, in particular, may have specific cultural meanings or be viewed as very personal by study participants (Pergament 1999). Consulting with community members about how to treat biospecimens respectfully, including acceptable procedures for storing or discarding samples, may improve acceptability (Wright et al. 2018). Other strategies, such as offering any excess hair collected as a keepsake for participants, may also be appropriate, especially if it’s the child’s first haircut.
Cultural practices may also influence the feasibility of biomarker collection. In our study, hair collection was less successful in Black children than Hispanic children, but this was not due to differences in parental refusal. Rather, boys of Black/African ancestry in our sample often had closely shaved hairstyles, which prohibited hair collection due to short hair length. This is similar to the findings of a hair collection feasibility study conducted by Ford et al. (2016), in which adolescents identifying as Black were less likely to participate than adolescents identifying as White due to insufficient hair length. When selecting biomarkers for a research study, cultural considerations should include factors like aesthetic preferences (e.g. preferred hairstyles), as well as cultural values that may affect feasibility (e.g. a child’s hair is cut only when they reach a specific age). Specific strategies, such as asking caregivers to postpone a child’s haircut until after data collection, or scheduling a research visit immediately before a scheduled haircut to obtain maximal hair length, may also be considered in order to avoid excluding specific groups from participation in biomarker studies.
Strengths and Limitations
Hair and salivary biomarker collection and analysis were largely successful in this study. While participation did not differ between former MTB intervention and control group families, this may have been influenced by previous long-term engagement with our research team by both groups. Results of this study may not be generalizable to other study designs, ages, geographic areas, or ethnic/cultural groups. For example, most Hispanic families in our study reported their ethnic background to be Puerto Rican or Dominican. Individuals of Puerto Rican and Dominican descent may have different cultural practices when compared to other Hispanic groups. These may affect hair practices and/or cultural beliefs about hair that ultimately influence research study participation. Given the heterogeneity of the Hispanic community and within individuals of Puerto Rican and Dominican ethnicity, nuanced research with other ethnic groups and within ethnic groups is needed to better understand cultural preferences and perspectives. Our findings may also not be generalizable to other biomarker collection methods, such as salivary cortisol, which requires collection of multiple samples over one or more days. However, despite these limitations, the findings of this study highlight the importance of communicating with participants about biomarker collection, even after informed consent is obtained. Additional studies with children and families who are underrepresented in research is necessary to fully understand the breadth of ethical issues and diverse cultural traditions that should be considered in pediatric biobehavioral research studies.
Conclusion
Past biobehavioral research has primarily been conducted with non-Hispanic White individuals with above-average socioeconomic status (Kuhlman et al. 2019). Thus, efforts to conduct research with diverse samples is critical, particularly among under-represented groups who are also often at highest risk for health inequities (Kuhlman et al. 2019). Documenting issues surrounding acceptability and feasibility of biomarker collection with ethnically, economically, and developmentally diverse groups is necessary to develop sensitive research methods, promote trust in biobehavioral research, and avoid repeating mistakes of prior research studies. Future research studies will greatly benefit from thoughtful protocol development and attention to potential ethical, developmental, and cultural issues.
Acknowledgements
We thank Andrea Miller and Priscilla Qinglan Ding for their assistance with recruitment and data collection, Monica Ordway for her comments on this manuscript, and the Yale School of Nursing Center for Biobehavioral Research for providing the resources necessary to conduct this research. We also gratefully acknowledge the families who participated in this study for contributing their time and expertise. This work was supported by the National Institute of Nursing Research (F31NR016385 and K99NR018876) and the National Institute of Mental Health (NIMH T32 MH018268) of the National Institutes of Health, the NAPNAP Foundation, the Connecticut Nurses Foundation, the Jonas Nurse Leaders Scholars Program, the Alpha Nu chapter of Sigma Theta Tau International, and the American Academy of Child and Adolescent Pilot Research Award.
Appendix A
Enrollment of participants from Minding the Baby® RCT to early school age follow up study (originally reported in Londono Tobon et al., under review).
Appendix B
Demographic characteristics of Minding the Baby® participants at early school age follow-up.
| Characteristic | Intervention group (n = 564S) | Control group (n = 54) | p value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age mother (years)a | 27 (2.9) | 26 (3.3) | 0.60 |
| Age child (years) | 6.6 (1.7) | 6.7 (2.1) | 0.82 |
| Time to follow-up (years) | 4.6 (1.7) | 4.7 (2.1) | 0.82 |
| Maternal education (years)c | 13 (1.7) | 13 (1.6) | 0.61 |
| N (%) | N (%) | ||
| Race/Ethnicity (Mother) | |||
| Black/African American | 7 (16) | 25 (46) | |
| Hispanic | 33 (77) | 27 (50) | < 0.01** |
| Other | 3 (7.0) | 2 (3.7) | |
| Race/Ethnicity (Child) | |||
| Black/African American | 8 (19) | 25 (46) | 0.02* |
| Hispanic | 33(77) | 28 (52) | |
| Other | 2 (4.7) | 1 (1.9) | |
| Female sex (child) | 22 (51) | 25 (46) | 0.63 |
| Socioeconomic statusb | |||
| Receiving public assistance | 39 (91) | 49 (91) | 0.99 |
| Marital status | |||
| Single/separated/divorced | 28 (65) | 37 (68) | |
| Married/living together | 15 (35) | 17 (32) | 0.72 |
Originally reported in Londono Tobon et al. (under review)
p value < 0.05,
p value < 0.01,
p value < 0.001
Independent samples t-test was used for analysis of this variable. Mann–Whitney U was used for all other continuous demographic variables
Fisher’s exact test was used for analysis of this variable. Pearson’s Chi-square was used for all others
Different distribution patterns between intervention and control groups
References
- Al-Delaimy W. (2002). Hair as a biomarker for exposure to tobacco smoke. Tobacco Control, 11(3), 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates RA, Salsberry PJ, & Dynia JM (2019). Feasibility of sampling hair for cortisol analysis in high-risk mothers and their toddlers. Paper presented at the Sigma Theta Tau Leadership Connection, Indianapolis, Indiana. [Google Scholar]
- Bates R, Salsberry P, & Ford J. (2017). Measuring stress in young children using hair cortisol: The state of the science. Biological Research for Nursing, 19(5), 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson MHE, Mensah F, Goldfeld S, & Price AM (2019). Using hair cortisol to examine the role of stress in children’s health inequalities at 3 years. Academic Pediatrics, 20, 193–202. [DOI] [PubMed] [Google Scholar]
- Condon EM (2016). Psychosocial influences on acceptability and feasibility of salivary cortisol collection from community samples of children. Research in Nursing & Health, 39, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon EM (2018). Chronic stress in children and adolescents: A review of biomarkers for use in pediatric research. Biological Research for Nursing, 20, 473–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon EM, Sadler LS, & Mayes L. (2018). Toxic stress and protective factors in multiethnic school age children: A research protocol. Research in Nursing & Health, 41, 97–106. 10.1002/nur.21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, & George DMMS (2002). Distrust, race, and research. Archives of Internal Medicine, 162(21), 2458–2463. [DOI] [PubMed] [Google Scholar]
- Ford JL, Boch SJ, & McCarthy D. (2016). Feasibility of hair collection for cortisol measurement in population research on adolescent health. Nursing Research, 65(3), 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. (2007). Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology & Behavior, 92(4), 583–590. [DOI] [PubMed] [Google Scholar]
- Greff MJ, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, & van Uum SH (2018). Hair cortisol analysis: An update on methodological considerations and clinical applications. Clinical Biochemistry, 63, 1–9. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B. (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Salas-Wright CP, Córdova D, Ugalde J, Todic J, & Mendoza Lua F. (2018). The acceptability of biobehavioral research with Latino youth in the United States. Journal of Adolescent Research, 34(5), 597–618. [Google Scholar]
- Johnson SB, Riley AW, Granger DA, & Riis J. (2013). The science of early life toxic stress for pediatric practice and advocacy. Pediatrics, 131(2), 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Gierens A, Hellhammer DH, Wüst S, & Schlotz W. (2012). Salivary cortisol in ambulatory assessment—some dos, some don’ts, and some open questions. Psychosomatic Medicine, 74(4), 418–431. [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Urizar GG Jr., Robles TF, Yim IS, & Schetter CD (2019). Testing plausible biopsychosocial models in diverse community samples: Common pitfalls and strategies. Psychoneuroendocrinology, 107, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono Tobon AL, Condon EM, Holland ML, Sadler LS, Mayes LC, & Slade A. School age effects of minding the baby—an attachment-based home visiting intervention—on parenting and child behaviors (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Campo P, BeLue R, Borenstein H, Reed-Vance M, Lanzi RG, Schafer P, et al. (2016). Issues and solutions for collecting biological specimen in longitudinal studies: Experience from the Community Child Health Network Research Network. Journal of Health Care for the Poor and Underserved, 27(1), 339–351. [DOI] [PubMed] [Google Scholar]
- Pergament D. (1999). It’s not just hair: Historical and cultural considerations for an emerging technology. Chi.-Kent L. Rev, 75, 41. [Google Scholar]
- Sadler LS, Slade A, Close N, Webb DL, Simpson T, Fennie K, & Mayes LC (2013). Minding the baby: Enhancing reflectiveness to improve early health and relationship outcomes in an interdisciplinary home-visiting program. Infant Mental Health Journal, 34(5), 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimetrics LLC (2020). Cytokine Saliva Collection. Salimetrics. Retrieved February 26, 2020, from https://salimetrics.com/salivary-panel/cytokine-panel/cytokine-saliva-collection/. [Google Scholar]
- Sauvé B, Koren G, Walsh G, Tokmakejian S, & Van Uum SH (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and Investigative Medicine, 30, E183–E191. [DOI] [PubMed] [Google Scholar]
- Shalowitz DI, & Miller FG (2005). Disclosing individual results of clinical research: Implications of respect for participants. JAMA, 294(6), 737–740. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–e246. [DOI] [PubMed] [Google Scholar]
- Slade A, Holland ML, Ordway MR, Carlson EA, Jeon S, Close N, et al. (2019). Minding the Baby®: Enhancing parental reflective functioning and infant attachment in an attachment-based, interdisciplinary home visiting program. Development and Psychopathology, 1–15. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. [DOI] [PubMed] [Google Scholar]
- Wright KD, Ford JL, Perazzo J, Jones LM, Mahari S, Sullenbarger BA, et al. (2018). Collecting hair samples for hair cortisol analysis in African Americans. JoVE (Journal of Visualized Experiments), 136, e57288. [DOI] [PMC free article] [PubMed] [Google Scholar]