Abstract
Background
Brix refractometry can be used to assess colostral immunoglobulin G (IgG) concentration, but studies identifying Brix percentages to detect high‐ and low‐IgG colostrum are lacking for beef cows and interlaboratory agreement is unknown.
Objectives
Evaluate Brix refractometer performance and interlaboratory agreement for assessing beef cow colostrum IgG concentration, including determination of thresholds to identify colostrum containing IgG concentrations <100 g/L and ≥150 g/L.
Animals
Beef cows (n = 416) from 11 cow‐calf operations in Alberta, Canada.
Methods
Colostral IgG concentrations were measured using radial immunodiffusion (RID) and estimated by Brix refractometry for this retrospective study. Spearman correlation coefficients were assessed between RID and Brix refractometry. Likelihood ratios and misclassification cost‐term analysis were used to determine optimal Brix percentages for detecting colostrum containing IgG concentrations <100 g/L and ≥150 g/L. Concordance correlation coefficient (CCC) and Bland‐Altman analyses were performed for Brix percentages obtained at 3 different laboratories.
Results
Brix percentages obtained at 3 laboratories were positively correlated with IgG results (r = 0.72, 0.68, and 0.76, respectively). Colostrum Brix percentages of <24% and ≥30% were optimal for indicating IgG concentrations of <100 g/L and ≥150 g/L, respectively. Interlaboratory agreement was substantial, with CCC ranging from 0.89 to 0.96 and Bland‐Altman analysis showing small mean differences (−1.2% to 0.09% Brix) and narrow limits of agreements (−4.8% to 2.4% Brix) among laboratories.
Conclusions and Clinical Importance
Brix refractometry shows good potential for reliably estimating IgG concentrations in beef cow colostrum across multiple laboratories and can be recommended to aid colostrum management decisions on farms.
Keywords: beef, Brix refractometer, colostrum quality, immunoglobulin G, transfer of passive immunity
Abbreviations
- AUC
area under the curve
- CCC
concordance correlation coefficient
- IgG
immunoglobulin G
- LHR
likelihood ratio
- MCT
misclassification cost‐term
- RID
radial immunodiffusion
- ROC
receiver operating characteristic
- SCCL
Saskatoon Colostrum Company Ltd
- TPI
transfer of passive immunity
1. INTRODUCTION
Calves are born agammaglobulinemic because of the epitheliochorial structure of the bovine placenta and consequently depend on transfer of passive immunity (TPI) through immunoglobulins in colostrum shortly after birth. 1 Inadequate TPI in beef calves, defined as serum immunoglobulin G (IgG) concentrations <24 g/L, 2 , 3 is an issue of particular importance because such calves are at higher risk for morbidity and mortality. 2 , 3 Approximately 33% of beef calves in western Canada experience inadequate TPI, 3 highlighting the need for improved colostrum management for beef calves.
Assessment of colostrum IgG concentration on the farm has the potential to improve TPI by guiding prompt decision‐making regarding colostrum intervention. Radial immunodiffusion (RID), 4 the current reference test for IgG quantification, requires expensive reagents, skilled laboratory technicians, and has a turnaround time of >24 hours, making it impractical for clinical use. Alternative methods of estimating colostral IgG concentration have been studied. 5 , 6 , 7 , 8 , 9 , 10 The Brix refractometer is practical and inexpensive. 11 It determines the total solids content in nonsucrose‐containing liquids such as colostrum, and subsequently IgG concentration can be estimated. 5 , 12 It has been tested extensively for use in dairy cow colostrum, 13 but performance evaluation for determination of beef cow colostrum IgG concentration is limited to a single study. 14
Brix percentages, and thus corresponding thresholds for IgG assessment, may vary as a result of within and between study variability, 13 which includes factors such as study population characteristics (eg, average colostrum IgG concentration) and study methodology (eg, sample handling and processing, statistical approach). Average IgG concentrations in beef cow colostrum are usually substantially higher than those of dairy cow colostrum. 15 Differences exist even among beef cow populations, with previous work conducted by our research group showing average colostral IgG concentrations of 143.3 7 and 154.7 g/L 16 compared with the averages of 60 17 and 95.9 g/L 14 reported in other recent studies. Investigation of Brix percentages that best predict colostral IgG concentration in various populations is necessary for appropriate on‐farm use. Herds with high overall colostral IgG concentration, such as can be found in western Canada, may benefit not only from a threshold to identify low‐IgG colostrum (<100 g/L) but also a threshold to identify high‐IgG colostrum (≥150 g/L). The traditional method of establishing a single cut point for diagnostic tests using only sensitivity (Se) and specificity (Sp) has many pitfalls, including loss of valuable information by dichotomizing the data 18 , 19 and the mistaken assumption that false‐negative and false‐positive results have the same cost. 20 Taking economic considerations into account is important for any diagnostic test but is especially crucial for tests that impact management decisions in production animal settings, such as colostrum intervention. To our knowledge, this approach has not been taken for Brix refractometry. Last, comparison of Brix performance among different laboratories using the same colostrum samples is needed to ensure the tool can be used reliably under different conditions.
Our objectives were to evaluate Brix refractometer performance as compared with RID testing and to evaluate interlaboratory agreement for assessing beef cow colostrum IgG concentrations, including determination of thresholds to identify colostrum containing IgG <100 and ≥150 g/L.
2. MATERIALS AND METHODS
2.1. Colostrum samples
Colostrum samples were collected during several studies, 16 , 21 , 22 , 23 all of which were approved by the University of Calgary Veterinary Sciences Animal Care Committee (AC13‐0324, AC15‐0150, AC16‐0209, AC18‐0204) and conducted in accordance with guidelines established by the Canadian Council on Animal Care.
A total of 416 beef cow colostrum samples were available for this retrospective study. Colostrum samples were collected during the spring calving seasons of 2013, 2014, 2015, 2016, 2017, and 2019. Samples originated from 11 cow‐calf operations located in Alberta, Canada that consisted of Angus, Simmental, Gelbvieh, Hereford, and crossbred cattle. Herd sizes ranged from 199 to 3742 calving dams. Although study designs varied among different years and studies, the colostrum sampling protocols were similar. Colostrum quality was not the focus of any of the studies, and none of the study protocols was expected to impact colostrum IgG concentration in any way. All colostrum samples were collected by farm or research personnel within 24 hours of parturition. Most samples were collected within 1 hour (n = 314/416) of parturition. Dams of different ages and parities were included. Producers were instructed to collect 20 mL of colostrum pooled from all teats into a clean plastic sampling cup. After collection, samples were refrigerated or frozen on farm before being transported to the laboratory at the University of Calgary, where they were maintained frozen at −20°C (2019) or −80°C (all other years) until analysis.
Data recorded for individual colostrum samples included dam factors (ie, parity, breed, calving date, calving ease score), farm, herd size, and year. Dam parity was categorized as heifers (ie, first parity), second parity cows, and mature cows (ie, third or higher parity). Breed was categorized as crossbred (ie, phenotype consistent with >1 breed), Angus, and other purebreds (ie, Hereford, Gelbvieh, and Simmental). Recorded calving dates ranged from January to May. A calving ease score was assigned to each cow‐calf pair at birth and was categorized as unassisted, easy assist, or difficult assist. An unassisted birth required no human intervention and the calf usually was born in a calving pasture or a maternity pen. An easy assisted birth was defined as 1 to 2 people extracting the calf manually. A difficult assisted birth required ≥2 people, a fetal extractor, or a Caesarian section to deliver the calf. Herd size was recorded for the respective year of sample collection based on electronic herd inventory data.
2.2. Laboratory analysis
Total colostral IgG concentration was measured by RID assay at the Saskatoon Colostrum Company Ltd Quality Assurance Laboratory (SCCL) upon completion of that year's sample collection (ie, the end of the calving season). The RID assay was performed as originally described, 24 with modifications as described previously. 25 Brix analysis was performed on subsets of colostrum samples at the SCCL (Lab A, n = 364), at the University of Calgary (Lab B; n = 271), and at the University of Prince Edward Island (Lab C, n = 220) to assess overall agreement with RID and interlaboratory agreement. Lab A was considered the reference laboratory throughout the study because Brix refractometer analysis was performed concurrently with RID analysis each year and Lab A evaluated the largest number of colostrum samples by Brix refractometry, whereas Labs B and C evaluated smaller subsets only. Personnel conducting the Brix analyses were blinded to RID results. Brix refractometry was performed at room temperature using 2 different digital Brix refractometers (Lab A: Misco Palm Abbe PA203, MISCO Refractometer, Solon, Ohio; Labs B and C: PAL‐1, Atago Co Ltd, Bellevue, Washington) with a detection range of 0% to 53%. Colostrum samples were thoroughly homogenized before testing according to the manufacturer's instructions. Immediately, 2 to 3 drops of colostrum then were used to fill the measuring disk and the Brix percentage (%) was recorded. The refractometer was calibrated using distilled water before use and washed with distilled water between samples.
2.3. Statistical analysis
Statistical analysis was performed using STATA 15.1 (StataCorp, College Station, Texas). A Shapiro‐Wilk test was performed on all continuous variables to assess normality of the data. Descriptive statistics were calculated and are presented as mean and SD or median and interquartile range for parametric and nonparametric distributions, respectively. Based on results reported previously by our research group, 7 , 20 colostrum samples were classified according to IgG concentration into low‐IgG colostrum (IgG < 100 g/L) and high‐IgG colostrum (IgG ≥ 150 g/L).
2.3.1. Correlation coefficients and areas under the receiver operating characteristic curve
Spearman correlation coefficients were calculated to assess the correlation between IgG concentration (g/L), as determined by RID and Brix percentage (%) for each individual laboratory. Receiver operating characteristic (ROC) curves were constructed for each of the 3 laboratories and each outcome in question (colostrum containing IgG <100 and ≥150 g/L). Areas under the curve (AUC) were examined to assess the overall accuracy of Brix refractometry to predict colostrum containing IgG <100 and ≥150 g/L across the range of potential Brix thresholds. An AUC of 0.7 to 0.9 traditionally has been viewed as moderately accurate, whereas an AUC of >0.9 has been considered highly accurate and an AUC of 1 would indicate a perfect test. 18
2.3.2. Thresholds
Analyses were conducted to determine thresholds for 2 clinically relevant applications: (1) detection of low‐IgG colostrum (<100 g/L), in which case veterinarians could advise producers to monitor sufficient intake closely or supplement with an alternative colostrum source, and (2) detection of high‐IgG colostrum (≥150 g/L IgG), in which case veterinarians could advise producers to have the calf consume maternal colostrum and to freeze any surplus colostrum for other calves needing supplementation in the future.
Interval likelihood ratios (LHRs) have an advantage over the traditional dichotomous ROC curve approach by using more of the information in a given dataset, therefore minimizing loss of information and distortion. 19 Interval LHRs were calculated for all 3 laboratories. A relatively narrow Brix interval range of 2% was chosen to investigate biologically relevant differences, while trying to avoid the lack of precision that can occur when too many intervals are created. 19 Interval LHRs were calculated for each interval range using the following modified equation (1) 18 :
| (1) |
where P (test result | target condition present) is the probability of a certain Brix percentage in colostrum given the IgG concentration is truly <100 g/L or ≥150 g/L, respectively, and P (test result | target condition absent) is the probability of certain a Brix percentage in colostrum given the IgG concentration is not <100 g/L or ≥150 g/L, respectively. Therefore, the LHR in a given interval range is the likelihood that colostrum with a Brix percentage in that range will truly contain IgG <100 g/L or ≥150 g/L, respectively. The confidence interval (CI) for LHR was calculated as described previously. 26 Whenever an interval range contained 0 samples, 1 unit was added to each category in that calculation. Likelihood ratios were interpreted as follows: LHRs of >1 were considered to support the classification of the sample as containing IgG <100 g/L or ≥150 g/L, respectively, whereas LHRs close to 0 were suggestive of the opposite outcome (≥100 or <150 g/L). 27 A LHR of 1 had no effect on the odds of a certain colostrum IgG concentration, and a LHR with CI including 1 was considered not statistically significant.
Misclassification cost‐term (MCT) analysis is a powerful tool to illustrate the optimal thresholds for different scenarios because it takes into account not only Se and Sp of a given test, but also the prevalence of the target condition in question. Additionally, the MCT can be plotted for different cost ratios of false‐negative to false‐positive results, making it possible to develop thresholds that take into consideration different costs associated with false test results. The MCT analysis was performed for different herd scenarios including low (10%), average (50%), and high (80%) prevalence of cows with colostrum IgG <100 g/L. This analysis was solely based on Brix percentages from Lab A, the reference laboratory. Identification of low‐IgG colostrum (IgG < 100 g/L) was considered most relevant for making immediate colostrum intervention decisions in cow‐calf operations. Therefore, the MCT was calculated for this application and for each specific cut point using the following equation (2) 28 :
| (2) |
where p is the prevalence of low‐IgG colostrum, Se is test sensitivity, Sp is test specificity, and r is the cost ratio of false‐negative to false‐positive results. Because the true cost for misclassification will vary depending on herd‐ and calf‐level factors and is currently unknown, the MCT was plotted against various Brix percentages for 3 different false‐negative‐to‐false‐positive cost ratios: 1 : 5, 1 : 1, and 5 : 1. The ratio 1 : 5 assumes the cost for a false‐positive is 5 times the cost of a false‐negative, 1 : 1 assumes the cost for a false‐negative and a false‐positive result are the same, and 5 : 1 assumes the cost of false‐negative is 5 times the cost of a false‐positive. These cost ratios were chosen to include somewhat extreme scenarios to evaluate the robustness of this model in various herd settings but also to include the most likely scenario in North America. A false‐negative test result would lead to consumption of low‐IgG colostrum by the calf, which could lead to failed TPI with an estimated cost of $90 to $147 USD per beef calf. 29 On the other hand, a false‐positive result would lead to the unnecessary expense of feeding a colostrum replacement product, estimated at $60 USD per beef calf, which is the price of commercially available colostrum product containing 200 g IgG, but does not consider the time and resources required to administer it. The exact cost for labor in cow‐calf settings varies substantially and is currently unknown. Overall, it was expected that the true cost ratio in North America would lie somewhere between 1 : 1 and 1 : 2. Not every calf fed colostrum falsely identified as being of sufficient IgG concentration will suffer from complete failed TPI, decreasing the cost of a false‐negative Brix result, and some producers may choose to supplement or replace >200 g total IgG in the event a good quality colostrum sample is falsely identified as poor, increasing the cost of a false‐positive Brix result.
Diagnostic test characteristics (Se, Sp, Youden index, and accuracy) were calculated for the thresholds established using MCT and LHR. Sensitivity was defined as the proportion of colostrum samples containing IgG <100 or ≥150 g/L, as determined by RID that were correctly predicted by Brix refractometry. Specificity was defined as the proportion of colostrum samples with IgG concentrations ≥100 or <150 g/L as determined by RID that were correctly predicted by Brix refractometry. The Youden index (J) was calculated by using the following equation (3):
| (3) |
Lastly, accuracy was defined as the percentage of colostrum samples correctly classified by Brix refractometry.
2.3.3. Interlaboratory agreement
Concordance correlation coefficients (CCCs) were calculated to quantify the agreement between paired test results obtained by Brix refractometry in the 3 different laboratories. A CCC of 1 would indicate perfect agreement. 30 The closer the reduced major axis is to the line of perfect concordance on the CCC plot, the better the agreement between 2 tests. To evaluate the limits of agreement further and to identify potential systematic errors, Bland‐Altman plots also were constructed.
The level of significance for all analyses was set at P < .05.
3. RESULTS
3.1. Descriptive statistics
The number of samples available from each herd ranged from 11 to 113. Colostrum sample characteristics are shown in Table 1. Descriptive statistics of colostrum IgG concentration and Brix percentages measured by RID and Brix refractometry, respectively, are presented in Table 2. The IgG concentrations as determined by RID followed a normal distribution, whereas the Brix percentages obtained in the 3 study laboratories were skewed to the right. A total of 38 colostrum samples (9.1%) contained IgG <100 g/L, whereas 207 samples (49.8%) contained IgG ≥150 g/L.
TABLE 1.
Characteristics of beef cow colostrum samples used for determination of immunoglobulin G concentrations by radial immunodiffusion assay and Brix refractometry
| Colostrum samples | |||
|---|---|---|---|
| n | % | ||
| Year (n = 416) | 2013 | 36 | 8.7 |
| 2014 | 77 | 18.5 | |
| 2015 | 52 | 12.5 | |
| 2016 | 60 | 14.4 | |
| 2017 | 146 | 35.1 | |
| 2019 | 45 | 10.8 | |
| Breed (n = 400) a | Angus | 122 | 30.5 |
| Other purebred | 35 | 8.8 | |
| Crossbred | 243 | 60.8 | |
| Parity (n = 380) a | Heifers | 214 | 56.3 |
| Second parity cows | 55 | 14.5 | |
| Mature cows | 111 | 29.2 | |
| Calving ease score (n = 342) a | Unassisted | 67 | 19.6 |
| Easy assist | 133 | 38.9 | |
| Difficult assist | 142 | 41.5 | |
Sample size does not equal 416 as information was missing for some samples.
TABLE 2.
Descriptive statistics of immunoglobulin G concentration and Brix refractometry percentages in western Canadian beef cow colostrum samples
| Test | n | Mean/median | SD/IQR | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Reference RID assay a (g/L) | 416 | 149.6 | 38.7 | 19.2 | 264.7 | |
| Digital Brix refractometer (%) | Lab A b | 364 | 28.6 | 26.1‐31.9 | 13.1 | 44.3 |
| Lab B b | 271 | 28.9 | 25.7‐31.9 | 16.0 | 43.2 | |
| Lab C b | 220 | 29.5 | 26.6‐31.8 | 13.7 | 44.9 | |
Abbreviations: IQR, interquartile range; RID, radial immunodiffusion assay.
Parametric data reported as mean and SD.
Nonparametric data reported as median and interquartile range.
3.2. Correlation coefficients and areas under the ROC curve
The IgG concentrations measured by RID were positively correlated with the Brix percentages measured by Brix refractometry in Lab A (r = 0.72; Figure 1A), Lab B (r = 0.68; Figure 1B), and Lab C (r = 0.76; Figure 1C). The AUC were 0.93, 0.95, and 0.94 for prediction of colostrum IgG <100 g/L, and 0.87, 0.82, and 0.88 for prediction of colostrum IgG ≥150 g/L for Labs A, B, and C, respectively.
FIGURE 1.
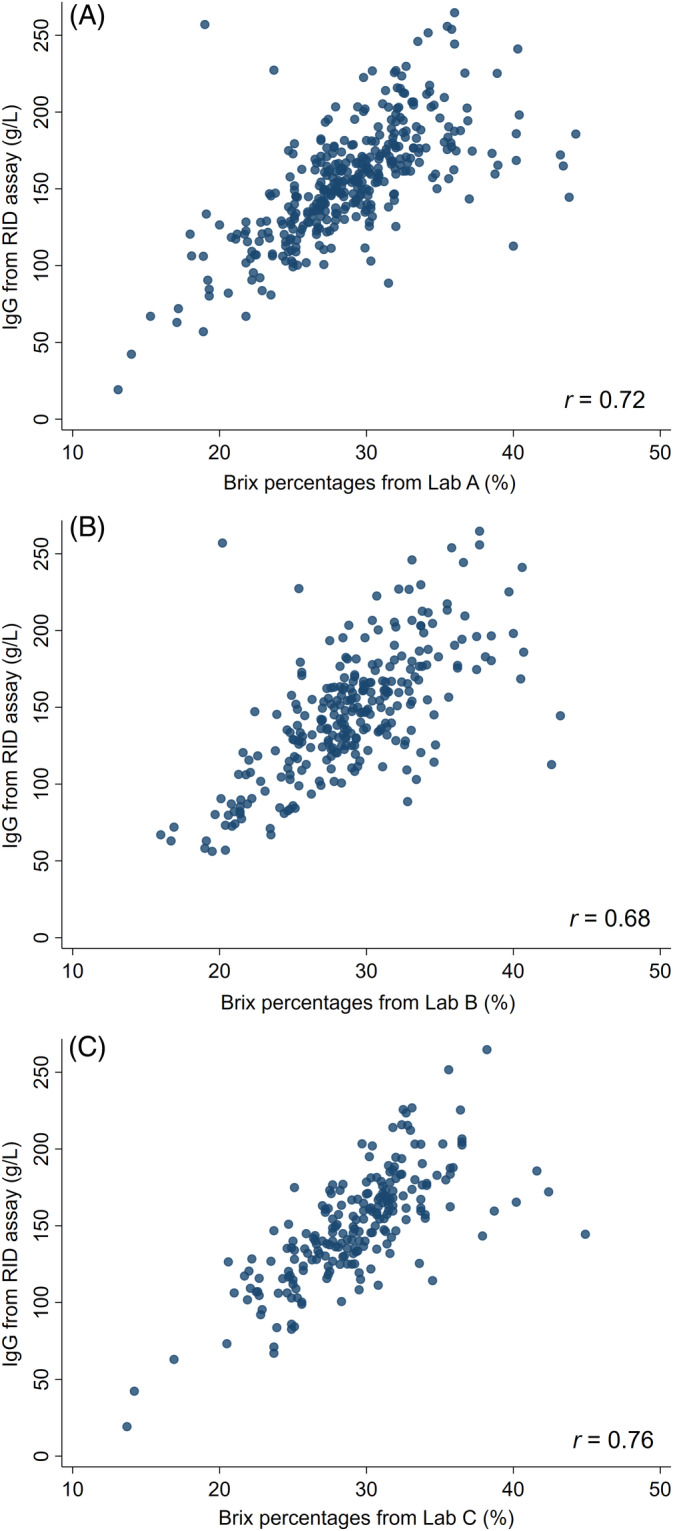
Scatter plots showing the correlation between colostral IgG concentrations obtained by the reference RID (g/L) and estimated by digital Brix refractometers (%) in three different laboratories: A, Lab A; B, Lab B; C, Lab C. r = Spearman correlation coefficient. IgG, immunoglobulin G; RID, radial immunodiffusion assay
3.3. Likelihood ratios
The LHRs for colostrum samples with different Brix percentages at each of the 3 laboratories are presented in Table 3. Samples with Brix percentages <22% were between 11.2 and 16.8 times more likely to contain IgG <100 g/L than they were to contain IgG ≥100 g/L, and samples with Brix percentages of 22% to 23.9% were between 3.8 and 6.7 more likely to contain IgG <100 g/L than they were to contain IgG ≥100 g/L. Brix percentages of <24% therefore were optimal to detect colostrum with IgG concentrations <100 g/L for all laboratories. Brix percentages of ≥30% were optimal to detect colostrum IgG concentrations ≥150 g/L for all laboratories, because those samples were between 3.3 and 5.3 times more likely to contain IgG ≥150 g/L than they were to contain IgG <150 g/L.
TABLE 3.
Frequency of beef cow colostrum samples in different strata and interval likelihood ratios for various Brix percentage (%) ranges
| Brix range (%) | Lab A (n = 364) | Lab B (n = 271) | Lab C (n = 220) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Colostrum IgG < 100 g/La | |||||||||
| Total n | Samples <100 g/L | Likelihood ratio (95% CI) | Total n | Samples <100 g/L | Likelihood ratio (95% CI) | Total n | Samples <100 g/L | Likelihood ratio (95% CI) | |
| ≤21.9 | 25 | 14 | 16.83 (8.82‐32.11) | 25 | 21 | 25.73 (10.74‐66.51) | 8 | 4 | 11.21 (3.56‐35.31) |
| 22‐23.9 | 21 | 5 | 4.75 (2.05‐11.02) | 11 | 4 | 3.80 (1.31‐11.03) | 14 | 5 | 6.73 (2.74‐16.49) |
| 24‐25.9 | 41 | 1 | 0.66 (0.17‐2.56) | 34 | 7 | 1.74 (0.85‐3.53) | 27 | 4 | 2.34 (1.01‐5.42) |
| 26‐27.9 | 73 | 0 | 0.18 (0.03‐1.26) | 33 | 2 | 0.57 (0.18‐1.77) | 31 | 0 | 0.35 (0.05‐2.42) |
| 28‐29.9 | 60 | 0 | 0.22 (0.03‐1.53) | 64 | 0 | 0.09 (0.01‐0.66) | 40 | 0 | 0.27 (0.05‐0.19) |
| ≥30 | 144 | 0 | 0.09 (0.01‐0.64) | 104 | 0 | 0.06 (0.01‐0.40) | 100 | 0 | 0.11 (0.02‐0.75) |
| Colostrum IgG ≥ 150 g/L a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total n | Samples ≥ 150 g/L | Likelihood ratio (95% CI) | Total n | Samples ≥ 150 g/L | Likelihood ratio (95% CI) | Total n | Samples ≥ 150 g/L | Likelihood ratio (95% CI) | |
| ≤21.9 | 25 | 1 | 0.04 (0.01‐0.25) | 25 | 0 | 0.05 (0.01‐0.35) | 8 | 0 | 0.12 (0.02‐0.91) |
| 22‐23.9 | 21 | 1 | 0.04 (0.01‐0.31) | 11 | 0 | 0.10 (0.01‐0.79) | 14 | 0 | 0.07 (0.01‐0.52) |
| 24‐25.9 | 41 | 6 | 0.14 (0.06‐0.33) | 34 | 6 | 0.30 (0.14‐0.66) | 27 | 2 | 0.12 (0.04‐0.39) |
| 26‐27.9 | 73 | 27 | 0.49 (0.32‐0.75) | 33 | 8 | 0.43 (0.21‐0.89) | 31 | 8 | 0.40 (0.19‐0.81) |
| 28‐29.9 | 60 | 40 | 1.66 (1.01‐2.72) | 64 | 30 | 1.10 (0.72‐1.68) | 40 | 13 | 0.53 (0.29‐0.95) |
| ≥30 | 144 | 124 | 5.14 (3.36‐7.86) | 104 | 76 | 3.30 (2.32‐4.73) | 100 | 84 | 5.27 (3.35‐8.28) |
Abbreviations: CI, confidence interval; IgG, immunoglobulin G.
As determined by radial immunodiffusion.
3.4. Misclassification cost‐term
Figure 2B to D shows the MCT for different scenarios of low‐IgG colostrum (IgG <100 g/L) herd prevalence (low, average, and high) and across various false‐negative‐to‐false‐positive cost ratios (1 : 5, 1 : 1, and 5 : 1), along with the overall Se and Sp across Brix thresholds (Figure 2A). Similar MCT patterns were observed for the different false‐negative‐to‐false‐positive cost ratios, but the magnitude of MCT decrease varied based on prevalence of low‐IgG colostrum. In all 3 prevalence scenarios, a significant decrease in MCT occurred up to a Brix percentage of 24% for all cost ratios, with the exception of a cost ratio of 5 : 1 in herds with a high prevalence of low‐IgG colostrum (Figure 2D). In this herd scenario, only a negligible decrease in MCT was evident. The initial decrease in MCT was followed by an additional but very small MCT decrease up to a Brix percentage of 25% only for herds with low prevalence of low‐IgG colostrum (Figure 2B). No further substantial decrease for any herd scenario was observed at Brix percentages >25%, and in herds with average or high prevalence of low‐IgG colostrum, MCT increased substantially at higher Brix percentages. The lowest MCT result can be considered as the Brix percentage that minimizes the cost. 31 The MCT decreased from 90% and 50% at a Brix percentage of 13% in low and average prevalence herds, respectively, to approximately 10% at a Brix percentage of 24%, indicating that a large proportion of misclassification cost could be avoided by using a Brix threshold of 24%. However, in high prevalence herds, the change in MCT was less pronounced with only a 10% decrease between Brix percentages of 13% and up to 24%, depending on the cost ratio. For beef herds with low prevalence of low‐IgG colostrum, such as the herds in our study, further decrease of misclassification cost can be achieved by using a Brix threshold of 25% for the classification of colostrum with IgG concentrations <100 g/L. Test characteristics were calculated for the selected Brix thresholds of 24%, 25%, and 30% and are presented in Table 4 for all 3 laboratories.
FIGURE 2.
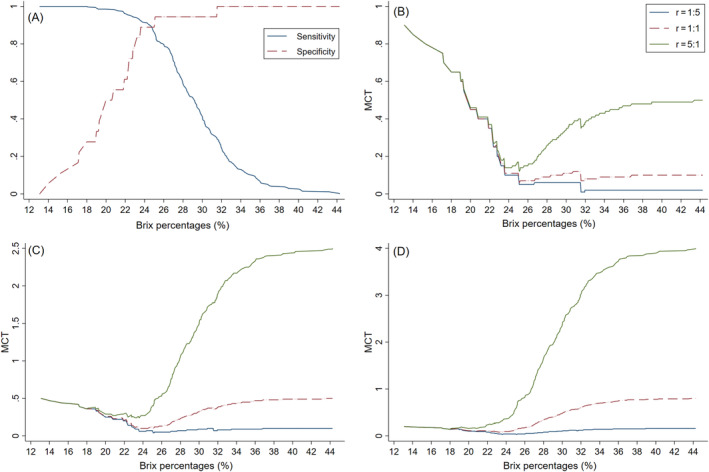
Sensitivity and specificity plotted across Brix percentages, A, and misclassification cost‐term plots of Brix thresholds to identify colostrum IgG concentrations of <100 g/L in herds with, B, low (10%), C, average (50%), and, D, high (80%) prevalence of low‐IgG colostrum. r: ratio of the cost of false‐negative to false‐positive test results. r = 1 : 5 shows the slope assuming the cost for a false‐positive is five times the cost for a false‐negative. r = 1 : 1 shows the slope assuming the cost for a false‐negative and a false‐positive result are the same. r = 5 : 1 shows the slope assuming the cost of false‐negative is five times the cost of a false‐positive. These graphs are based on Brix percentages obtained from Lab A. IgG, immunoglobulin G
TABLE 4.
Test characteristics of selected Brix thresholds for identification of low‐IgG (IgG < 100 g/L) and high‐IgG (IgG ≥ 150 g/L) beef cow colostrum using Brix refractometry in three different laboratories
| Colostrum IgG (g/L) a | Brix threshold (%) | Test characteristics | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab A (n = 364) | Lab B (n = 271) | Lab C (n = 220) | |||||||||||||||||
| P | Se | Sp | Acc. | J | AUC | P | Se | Sp | Acc. | J | AUC | P | Se | Sp | Acc. | J | AUC | ||
| <100 | <24 | 4.9 | 88.9 | 91.3 | 91.2 | 0.80 | 0.93 | 12.9 | 71.4 | 95.3 | 92.2 | 0.67 | 0.95 | 5.9 | 69.2 | 93.7 | 92.3 | 0.63 | 0.94 |
| <25 | 4.9 | 88.9 | 86.1 | 86.3 | 0.75 | 0.93 | 12.9 | 82.9 | 91.9 | 90.8 | 0.75 | 0.95 | 5.9 | 84.6 | 88.9 | 88.6 | 0.74 | 0.94 | |
| ≥150 | ≥30 | 54.7 | 62.3 | 87.9 | 73.9 | 0.5 | 0.87 | 44.6 | 62.6 | 81.3 | 73.1 | 0.44 | 0.82 | 48.6 | 78.5 | 85.8 | 82.3 | 0.64 | 0.88 |
Note: P, prevalence of low (IgG < 100 g/L) and high (IgG ≥ 150 g/L) IgG colostrum in study population; Se, sensitivity; Sp, specificity; Acc, accuracy; J, Youden index (Se + Sp − 1).
Abbreviations: AUC, area under the curve; IgG, immunoglobulin G.
As determined by radial immunodiffusion.
3.5. Interlaboratory agreement
Concordance correlation coefficients among Brix percentages from the 3 laboratories ranged from 0.89 to 0.96 (Figure 3). Minor differences in the reduced major axis in relation to the line of perfect concordance were observed among laboratories, but were considered clinically irrelevant. Bland‐Altman plots illustrate the limits of agreement between the Brix percentages obtained in 3 different laboratories (Figure 4). Mean differences between Lab A and Lab C as well as between Lab B and Lab C were negligible (−0.36% and 0.09% Brix, respectively) and the limits of agreement were small (−2.9% to 2.2% Brix and −2.3% to 2.4% Brix, respectively). Although the mean difference between Lab A and Lab B and the limits of agreement were slightly higher (−1.2% Brix and −4.8% to 2.4% Brix), these findings were considered unimportant for clinical application.
FIGURE 3.
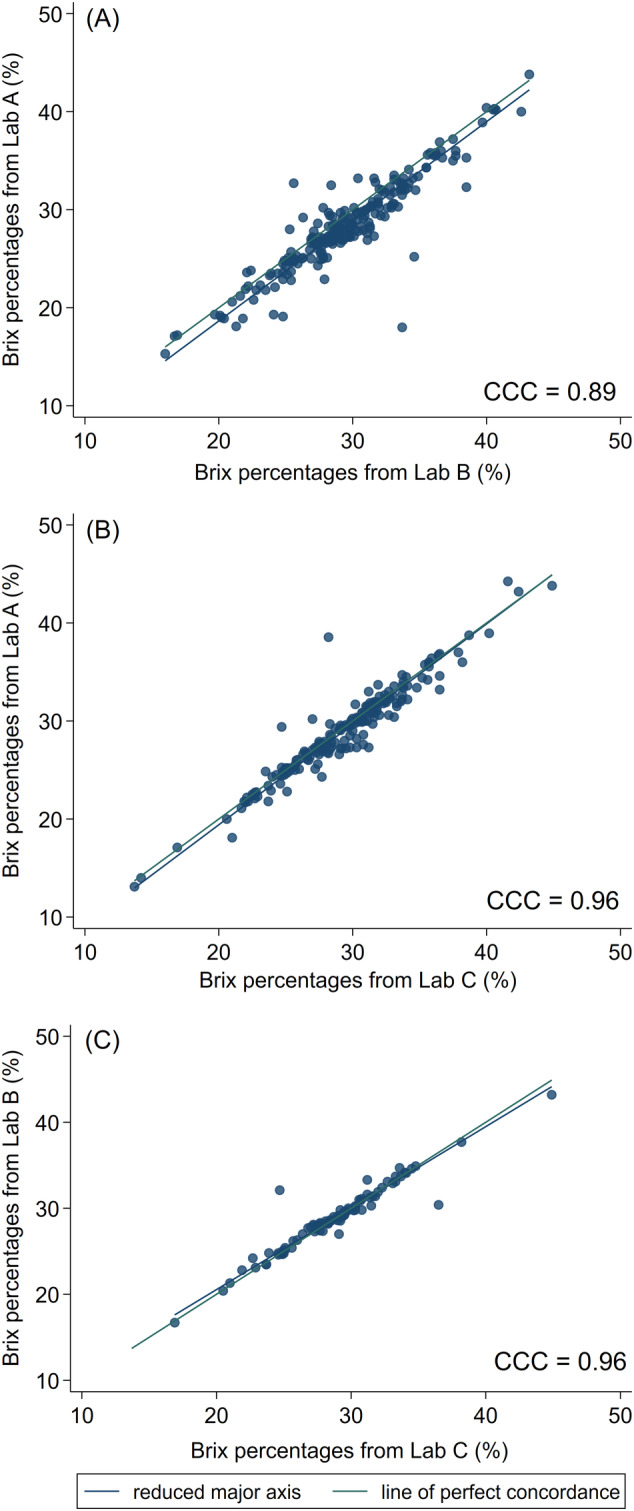
Concordance correlation coefficient plots of Brix percentages (%) measured by Brix refractometers in, A, Lab A and Lab B, B, Lab A and Lab C, and, C, Lab B and Lab C, to estimate IgG concentrations in beef cow colostrum. IgG, immunoglobulin G
FIGURE 4.
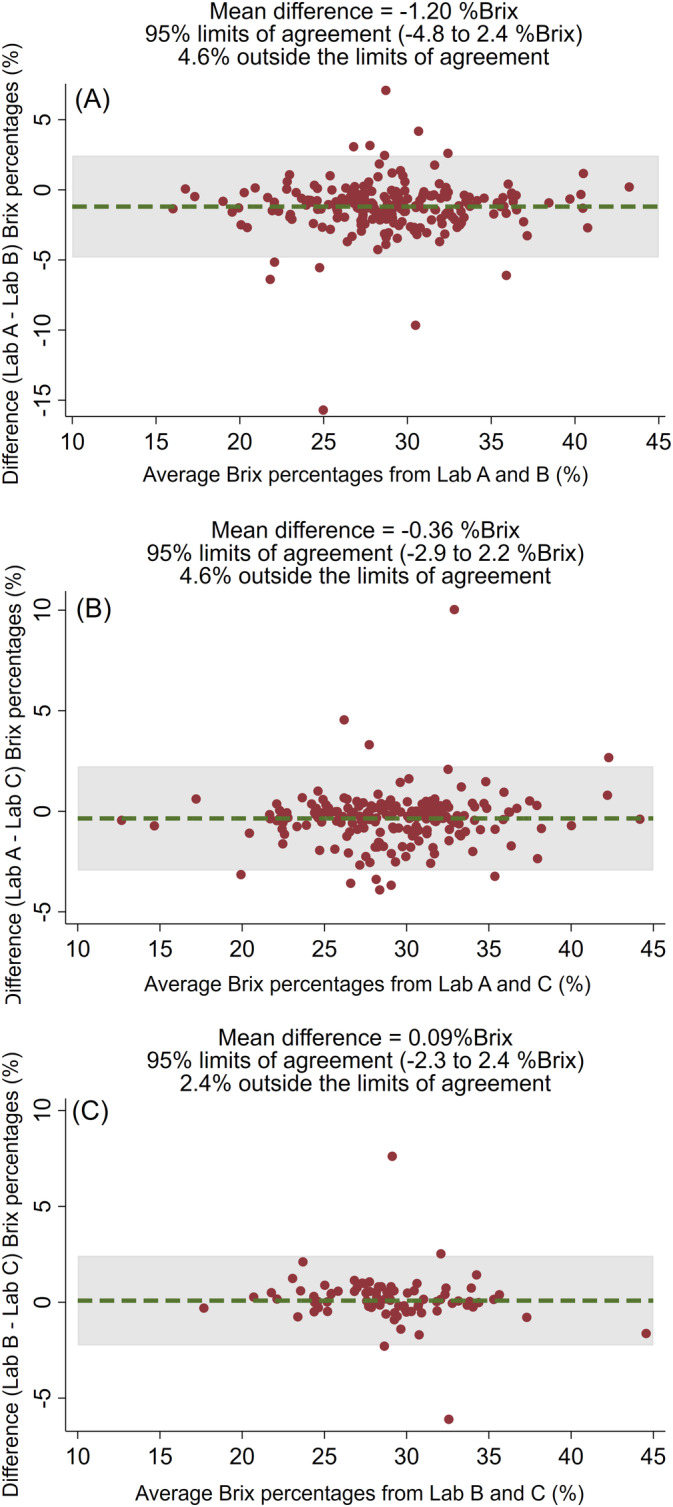
Interlaboratory agreement demonstrated by Bland‐Altman plots comparing the mean differences of Brix percentages (%) measured by Brix refractometers in, A, Lab A and Lab B, B, Lab A and Lab C, and, C, Lab B and Lab C to estimate IgG concentrations in beef cow colostrum. IgG, immunoglobulin G
4. DISCUSSION
We assessed Brix refractometer performance to estimate IgG concentrations in beef cow colostrum and determined Brix thresholds for detection of colostrum containing IgG <100 and ≥150 g/L. The Brix percentages in our study showed good correlation with the reference test RID as well as substantial interlaboratory agreement. Although samples with Brix percentages of <24% generally can be considered to contain IgG concentrations <100 g/L, the MCT approach shows that, depending on the context, some herds may benefit by using the slightly higher threshold of <25%. Colostrum with Brix percentages ≥30% can be considered to contain IgG concentrations ≥150 g/L. To our knowledge, ours is the first study to consider the cost of misclassification for the determination of suggested Brix thresholds as well as an assessment of interlaboratory agreement.
Only 38 of 416 samples had colostrum IgG concentrations <100 g/L in our study population, leading to a very low prevalence of low‐IgG colostrum. The mean IgG concentration of 149.7 g/L in our study population was higher than the 95.9 g/L reported in the only other study assessing Brix refractometry for beef cow colostrum. 14 Similarly, the average Brix percentages found in our study for the 3 laboratories (28.6%‐29.5%) were slightly higher than the 26.3% ± 5.2% reported previously. 14 Most of the colostrum samples evaluated for our study were collected within 1 hour of calving, which is very similar to the colostrum collection time in the previous study 14 and makes timing an unlikely contributor to the differences. Breed has been confirmed as a predictor variable for colostrum IgG concentration in some studies, 32 , 33 but not in others. 14 The breeds represented in our study and a previous study 14 differ substantially as did the peri‐parturient management of enrolled animals. It is likely that many factors, including genetics, colostrum yield, management, nutrition, environmental factors, study design, and testing methods, impacted the differences in outcome in our study population as compared to other studies. 14 , 33 The wide range of colostrum IgG concentrations (19.2‐264.7 g/L) in our study population further emphasizes the wide variability in beef cow colostrum IgG concentration, a trend that also has been observed for dairy cow colostrum. 11 The correlation between Brix and RID found in our study is similar to findings of dairy studies (r = 0.64‐0.75), 5 , 11 , 12 but slightly lower than reported previously for beef colostrum (r = 0.8). 14
The limited clinical utility of the traditional, dichotomous approach of determining cut points for diagnostic tests led to the multimethod approach used in our study. 18 Based on both LHR and MCT analysis, colostrum samples with Brix percentages <24% generally can be classified as colostrum with IgG <100 g/L. This percentage is lower than the 26.9% recommended previously. 14 Direct comparison of results obtained in the 2 studies is difficult because of the different characteristics of the colostrum samples (see above), the different IgG isotypes evaluated (ie, IgG1 versus total IgG), and, most importantly, different statistical approaches. The suggested threshold of 24% Brix in our study showed slightly better test characteristics than the 26.9% reported previously. 14 The Youden index, a measure of combined Se and Sp, ranged from 0.63 to 0.80 depending on the laboratory in our study, which is slightly higher than the 0.62 reported previously. 14 Brix refractometry was highly accurate (AUC = 0.93‐0.95) to detect low‐IgG colostrum (IgG < 100 g/L) in our study population, which also was slightly better than the AUC of 0.89 reported previously. 14
Overall, our results indicate that most herds would benefit from using Brix percentages <24% to identify colostrum containing IgG <100 g/L. This knowledge was gained using 2 different statistical approaches, indicating that this threshold does not only have good test characteristics, but also has the benefit of decreasing misclassification cost. However, the MCT approach showed that different circumstances may require adaptations of the thresholds used. In herds with low prevalence (10%) of low‐IgG colostrum (IgG < 100 g/L), the use of a slightly higher Brix percentage (25%) could further decrease the MCT, independent of the cost ratio of false‐negative to false‐positive test results (Figure 2B). Although the MCT in our study also was modeled for herds with a high prevalence (80%) of low‐IgG colostrum (IgG < 100 g/L), determination of appropriate thresholds should be undertaken based on populations with lower average IgG concentrations. Generally, veterinarians and producers in production settings similar to those evaluated in our study should be advised to ensure appropriate colostrum yield in cows with colostrum showing <24% Brix and to manage the calf to ensure sufficient nursing or to hand‐feed supplemental colostrum.
We also evaluated the Brix refractometer for its ability to classify colostrum containing IgG concentrations ≥150 g/L. This application could be useful in herds with typically high colostrum IgG concentrations, such as the cow‐calf operations enrolled in our study. Although the AUC and other test characteristics for the selected threshold based on LHR were lower compared to detection of low‐IgG colostrum, Brix refractometry seems to be a useful tool for this application as well. Brix percentages ≥30% were considered optimal to predict colostrum IgG concentrations of ≥150 g/L. This information could aid management decisions in 2 ways: calves of these cows should consume this maternal colostrum either by nursing or human intervention, and excess colostrum could be collected and frozen for calves requiring supplemental colostrum.
Agreement of the different laboratories as assessed by CCC and Bland‐Altman analysis was good. Although small statistical differences in agreement were observed, these were considered of minimal biological importance to the practical applications of Brix refractometry. Our results indicate that even when different models of Brix refractometers were used by different personnel in different environments, the results generally were comparable.
The main limitation of our study is its retrospective design and the consequent lack of control over animal enrollment, colostrum sampling, and sample handling and storage. Most animals enrolled in our study were Angus or crossbred cattle, which is representative of the local breed distribution in Alberta. Breed could have influenced colostral IgG concentration and therefore the calculated thresholds. As indicated by the wide range of published mean IgG concentrations in beef cow colostrum, 15 a single recommendation for appropriate thresholds may not be appropriate, and further research should be encouraged to determine guidelines for different study populations. Many colostrum samples were collected from cows needing some degree of assistance at birth because of the study designs of the projects, 16 , 21 , 22 , 23 and the distribution of IgG concentrations may be different in a population consisting only of unassisted cows. No studies to date have investigated the potential association between calving ease and colostrum IgG concentration of beef cows. However, colostrum samples of cows needing assistance are more likely to be evaluated by producers and veterinarians, given the higher likelihood of their calves needing some degree of colostrum intervention, 16 which makes them an appropriate sample population to study. The exact colostrum collection time was not known for 102 samples, which were not removed from evaluation of Brix refractometry performance because colostrum may be evaluated on farm at various times after calving. Particularly when parturition is not observed or assisted, colostrum IgG concentration may not be assessed immediately, and only when a concern is noted for the cow or the calf will a sample be collected for testing. The lowest IgG concentration measured in our study was 19.2 g/L, and although it might be suspected that this was transition milk, this particular sample was known to have been collected within 10 minutes of parturition during an on‐farm research study. 21 By including a wide range of colostrum IgG concentrations, the internal and external validity of the findings of our study was increased. Although all samples were shipped on ice in the same manner and Brix refractometry was performed similarly in all laboratories, slight differences in sample handling during transport and in the respective laboratories as well as different time spans and freeze‐thaw cycles from colostrum collection to analysis may have contributed to some of the observed variation reported in our study. Last, the examined plausible cost ratios for our study were chosen based on limited available literature. Estimates of false‐negative results (cost of failed TPI) were extrapolated from results of a meta‐analysis referring to European production systems, 29 which may vary substantially from Canadian cow‐calf settings. Unfortunately, economic models assessing the impact of TPI in beef calves based on North American cattle populations are lacking. No published estimates of labor cost in cow‐calf operations were available at the time of analysis.
In conclusion, Brix refractometry is a promising tool for the determination of colostrum IgG concentration in beef cow colostrum, showing positive correlation with RID and substantial agreement among laboratories. Our results suggest that, in herds similar to those enrolled here, a Brix percentage threshold of <24% can be used to detect low‐IgG colostrum (IgG < 100 g/L) and Brix percentages of ≥30% are indicative of high‐IgG colostrum (IgG ≥ 150 g/L). Producers may choose to supplement or replace colostrum in cows with colostrum <24% Brix, and to freeze colostrum ≥30%. Colostrum yielding Brix results between 24% and 30% Brix likely will be appropriate for consumption by the calf, given sufficient volume of colostrum is available.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Colostrum samples were collected during several studies (cited in manuscript), all of which were approved by the University of Calgary Veterinary Sciences Animal Care Committee (AC13‐0324, AC15‐0150, AC16‐0209, AC18‐0204) and conducted in accordance with guidelines established by the Canadian Council on Animal Care.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
We thank the Saskatoon Colostrum Company Ltd for their in‐kind contribution of RID and Brix testing and Dr Elizabeth Homerosky for her efforts in collecting a subset of samples used for this study. Additionally, we also thank Maggie Grover, Alycia Webster, Jackie Annis, Jackie Elgert, Zeanna Janmohamed, and Nicole Hawe (University of Calgary) and Cynthia Mitchell (Maritime Quality Milk Laboratory, University of Prince Edward Island) for their technical assistance with Brix refractometry.
Gamsjäger L, Elsohaby I, Pearson JM, et al. Assessment of Brix refractometry to estimate immunoglobulin G concentration in beef cow colostrum. J Vet Intern Med. 2020;34:1662–1673. 10.1111/jvim.15805
Funding information Alberta Agriculture and Forestry; American College of Veterinary Internal Medicine Fellowship in Advanced Research Training; University of Calgary Faculty of Veterinary Medicine Simpson Ranch Research Grant
REFERENCES
- 1. Godden SM, Lombard JE, Woolums AR. Colostrum management for dairy calves calf. Vet Clin North Am. 2019;35:535‐556. 10.1016/j.cvfa.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dewell RD, Hungerford LL, Keen JE, et al. Association of neonatal serum immunoglobulin G1 concentration with health and performance in beef calves. J Am Vet Med Assoc. 2006;228(6):914‐921. 10.2460/javma.228.6.914. [DOI] [PubMed] [Google Scholar]
- 3. Waldner CL, Rosengren LB. Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes. Can Vet J. 2009;50:275‐281. [PMC free article] [PubMed] [Google Scholar]
- 4. Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2(3):235‐254. 10.1016/0161-5890(65)90004-0. [DOI] [PubMed] [Google Scholar]
- 5. Chigerwe M, Tyler JW, Middleton JR, Spain JN, Dill JS, Steevens BJ. Comparison of four methods to assess colostral IgG concentration in dairy cows. J Am Vet Med Assoc. 2008;233(5):761‐766. 10.2460/javma.233.5.761. [DOI] [PubMed] [Google Scholar]
- 6. Elsohaby I, Hou S, McClure JT, Riley CB, Shaw RA, Keefe GP. A rapid field test for the measurement of bovine serum immunoglobulin G using attenuated total reflectance infrared spectroscopy. BMC Vet Res. 2015;11(1):218 10.1186/s12917-015-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elsohaby I, Windeyer MC, Haines DM, et al. Application of transmission infrared spectroscopy and partial least squares regression to predict immunoglobulin G concentration in dairy and beef cow colostrum. J Anim Sci. 2018;96(2):771‐782. 10.1093/jas/sky003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drikic M, De Buck J, Doepel L, Fu Y, Windeyer C, Olsen S. Determining the IgG concentrations in bovine colostrum and calf sera with a novel enzymatic assay. J Anim Sci Biotechnol. 2018;9(1):1‐9. 10.1186/s40104-018-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lemberskiy‐Kuzin L, Lavie S, Katz G, Merin U, Leitner G. Determination of immunoglobulins levels in colostrum by using an on‐line milk analyzer. Can J Anim Sci. 2019;3(3):1‐3. 10.1139/cjas-2018-0178. [DOI] [Google Scholar]
- 10. Chigerwe M, Dawes ME, Tyler JW, Middleton JR, Moore MP, Nagy DM. Evaluation of a cow‐side immunoassay kit for assessing IgG concentration in colostrum. J Am Vet Med Assoc. 2005;227(1):129‐131. 10.2460/javma.2005.227.129. [DOI] [PubMed] [Google Scholar]
- 11. Quigley JD, Lago A, Chapman C, Erickson P, Polo J. Evaluation of the Brix refractometer to estimate immunoglobulin G concentration in bovine colostrum. J Dairy Sci. 2013;96(2):1148‐1155. 10.3168/jds.2012-5823. [DOI] [PubMed] [Google Scholar]
- 12. Bielmann V, Gillan J, Perkins NR, Skidmore AL, Godden S, Leslie KE. An evaluation of Brix refractometry instruments for measurement of colostrum quality in dairy cattle. J Dairy Sci. 2010;93(8):3713‐3721. 10.3168/jds.2009-2943. [DOI] [PubMed] [Google Scholar]
- 13. Buczinski S, Vandeweerd JM. Diagnostic accuracy of refractometry for assessing bovine colostrum quality: a systematic review and meta‐analysis. J Dairy Sci. 2016;99(9):7381‐7394. 10.3168/jds.2016-10955. [DOI] [PubMed] [Google Scholar]
- 14. Vandeputte S, Detilleux J, Rollin F. Investigation of colostrum quality in beef cattle by radial immunodiffusion and brix refractometry. Vet Rec. 2014;175(14):353 10.1136/vr.101590. [DOI] [PubMed] [Google Scholar]
- 15. McGee M, Earley B. Review: passive immunity in beef‐suckler calves. Animal. 2019;13(4):810‐825. 10.1017/s1751731118003026. [DOI] [PubMed] [Google Scholar]
- 16. Homerosky ER, Timsit E, Pajor EA, Kastelic JP, Windeyer MC. Predictors and impacts of colostrum consumption by 4 h after birth in newborn beef calves. Vet J. 2017;228:1‐6. 10.1016/j.tvjl.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 17. Earley B, Tiernan K, Duffy C, et al. Effect of suckler cow vaccination against glycoprotein E (gE)‐negative bovine herpesvirus type 1 (BoHV‐1) on passive immunity and physiological response to subsequent bovine respiratory disease vaccination of their progeny. Res Vet Sci. 2018;118:43‐51. 10.1016/j.rvsc.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gardner IA, Greiner M. Receiver‐operating characteristic curves and likelihood ratios: improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet Clin Pathol. 2006;35(1):8‐17. 10.1111/j.1939-165X.2006.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 19. Brown MD, Reeves MJ. Interval likelihood ratios: another advantage for the evidence‐based diagnostician. Ann Emerg Med. 2003;42(2):292‐297. 10.1067/mem.2003.274. [DOI] [PubMed] [Google Scholar]
- 20. Greiner M. Two‐graph receiver operating characteristic (TG‐ROC): update version supports optimisation of cut‐off values that minimise overall misclassification costs. J Immunol Methods. 1996;191(1):93‐94. 10.1016/0022-1759(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 21. Pearson JM, Pajor EA, Campbell JR, et al. Clinical impacts of administering a nonsteroidal anti‐inflammatory drug to beef calves after assisted calving on pain and inflammation, passive immunity, health, and growth. J Anim Sci. 2019;97(5):1996‐2008. 10.1093/jas/skz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearson JM, Pajor E, Campbell J, Levy M, Caulkett N, Windeyer MC. A randomised controlled trial investigating the effects of administering a non‐steroidal anti‐inflammatory drug to beef calves assisted at birth and risk factors associated with passive immunity, health, and growth. Vet Rec Open. 2019;6(1):1‐10. 10.1136/vetreco-2019-000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gamsjäger L, Windeyer MC. An Assessment of Beef Cattle Colostrum and Intervention Strategies to Optimize Colostrum Management in Beef Calves. Unpubl data; 2020.
- 24. Chelack BJ, Morley PS, Haines DM. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can Vet J. 1993;34(7):407‐412. [PMC free article] [PubMed] [Google Scholar]
- 25. Shivley CB, Lombard JE, Urie NJ, et al. Preweaned heifer management on US dairy operations: part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J Dairy Sci. 2018;101(10):9229‐9244. 10.3168/jds.2017-14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44(8):763‐770. 10.1016/0895-4356(91)90128-V. [DOI] [PubMed] [Google Scholar]
- 27. Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing studies of medical tests In: Hulley SB, Cummings SR, Browner WS, Grady D, Newman TB, eds. Designing Clinical Research. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2007:124. [Google Scholar]
- 28. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver‐operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23‐41. 10.1016/0009-2797(70)90001-3. [DOI] [PubMed] [Google Scholar]
- 29. Raboisson D, Trillat P, Cahuzac C. Failure of passive immune transfer in calves: a meta‐analysis on the consequences and assessment of the economic impact. PLoS One. 2016;11(3):e0150452 10.1371/journal.pone.0150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Altman DG. Practical Statistics for Medical Research. London, England: Chapman Hall; 1991. [Google Scholar]
- 31. Buczinski S, Fecteau G, Dubuc J, Francoz D. Validation of a clinical scoring system for bovine respiratory disease complex diagnosis in preweaned dairy calves using a Bayesian framework. Prev Vet Med. 2018;156(5):102‐112. 10.1016/j.prevetmed.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guy MA, McFadden TB, Cockrell DC, Besser TE. Regulation of colostrum formation in beef and dairy cows. J Dairy Sci. 1994;77:3002‐3007. 10.3168/jds.s0022-0302(94)77241-6. [DOI] [PubMed] [Google Scholar]
- 33. Murphy BM, Drennan MJ, O'Mara FP, Barley B. Cow serum and colostrum immunoglobulin (IgG1) concentration of five suckler cow breed types and subsequent immune status of their calves. Ir J Agric Food Res. 2005;44(2):205‐213. [Google Scholar]


