Abstract
Background
Middle ear effusion is common in brachycephalic dogs with similarities to otitis media with effusion in children. Association with the cranial and eustachian tube morphology and bacterial infection is suspected in both species.
Hypothesis/objectives
To determine cytological and bacteriological features of middle ear effusions in dogs, provide information on histological features, and further assess the dog as a model of the human disease.
Animals
Sixteen live dogs, 3 postmortem cases of middle ear effusion, and 2 postmortem controls.
Methods
Prospective; clinical investigation using computed tomography, magnetic resonance imaging, video‐otoscopy, myringotomy; cytological assessment of 30 and bacteriology of 28 effusions; histology and immunohistochemistry (CD3 for T‐lymphocytes, Pax5 for B lymphocytes and MAC387 for macrophages) of 10 middle ear sections.
Results
Effusions were associated with neurological deficits in 6/16 (38%) and concurrent atopic dermatitis and otitis externa in 9/16 (56%) of live cases. Neutrophils and macrophages predominated on cytology (median 60 [range 2%‐95.5%] and 27 [2%‐96.5%]) whether culture of effusions was positive or not. In histology sections, the mucosa was thickened in affected dogs but submucosal gland dilatation occurred in affected and unaffected dogs. There was no bacterial growth from 22/28 (79%) of effusions. Bacteria isolated from the other 6 (21%) were predominantly Staphylococcus pseudintermedius (4/6, 67%).
Conclusions and Clinical Importance
Clinical, morphological, and cytological findings in middle ear effusions of dogs and people suggest similar pathogeneses. Middle ear effusion of dogs could be a useful model of human otitis media with effusion. Such comparisons can improve understanding and management across species.
Keywords: canine, Cavalier King Charles spaniel, immunohistochemistry, middle ear, otitis media with effusion
Abbreviations
- AD
atopic dermatitis
- BAER
brainstem auditory evoked response
- BOAS
brachycephalic obstructive airway syndrome
- CKCS
Cavalier King Charles spaniel
- CT
computed tomography
- ET
eustachian tube
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- MEE
middle ear effusion
- MRI
magnetic resonance imaging
- OME
otitis media with effusion
- PM
postmortem
- PSOM
primary secretory otitis media
- WBC
white blood cell
1. INTRODUCTION
Middle ear effusion (MEE) of dogs is characterized by the presence of fluid in the tympanic bullae. MEE can be asymptomatic but clinical signs reported include head and neck pain, impaired hearing, facial paralysis, vestibular signs, head tilt, and otic pruritus. 1 Diagnosis is based on demonstration of fluid in the tympanic bullae by computed tomography (CT) or magnetic resonance imaging (MRI), examination of the tympanic membrane by video‐otoscopy, and collection of effusion by myringotomy. 1 , 2 , 3 , 4 , 5 , 6 In addition, hearing impairment can be assessed by brainstem auditory evoked response (BAER) testing. 7 , 8
MEE is most common in Cavalier King Charles spaniels (CKCS) 1 , 9 , 10 but occurs in other brachycephalic breeds, for example, boxers and bulldogs. 6 , 11 The pathogenesis of MEE in dogs is likely to be multifactorial, and potential causes include brachycephaly resulting in obstruction of the eustachian tube (ET), 12 , 13 hypersecretion of mucus from the mucosa of the bullae, and middle ear infection. In 1 study, increased soft palate thickness and decreased nasopharyngeal dimensions are associated with bilateral MEE in CKCS but not in Boxers. 13
The condition is also referred to as primary secretory otitis media (PSOM) or otitis media with effusion (OME), but the term MEE has been advocated for dogs as inflammation is not invariably present. 14 The nomenclature is also influenced by the human manifestation of OME, which is most prevalent in children and commonly known as “glue ear.” This shares many of the features of MEE in dogs. 15 , 16 Although cranial and palatine morphology are also considered to be important factors in children, 17 acute viral or bacterial otitis media usually precedes the development of chronic OME. 16 Whether primary bacterial infection is a factor in dogs remains unclear. 18
There are few reports of the cytological findings in the fluid or the histopathology of middle ears in affected dogs. Cytological descriptions have included the presence or absence of bacteria 18 and the presence of mucus with no evidence for an inflammatory infiltrate, although only a small number of cases was studied. 11 In children with chronic OME, neutrophils predominate in effusions that have culturable pathogenic bacteria, 19 while white blood cell (WBC) populations differ in mouse models depending on the underlying pathogenesis 20 but similar detailed studies of the cytology and histopathology in canine MME are lacking.
The objectives of this study were to determine the nature of the inflammatory cell population and other cytological features of the effusion in dogs with MEE and compare them with other species, and to provide preliminary information on the histological features of MEE. A secondary objective was to further assess the dog as a naturally occurring animal model of human OME.
2. MATERIALS AND METHODS
2.1. Animals
All dogs were recruited prospectively from the hospital's referred population of dogs except for 1 postmortem (PM) case from an external practice. The study was approved by the hospital's Veterinary Ethical Review Committee (approval no. 36‐16) and clients gave informed consent for the use of excess samples and anonymized case data. Inclusion criteria were brachycephalic breed, 21 and detection of a uni‐ or bilateral MEE by CT, MRI, video‐otoscopy, or a combination of these modalities in live cases, or at PM examination in dead cases. The latter were euthanized for reasons unconnected with MEE. Exclusion criteria for MEE analysis were purulent otitis media, recent previous flushing of the tympanic bulla, or excessive hemorrhage at sample collection.
2.2. Clinical diagnostic investigation
No diagnostic investigation was carried out purely for the study, and therefore not all cases were investigated identically. All cases were examined by recognized specialists in neurology or dermatology (“live” cases) except for 5 additional PM cases, 3 of which on examination had MEE. Postmortem samples were collected within 2 hours of death. The live cases were subject to general, neurological, and dermatological examination, and if clinically indicated, CT, MRI scanning, or both modalities. Some cases also underwent BAER testing for deafness; the BAER tests were performed under general anesthesia before and after myringotomy and ear flushing using a TRuTraceEMG system (Deymed Diagnostic, Hronov, Czech Republic). The stimulus was an alternating click delivered via headphones at a rate of 10/s. Recordings were made at intensity levels of 100 “decibels relative to normal hearing level” (dB nHL). 7 Masking noise was applied to the contralateral ear at 20 to 30 dB less than the test stimulus. Responses were recorded at an amplifier sensitivity of 1 nV/division, analysis time of 10 ms/division, 32 to 3200 Hz bandpass, and automatic artifact rejection. A minimum of 1000 time‐locked responses was averaged to produce each trace. In the majority of the dogs, the display gain was increased to help the identification of the waveforms. 22
Video‐otoscopy was carried out using a Karl‐Storzvideo‐otoscope(Karl‐Storz GmBH, Tuttlingen, Germany) under general anesthesia and the ears were assessed for abnormalities of the external ear canal (discharge, narrowness or thickening of the lumen, presence of masses), abnormalities of the tympanic membrane (intact, perforated, bulging or flat), and the appearance of MEE during myringotomy. Myringotomy was performed as previously described if the tympanic membrane was intact but appeared abnormal, or a MEE was suspected after diagnostic imaging. 23 Briefly, the external ear canals were cleaned before making an incision in the caudoventral quadrant of the tympanic membrane with the cut end of a fresh 5‐Fr gauge Argyle catheter passed through the operating channel of the video‐otoscope. Samples of the MEEs were aspirated and collected into plain, sterile, plastic tubes. Where material could not be directly aspirated, 1 mL of sterile 0.9% saline was flushed into the middle ear and aspirated.
2.3. Cytology and bacteriology
After immediate delivery to the laboratory, aliquots of the effusions were taken for bacteriology and 2 direct cytology smears. Where the fluid was dilute, mucus clumps were selected for smearing. Smears were stained with May‐Grünwald‐Giemsa using standard methods. For consistency, the smears were evaluated by the first author who is a board‐certified veterinary clinical pathologist, scoring the cellularity and the amount of mucus and RBCs, performing a 200‐cell leukocyte differential count, and evaluating neutrophil morphology. This is summarized in Table S1. Briefly, an ordinal scale of 1, 2, and 3 was used for mucus thickness, average number of RBCs, and nucleated cells/×40 field and degree of neutrophil karyolysis, karyorrhexis, and pyknosis. Differential cell counts of nucleated cells (neutrophils, lymphocytes, macrophages, and eosinophils) were also performed and expressed as a percentage of each cell type.
The samples for bacteriology were inoculated onto 2 plates of Columbia Agar with 5% horse blood, 1 MacConkey agar plate, and into mycoplasma broth (Thermo Fisher Oxoid Ltd, Basingstoke, UK). One horse blood agar plate and the MacConkey agar plate were incubated aerobically at 37°C and the second horse blood agar plate was incubated anaerobically at 37°C. The mycoplasma broth was incubated aerobically at 37°C for 5 days. The agar plates were monitored for bacterial growth for up to 48 hours. Single colonies were subcultured onto horse blood agar to obtain pure cultures. These were stained with gram stain, identified using the Vitek 2 60 automated bacterial identification and antimicrobial sensitivity instrument (bioMérieux S.A., Marcy l'Etoile, France) and interpreted in accordance with international standards. 24 The mycoplasma broth was inoculated onto mycoplasma/ureaplasma agar (Thermo Fisher Oxoid Ltd) and incubated anaerobically at 37°C for 5 days. Suspicious colonies were stained by Dienes stain and examined microscopically. Malassezia sp. grew on horse blood agar plates or were identified on gram‐stained smears of the fluids.
2.4. Histology and immunohistochemistry
Histology and immunohistochemistry (IHC) were carried out PM on 3 cases with bilateral MEEs as an incidental finding and 2 unaffected dogs. Blocks from each ear, incorporating the terminal horizontal ear canal, tympanic membrane, tympanic bulla, and part of the inner ear were fixed in 10% phosphate buffered formalin solution. If present, MEE was evident during initial sample collection; some fluid was lost during processing but sufficient was present to allow subsequent microscopy. After fixation for 1 to 2 months, they were decalcified for 4 days in Decal II solution (3800461E, Leica Microsystems Ltd, Milton Keynes, UK) followed by Decal I solution (3800441E, Leica Microsystems Ltd) until soft (approximately 6 months). After routine processing to paraffin wax‐embedded blocks, sections (5 μm) were cut transversely and sections stained with hematoxylin and eosin (H&E) and Alcian blue using standard techniques.
Immunohistochemistry for T lymphocytes (CD3), B lymphocytes (Pax5), and activated macrophages (MAC387) was carried out; see Table S2 for reagents and protocols. Positive controls were canine tonsil, spleen, and lymph node (CD3 and Pax 5) and Mycobacterium‐infected tissue (MAC387), and negative controls were processed with antibody diluent in place of primary antibody. All IHC had been previously validated in our laboratory, including use on tissue subjected to prolonged formalin fixation and decalcification.
The histological and IHC sections were first evaluated subjectively by the first and last authors (board‐certified veterinary clinical and anatomic pathologists, respectively) for the presence of fluid in the tympanic bulla, presence or absence of inflammation in the mucosa and submucosa, cell types present in the fluid and tissues, and any other features.
Bright field images were acquired using a Hamamatsu NanoZoomer‐XR slide scanner (Hamamatsu City, Japan), or on an Olympus BX41 microscope (Olympus Industrial, Southend‐on‐Sea, UK) equipped with an Olympus DP72 camera and Cell^D software (Olympus Industrial). Image analysis of the bulla mucosa was then undertaken to assess its thickness. To standardize the morphometric analysis, representative 1 to 2 mm lengths of well‐preserved bulla mucosa from the medial surface but avoiding the cochlea promontory were chosen. The thickness of the mucosa was calculated by dividing the mucosa area (epithelium and submucosal connective tissue, blood vessels, and if present submucosal glands) by the corresponding length of the underlying bulla bone. Areas were measured with the “brush” tool (Qu‐Path software https://qupath.github.io/) 25 and lengths with the “freehand” line tool (Hamamatsu NanoZoomer slide scanner software).
2.5. Statistical analysis
The ordinal cytological scores were compared between effusions from which bacteria were isolated (culture‐positive group) and those from which no bacteria were isolated (culture‐negative group) using Mann‐WhitneyU tests. D'Agostino and Pearson omnibus normality tests on the bulla fluid WBC differential counts showed the data were not normally distributed so Mann‐Whitney tests were also performed on these data. For each dog, matched data for WBC classes (neutrophils, macrophages, lymphocytes, and eosinophils) were compared using 1‐way nonparametric ANOVA (Friedman test and Dunns multiple comparison tests). Two‐tailed tests were used throughout and test values P < .05 were considered to be statistically significant. The group size for bulla histological analysis was too small to assess normality, so Mann‐WhitneyU tests were performed to compare mucosal thickness data. Graphs and statistics were generated using Prism GraphPad (v6.0c, GraphPad Software, San Diego, California).
3. RESULTS
3.1. Animals
The signalment and location of the effusion (unilateral or bilateral) in the cases of MEE are shown in Table 1. In total, effusion samples were collected from 16 live cases and 2 PM cases, and 1 further PM case had a small effusion that could not be collected. A further 2 PM cases without effusions were used for histological comparison with the 3 affected PM cases. CKCS predominated with smaller numbers of French Bulldogs and Boxers, and 1 English Bulldog.
TABLE 1.
Signalment and site of effusion(s) in live and PM cases of MEE, and 2 normal brachycephalic dogs
| Group | Breed | Age (years) | Sex | Middle ear effusion |
|---|---|---|---|---|
| Cytology cases (18 [16 live, 2 PM]) | Cavalier King Charles spaniel (8), French Bulldog (5), Boxer (4), English Bulldog (1) | 5.5 ± 2.9 a (range 1‐10) | M (6), MN (2), FN (10) | Unilateral (6), bilateral (12) |
| Histology cases with effusions (3, of which 2 included above) |
Boxer (2) French Bulldog (1) |
6, 8 1 |
FN (2) MN |
Bilateral (2) Bilateral (1) |
| Histology normal (2) |
Boxer (1) English Bulldog (1) |
6 10 |
FN M |
Absent |
Mean ± SD.
Abbreviations: FN, neutered female; M, male; MEE, middle ear effusion; MN, neutered male; PM, postmortem.
3.2. Clinical signs and diagnostic investigation
The 16 live cases had a range of clinical signs: vestibular signs (including vestibular ataxia) (8), facial paresis or paralysis (6), pain localized to head or neck, or both (4), hearing loss (3), head shaking/scratching (3), seizure‐like episodes (described as collapsing episodes with/without head/neck scratching) (3), generalized tonic‐clonic seizures (2), and generalized proprioceptive ataxia in 1 dog. Some dogs presented with more than 1 clinical sign, as in the case of facial paresis/paralysis that was always associated with vestibular signs. BAER testing was performed in only 4 cases and revealed bilateral conductive hearing loss in 3 dogs. In the fourth dog, wave V could be detected in the left ear after myringotomy at the maximal stimulus intensity of 100 dB nHL suggestive of conductive deafness but no wave could be identified in the contralateral ear suggestive of a sensorineural hearing loss. The amplitude and latency of the waves showed a mild improvement in 2 dogs with conductive hearing loss following myringotomy and ear flushing.
Of the 16 live cases, CT scanning only was carried out in 5, MRI only in 9, and MRI plus CT in 2 dogs. The main CT findings were unilateral or bilateral fluid in the tympanic bullae (6), thickening with or without irregular lysis/proliferation of the bullae walls (2), stenotic or thickened external ear canal (3), or material accumulated in the external ear canal, or both (2). Postcontrast findings included contrast enhancement of the lining of the bullae (2) and ear canals (3). Analysis of the Hounsfield units was consistent with accumulation of fluid/mucus. The MRI observations relating mainly to the ears were fluid in the bulla(e) (10) and contrast enhancing of the mucosa of the bulla consistent with mucosal inflammatory thickening (5).
Video‐otoscopy of both ears and myringotomy of all affected ears were carried out in all 16 live cases. This revealed narrow or thickened external ear canals (9 cases), otitis externa (7), bulging tympanic membranes (6), opacity (6), perforated tympanic membranes (3), or a combination of these features. Middle ear effusion was evident in all 16 live cases on myringotomy or aspiration of the middle ear contents; of these, the fluid was macroscopically mucoid in 12 cases and bloody in 3 (whether unilateral or bilateral). In 1 case, the fluid was mucoid in 1 ear and bloody in the other.
In the live cases, the final diagnoses in the 8 CKCS were MEE only in 1 case, and MEE with concurrent Chiari‐like malformation and syringomyelia in 7 cases; 2 of these cases were also diagnosed with idiopathic epilepsy, 2 with diabetes mellitus, and 1 with brachycephalic obstructive airway syndrome (BOAS). In the 4 French Bulldogs, the diagnoses were MEE only in 1 ear and bacterial otitis media‐interna in the other ear (1), MEE and congenital cerebellar malformation and cervical spinal arachnoid cyst (1), MEE with left external ear canal polyp and otitis media‐interna, right otitis media, and atopy (1), MEE plus atopy, aural hematoma, BOAS, and esophageal dysmotility in the last case. In the 4 Boxers, the diagnoses were MEE and pituitary tumor (2), MEE and idiopathic epilepsy (1), and MEE only (1). The English Bulldog had MEE, unilateral aural cholesteatoma, and a suspected immune‐mediated encephalitis and myositis (1). Overall, it was considered that effusions were directly associated with neurological deficits in 6/16 live cases.
Concurrent atopic dermatitis (AD) and otitis externa was present in 9/16 live dogs and 1/5 of the PM cases. All 5 PM cases had been euthanized for reasons unconnected with middle ear disease and where present, the MEEs were an incidental finding.
3.3. Cytology
Thirty MEEs were obtained from 18 cases of MEE although not all were suitable for all aspects of the cytological assessment (eg, excessive dilution during flushing defined by complete absence of mucus, or poor cell preservation). Of these 30 effusions, 14 were from the right ear, 15 were from the left ear and in 1 unilateral effusion the side was not recorded. The smears consisted of a varying amount and thickness of homogeneous, pale to deep blue‐staining mucus (Figure 1A‐C), rarely forming Curschmann's spirals (Figure 1B). Inflammatory cells were embedded in the mucus, or spread more diffusely in the background, or both (Figure 1A,C). Keratinized squames were present in some cases as individuals and small aggregates. Neutrophils, macrophages, and a small number of lymphocytes could usually be identified although in some cases they were too pyknotic to identify, especially where mucus was thick. Eosinophils were rarely seen; basophils and mast cells were not observed. The macrophages were often vacuolated (16/23 [70%] cases suitable for cellular assessment), and many contained hemosiderin (10/23 [43%]), or showed erythrophagocytosis (9/23 [39%]), or leukophagocytosis (6/23 [26%]) (Figure 1D). In 2/30 (7%) smears, cholesterol crystals were observed.
FIGURE 1.
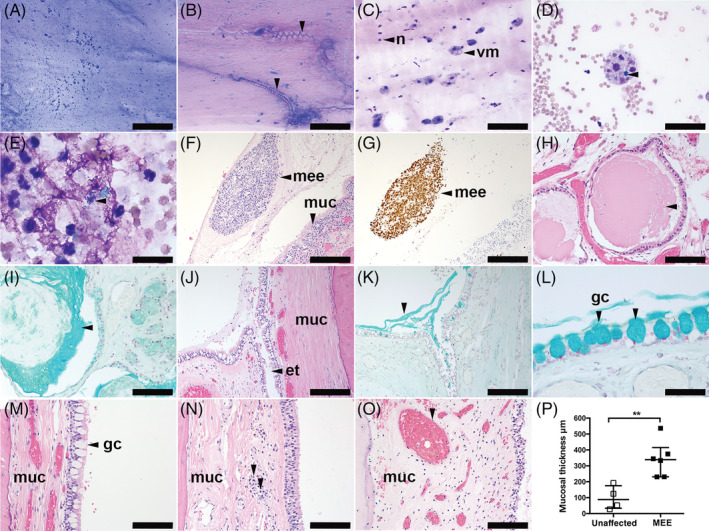
A‐E, Cytology of canine middle ear effusions (MMEs) from cases of MEE. A, Thick mucus with enmeshed cells; B, thick mucus forming Curschmann's spirals (arrowheads). C, Vacuolated macrophages and neutrophils. D, Hemorrhage, erythrophagocytosis, leukophagocytosis, and intracytoplasmic hemosiderin (arrowhead) in a vacuolated macrophage. E, Degenerate neutrophils with intracellular bacteria (arrowhead), May‐Grünwald Giemsa stain. F‐O, Histological sections of canine tympanic bulla. F, Middle ear effusion fluid (arrowhead) containing mucus and many cells adjacent to the hypercellular inflamed mucosa (muc), hematoxylin and eosin (H&E). G, Macrophages predominate in the MEE fluid shown in (F), MAC387 immunohistochemistry. H, Dilatation and accumulation of secretion in the submucosal glands (arrowhead), H&E, and (I) mucus, Alcian blue. J‐L, Unaffected case with anatomically normal goblet cells adjacent to the eustachian tube (ET) ostium (arrowhead), H&E. K, Mucus accumulation at the ET ostium of the same case as (J), Alcian blue (arrowhead). L, Higher magnification image of goblet cells in the same case as (J) and (K), Alcian blue. M, Mucosa in unaffected dog has normal goblet cells (arrowhead), (N,O) thickened mucosa in different dogs with MEE; N, illustrates light infiltration of lymphocytes and plasma cells (arrowheads); and O, with congested (dilated) blood vessels (arrowhead). P, Morphometric analysis shows that the bulla mucosa is significantly thickened in bullae of dogs with MEE. Data in graph (P) are represented as points, the bar represents the median, and the error bars represent the interquartile range. Data were analyzed with a Mann‐Whitney U test. **P < .01 for 2‐tailed tests. et, eustachian tube; gc, goblet cell; n, neutrophil leukocyte; mee, middle ear effusion; muc, mucosa; vm, vacuolated macrophage. Scale bars = (A) 500 μm; (F,G,H,J,K) 200 μm; (B,C,I,M,N,O) 100 μm; (D,L) 50 μm; (E) 20 μm
The scores for the cytological assessment in effusions from which bacteria were isolated on culture (culture‐positive, n = 6) and those from which there was no bacterial growth (culture‐negative, n = 24) are shown in Figure 2. The mucus was significantly thicker in the culture‐negative group compared with the culture‐positive group, and the number of RBCs was significantly higher in the culture‐positive group. There was no significant difference between culture‐negative and culture‐positive groups for the scores for cellularity, or neutrophil karyolysis, karyorrhexis, or pyknosis. Cellularity was low in most effusions and only exceeded a score of 1 in 3 effusions from the culture‐negative group and 2 from the culture‐positive group.
FIGURE 2.
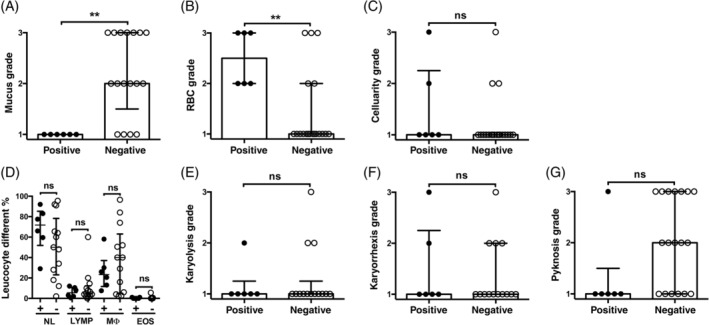
Middle ear cytology. A, Mucus is significantly decreased and B, RBCs are significantly increased in culture‐positive middle ear fluids. The cellularity (C), WBC differential counts (D), and neutrophil karyolysis (E), karyorrhexis (F), and pyknosis grades (G) are not significantly different between culture‐positive and culture‐negative middle ear fluids. Note basophils and mast cells were not detected in the bulla fluid cytology preparations. Data in the graphs are represented as points. In graph (D) the bar, and in graphs (A‐C) and (E‐G), the histogram bar represent the median. The error bars represent the interquartile range. Data were analyzed with Mann‐WhitneyU tests. ns P > .05, **P < .01 for 2‐tailed tests were used. +, culture‐positive; −, culture‐negative; EOS, eosinophils; LYMP, lymphocytes; MØ, macrophages; NL, neutrophils
Overall, neutrophils and macrophages predominated in culture‐positive and culture‐negative fluids, with smaller numbers of lymphocytes and rare eosinophils. When the percentage of each nucleated cell type was compared with each other type across all cases, neutrophils (P < .0001, P < .001) and macrophages (P < .001, P < .05) were greater than eosinophils for both culture‐negative and culture‐positive bulla fluids, respectively, but the percentages of neutrophils and macrophages in each fluid varied widely and were not significantly different between culture‐negative and culture‐positive groups.
Bacteria (cocci, bacilli, or both) were seen in 10/30 (33%) of effusion smears; in 2 cases the bacteria were bilateral. They were extracellular only in 5 effusions and intracellular ± extracellular in 5 (Figure 1E). Five of the 10 smears with bacteria seen on cytology had no growth and 5 were positive on culture; in only 1 case were bacteria isolated on culture but not seen on smears. Malassezia sp. were also seen in 9 effusions, usually attached to keratinized squames, but only 2 of these were positive for this organism on culture.
3.4. Postmortem histology and IHC
The 3 cases in which effusions were identified PM had a small amounts of pale pink proteinaceous material (fluid) with enmeshed cells, mainly macrophages or neutrophils, or both, attached to the mucosa of the bullae in H&E‐stained sections (Figure 1F,G). The bulla submucosae were variably congested; 1 case had moderate submucosal inflammation comprising macrophages and neutrophils (Figure 1F). Anatomically normal goblet cells were evident in 2/3 affected cases. Immunohistochemistry confirmed that the non‐neutrophil inflammatory cells in the effusions were predominantly MAC387‐positive macrophages (Figure 1G); the submucosa had infiltrates of CD3‐positive T‐lymphocytes and plasma cells but Pax5‐positive B‐lymphocyte numbers were very low. Dilatation of the submucosal glands was associated with accumulation of Alcian blue‐positive mucus and was present bilaterally in 1 case (Figure 1H,I) and in a second case dilatation of the submucosal glands was present unilaterally and the contralateral bulla had infolding of epithelium. The third affected case had moderate numbers of submucosal plasma cells and mucosal polyps. The bulla mucosa in dogs with MEE was significantly thicker than unaffected dogs (Figure 1M‐P).
The 2 PM cases without MEE also showed submucosal congestion. One case had moderate unilateral submucosal gland dilatation and the contralateral bulla had epithelial infolding, a small focus of neutrophils, macrophages, plasma cells, and small lymphocytes (comprising rare T and B lymphocytes) in the submucosa and anatomically normal goblet cells near the opening (ostium) of the ET into the bulla (Figure 1J‐M). The other case had unremarkable slender mucosa.
3.5. Bacteriology
There was no aerobic, anaerobic, or mycoplasma bacterial growth from 22 of the 28 (79%) effusions that were cultured. Bacteria isolated from the other 6 (21%) samples consisted of S pseudintermedius (4), Escherischia coli (1), and a mixed growth (1). One case of S pseudintermedius infection was bilateral. Malassezia pachydermatis was isolated on culture from effusions from 2 cases, both unilaterally. Of the 3 dogs with unilateral tympanic membrane perforation, culture was positive for S pseudintermedius in 1 effusion and negative in the other 2.
4. DISCUSSION
In this study, findings typical of MEE were identified on clinical examination, diagnostic imaging, video‐otoscopy, and myringotomy, and when performed, BAER testing. Effusions had varying amounts of mucoid material and a predominance of neutrophils and macrophages in culture‐positive and culture‐negative effusions. Mucus was thicker and the number of RBCs was lower in fluids from which no bacteria were cultured. Bacteria were often observed in smears, and less often yeasts, but the presence of these microbes in smears did not always correspond to positive cultures, in keeping with the finding with human studies that PCR detection of otopathogens is more sensitive than bacterial culture. 26 Cultures were positive in a minority of fluids (21%), with S pseudintermedius predominating.
The variation in bulla mucosa histology in dogs with MEE might be attributable to the indeterminate duration and the severity of otitis media. Metaplasia of submucosal glands found in some bullae might contribute to accumulation of mucus in the bulla fluid, but this change, albeit less pronounced, was found in 1 case without MEE, suggesting that this feature could be a normal age‐related change or that MEE was at an early stage in this dog. The possibility that metaplasia of the submucosal glands is a predisposing factor to development of MEE in susceptible breeds should also be considered. T‐lymphocytes and plasma cells, as well as neutrophils and macrophages were detected in affected bulla mucosae, but small numbers of these cells can be resident in the submucosa of unaffected dogs. Although the number of cases examined was small (n = 3 with MEE, n = 2 without MEE), there was a statistically significant thickening of bulla mucosa in the affected group. Further PM evaluation of symptomatic and nonsymptomatic cases might reveal further qualitative and quantitative histological differences between dogs with MEE and those without. Metaplasia of submucosal glands in dogs resembles that found in rats 27 and humans 28 with chronic otitis media; however, submucosal glandular metaplasia is not documented in mouse models of otitis media.
As expected, CKCS were among those with MEE 1 , 9 , 10 although in the current study other brachycephalic breeds (including boxers and bulldogs) made up more than half of the total cases as is the case in other studies. 6 , 11 No cases of primary MEE were identified in the many dogs of mesocephalic or dolichocephalic breeds referred to the hospital during the study; the few cases with effusions in such breeds arose secondary to changes in the middle ear and pharynx following radiotherapy, space occupying lesions, and surgery. The clinical signs of MEE in the brachycephalic dogs were typical of those previously described, 1 , 3 and all the live cases were referred for evaluation of problems consistent with MEE. However, it is possible that some clinical signs such as pain localized to head or neck and head shaking/scratching were, at least partly, attributable with other concurrent conditions such as Chiari‐like malformation/syringomyelia and AD. 29 , 30 A large‐scale clinical study with CT or MRI would be required to determine the true prevalence of symptomatic and asymptomatic MEE in brachycephalic breeds.
The diagnosis of MEE was based on identification of fluid in the tympanic bullae by CT or MRI, video‐otoscopic examination of the tympanic membrane, myringotomy (where necessary), and collection of the effusion material, as previously reported. 1 , 2 , 4 , 5 , 6 The main abnormalities on video‐otoscopy related to MEE were bulging, opacity, and in a few cases, perforation of the tympanic membrane, as in other studies. 10 , 11 The proportion of unilateral and bilateral effusions in the present study were also similar to previous studies, 3 , 18 with the majority being bilateral. BAER test results were also similar to previous reports. 7 , 8
Brachycephalic morphology is a major risk factor for MEE, probably because of obstruction or dysfunction, or both, of the ET. 12 , 13 In addition, increased soft palate thickness and decreased nasopharyngeal dimensions have been associated with bilateral MEE in CKCS but not Boxers. 13 Other studies show an association with trigeminal nerve mass lesions resulting in dysfunction of the tensor veli palatini muscle involved in opening the pharyngeal orifice of the ET. 31 , 32 However, this is unlikely to be a common cause of ET dysfunction and no such nerve lesions were identified in our study. Cranial and palatine morphology are also important factors in humans. Infants have a relatively small and shortened face with a large domed cranium, and the low prevalence in adults is likely to be at least partly because of maxillary‐facial maturation. 17 In some mouse models of OME, there is malformation of basicranial anatomy, shortening and abnormal angulation of the ET, and cilia abnormalities that might affect clearance of fluid from the middle ear. 33 , 34 The domed heads and brachycephaly in some OME mouse mutants result in an increased angle between the midline of the skull base and the ET leading to a more horizontal position of the ET. 33 Comparison of human infants, some mouse models, and brachycephalic dogs suggests that similar anatomical predisposing factors might be present in all 3 species.
The importance of the bacteria isolated from cases of canine MEE is uncertain. Most samples were culture‐negative (79%), similar to a recent report. 18 In the 21% that were culture‐positive, S pseudintermedius was the main isolate. In 1 study, 18 coagulase‐negative staphylococci were the main bacteria followed by S pseudintermedius, although differences in prevalence might depend on the organisms predominating in the general population. Interpretation of culture results is further complicated by the fact that bacteria, especially coliforms, Branhamella and staphylococci, have been cultured from at least 48% of healthy canine middle ears. 35 In addition, all of the samples with positive cytology, positive cultures, or both came from dogs with concurrent AD and otitis externa, most of which had chronic changes in the external ear canals. The organisms could therefore be contaminants from the external ear canals or from otitis media associated with tympanic perforation or otitis externa, although of the 3 dogs with unilateral tympanic membrane perforation, culture was only positive for S pseudintermedius in 1 and negative in the other 2 effusions. The association of AD with MEE is difficult to determine, as brachycephalic dogs are predisposed to AD, 36 and both are common, arise in young dogs, and are chronic, slowly progressive conditions. This makes it difficult to determine whether one preceded the other. In children, viral or bacterial infection resulting in acute otitis media usually precedes development of OME. However, prior infection is not necessary in mouse models 16 and it is unclear whether this is the case in dogs. In the present study, the effusions were classified as culture‐positive or negative. However, there is currently debate regarding the significance of nonculturable bacteria identified by molecular techniques such as PCR in human MEEs 26 and culture‐negative effusions are not necessarily free of pathogens. This hypothesis is supported by the finding of enhancement of the mucosal lining of the bulla (interpreted as inflammatory thickening) on MRI in 3 dogs with negative cultures in our study. As PCR is not routinely performed on MEEs from canine patients, the presence and importance of bacteria in canine MEE might therefore be underestimated. The role of M pachydermatis is also unclear but as they were mainly found attached to keratinized squames, it is likely that they were contaminants from the external ear or tympanic membrane.
The cytological findings were a major focus of the present study as previous reports are limited and have not described many of the features. One case series of 7 Boxer dogs described the presence of mucus but found no evidence for an inflammatory infiltrate. 11 In our study, mucus viscosity was lower (ie, more serous) in effusions from which bacteria were isolated. The mucus occasionally formed Curschmann's spirals. These structures consist of inspissated mucus and are most commonly seen in bronchoalveolar lavage samples although they have also been reported in dermal lesions. 37 Viscosity of mucins is dependent on their binding to each other and to other glycoproteins, and in human OME, mucin content could be affected by the presence of bacterial DNA. 38 Furthermore, the type of effusion could reflect underlying immunological processes. For example, molecular signatures of effusions in children suggest that serous effusions are more consistent with humoral immunity and mucoid effusions with cell‐mediated immunity. 38 The predominant type of immunity might therefore vary in dogs depending on the presence or absence of bacteria. RBCs were more numerous in culture‐positive ears, although this could reflect a greater tendency of the mucosa to bleed during myringotomy and flushing. However, macrophages showing erythrophagocytosis or intracellular hemosiderin, confirming recent and previous hemorrhage, respectively, were present in both groups and therefore mild preexisting hemorrhage is likely to be a feature whether infected or not. This has also been reported in a small proportion (12.5%) of children with OME. 39
The cellularity was low (<15 cells/×40 field) and therefore only mildly inflammatory in most of the present cases, irrespective of whether bacteria were cultured. The differential cell counts varied considerably between individual dogs, but a consistent marked predominance of neutrophils and macrophages was observed. Neutrophils predominate in chronic OME effusions of children that have culturable pathogenic bacteria and culture‐negative effusions. However, lymphocyte differential counts were higher in culture‐negative effusions even though neutrophils still predominate. 19 Proteomic studies show neutrophils and products such as neutrophil extra cellular traps are abundant in chronic OME effusions. 40 Furthermore, neutrophils and macrophages predominate in both mucoid and serous chronic OME effusions in children but serous effusions had higher lymphocyte differential counts; transcriptome data indicate CD4+ and CD8+ T‐lymphocyte and NK cell signatures. 39 A study that investigated the relationship between microbiome and mucin production showed high prevalence of Haemophilus spp. in chronic OME samples containing both MUC5AC and MUC5B. 41 Inflammatory cell populations in mouse models of chronic otitis media depend on the underlying pathogenesis; in the MECOM model with dysregulated inflammatory responses 42 neutrophils and macrophages predominate, whereas macrophages and lymphocytes predominate in the FBXO11 model where chronic otitis media is initiated by a bulla cavitation defect. 20 In the MECOM mouse model, the predominant population of neutrophils and macrophages showed evidence of cellular hypoxia. Increased oxygen demand by inflammatory cells might contribute to mucosal hypoxia. 43 This could be a contributing factor for OME in children, where upregulation of hypoxia pathways, as evidenced by the upregulation of vascular endothelial growth factor, has been reported. 38 , 39 , 44 , 45 Given the similarities among the cells present in dogs, mouse models, and children, it is possible that such mechanisms of development and response to MEE could also be similar.
In conclusion, this study has demonstrated broadly similar clinical, morphological, and cytological findings between MEE in dogs, OME in people and mouse models, suggesting that these conditions share similar etiological factors and pathogeneses. The impact of microbial status and the microbiome on WBC differential counts and mucin content in MEE in dogs remain to be determined, but might reveal further similarities with human chronic OME. The interpretation of these correlates will require rigorous evaluation of metadata from dogs such as the duration of MEE, and confounding effects such steroid and antibiotic treatment, immune status, and intercurrent disease. Nevertheless, there are grounds to consider that MEE in dogs might be a useful naturally occurring model of OME in children and that more detailed comparison at a molecular level is warranted. Human OME differs from chronic otitis media in mouse models in several important respects; mice do not form the tenacious mucoid effusions seen in the common form of human OME, and human OME resolves spontaneously as well as after successful surgery to place grommets (ventilation tubes) in the tympanic membrane. In comparison, spontaneous resolution of chronic otitis media has not been documented in mice. 16 Dogs with MEE can have mucoid effusions. 1 , 3 , 14 Although surgical biopsy would be needed to study the histology of OME in live dog cases, the alternative, less invasive, approach of using bulla cytology (and microbial detection) to complement otoscopy and CT or MRI imaging employed in this and other dog studies 1 , 3 , 14 has the potential to conduct longitudinal investigations of the response to treatment such as grommet placement 14 as well as determining whether spontaneous resolution also occurs in dogs. Such comparisons have the potential to improve understanding and management of this common condition across the species.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was approved by the Royal (Dick) School of Veterinary Studies Veterinary Ethical Review Committee (approval no. 36‐16).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Criteria for cytological scoring of middle ear effusions.
Table S2 Immunohistochemistry reagents and protocols
ACKNOWLEDGMENTS
The authors thank the Fiona and Ian Russell Seed Corn Fund for generous financial support. The authors are also grateful to the owners, clinicians, and the staff of the Hospital for Small Animals and Pathology Department for their invaluable help.
Milne E, Nuttall T, Marioni‐Henry K, et al. Cytological and microbiological characteristics of middle ear effusions in brachycephalic dogs. J Vet Intern Med. 2020;34:1454–1463. 10.1111/jvim.15792
Funding information The Fiona and Ian Russell Seed Corn Fund for Companion Animal Research, Grant/Award Number: N/A
REFERENCES
- 1. Stern‐Bertholtz W, Sjöström L, Hårkanson NW. Primary secretory otitis media in the Cavalier King Charles spaniel: a review of 61 cases. J Small Anim Pract. 2003;44:253‐256. [DOI] [PubMed] [Google Scholar]
- 2. Dickie AM, Doust R, Cromarty L, Johnson VS, Sullivan M, Boyd JS. Comparison of ultrasonography, radiography and a single computed tomography slice for the identification of fluid within the canine tympanic bulla. Res Vet Sci. 2003;75:209‐216. [DOI] [PubMed] [Google Scholar]
- 3. Cole LK, Samii VF, Wagner SO, Rajala‐Schultz PJ. Diagnosis of primary secretory otitis media in the Cavalier King Charles spaniel. Vet Dermatol. 2015;26:459‐466. e106‐457. [DOI] [PubMed] [Google Scholar]
- 4. Foster A, Morandi F, May E. Prevalence of ear disease in dogs undergoing multidetector thin‐slice computed tomography of the head. Vet Radiol Ultrasound. 2015;56:18‐24. [DOI] [PubMed] [Google Scholar]
- 5. Classen J, Bruehschwein A, Meyer‐Lindenberg A, Mueller RS. Comparison of ultrasound imaging and video otoscopy with cross‐sectional imaging for the diagnosis of canine otitis media. Vet J. 2016;217:68‐71. [DOI] [PubMed] [Google Scholar]
- 6. Owen MC, Lamb CR, Lu D, Targett MP. Material in the middle ear of dogs having magnetic resonance imaging for investigation of neurologic signs. Vet Radiol Ultrasound. 2004;45:149‐155. [DOI] [PubMed] [Google Scholar]
- 7. Harcourt‐Brown TR, Parker JE, Granger N, Jeffery ND. Effect of middle ear effusion on the brain‐stem auditory evoked response of Cavalier King Charles spaniels. Vet J. 2011;188:341‐345. [DOI] [PubMed] [Google Scholar]
- 8. Mason CL, Paterson S, Cripps PJ. Use of a hearing loss grading system and an owner‐based hearing questionnaire to assess hearing loss in pet dogs with chronic otitis externa or otitis media. Vet Dermatol. 2013;24:512‐e121. [DOI] [PubMed] [Google Scholar]
- 9. Corfield GS, Burrows AK, Imani P, Bryden SL. The method of application and short term results of tympanostomy tubes for the treatment of primary secretory otitis media in three Cavalier King Charles spaniel dogs. Aust Vet J. 2008;86:88‐94. [DOI] [PubMed] [Google Scholar]
- 10. Cole LK. Primary secretory otitis media in Cavalier King Charles spaniels. Vet Clin North Am Small Anim Pract. 2012;42:1137‐1142. [DOI] [PubMed] [Google Scholar]
- 11. Paterson S. Otitis media with effusion in the boxer: a report of seven cases. J Small Anim Pract. 2017;59:646–650. [DOI] [PubMed] [Google Scholar]
- 12. Tojo M, Matsuda H, Fukui K, et al. Experimental induction of secretory and purulent otitis media by the surgical obstruction of the Eustachian tube in dogs. J Small Anim Pract. 1985;26:81‐89. [Google Scholar]
- 13. Hayes GM, Friend EJ, Jeffery ND. Relationship between pharyngeal conformation and otitis media with effusion in Cavalier King Charles spaniels. Vet Rec. 2010;167:55‐58. [DOI] [PubMed] [Google Scholar]
- 14. Guerin V, Hampel R, Ter Haar G. Video‐otoscopy‐guided tympanostomy tube placement for treatment of middle ear effusion. J Small Anim Pract. 2015;56:606‐612. [DOI] [PubMed] [Google Scholar]
- 15. Schilder AG, Chonmaitree T, Cripps AW, et al. Otitis media. Nat Rev Dis Primers. 2016;2:16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhutta MF, Thornton RB, Kirkham LS, et al. Understanding the aetiology and resolution of chronic otitis media from animal and human studies. Dis Model Mech. 2017;10:1289‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bluestone CD, Swarts JD. Human evolutionary history: consequences for the pathogenesis of otitis media. Otolaryngol Head Neck Surg. 2010;143:739‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cole LK, Rajala‐Schultz PJ, Lorch G, Daniels JB. Bacteriology and cytology of otic exudates in 41 Cavalier King Charles spaniels with primary secretory otitis media. Vet Dermatol. 2019;30:151‐e144. [DOI] [PubMed] [Google Scholar]
- 19. Lim DJ, Lewis DM, Schram JL, Birck HG. Otitis media with effusion. Cytological and microbiological correlates. Arch Otolaryngol. 1979;105:404‐412. [DOI] [PubMed] [Google Scholar]
- 20. Del‐Pozo J, MacIntyre N, Azar A, et al. Chronic otitis media is initiated by a bulla cavitation defect in the FBXO11 mouse model. Dis Model Mech. 2019;12:dmm038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marchant TW, Johnson EJ, McTeir L, et al. Canine brachycephaly is associated with a retrotransposon‐mediated missplicing of SMOC2. Curr Biol. 2017;27:1573‐1584. e1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steiss JE, Cox NR, Hathcock JT. Brain stem auditory‐evoked response abnormalities in 14 dogs with confirmed central nervous system lesions. J Vet Intern Med. 1994;8:293‐298. [DOI] [PubMed] [Google Scholar]
- 23. Nuttall T, Cole LK. Ear cleaning: the UK and US perspective. Vet Dermatol. 2004;15:127‐136. [DOI] [PubMed] [Google Scholar]
- 24. Institute CaLS . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard VET01‐A4. 4th ed. Wayne, PA; 2013. [Google Scholar]
- 25. Bankhead P, Loughrey MB, Fernandez JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ngo CC, Massa HM, Thornton RB, Cripps AW. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One. 2016;11:e0150949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Del‐Pozo J, MacIntyre N, Azar A, et al. Role of ectodysplasin signalling in middle ear and nasal pathology in rat and mouse models of hypohidrotic ectodermal dysplasia. Dis Model Mech. 2019;12:dmm037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magliocca KR, Vivas EX, Griffith CC. Idiopathic, infectious and reactive lesions of the ear and temporal bone. Head Neck Pathol. 2018;12:328‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rushbridge C. Primary secretory otitis media with effusion in Cavalier King Charles spaniels [letter]. J Small Anim Pract. 2004;45:222. [PubMed] [Google Scholar]
- 30. Volk HA, Davies ES. Middle ear effusions in dogs: an incidental finding? Vet J. 2011;188:256‐257. [DOI] [PubMed] [Google Scholar]
- 31. Kent M, Glass EN, de Lahunta A, Platt SR, Haley A. Prevalence of effusion in the tympanic cavity in dogs with dysfunction of the trigeminal nerve: 18 cases (2004‐2013). J Vet Intern Med. 2013;27:1153‐1158. [DOI] [PubMed] [Google Scholar]
- 32. Wessmann A, Hennessey A, Goncalves R, Benigni L, Hammond G, Volk HA. The association of middle ear effusion with trigeminal nerve mass lesions in dogs. Vet Rec. 2013;173:449. [DOI] [PubMed] [Google Scholar]
- 33. Yang B, Tian C, Zhang ZG, et al. Sh3pxd2b mice are a model for craniofacial dysmorphology and otitis media. PLoS One. 2011;6:e22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Yu H, Xu M, et al. Pathological features in the LmnaDhe/+ mutant mouse provide a novel model of human otitis media and laminopathies. Am J Pathol. 2012;181:761‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuda H, Tojo M, Fukui K, et al. The aerobic bacterial flora of the middle and external ears in normal dogs. J Small Anim Pract. 1984;25:269‐274. [Google Scholar]
- 36. Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet Res. 2015;11:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raskin RE. General categories of cytologic interpretation In: Raskin RE, Meyer DJ, eds. Canine and Feline Cytology: A Color Atlas and Interpretation Guide. 3rd ed. St. Louis, MO: Elsevier; 2016:29‐30. [Google Scholar]
- 38. Val S, Poley M, Anna K, et al. Characterization of mucoid and serous middle ear effusions from patients with chronic otitis media: implication of different biological mechanisms? Pediatr Res. 2018;84:296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhutta MF, Lambie J, Hobson L, et al. Transcript analysis reveals a hypoxic inflammatory environment in human chronic otitis media with effusion. Front Genet. 2020;10(Article 1327):1–10. 10.3389/fgene.2019.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Val S, Poley M, Brown K, et al. Proteomic characterization of middle ear fluid confirms neutrophil extracellular traps as a predominant innate immune response in chronic otitis media. PLoS One. 2016;11:e0152865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krueger A, Val S, Perez‐Losada M, et al. Relationship of the middle ear effusion microbiome to secretory mucin production in pediatric patients with chronic otitis media. Pediatr Infect Dis J. 2017;36:635‐640. [DOI] [PubMed] [Google Scholar]
- 42. Xu X, Woo CH, Steere RR, et al. EVI1 acts as an inducible negative‐feedback regulator of NF‐kappaB by inhibiting p65 acetylation. J Immunol. 2012;188:6371‐6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheeseman MT, Tyrer HE, Williams D, et al. HIF‐VEGF pathways are critical for chronic otitis media in Junbo and Jeff mouse mutants. PLoS Genet. 2011;7:e1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jung HH, Kim MW, Lee JH, et al. Expression of vascular endothelial growth factor in otitis media. Acta Otolaryngol. 1999;119:801‐808. [DOI] [PubMed] [Google Scholar]
- 45. Sekiyama K, Ohori J, Matsune S, Kurono Y. The role of vascular endothelial growth factor in pediatric otitis media with effusion. Auris Nasus Larynx. 2011;38:319‐324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Criteria for cytological scoring of middle ear effusions.
Table S2 Immunohistochemistry reagents and protocols


