Abstract
Background
Reflux and aspiration in people are associated with respiratory disease, whereas approximately 50% of healthy adults microaspirate without apparent consequence. In dogs, analogous information is lacking.
Hypothesis
Healthy dogs commonly have gastroesophageal reflux and a proportion of these dogs will have laryngopharyngeal reflux with silent aspiration.
Animals
Twelve healthy, client‐owned dogs.
Methods
Prospective study: Dogs were free‐fed a meal containing (111 MBq) colloidal 99m‐technetium phytate. Dynamic‐scans were performed 5 and 30 minutes postingestion. Time‐activity curves, reflux margination, volume, frequency, and duration were evaluated over 7 regions of interest in dorsal ± left‐lateral recumbency. Static scans (dorsal recumbency) were performed 2 and 18 hours postfeeding to detect aspiration. Reflux and aspiration were defined as counts ≥200% background activity ± decreased gastric counts. Between‐group comparisons were performed by Wilcoxon rank‐sum test or one‐way ANOVA on ranks with significance of P < .05.
Results
In this study, reflux of variable magnitude was detected in 12/12 dogs. No significant differences in outcome parameters were detected with recumbency (P > .05). Margination to the pharynx and proximal, middle, and distal esophagus was identified in 5/12, 2/12, 3/12, and 2/12 dogs, respectively. Median (IQR) reflux frequency and duration were 2 events/5 minutes (1‐3.3 events/5 minutes) and 6 seconds (4‐9 seconds) respectively. No dog had detectable aspiration.
Conclusions and Clinical Importance
Nuclear scintigraphy can document reflux in dogs. Reflux, but not aspiration, is common in healthy dogs and must be considered when interpreting results in clinically affected dogs.
Keywords: laryngopharyngeal, oropharyngeal, technetium, time‐activity curve
Abbreviations
- AARS
aspiration associated respiratory syndrome
- GER
gastroesophageal reflux
- LPR
laryngopharyngeal reflux
- MBq
megabecquerel
- MU‐VHC
University of Missouri Veterinary Health Center
- ROI
regions of interest
- TAC
time‐activity curves
- Tc‐phytate
colloidal 99m‐technetium phytate
- VFSS
videofluoroscopic swallow study
1. INTRODUCTION
Reflux, inclusive of gastroesophageal and laryngopharyngeal reflux, is a known source of acute and chronic respiratory disease in people. 1 , 2 , 3 , 4 , 5 Reflux and microaspiration occur in healthy adult humans without obvious clinical consequence. 4 , 6 Clinically silent microaspiration highlights the importance of normative data before ascribing clinical significance to reflux, microaspiration, or both in the pathogenesis of respiratory disease in dogs. Repetitive microaspiration is a strong contributor to respiratory disease in people. 7 , 8 , 9 Importantly, treatment of gastroesophageal reflux (GER) and laryngopharyngeal reflux (LPR), reflux extending beyond the esophagus into the laryngopharynx, reduces frequency of disease exacerbations and slows the rate of decline in lung function in people with asthma, chronic obstructive pulmonary disease, and idiopathic pulmonary fibrosis. 1 As in people, a relationship between reflux, microaspiration, and respiratory disease is suspected in dogs based on a few clinical studies, case reports, and studies in animal models. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 However, the frequency of reflux and microaspiration in healthy dogs has not been clearly established. The prevalence of gastroesophageal reflux (GER) in healthy, free‐feeding dogs is 41% as detected by videofluoroscopic swallow study (VFSS). 18 However, this technique lacks the sensitivity to detect reflux associated with microaspiration. The ability to document reflux and microaspiration in dogs with respiratory disease has the potential to open doors for novel avenues of therapeutic intervention. As such, characterizing reflux and aspiration in normal dogs, and subsequently in dogs with aspiration associated respiratory disease (AARS), is an area of significant clinical relevance.
Current diagnostic methods for reflux and aspiration in dogs lack sensitivity and specificity because of intermittent reflux events, variable client vigilance, small volumes of refluxate, and the presence of nonacidic refluxate, which can account for as many as 90% of events in people. 19 , 20 , 21 , 22 , 23 Nuclear scintigraphy has potential to address many of these limitations and has been used successfully to detect reflux and aspiration people. 24 , 25 , 26 , 27 , 28 This technique has been used to safely evaluate pulmonary aspiration in medically fragile human infants. 24 , 25 In dogs, this technique is postulated to be able to detect reflux events missed by owner observation and that might be missed by VFSS because of small volume, detect nonacidic reflux events, broaden effective time of data collection by looking at additive radio‐nuclide activity in several anatomic regions, and help establish the normative data necessary to determine the significance of positive results obtained in clinical patients. 24 , 25 , 26 , 27 , 28 Nuclear scintigraphy might therefore represent a novel and sensitive means to help in the diagnosis of elusive reflux and AARS in dogs.
The objectives of this study were to evaluate the utility of nuclear scintigraphy for detection of reflux in dogs, characterize and determine the prevalence of reflux and aspiration events in healthy mesocephalic dogs, and to obtain clinically relevant normative data to allow further exploration of nuclear scintigraphy as a diagnostic tool for dogs with suspected reflux and AARS. We hypothesize that healthy dogs will commonly have GER and a proportion of these dogs will have LPR with silent aspiration into their respiratory tract.
2. MATERIALS AND METHODS
2.1. Animals
Twelve clinically healthy, adult, mesocephalic companion dogs were enrolled with informed consent (University of Missouri Animal Care and Use Committee, #9871). Dogs were determined to be healthy based on normal physical examinations and the absence of clinical respiratory and GI disease within the preceding 6 months. Brachycephalic breeds and dogs receiving prokinetic medications were excluded.
2.2. Reflux scintigraphy
After a 12‐hour fast, dogs were free‐fed a standardized meal impregnated with (111 MBq) colloidal 99m‐technetium phytate (Tc‐phytate) (Mid‐America Isotopes, Ashland Missouri). The test meals consisted of 1/4 to 1/2 of a 13 oz can of a commercially available maintenance dog food. Volume was tailored by body size. Diets were formulated to meet AAFCO standards. The fat content for each diet was between 17.3% and 18.2% on a dry matter basis. Each meal was followed by water (10‐20 mL) to ensure clearance of radioactive material from the pharynx and esophagus. Additional food was withheld for the remainder of the study. Water was available ad libitum.
Camera standardization was performed by a cobalt‐57 sheet source with uniformity flooding for 3 million counts. The matrix dimensions were 256 by 256. Motion correction was not used in this study to maximize the number of evaluated frames. During data collection, noninvasive temporary markers were placed at the level of the mandible and stomach to ensure regions of interest (ROI) were maintained in the area of detection. Images were collected by a gamma camera with 140 keV parallel hole collimator (Equistand, Middlesex, New Jersey) with Mirage computer system (Medical Imaging technologies, Akron, Ohio) at 2 seconds/frame (frame duration). 29 , 30 Dynamic studies were collected over 5 minutes (150 frames; 1 frame/2 seconds) in left‐lateral and dorsal recumbency (n = 6) or in dorsal recumbency alone (n = 6). Data were collected 5‐ and 30‐minutes postingestion of Tc‐phytate. Static studies were collected for all 12 dogs in dorsal recumbency in order to visualize both lung fields (right and left). Static data were collected over 2 minutes (60 frames; 1 frame/2 seconds) at 2 and 18 hours postingestion of Tc‐phytate. No abdominal pressure was applied. Dogs were housed in accordance with University of Missouri Environmental Health and Safety regulations between scans. All studies were performed without anesthesia or sedation.
2.3. Data analysis
Data analysis was performed by 3DSlicer (version 4.10.1) and Fiji (imageJ) 31 analysis software. For dynamic and static studies performed in dorsal recumbency, ROI were drawn over the pharynx; proximal, middle, and distal esophagus; stomach; and right and left lung fields (Figure 1A). For dynamic studies performed in left‐lateral recumbency, lung ROI were not evaluated because of summation of the right and left lung fields (Figure 1B). For dynamic studies, time‐activity‐curves(TAC), maximal reflux margination, volume, frequency, and duration were evaluated for each ROI. 30 Reflux events (displayed on TAC) were defined as counts ≥200% background activity with a concurrent decrease in gastric counts (Figure 2). 24 Reflux TAC were subcategorized as rising (ie, repeated reflux events with failure of clearance), flat (ie, no reflux or reflux events with return to baseline between events) or falling (ie, reflux with delayed clearance) (Figure 3). 24 Dogs with rising TAC were determined to have cumulative reflux events even if an exact number could not be quantified provided cumulative counts exceeded 200% of background (calculated from paraesophageal soft tissue). Maximal reflux margination was recorded as the maximal distance traveled (ie, most distal ROI from the stomach) by reflux for each dog. Reflux volume was calculated as previously described. 32 Reflux volume was depicted as the percent of gastric counts refluxed during each event. Reflux frequency was calculated as the number of discrete events occurring over the 5‐minute collection period. Reflux duration was determined by the number of seconds counts remained ≥200% background. Static studies were used to confirm complete clearance of reflux after feeding (ie, baseline), persistent ROI contamination and aspiration. Aspiration was defined as counts ≥200% baseline though a concurrent a drop in gastric counts were not considered necessary for diagnosis. 24
FIGURE 1.
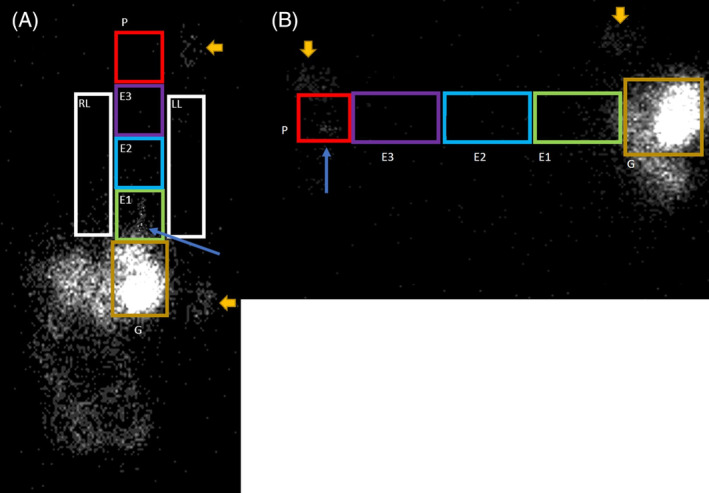
A,B, Regions of interest (ROI) are drawn over dynamic images collected in A, dorsal and B, left‐lateral recumbency 30‐minutes postingestion of a meal containing (111 MBq) colloidal 99m‐technetium phytate (Tc‐phytate). Lung ROI were not collected for dogs in lateral recumbency because of summation of the right and left lung fields. Position markers at the level of the mandible and stomach are denoted by the large gold arrows. Distal esophageal (E1) and pharyngeal (P) reflux are denoted by blue arrows in panels A and B, respectively. Distal third of the esophagus (E1), middle third of the esophagus (E2), and proximal third of the esophagus (E3), pharynx (P), right lung field (RL), left lung field (LL), gastric (G)
FIGURE 2.

Time‐activity curves are displayed for the distal esophagus (E1) (top) and stomach (bottom) respectively. A discrete reflux event representing counts ≥200% background is present between frames 128 and 131 (*) in the distal esophagus. Data were collected at 2 seconds/frame. The duration of this reflux event is 3 seconds. This event corresponds to a decrease in gastric counts over the same time period (**) consistent with true gastroesophageal reflux
FIGURE 3.

Representative reflux time‐activity‐curves (TAC) from the esophagus (rising and falling) and pharynx (flat) are depicted above. Reflux TAC are classified as 1 of the following: rising (reflecting repeated reflux events with failure of clearance), flat (no reflux or reflux events with return to baseline between events) or falling (reflux with delayed clearance)
2.4. Statistical evaluation
Statistical analysis was performed by SigmaPlot (version 14.0) data analysis software. Descriptive statistics were applied where appropriate. Between groups comparisons were made by a one‐way ANOVA on Ranks or Wilcoxon signed‐rank test with a P ≤ .05 significance level. Post hoc analysis (Dunn's analysis for multiple comparisons) was performed where appropriate.
3. RESULTS
3.1. Animals
Twelve healthy companion dogs were enrolled with informed consent. Breeds represented included mixed breed (n = 3), Beagle (n = 2), Labrador Retriever (n = 2), and 1 each for miniature Dachshund, Jack Russel terrier, Brittany spaniel, Feist terrier, and West Highland white terrier. Seven dogs were castrated males and 5 were spayed females. Ages ranged from 3 to 13 years with a median (IQR) age of 9.5 years (6‐10.25 years). Weights ranged from 7.1 to 26.0 kg with a median (IQR) weight of 9.6 kg (8.2‐20.4 kg). Body condition score (9‐point scale) ranged from 4 to 6 with a median (IQR) BCS of 5 (5).
3.2. Scintigraphy
Baseline scans demonstrated complete clearance of Tc‐phytate from the pharynx and esophagus at the start of the study. Reflux events were detected in all 12 dogs by reflux scintigraphy. A total of 144 TAC were further characterized as rising (n = 27), flat (n = 105), and falling (n = 12). No significant differences were detected for demographic data (age, weight, or BCS), recumbency (left‐lateral or dorsal), collection time (5 or 30 minutes), or ROI for TAC subcategory, maximal reflux extension, reflux frequency (events/5 minutes), or reflux duration (sec) (P > .05 for all). As such, groups were combined for further evaluation. Thirty‐three discrete esophageal and 6 pharyngeal reflux events were identified. Pharyngeal reflux was identified in 5/12 dogs. Reflux marginated maximally to the pharynx (5/12 dogs), proximal esophagus (2/12 dogs), middle esophagus (3/12 dogs), and distal esophagus (2/12 dogs). The total number of discrete reflux events identified within the esophageal ROI were 4/33, 6/33, and 23/33 for the proximal, middle, and distal esophagus respectively. The median (IQR) reflux volumes for esophageal and pharyngeal ROI are displayed in Table 1. Distal esophageal reflux volume was significantly greater than for pharyngeal reflux volume (P < .001). Reflux events detectable in the middle and proximal esophagus were not significantly greater in volume than pharyngeal reflux (P > .05). Median (IQR) frequency of reflux was 2 events/5 minutes (1‐3.25 events/5 minutes). Median (IQR) duration (seconds) of reflux was 6 seconds (4‐9 seconds). Static scans showed no evidence of persistent contamination (counts ≥200% of background) within the pharyngeal or esophageal ROI for any dog regardless of time point (2 or 18 hours). Likewise, lung ROI showed no evidence of pulmonary aspiration for any healthy dog regardless of time point (2 or 18 hours). Median (IQR) counts for the baseline, 2‐ and 18‐hour static scans are provided in Table 2.
TABLE 1.
Median (IQR) reflux volume displayed for esophageal (E1‐E3) and pharyngeal (P) regions of interest (ROI). Volume is displayed as a percent of gastric counts. 32 The volume of refluxate in the E1 ROI was significantly increased compared to E2, E3, and P ROI
| Region of interest (ROI) | Median (IQR) |
|---|---|
| P | 1.2% (0.6%‐1.6%) |
| E3 | 0.8% (0.5%‐0.9%) |
| E2 | 0.7% (0.6%‐1.0%) |
| E1 a | 14.4% (7.7%‐27.4%) |
Abbreviations: E1, distal third of esophagus; E2, middle third of esophagus; E3, proximal third of esophagus.
Statistical significance.
TABLE 2.
Median (IQR) counts per region of interest (ROI) for the baseline, 2‐hour, and 18‐hour static scans are provided
| Region of interest (ROI) | Median (IQR) baseline | Median (IQR) 2 h | Median (IQR) 18 h |
|---|---|---|---|
| P | 442 (344‐508.5) | 437 (403‐461) | 554 (473‐598) |
| E3 | 667 (528‐857) | 722 (570‐830) | 726 (687‐1125) |
| E2 | 684 (591‐850) | 704 (614‐849) | 945 (815‐1160) |
| E1 | 683 (545‐786) | 680 (656‐765) | 837 (752‐837) |
| Left lung field | 2143 (1695‐2865) | 2457 (2058‐2735) | 2625 (2392‐3312) |
| Right lung field | 2324 (1735‐2861) | 2350 (2057‐3016) | 3122 (2292‐3731) |
Note: Aspiration was not detected in any dog as denoted by counts ≥200% of background.
Abbreviations: E1, distal third of esophagus; E2, middle third of esophagus; E3, proximal third of esophagus; P, pharynx.
4. DISCUSSION
In this study, reflux scintigraphy was used to successfully identify and characterize reflux events in clinically healthy mesocephalic dogs. Contrary to our hypothesis, reflux (GER and LPR) but not pulmonary aspiration was a common finding in healthy dogs. This provides normative data allowing further investigation into the role of reflux in the pathogenesis of respiratory disease. Investigating the relationship between reflux and aspiration might open new avenues of therapeutic intervention in affected dogs with a variety of respiratory disorders.
People both reflux and aspirate without apparent clinical consequence because of the presence of functional protective and clearance mechanisms. 4 , 6 , 33 Reflux is associated with the pathogenesis and progression of respiratory disease in human medicine with a prevalence of 50% in patients with chronic cough, asthma, COPD, and pulmonary fibrosis. 1 , 2 , 3 , 4 , 5 Therefore, the development of disease is likely dictated by frequency, margination, volume, duration and content of reflux, and aspiration rather than the presence or absence of such events. As such, data documenting the prevalence of reflux and aspiration in normal dogs and objective characterization of these events is necessary before ascribing clinical significance to reflux and aspiration in clinically affected dogs.
Airway‐associated reflux can be subcategorized into GER and LPR. Laryngopharyngeal reflux is considered a supraesophageal manifestation of GER with a demonstrated correlation between the severity of GER and prevalence of LPR. 34 Laryngopharyngeal reflux also increases risk of macro‐ and microaspiration, laryngeal dysfunction and exacerbation of pulmonary pathology. 34 , 35 Treatment of GER and LPR reduces the frequency of disease exacerbations and slow the rate of decline in lung function in people with airway and pulmonary parenchymal disease. 1 Treatment for reflux before brachycephalic airway surgery resulted in decreased postoperative complications in brachycelphalic dogs. 10 Treatment for GER and LPR might therefore reflect a potential avenue of therapeutic intervention for dogs with AARS. Unfortunately, studies evaluating the relationship between GER, LPR, aspiration, and the development of respiratory disease are conspicuously absent in the veterinary literature. This due in part because diagnostics capable of identifying dogs with naturally occurring GER, LPR, and microaspiration are lacking.
In this study, reflux was identified in all dogs, compared with 41% in healthy dogs evaluated by VFSS. 18 This might be attributed to the increased sensitivity of scintigraphy compared to fluoroscopy for small volume reflux as well as the longer collection period of scintigraphy compared to VFSS. 36 Increased intragastric pressure during recumbency might also be contributory and clinically relevant in that it is common for a dog to lay down after eating). There are decreased numbers of transient lower esophageal sphincter relaxations in dogs and people in supine compared to upright posture. 37 , 38 , 39 As such, increased frequency of transient lower esophageal sphincter relaxations, because of dorsal and lateral recumbency are unlikely to contribute to the increased number of dogs with reflux observed in this study. The majority of dogs in this study had an ideal or near ideal body condition score that was similar to the population of dogs evaluated in the VFSS study. 18 As such, body condition is unlikely to contribute to the increased prevalence of reflux noted with scintigraphy. In both the study by fluoroscopy to document reflux 18 and in the current study, dogs were fed a commercially available wet food. Further, the polar surface areas for Tc‐phytate and iohexol are 202 Å2 and 200 Å2, respectively. 18 , 40 As such, particle sizes are considered unlikely to contribute to the difference in the prevalence of reflux between these 2 techniques. The presence of detectable reflux in 100% of healthy dogs evaluated by reflux scintigraphy highlights the need for additional parameters to discriminate between healthy dogs and those with clinically significant reflux.
The use of TACs allows for quantification of repeat reflux events and are routinely evaluated in reflux scintigraphy studies in people. 24 , 41 The presence of a rising TAC is considered supportive of cumulative reflux events though individual reflux events cannot necessarily be visualized because of superimposition. Further motion correction was not performed to increase the number of usable frames. Individual reflux events were determined by counts ≥200% with concurrent drops in gastric counts (Figure 2). Corresponding drops in gastric counts help decrease the likelihood that reflux events were the result of motion artifact. Reflux events were not always traceable to their maximal extent in each ROI (eg, reflux events detectable in ROI E1 and E3 but not E2). This was likely attributable to rapid transit times compared to sampling rate. Similarly discontinuous tracings may be seen in people with reflux detected by impedance manometry as well as scintigraphy. 42 , 43 Such discontinuous tracings precluded correlation of total reflux volume with extent of margination. As such, reflux volume was calculated according the volume calculated within each ROI. A higher frame rate might improve detection of individual reflux events and additional studies in dogs are needed to establish a correlation between severity of GER and the presence of LPR as is seen in people. 29 , 30 , 34 Despite this limitation, the frame rate selected for this study is otherwise considered sufficient. Sampling rates 1 to 6 seconds/frame are used for pediatric gastric scintigraphy studies. Esophageal pH studies demonstrate that increased sampling rate does not correlate with increased acid exposure time, and it has been postulated that brief reflux events are less likely to be clinically relevant than prolonged esophageal contact with refluxate. 27 , 30 , 44 , 45 As a rising TAC curve is indicative of esophageal exposure to gastric contents, the sampling rate in this study is not considered a significant limitation. In this study all TAC subcategories (rising, flat, and falling) were represented. The presence of LPR in this study was not associated with a rising TAC as has been demonstrated in some studies in people though the influence of type 2 error cannot be ruled out. 24 Studies in clinically affected dogs are needed to determine the clinical significance of flat and falling TAC.
Pharyngeal reflux (ie, LPR) was identified in nearly 42% (5/12) of dogs representing approximately 15% of total discrete reflux events detected. This mirrors findings in people where LPR events were detectable in 10% to 30% of asymptomatic adults evaluated by impedance manometry. 46 Laryngopharyngeal reflux, is clinically important as laryngeal structures are susceptible to damage by acid and digestive enzymes. 47 , 48 , 49 In the esophagus, up to 40 reflux episodes per day is considered normal in people; however, as few as 3 reflux episodes reaching the larynx are sufficient to cause detectable damage. 49 In dogs without LPR, maximal esophageal margination for the proximal, middle, and distal esophagus was approximately 16.7% (2/12), 25% (3/12), and 16.7% (2/12) accounting for 70% (23/33), 18% (6/33), and 12% (4/33) of detected individual esophageal reflux events, respectively. This is a departure from VFSS where the majority of reflux events appeared to be contained within the distal esophagus. 18 The frequency of distal esophageal reflux may explain the soft tissue opaque structure in the distal esophagus frequently observed on left‐lateral thoracic radiographs. 50 In healthy adults, proximal esophageal reflux was identified in 34% of upright reflux events by esophageal impedance pH monitoring. Additional studies evaluating the reflux margination in people with gastroesophageal reflux disease and laryngopharyngeal reflux disease, suggest that symptomatic patients have a higher median proximal margination of reflux events, larger reflux volumes, and longer reflux durations than healthy controls. 51
The volume of refluxate is considered important as it reflects the total amount of potentially damaging substances. Absolute quantification of reflux volumes is technically challenging but may be performed by calculating the percent of gastric counts that are detected in the esophagus during reflux events. Large‐volume reflux events (>4%) were occasionally detected in this population. The volume of reflux in the distal esophagus was considerably greater than in the other esophageal and pharyngeal ROI suggesting decreasing volumes of refluxate with increased margination from the stomach. Frequency and duration of reflux events were able to be characterized by reflux scintigraphy suggesting utility in clinical patients; however, controlled studies comparing objective parameters in healthy and clinically affected dogs are needed.
Aspiration was not identified in any healthy dog, which is contrary to the human literature. Species differences might account for this variation, however type 2 statistical error cannot be ruled out and further studies are needed. Lack of sensitivity for detecting aspiration is considered unlikely. Computer acquisition of data demonstrated that as little as 0.1 MBq of activity aspirated into the lungs can be detected by the gamma camera. 52 This suggests that microaspiration is likely to be detected where present.
Limitations of this study include small sample size. Further, not all dogs were evaluated both in left‐lateral and dorsal recumbency. Importantly, no differences were identified with recumbency, which is important as dorsal recumbency allows independent visualization of the right and left lung fields. Ultimately these do not alter the conclusion that reflux scintigraphy represents a viable adjunctive diagnostic for dogs with reflux and AARS especially when repetitive microaspiration is suspected. Finally, only mesocephalic dogs were evaluated in this study.
5. CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
6. OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
7. INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
University of Missouri Animal Care and Use Committee (#9871).
8. HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Dr Grobman's salary is supported in part by the Boehringer Ingelheim Resident‐Postdoctoral Scholar Program. We thank Riley Butler RT (Radiology) for her technical assistance during this project. Preliminary results were presented as an Abstract at the 27th ECVIM‐CA annual congress St. Julian's, Malta, 14‐16 September 2017, and the 35th Veterinary Comparative Respiratory Society Forum, Urbana, Illinois, 1‐4 October 2017. The study was supported by the University of Missouri College of Veterinary Medicine Clinician Scientist Award.
Grobman ME, Maitz CA, Reinero CR. Detection of silent reflux events by nuclear scintigraphy in healthy dogs. J Vet Intern Med. 2020;34:1432–1439. 10.1111/jvim.15798
Funding information University Of Missouri College Of Veterinary Medicine Clinician Scientist Award; Boehringer Ingelheim Resident‐Postdoctoral Scholar Program
REFERENCES
- 1. Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332‐338. [DOI] [PubMed] [Google Scholar]
- 2. Gaude GS. Pulmonary manifestations of gastroesophageal reflux disease. Ann Thorac Med. 2009;4:115‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuksel ES, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease: cough, asthma, laryngitis, chest pain. Swiss Med Wkly. 2012;142:w13544. [DOI] [PubMed] [Google Scholar]
- 4. Molyneux ID, Morice AH. Airway reflux, cough and respiratory disease. Ther Adv Chronic Dis. 2011;2:237‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irwin RS, Zawacki JK, Curley FJ, French CL, Hoffman PJ. Chronic cough as the sole presenting manifestation of gastroesophageal reflux. Am Rev Respir Dis. 1989;140:1294‐1300. [DOI] [PubMed] [Google Scholar]
- 6. Smith JA, Houghton LA. The oesophagus and cough: laryngo‐pharyngeal reflux, microaspiration and vagal reflexes. Cough (London, England). 2013;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Decalmer S, Stovold R, Houghton LA, et al. Chronic cough: relationship between microaspiration, gastroesophageal reflux, and cough frequency. Chest. 2012;142:958‐964. [DOI] [PubMed] [Google Scholar]
- 8. Harding SM, Schan CA, Guzzo MR, et al. Gastroesophageal reflux‐induced bronchoconstriction. Is microaspiration a factor? Chest. 1995;108:1220–1227. [DOI] [PubMed] [Google Scholar]
- 9. Houghton LA, Lee AS, Badri H, DeVault KR, Smith JA. Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat Rev Gastroenterol Hepatol. 2016;13:445‐460. [DOI] [PubMed] [Google Scholar]
- 10. Poncet CM, Dupre GP, Freiche VG, Bouvy BM. Long‐term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J Small Anim Pract. 2006;47:137‐142. [DOI] [PubMed] [Google Scholar]
- 11. Lecoindre P, Richard S. Digestive disorders associated with the chronic obstructive respiratory syndrome of brachycephalic dogs: 30 cases (1999‐2001). Rev Med Vet. 2004;155:141‐146. [Google Scholar]
- 12. Poncet C, Dupre G, Freiche V, et al. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract. 2005;46:273‐279. [DOI] [PubMed] [Google Scholar]
- 13. Määttä OM, Laurila HP, Holopainen S, et al. Reflux aspiration in lungs of dogs with respiratory disease and in healthy West Highland White Terriers. J Vet Intern Med. 2018;32:2074‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lux CN, Archer TM, Lunsford KV. Gastroesophageal reflux and laryngeal dysfunction in a dog. J Am Vet Med Assoc. 2012;240:1100‐1103. [DOI] [PubMed] [Google Scholar]
- 15. Ishikawa T, Sekizawa S‐I, Sant'Ambrogio FB, et al. Larynx vs esophagus as reflexogenic sites for acid‐induced bronchoconstriction in dogs. J Appl Physiol. 1999;86:1226‐1230. [DOI] [PubMed] [Google Scholar]
- 16. Nafe LA, Grobman M, Reinero C, et al. Aspiration‐related respiratory syndromes in the dog. J Am Vet Med Assoc. 2018;253(3):292‐300. [DOI] [PubMed] [Google Scholar]
- 17. Grobman M, Masseau I, Reinero C. Aerodigestive disorders in dogs evaluated for cough using respiratory fluoroscopy and videofluoroscopic swallow studies. Vet J. 2019;251:105344. [DOI] [PubMed] [Google Scholar]
- 18. Harris R, Grobman M, Mitchell A, et al. Standardizing a freely‐behaving canine videofluoroscopic swallow study protocol to investigate dysphagia in dogs. J Vet Intern Med. 2017;31(2):383‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kook P, Kempf J, Ruetten M, et al. Wireless ambulatory esophageal pH monitoring in dogs with clinical signs interpreted as gastroesophageal reflux. J Vet Intern Med. 2014;28:1716‐1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Postma GN, Belafsky PC, Aviv JE, et al. Laryngopharyngeal reflux testing. Ear Nose Throat J. 2002;81:14‐18. [PubMed] [Google Scholar]
- 21. Vaezi MF. New tests for the evaluation of laryngopharyngeal reflux. Gastroenterol Hepatol. 2013;9:115‐117. [PMC free article] [PubMed] [Google Scholar]
- 22. Sidhwa F, Moore A, Alligood E, et al. Diagnosis and Treatment of the Extraesophageal Manifestations of Gastroesophageal Reflux Disease. Ann Surg. 2017;265 63–26567. [DOI] [PubMed] [Google Scholar]
- 23. Faruqi S, Molyneux ID, Fathi H, et al. Chronic cough and esomeprazole: a double‐blindplacebo‐controlled parallel study. Respirology. 2011;16:1150‐1156. [DOI] [PubMed] [Google Scholar]
- 24. Falk GL, Beattie J, Ing A, et al. Scintigraphy in laryngopharyngeal and gastroesophageal reflux disease: a definitive diagnostic test? World J Gastroenterol. 2015;21:3619‐3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elbl B, Birkenfeld B, Walecka A, et al. Upper gastrointestinal tract scintigraphy and ultrasonography in diagnosis of gastroesophageal reflux in children. Pol J Radiol. 2011;76:63. [PMC free article] [PubMed] [Google Scholar]
- 26. Ravelli AM, Panarotto MB, Verdoni L, Consolati V, Bolognini S. Pulmonary aspiration shown by scintigraphy in gastroesophageal reflux‐related respiratory disease. Chest J. 2006;130:1520‐1526. [DOI] [PubMed] [Google Scholar]
- 27. Songür N, Songür Y, Cerci SS, et al. Gastroesophageal scintigraphy in the evaluation of adult patients with chronic cough due to gastroesophageal reflux disease. Nucl Med Commun. 2008;29:1066‐1072. [DOI] [PubMed] [Google Scholar]
- 28. Silver KH, Van Nostrand D. The use of scintigraphy in the management of patients with pulmonary aspiration. Dysphagia. 1994;9:107‐115. [DOI] [PubMed] [Google Scholar]
- 29. Mariani G, Boni G, Barreca M, et al. Radionuclide gastroesophageal motor studies. J Nucl Med. 2004;45:1004‐1028. [PubMed] [Google Scholar]
- 30. Radiology ACo. Society of Nuclear Medicine, Society for Pediatric Radiology . ACR‐SNM‐SPR Practice Guideline for the Performance of Gastrointestinal Scintigraphy in 2011; American College of Radiology; 2010. [Google Scholar]
- 31. Schindelin J, Arganda‐Carreras I, Frise E, et al. Fiji: an open‐source platform for biological‐image analysis. Nat Methods. 2012;9:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puranik AD, Nair G, Aggarwal R, et al. Scintigraphic scoring system for grading severity of gastro‐esophageal reflux on 99mTc sulfur colloid gastro‐esophageal reflux scintigraphy: a prospective study of 39 cases with pre and post treatment assessment. Indian J Nucl Med. 2013;28:79‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Troche MS, Brandimore AE, Godoy J, et al. A framework for understanding shared substrates of airway protection. J Appl Oral Sci Revista FOB. 2014;22:251‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Groome M, Cotton JP, Borland M, McLeod S, Johnston DA, Dillon JF. Prevalence of laryngopharyngeal reflux in a population with gastroesophageal reflux. Laryngoscope. 2007;117:1424‐1428. [DOI] [PubMed] [Google Scholar]
- 35. Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J. 2002;81:7‐9. [PubMed] [Google Scholar]
- 36. Padhy A, Gopinath P, Sharma S, et al. Radionuclide detection of gastroesophageal reflux in children suffering from recurrent lower respiratory tract infection. Indian J Pediatr. 1990;57:517‐525. [DOI] [PubMed] [Google Scholar]
- 37. Little AF, Cox MR, Martin CJ, et al. Influence of posture on transient lower oesophageal sphincter relaxation and gastro‐oesophageal reflux in the dog. J Gastroenterol Hepatol. 1989;4:49‐54. [DOI] [PubMed] [Google Scholar]
- 38. Mittal RK, Holloway RH, Penagini R, Ashley Blackshaw L, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601‐610. [DOI] [PubMed] [Google Scholar]
- 39. Cho YK. How to interpret esophageal impedance pH monitoring. J Neurogastroenterol Motil. 2010;16:327‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Information NCfB . PubChem Database. CID 76957057.
- 41. Caglar M, Volkan B, Alpar R. Reliability of radionuclide gastroesophageal reflux studies using visual and time‐activity curve analysis: inter‐observer and intra‐observer variation and description of minimum detectable reflux. Nucl Med Commun. 2003;24:421‐428. [DOI] [PubMed] [Google Scholar]
- 42. Mousa HM, Rosen R, Woodley FW, et al. Esophageal impedance monitoring for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2011;52:129‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uslu Kızılkan N, Bozkurt MF, Saltık Temizel IN, et al. Comparison of multichannel intraluminal impedance‐pH monitoring and reflux scintigraphy in pediatric patients with suspected gastroesophageal reflux. World J Gastroenterol. 2016;22:9595‐9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lutsi B, Hirano I. Ambulatory pH monitoring: new advances and indications. Gastroenterol Hepatol. 2006;2:835‐842. [PMC free article] [PubMed] [Google Scholar]
- 45. Emde C, Garner A, Blum A. Technical aspects of intraluminal pH‐metry in man: current status and recommendations. Gut. 1987;28:1177‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Merati AL, Ulualp SO, Lim HJ, Toohill RJ. Meta‐analysis of upper probe measurements in normal subjects and patients with laryngopharyngeal reflux. Ann Otol Rhinol Laryngol. 2005;114:177‐182. [DOI] [PubMed] [Google Scholar]
- 47. Patel DA, Blanco M, Vaezi MF. Laryngopharyngeal reflux and functional laryngeal disorder: perspective and common practice of the general gastroenterologist. Gastroenterol Hepatol. 2018;14:512‐520. [PMC free article] [PubMed] [Google Scholar]
- 48. Campagnolo AM, Priston J, Thoen RH, Medeiros T, Assunção AR. Laryngopharyngeal reflux: diagnosis, treatment, and latest research. Int Arch Otorhinolaryngol. 2014;18:184‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129‐2134. [DOI] [PubMed] [Google Scholar]
- 50. Thrall DE, Robertson ID. Atlas of Normal Radiographic Anatomy and Anatomic Variants in the Dog and Cat‐E‐Book. Elsevier Health Sciences; 2015. [Google Scholar]
- 51. Sifrim D. Relevance of volume and proximal extent of reflux in gastro‐oesophageal reflux disease. Gut. 2005;54:175‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruth M, Carlsson S, Månsson I, Bengtsson U, Sandberg N. Scintigraphic detection of gastro‐pulmonary aspiration in patients with respiratory disorders. Clin Physiol. 1993;13:19‐33. [DOI] [PubMed] [Google Scholar]


