Abstract
Background
Dogs with hypoadrenocorticism (HA) frequently show signs of gastrointestinal disease (SGD). The prevalence of dogs presented for chronic SGD with HA is unknown.
Objectives
The aims of this study were to determine the prevalence of HA in dogs with chronic SGD and to identify clinical and laboratory variables for HA in this population.
Animals
One hundred fifty‐one dogs with chronic SGD.
Methods
In this multicentered prevalence study a standardized workup was performed in prospectively enrolled dogs with SGD > 3 weeks duration. Basal serum cortisol concentration was measured in every dog with ACTH stimulation test (ACTHST) if basal serum cortisol concentration was <3 μg/dL.
Results
Basal serum cortisol concentration was <3 μg/dL in 80/151 (53%) dogs, <2 μg/dL in 42/151 (28%) dogs, and < 1 μg/dL in 9/151 (6%) dogs. In 6/151 dogs HA was diagnosed based on ACTHST (stimulated serum cortisol concentration < 2 μg/dL), a prevalence of 4%. There was no difference in history, physical examination, and laboratory variables between dogs with HA and those with other causes of chronic SGD. In 4/6 dogs with HA, there was melena or hematochezia indicating gastrointestinal blood loss. Hyperkalemia, hyponatremia, or both was not observed in any dog.
Conclusion and Clinical Importance
The prevalence of HA among dogs with chronic SGD is higher than in the general population. Based on these results, testing adrenal function should be performed as a standard screening test in dogs with chronic SGD to differentiate between HA and chronic enteropathies.
Keywords: Addison's disease, atypical hypoadrenocorticism, chronic diarrhea, cortisol
Abbreviations
- ACTHST
ACTH stimulation test
- ALT
alanine aminotransferase
- CI
confidence interval
- GDH
glucocorticoid‐deficient hypoadrenocorticism
- GI
gastrointestinal
- GIS
gastrointestinal signs
- GSAVA
German Small Animal Veterinary Association
- HA
hypoadrenocorticism
- HPA
hypothalamus‐pituitary‐adrenal axis
- MGDH
mineralocorticoid‐ and glucocorticoid deficient hypoadrenocorticism
- SGD
signs of gastrointestinal disease
1. INTRODUCTION
Hypoadrenocorticism (HA) is an uncommon endocrinopathy in dogs with prevalence ranges from 0.06 to 0.28% with 0.09% of an insured dog population in Sweden being affected.1, 2 There is an increased risk for HA in Portuguese Water Dogs, Standard Poodles, Bearded Collies, Cairn Terrier, and Cocker Spaniels.2 There is also a genetic predisposition for HA in breeds such as the Nova Scotia Duck Tolling Retriever, Leonberger and Pomeranian, with the highest prevalence (9.73%) in Great Pyrenees dogs.3, 4
It is assumed that primary HA results from a slowly progressing immune‐mediated destruction and consecutive atrophy of the adrenal cortex, which is also the most common cause for Addison's disease in humans.5, 6, 7 Rare other causes include trauma and infiltrative damage by neoplasia, abscess, and granulomatous inflammation.8, 9, 10
In most cases of HA gradual destruction of all 3 layers of the adrenal cortex results in an inadequate secretion of both mineralocorticoid and glucocorticoid hormones (MGDH), leading to typical electrolyte imbalances (namely hyperkalemia, hyponatremia, and often also hypochloremia) with sometimes dramatic clinical consequences.11, 12 However, up to 30% of dogs with HA have what has been termed atypical or glucocorticoid deficient HA (GDH) where electrolyte concentrations remain within the reference range.11 The absence of typical laboratory abnormalities makes GDH more difficult to diagnose in a clinical setting.
Dogs with HA are frequently presented with vague, episodic, and nonspecific clinical signs including anorexia (89%), vomiting (72%), weight loss (42%), and diarrhea (35%).11, 13
Signs of gastrointestinal disease (SGD) secondary to a lack of glucocorticoids are indistinguishable from clinical signs caused by primary GI disorders.11, 14, 15, 16 In the absence of electrolyte abnormalities, subtle laboratory abnormalities might lead to a suspicion of HA. These can include the lack of a stress leukogram, relative or absolute lymphocytosis, eosinophilia, prerenal azotemia, hypoalbuminemia, hypoglycemia or hypercalcemia.11, 13, 17 However, these changes are nonspecific, have a number of possible causes and do not help to discriminate primary GI disease from HA.
Hence, diagnosis of HA, especially GDH, is dependent on adrenal gland function testing, such as ACTH‐stimulation test (ACTHST).11, 12, 17 This multicenter study was conducted in order to evaluate the necessity of performing this relatively expensive and time‐consuming test in dogs with chronic SGD. The primary aim was to assess the prevalence of HA in dogs with chronic SGD. The secondary aim was to identify clinical and laboratory variables that might help to identify or exclude HA in this group of dogs before performing ACTHST, especially in comparison to other dogs with chronic SGD that do not have HA.
2. MATERIALS AND METHODS
2.1. Study design and general diagnostic workup
This study was conducted prospectively by members of the German Small Animal Veterinary Association (GSAVA) Working Group for Gastroenterology at 6 different private or university referral hospitals. Ethical approval was obtained according to German animal welfare laws (approval no.: 37‐24‐11‐2014). Each owner was informed about the purpose of the study and signed a consent form stating that blood samples were used for scientific research projects.
Between November 2014 and December 2015, dogs older than 6 months of age presenting with chronic (>3 weeks) SGD such as vomiting, regurgitation, diarrhea, weight loss, or anorexia were offered enrolment. Dogs treated with steroids were only included if (1) short‐acting corticosteroids (<12 hours; eg, hydrocortisone) were given for not more than 1 week and had been discontinued for at least 1 week before enrolment; (2) intermediate‐acting corticosteroids (12‐36 hours; eg, prednisolone) were given for not more than 1 week and had been discontinued for at least 4 weeks before enrolment; and (3) long‐acting corticosteroids (>36 hours; eg, dexamethasone) had been discontinued for at least 3 months before enrolment. These criteria were selected on the basis of a consensus between 3 endocrinologists and the authors. There are no published guidelines about the delay required for adequate adrenal function recovery after administration of different corticosteroids, and the best timing for performing an ACTH stimulation.
Clinical signs were quantified using the canine chronic enteropathy clinical activity index (CCECAI).18 Diagnostic workup consisted of a complete blood count, routine biochemistry profile including electrolytes, and determination of serum TLI, cPL, folate and cobalamin concentration in all dogs.
Clinicians were given the choice to send blood count and biochemistry to 1 of 2 laboratories (IDEXX Laboratories, Ludwigsburg, Germany and Central Laboratory Animal Research Facility, University Utrecht). At Idexx Laboratories blood count was measured using Sysmex XT and biochemistry was measured using Beckman AU5800.19
In Utrecht blood count was measured using Siemens ADVIA 2120 and biochemistry was measured using Hitachi 912 (Roche Diagnostics GmbH, Germany).
Fecal parasitology (flotation, standard egg count) was performed in every dog if recent fecal testing results from the referring veterinary surgeon were not available. Further diagnostic workup including diagnostic imaging modalities was performed based on individual clinician's decisions and availability.
2.2. Endocrine testing and cortisol measurements
Basal serum cortisol concentration was determined in all dogs. An ACTHST was performed if basal serum cortisol concentration was <3 μg/dL (<83 nmol/L) or without waiting for the result of the basal cortisol dictated by the dog's clinical signs or the owner's willingness to return for the test. A basal cortisol level of 3 μg/dL as cutoff for adrenal testing was empirically chosen to ensure not to miss any dog with hypoadrenocorticism.20, 21
For the ACTHST 5 μg/kg synthetic ACTH (Synacthen, Alfasigma S.p.A., Milan, Italy) was administered by intravenous injection and serum cortisol concentration determined before and 1 hour after ACTH administration.22 The diagnosis of HA was defined by a stimulated serum cortisol concentration ≤2 μg/dL.23
Cortisol concentration was measured using a solid phase, competitive chemiluminescent enzyme immunoassay (IMMULITE 2000 Cortisol, Siemens) validated for dogs.24, 25
Clinicians were given the choice to send samples for cortisol determination to 1 of 2 laboratories (IDEXX Laboratories, Ludwigsburg, Germany and Central Laboratory Animal Research Facility, University Utrecht), both using the same methodology.
2.3. Statistical evaluation and presentation of the data
A sample size analysis was performed using G*Power 3. and BIAS for Windows 11.01 g software.26 For evaluation of the HA prevalence a maximum prevalence of 5% was postulated and the maximum range of the 95% confidence interval was defined as 7%. For evaluation of the laboratory findings an effective size Cohen's d > 1, a P‐value <.05 and a minimum power of 80% was predetermined.
Statistical analyses were conducted with IBM SPSS 23.0 (IBM Germany GmbH, Ehningen, Germany), MedCalc Statistical Software version 17.9.7 (MedCalc Software bvba, Ostend, Belgium), and BIAS for Windows 11.01(Epsilon Ed., Frankfurt, Germany). Normal distribution of data was determined by Kolmogorov‐Smirnoff test. Data were compared using Fisher's exact and Mann‐Whitney U test. Statistical significance was defined as P < .05.
The prevalence and its 95% confidence interval (CI) according to Wilson were calculated. A binomial test was used to compare the prevalence of HA with the 2 reported values of 0.06 and 0.28%.1
3. RESULTS
Of 151 dogs (41 dogs from the Clinic of Small Animal Medicine LMU Munich Germany, 70 dogs from Small Animal Hospital Internal Medicine, Justus Liebig University Gießen, Germany and 40 dogs from Division of Small Animal Internal Medicine, Department of Clinical Sciences of Companion Animals, Faculty of Veterinary Medicine, Utrecht University, Netherlands) enrolled in the study, 6 dogs were diagnosed with HA (all of them GDH). The calculated prevalence was 4.0% (95% CI: 1.8%‐8.4%). A binomial test indicated that the proportion of dogs with SGD was significantly higher (P < .001) than the expected prevalence of 0.06 or 0.28% (1‐sided).1, 2
3.1. Signalment and history
3.1.1. Dogs without HA (n = 145)
Most common breeds of dogs without HA were mixed breed dogs (n = 35), Golden Retriever (n = 8), Labrador Retriever (n = 7), Poodle (n = 7), Dachshund (n = 7), German Shepherd (n = 6), Jack Russel Terrier (n = 5), Yorkshire Terrier (n = 5), French Bulldog (n = 5), Collie (n = 3), Hovawart (n = 3), Rottweiler (n = 3) and 51 other purebred dogs of 42 different breeds. Eighty‐two dogs were male, of which 41 were neutered, and 63 female, of which 34 were spayed. Median age was 4.0 years (range: 1 year to 15 years) and median body weight was 17.7 kg (range: 1.8‐71.7 kg).
Median duration of SGD was 5 months (range: 1‐96 months). Most frequent presenting complaints were abnormal fecal or stool consistency (106/141; 75%), increased defecation frequency (85/135; 63%), mucoid feces (49/140; 35%), tenesmus (32/140; 23%), hematochezia (35/140; 32%), and melena (9/141; 6%). Fifty‐two percent of dogs had a history of chronic vomiting (17% 1x/week; 12% 2‐3x/week; 23% >3x/week). Median CCECAI was 5 (range: 1‐20), which reflects mild disease activity.
3.1.2. Dogs with HA (n = 6)
Dogs with HA consisted of 3 mixed breed dogs, and 1 each of the following breeds: Standard Poodle, Basset hound, and German Shepherd dog. Three dogs were male, of which 1 was neutered, and 3 were spayed females. Median age was 5 years (range: 3‐11 years) and median body weight was 23.9 kg (range: 8.5‐31.0 kg). Median duration of SGD until presentation was 3 months (range: 1‐36 months). Signs of gastrointestinal disease were abnormal fecal or stool consistency (5/6), increased defecation frequency (5/6), mucoid feces (3/5), melena (2/6), hematochezia (4/6), and tenesmus (1/5). Four dogs had a history of chronic vomiting (1 dog 1x/week; 3 dogs 2‐3x/week). The median CCECAI in the 6 dogs with HA was 6.5 (range: 3‐10), reflecting a moderate disease activity.
No statistical difference concerning breed (P = .12), age (P = .31), sex (P = .75), body weight (P = .68), and severity of clinical signs (P = .67) between dogs with and without HA was detected.
3.2. Laboratory findings
Laboratory variables of dogs with and without HA are shown in Tables 1, 2, 3. There was no significant difference in any laboratory variable between groups. In dogs without HA 6 dogs had hyponatremia, 2 dogs had hyperkalemia but none of those dogs had a combination of hyponatremia and hyperkalemia. None of the 6 dogs with HA had hyperkalemia or hyponatremia, but 2 dogs with signs of gastrointestinal bleeding had combined laboratory changes of anemia, hypoalbuminemia, and increased urea.
Table 1.
Hematology parameters of 151 dogs with chronic gastrointestinal signs grouped by presence or absence of hypoadrenocorticism (HA)
| Variable | Dogs without HA | Dogs with HA | P value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Hematocrit (l/L) | 0.50 (n = 143) | 0.34‐0.66 | 0.46 (n = 6) | 0.35‐0.62 | .20 |
| MCV (f/L) | 71 (n = 141) | 57‐88 | 68 (n = 6) | 66‐77 | .53 |
| Leukocytes (×109/L) | 9.3 (n = 141) | 4.6‐63.5 | 13.0 (n = 6) | 5.5‐26.5 | .18 |
| Monocytes (×109/L) | 0.41 (n = 138) | 0.14‐1.48 | 0.53 (n = 6) | 0.10‐1.80 | .33 |
| Lymphocytes (×109/L) | 1.71 (n = 139) | 0.28‐25.40 | 2.57 (n = 6) | 0.99‐5.97 | .12 |
| Neutrophils (×109/L) | 6.37 (n = 137) | 2.46‐44.27 | 6.77 (n = 6) | 3.86‐22.30 | .73 |
| Eosinophils (×109/L) | 0.46 (n = 135) | 0.03‐1.69 | 0.40 (n = 6) | 0.03‐1.40 | .86 |
| Thrombocytes (×109/L) | 252 (n = 139) | 77‐875 | 224 (n = 6) | 115‐421 | .54 |
Table 2.
Serum biochemistry parameters of 151 dogs with chronic gastrointestinal signs grouped by presence or absence of hypoadrenocorticism (HA)
| Variables | Dogs without HA | Dogs with HA | P value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| ALT (U/L) | 48 (n = 145) | 9‐531 | 91 (n = 6) | 52‐581 | .02 |
| AP (U/L) | 41 (n = 145) | 11‐590 | 46 (n = 6) | 26‐678 | .52 |
| Bilirubin (μmol/L) | 3.42 (n = 144) | 0.10‐6.84 | 3.42 (n = 6) | 2.10‐5.13 | .70 |
| Cholesterol (mmol/L) | 5.68 (n = 142) | 1.12‐14.14 | 2.49 (n = 6) | 0.90‐7.59 | .05 |
| Triglyceride (mmol/L) | 0.58 (n = 104) | 0.24‐2.75 | 0.36 (n = 4) | 0.17‐0.73 | .06 |
| Total protein (g/L) | 61 (n = 145) | 25‐86 | 62 (n = 6) | 53‐85 | .66 |
| Albumin (g/L) | 32.50 (n = 145) | 7.70‐39.50 | 35.00 (n = 6) | 21.00‐42.10 | .38 |
| Urea (mmol/L) | 3.16 (n = 145) | 1.00‐18.48 | 4.41 (n = 6) | 1.33‐24.00 | .42 |
| Creatinine (μmol/L) | 79.56 (n = 144) | 26.52‐282.89 | 77.28 (n = 6) | 44.20‐97.24 | .76 |
| Glucose (mmol/L) | 5.11 (n = 144) | 2.61‐9.10 | 4.44 (n = 6) | 3.77‐6.77 | .16 |
| Folic acid (ng/mL) | 10.90 (n = 131) | 2.16‐43.70 | 13.70 (n = 6) | 10.00‐18.20 | .11 |
| Cobalamin (pg/mL) | 352 (n = 138) | 106‐3362 | 295 (n = 6) | 175‐363 | .12 |
Table 3.
Electrolyte values of 151 dogs with signs of chronic gastrointestinal disease grouped by presence or absence of hypoadrenocorticism (HA)
| Variables | Dogs without HA | Dogs with HA | P value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Sodium (mmol/L) | 147 (n = 144) | 114‐152 | 146 (n = 6) | 142‐150 | .65 |
| Potassium (mmol/L) | 4.3 (n = 144) | 2.6‐6.1 | 4.5 (n = 6) | 3.9‐4.9 | .30 |
| Calcium (mmol/L) | 2.50 (n = 144) | 1.26‐3.10 | 2.55 (n = 6) | 2.30‐2.80 | .90 |
| Chloride (mmol/L) | 113 (n = 143) | 103‐121 | 113 (n = 6) | 95‐121 | .90 |
| Phosphate (mmol/L) | 1.1 (n = 143) | 0.5‐2.2 | 1.3 (n = 6) | 1.0‐2.1 | .07 |
Basal serum cortisol concentration was ≤3 μg/dL (≤83 nmol/L) in 80/151 dogs (53%), ≤2 μg/dL (≤55 nmol/L) in 42/151 dogs (28%), and ≤1 μg/dL (≤28 nmol/L) in 9/151 dogs (6%) including all 6 dogs with HA. The basal and stimulated serum cortisol concentrations of the ACTH stimulation testing comparing dogs with HA (n = 6) and without HA (n = 145) are shown in Figures 1 and 2. Serum cortisol concentrations before and after the stimulation with ACTH for each individual group (dogs with and without hypoadrenocorticism) are presented in Table 4, as well as in Figure 3 and 4.
Figure 1.
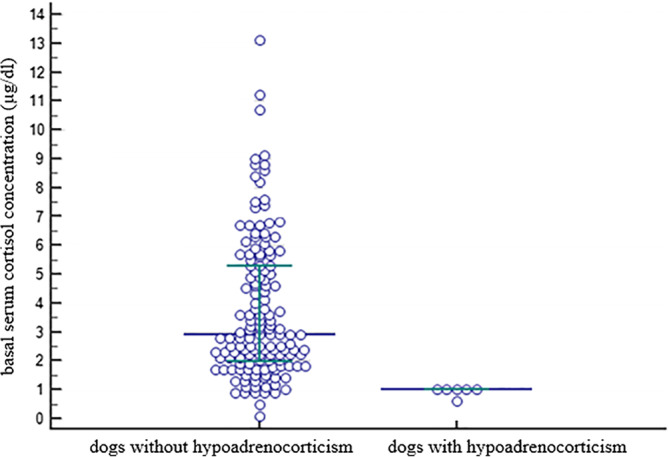
Scatter plot showing basal serum cortisol concentrations in 145 dogs without hypoadrenocorticism and 6 dogs with hypoadrenocorticism. The horizontal bars represent the 25% percentile, the median and the 75% percentile
Figure 2.
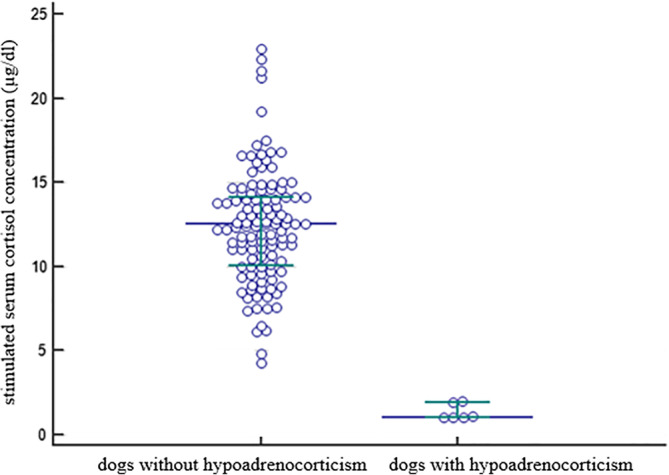
Scatter plot showing stimulated serum cortisol concentrations in 145 dogs without hypoadrenocorticism and 6 dogs with hypoadrenocorticism. The horizontal bars represent the 25% percentile, the median and the 75% percentile
Figure 3.
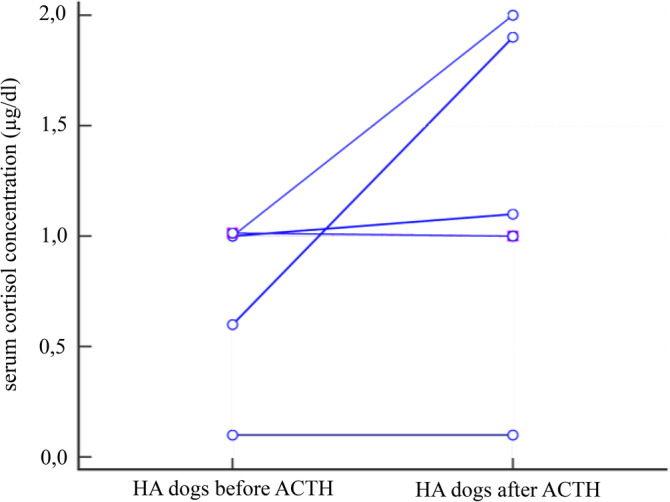
Scatter plot showing serum cortisol concentrations in 6 dogs with hypoadrenocorticism before and after the stimulation with ACTH
Figure 4.
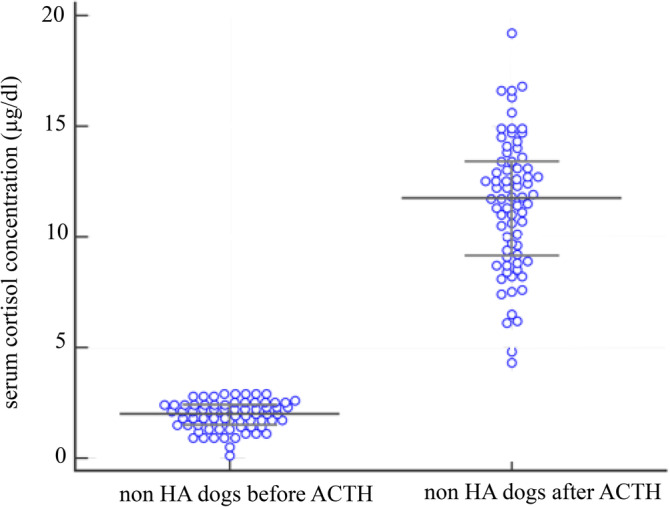
Scatter plot showing serum cortisol concentrations before and after the stimulation with ACTH in 74 dogs without hypoadrenocorticism and basal cortisol values < 3 μg/dL
4. DISCUSSION
This multicenter prospective study demonstrates a higher prevalence of HA in dogs with chronic SGD presented to several referral centers in Germany and in the Netherlands (4%) in comparison to the estimated prevalence in the general canine population (between 0.06 and 0.28%).1, 2
It is difficult to determine if the present study under‐ or over‐estimates the true prevalence of HA in dogs with chronic SGD. On the one hand, underestimation is possible as not all dogs suffering from HA might get referred or tested for HA. On the other hand, a referral population might be biased toward rare diseases, as it is more likely that cases that are difficult to diagnose or treat are referred, hence overestimating true prevalence in the population. In this context, it is important to stress that all dogs diagnosed with HA in the present study had GDH. The lack of typical electrolyte abnormalities decreases the suspicion for the disease and testing is less likely to take place.
The signalment of the dogs diagnosed with HA in the present study is somewhat different from previous studies, which is likely because of the fact that classical presentations with typical electrolyte changes are missing in the present population.2, 27, 28
Table 4.
Serum cortisol concentrations before (basal) and after ACTH stimulation
| Dogs without HA | Dogs with HA | |||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Basal cortisol μg/dL | 2.9 | 0.1‐13.1 | 1.0 | 0.1‐1.0 |
| Basal cortisol nmol/L | 80.04 | 2.76‐361.43 | 27.60 | 2.76‐28.00 |
| ACTH Stimulation (1 h) μg/dL | 12.5 | 4.3‐22.9 | 1.05 | 0.1‐2.0 |
| ACTH Stimulation (1 h) nmol/L | 358.65 | 237.27‐615.50 | 29.18 | 2.76‐55.20 |
Absence of mineralocorticoid deficiency puts these dogs in a stable hemodynamic state, which can explain why they are diagnosed later compared to dogs with typical features of HA (MGDH). However, also dogs with proven mineralocorticoid deficiency can potentially maintain normal electrolyte concentrations for an extended period of time, if conditions causing hypovolemia do not occur.29
Five of six dogs with HA had signs of GI blood loss. In 4/6 dogs melena or hematochezia was noted by the owner and 1/6 dogs had anemia and hypalbuminemia most likely because of GI loss. As glucocorticoids have an important influence on epithelial integrity and vascular permeability and they can act as trophic factors for the GI mucosa, it is possible that the lack of glucocorticoids predisposes individuals to mucosal erosions and ulcers and hence hemorrhage.30, 31
Neither historical information nor clinicopathological data were helpful to distinguish dogs with and without HA in the examined population. Moreover, no clinicopathological data were helpful to discriminate HA from other causes of chronic SGD.
28% of all dogs in this study had a basal serum cortisol concentration ≤2 μg/dL (≤55 nmoL/L). Dogs with chronic disease might be expected to have higher cortisol concentrations. Our results however show that chronic SGD are not generally associated with an increased stimulation of the hypothalamus‐adrenal axis. This finding is consistent with other reports demonstrating that 33% of dogs with nonadrenal illness have a serum basal cortisol concentrations ≤2 μg/dL (≤55 nmoL/L).32 On the other hand, a serum basal cortisol concentration >2 μg/dL (>55 nmoL/L) is considered useful in excluding a diagnosis of HA.21, 23 In most dogs with HA serum basal cortisol concentrations are <1 μg/dL, but few dogs can have a value between 1 and 2 μg/dL. Therefore, further adrenal function testing (eg, ACTHST, cortisol:endogenous ACTH ratio) is recommended in every dog with a value ≤2 μg/dL, which according to our results might be necessary in about 1/3 of dogs with chronic SGD to definitively diagnose or rule out HA.
The authors believe all 6 dogs with inappropriate response to ACTH have true “atypical” HA. Laboratory error (both assay variation or failure as well as a clerical error) cannot be fully excluded, but ACTH stimulation tests were performed as per standard operating procedure and with due diligence. By having stringent inclusion and exclusion criteria, every effort was made to eliminate dogs from the study that might have any residual glucocorticoid effects and hence persistent suppression of the HPA from any previous treatments. However, as currently no guidelines exist regarding the required time span until ACTH testing can be performed after a dog has been treated with different steroid formulations, a possible influence of prior steroid administration cannot be fully excluded.
This study was not set up to differentiate primary HA from secondary HA, hence endogenous ACTH was not measured. Although secondary HA is rare,33 it needs to be considered in dogs with HA without electrolyte abnormalities. Determination of endogenous ACTH would also have been advantageous to rule out prior administration of glucocorticoids, which would likely result in an ACTH‐stimulation mimicking hypoadrenocorticism. However, as a thorough drug history was taken for each dog it seems unlikely that the dogs identified to have no stimulation of cortisol production after ACTH administration were misdiagnosed.
Equally, aldosterone concentrations were not measured that would be necessary to document presence or absence of mineralocorticoid deficiency. Decreased aldosterone concentration could be present even in the absence of electrolyte abnormalities in some dogs with HA.29
In conclusion, the prevalence of HA among dogs with chronic SGD seen in Germany and the Netherlands is significantly higher compared to the reported prevalence in the general dog population.1, 2 Because approximately 1 in 25 dogs with chronic SGD might have HA, ruling out HA should be an important additional step in the standard approach of dogs with chronic SGD. Episodes of GI bleeding should specifically raise the suspicion; however, no differentiation between HA and other causes leading to chronic SGD is possible based on history, physical examination, or routine blood work. Determination of basal serum cortisol concentration should be performed as a standard screening test for HA, followed by further endocrine testing if baseline cortisol concentration is ≤2 μg/dL.
CONFLICT OF INTEREST DECLARATION
Some of the hematology, serum biochemistry profiles, and serum cortisol measurements were performed free of charge by IDEXX Laboratories, Ludwigsburg, Germany.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approval was obtained according to German animal welfare laws (approval no.: November 24, 2014).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
We gratefully acknowledge the referral hospitals for their contribution of cases and thank all the dog owners for their assistance and willingness to take part in the study. The authors thank Dr. Elke Huisinga for organizing free shipment and determination of laboratory variables free of charge for samples included in this study.
Hauck C, Schmitz SS, Burgener IA, et al. Prevalence and characterization of hypoadrenocorticism in dogs with signs of chronic gastrointestinal disease: A multicenter study. J Vet Intern Med. 2020;34:1399–1405. 10.1111/jvim.15752
This study was presented as an abstract at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, Maryland.
Funding information IDEXX Laboratories
REFERENCES
- 1. Kelch WJ. Canine Hypoadrenocorticism (Canine Addison's Disease): History, Contemporary Diagnosis by Practicing Veterinarians, and Epidemiology. University of Tennessee, 1996.
- 2. Hanson JM, Tengvall K, Bonnett BN, Hedhammar Å. Naturally occurring adrenocortical insufficiency ‐ an epidemiological study based on a Swedish‐insured dog population of 525,028 dogs. J Vet Intern Med. 2016;30:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Decome M, Blais MC. Prevalence and clinical features of hypoadrenocorticism in Great Pyrenees dogs in a referred population: 11 cases. Can Vet J. 2017;58:1093‐1099. [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes AM, Bannasch DL, Kellett K, Oberbauer AM. Examination of candidate genes for hypoadrenocorticism in Nova Scotia Duck Tolling Retrievers. Vet J. 2011;187:212‐216. [DOI] [PubMed] [Google Scholar]
- 5. Schaer M, Riley W, Buergelt C, et al. Autoimmunity and Addison's disease in the dog. J Am Anim Hosp Assoc. 1986;22:789‐794. [Google Scholar]
- 6. Boujon CE, Bornand‐Jaunin V, Scharer V, et al. Pituitary gland changes in canine hypoadrenocorticism: a functional and immunocytochemical study. J Comp Pathol. 1994;111:287‐295. [DOI] [PubMed] [Google Scholar]
- 7. Frank CB, Valentin SY, Scott‐Moncrieff JC, et al. Correlation of inflammation with adrenocortical atrophy in canine adrenalitis. J Comp Pathol. 2013;149:268‐279. [DOI] [PubMed] [Google Scholar]
- 8. Kook PH, Grest P, Raute‐Kreinsen U, Leo C, Reusch CE. Addison's disease due to bilateral adrenal malignancy in a dog. J Small Anim Pract. 2010;51:333‐336. [DOI] [PubMed] [Google Scholar]
- 9. Labelle P, De Cock HE. Metastatic tumors to the adrenal glands in domestic animals. Vet Pathol. 2005;42:52‐58. [DOI] [PubMed] [Google Scholar]
- 10. Korth R, Wenger M, Grest P, et al. Hypoadrenocorticism due to a bilateral abscessing inflammation of the adrenal cortex in a Rottweiler. Kleintierpraxis. 2008;53:479‐483. [Google Scholar]
- 11. Scott‐Moncrieff JC. Hypoadrenocorticism In: Feldman EC, Nelson RW, Reusch C, Scott‐Moncrieff JC, eds. Canine and Feline Endocrinology. St. Louis, Missouri: Elsevier Health Sciences; 2014:485‐520. [Google Scholar]
- 12. Kintzer PP, Peterson ME. Primary and secondary canine hypoadrenocorticism. Vet Clin North Am Small Anim Pract. 1997;27:349‐357. [DOI] [PubMed] [Google Scholar]
- 13. Scott‐Moncrieff JC. Hypoadreocorticism in dogs In: Ettinger SJ, Feldman EC, Cote E, eds. Textbook of Veterinary Internal Medicine. St. Louis, Missouri: Elsevier Health Sciences; 2017:1795‐1810. [Google Scholar]
- 14. Ruckstuhl N, Hoerauf A, Tomsa K, Reusch C. Pseudohypoadrenocorticism in two Siberian huskies with gastrointestinal parasitoses. Schweiz Arch Tierheilkd. 2002;144:75‐81. [DOI] [PubMed] [Google Scholar]
- 15. Graves TK, Schall WD, Refsal K, Nachreiner RF. Basal and ACTH‐stimulated plasma aldosterone concentrations are normal or increased in dogs with trichuriasis‐associated pseudohypoadrenocorticism. J Vet Intern Med. 1994;8:287‐289. [DOI] [PubMed] [Google Scholar]
- 16. DiBartola SP, Johnson SE, Davenport DJ, Prueter JC, Chew DJ, Sherding RG. Clinicopathologic findings resembling hypoadrenocorticism in dogs with primary gastrointestinal disease. J Am Vet Med Assoc. 1985;187:60‐63. [PubMed] [Google Scholar]
- 17. Kintzer PP, Peterson ME. Diagnosis and management of primary spontaneous hypoadrenocorticism (Addison's disease) in dogs. Semin Vet Med Surg (Small Anim). 1994;9:148‐152. [PubMed] [Google Scholar]
- 18. Allenspach K, Wieland B, Grone A, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700‐708. [DOI] [PubMed] [Google Scholar]
- 19. Mathers RA, Evans GO, Bleby J, Tornow T. Evaluation of the Sysmex XT‐2000iV haematology analyser for rat, dog and mouse whole blood samples. Comp Clin Pathol. 2008;17:137‐144. [Google Scholar]
- 20. Baumstark ME, Nussberger J, Boretti FS, et al. Use of plasma renin activity to monitor mineralocorticoid treatment in dogs with primary hypoadrenocorticism: desoxycorticosterone versus fludrocortisone. J Vet Intern Med. 2014;28:1471‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bovens C, Tennant K, Reeve J, Murphy KF. Basal serum cortisol concentration as a screening test for hypoadrenocorticism in dogs. J Vet Intern Med. 2014;28:1541‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lathan P, Moore GE, Zambon S, Scott‐Moncrieff JC. Use of a low‐dose ACTH stimulation test for diagnosis of hypoadrenocorticism in dogs. J Vet Intern Med. 2008;22:1070‐1073. [DOI] [PubMed] [Google Scholar]
- 23. Lennon EM, Boyle TE, Hutchins RG, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000‐2005). J Am Vet Med Assoc. 2007;231:413‐416. [DOI] [PubMed] [Google Scholar]
- 24. Proverbio D, Groppetti D, Spada E, Perego R. Comparison of the VIDAS and IMMULITE‐2000 methods for cortisol measurement in canine serum. Vet Clin Pathol. 2009;38:332‐336. [DOI] [PubMed] [Google Scholar]
- 25. Russell NJ, Foster S, Clark P, Robertson ID, Lewis D, Irwin PJ. Comparison of radioimmunoassay and chemiluminescent assay methods to estimate canine blood cortisol concentrations. Aust Vet J. 2007;85:487‐494. [DOI] [PubMed] [Google Scholar]
- 26. Faul F, Erdfelder E, Lang A‐G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175‐191. [DOI] [PubMed] [Google Scholar]
- 27. Sadek D, Schaer M. Atypical Addison's disease in the dog: a retrospective survey of 14 cases. J Am Anim Hosp Assoc. 1996;32:159‐163. [DOI] [PubMed] [Google Scholar]
- 28. Famula TR, Belanger JM, Oberbauer AM. Heritability and complex segregation analysis of hypoadrenocorticism in the standard poodle. J Small Anim Pract. 2003;44:8‐12. [DOI] [PubMed] [Google Scholar]
- 29. Baumstark ME, Sieber‐Ruckstuhl NS, Muller C, et al. Evaluation of aldosterone concentrations in dogs with hypoadrenocorticism. J Vet Intern Med. 2014;28:154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Filaretova L. Glucocorticoids are gastroprotective under physiologic conditions. Ther Adv Chronic Dis. 2011;2:333‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McNeill JR, Stark RD, Greenway CV. Intestinal vasoconstriction after hemorrhage: roles of vasopressin and angiotensin. Am J Physiol. 1970;219:1342‐1347. [DOI] [PubMed] [Google Scholar]
- 32. Gold AJ, Langlois DK, Refsal KR. Evaluation of basal serum or plasma cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs. J Vet Intern Med. 2016;30:1798‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peterson ME, Kintzer PP, Kass PH. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979‐1993). J Am Vet Med Assoc. 1996;208:85‐91. [PubMed] [Google Scholar]


