Abstract
Objective
Inflammation is crucial to limiting vascular disease. Previously we reported that acrolein, a known toxin in tobacco smoke, might play an important role in the progression of atherosclerosis via an inflammatory response involving cyclooxygenase-2 (COX-2) and prostaglandin production in human umbilical vein endothelial cells (HUVECs). Curcumin has been known to improve vascular function and have anti-inflammatory properties. In this study, we investigated whether curcumin prevents the induction of inflammatory response caused by acrolein.
Methods
Anti-inflammatory effects of curcumin were examined in acrolein-stimulated HUVECs. Induction of proteins, mRNA, prostaglandin and reactive oxygen species (ROS) were measured using immunoblot analysis, real-time reverse-transcription polymerase chain reaction, enzyme-linked immunosorbent assay and flow cytometry, respectively.
Results
Curcumin attenuates inflammatory response via inhibition of COX-2 expression and prostaglandin production in acrolein-induced human endothelial cells. This inhibition by curcumin results in the abolition of phosphorylation of protein kinase C, p38 mitogen-activated protein kinase, and cAMP response element-binding protein. Furthermore, curcumin suppresses the production of ROS and endoplasmic reticulum stress via phosphorylation of eukaryotic initiation factor-2α caused by acrolein.
Conclusion
These results suggest that curcumin might be a useful agent against endothelial dysfunction caused by acrolein-induced inflammatory response.
Keywords: Inflammation, Acrolein, Curcumin, Cyclooxygenase-2, Human umbilical vein endothelial cells
INTRODUCTION
Vascular cell homeostasis is crucial since, inflammatory response in vascular cells is considered as an important limiting factor in the development of arteriosclerosis.1 There is increasing evidence that atherosclerosis is a chronic disease caused by inflammatory response and oxidative stress within the vessel wall.2
Smoking is considered the most important risk factor for the progression of arteriosclerosis,3 however the precise mechanism is not known. Among the toxic substances present in tobacco smoke, α,β-unsaturated aldehyde substances are well known.4 Acrolein is the most reactive α,β-unsaturated aldehyde, and a toxic substance present in tobacco (about 200–460 µg per cigarette).5 Acrolein is also present in airborne pollutants caused by automobile combustion and might be produced as an end product of lipid peroxidation in vivo. There is increasing evidence that acrolein is involved in diseases such as arteriosclerosis, diabetes and Alzheimer's disease. Although it is not yet known as to which components of tobacco directly cause arteriosclerosis, it is believed that some of the 4,000 chemical components present in tobacco, work in combination to harm vascular endothelial cells. The toxic components present in the cigarette smoke are believed to pass through the blood vessels, damaging the blood vessel walls and causing cholesterol deposits.6
Our previous study showed that acrolein might play an important role in the development of atherosclerosis through inflammatory response involving cyclooxygenase-2 (COX-2) and prostaglandin production in endothelial cells.7,8 COX exists in 2 distinct forms, COX-1 and COX-2, which have different distributions and functions, and are the most important enzymes for the synthesis of prostaglandin (PG). COX-1 is invariably present in the body and is expressed in most tissues and plays an important role in normal physiology. In contrast, COX-2 is rarely expressed in normal conditions, but it is rapidly induced by stimuli such as mitogens, cytokines, and lipopolysaccharides, and plays a very important role in the conversion of arachidonic acid to prostanoid.9 In particular, COX-2 is thought to play an important role during inflammatory responses in various vascular diseases. Thus, inhibition of COX-2 is considered as a therapeutic strategy for inflammatory diseases such as arteriosclerosis.
Curcumin is a polyphenol found in the dietary spice turmeric.10 The unique yellow color in turmeric is due to curcumin. It is one of the compounds that have gained attention recently due to studies reporting its anti-cancer properties.11 Curcumin has a remarkable effect in inhibiting angiogenesis essential for cancer cell growth and has been shown to inhibit the development of various cancers.12 It is non-toxic to normal cells and is effective in inducing cancer cells to kill themselves.13 Curcumin is also believed to prevent or delay dementia and is known to inhibit cellular senescence.14 Its positive role in blood vessels is also known. Curcumin attenuates inflammatory responses in endothelial cells via inhibition of adhesion molecules.15 Anti-inflammatory effect of curcumin also involves inhibition of production of cytokines such as interferon-γ.16 The protective mechanism of curcumin associated with anti-inflammation, is thought to be related to the regulation of COX-2. Inflammatory mediators such as COX-2 and cytokines play a crucial role in chronic inflammation process.17,18
In this study, we investigated whether curcumin prevents the induction of inflammatory response caused by acrolein in endothelial cells. We determined that curcumin suppresses acrolein-induced COX-2 expression at the protein level, and this inhibition is results in the abolition of oxidative stress and endoplasmic reticulum (ER) stress. We also demonstrated that p38 mitogen-activated protein kinase (MAPK) and cAMP response element-binding protein (CREB) pathways are important signaling pathways in this process.
MATERIALS AND METHODS
1. Materials
Acrolein, curcumin and β-actin antibody (Ab) were purchased from Sigma Co. (St. Louis, MO, USA). Abs against phospho-specific p38 MAPK, protein kinase C (PKC) δ, CREB, and Abs against total p38 MAPK, PKCδ, and CREB were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). Anti-COX-1 and COX-2 polyclonal Abs were purchased from Santa Cruz Biotechnology (San Diego, CA, USA). Prostaglandin E2 (PGE2) assay kit was purchased from Cayman Co. (Ann Arbor, MI, USA). Other chemicals were of the highest grade available.
2. Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from STEMCELL Technologies (Vancouver, Canada). HUVECs were cultured in M199 medium with 10% fetal bovine serum, 10 ng/mL basic fibroblast growth factor, 5 units/mL heparin, 50 units/mL penicillin, and 50 µg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The cells were passaged every 3–4 days. HUVECs were cultured to about 80% confluency and then further incubated with fresh medium containing the above reagents. Cells within passages 4–9 were used for all experiments.19
3. PGE2 production assay
PGE2 in the culture medium was measured using an enzyme immunoassay kit (Cayman Chemical Co. Inc., Ann Arbor, MI, USA). The assay was performed according to the manufacturer's instructions. Briefly, 25 or 50 µL of the medium along with a serial dilution of PGE2 standard samples were mixed with appropriate amounts of acetylcholinesterase-labeled tracer and PGE2 antiserum, followed by incubation at room temperature for 18 hours. After the wells were emptied, cells were rinsed with wash buffer and 200 µL of Ellman's reagent containing substrate for acetylcholinesterase was added. The enzyme reaction was carried out on a slow shaker at room temperature. The plate was read at 405 nm in an enzyme-linked immunosorbent assay reader after 1 hour incubation.
4. Western blotting
HUVECs were washed with phosphate-buffered saline, harvested in lysis buffer (radioimmunoprecipitation assay) with protein inhibitor cocktail (Roche Co., Basel, Switzerland). After protein quantification, equal amount of protein (cell lysates 20–50 µg) was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then transferred onto a PVDF membrane. After blocking by incubation with 5% skim milk in Tris-buffered saline (TBS) for 1 hour at room temperature, the membrane was incubated with primary Abs at 4ºC overnight and then washed with TBS containing 0.05% Tween 20. The membrane was further incubated with peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:3,000; Organon Teknika Co., Durham, NC, USA) for 1 hour and then washed. The peroxidase activity was detected by the chemiluminescence method using WESTSAVE Up™ (Ab Frontier, Seoul, Korea).
5. Real-time quantitative polymerase chain reaction (PCR)
Total RNA was isolated from cells using TRIzol, according to the manufacturer's instructions. Real-time reverse-transcriptase PCR was performed as previously described,20 cDNA was synthesized using random primers and Moloney murine leukaemia virus reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Real-time reverse-transcriptase PCR analysis was performed using an StepOnePlus Real-Time PCR Instrument (Applied Biosystems, Foster City, CA, USA). The primer sequences for human COX-2 were GAATGGGGTGATGAGCAGTT (forward) and GCCACTCAAGTGTTGCACAT (reverse).
6. Promoter assay
COX-2 promoter-luciferase plasmid DNA was generated by transfer of enhancer 2 and minimal promoter sequences into the luciferase reporter plasmid pGL3-Basic. ARE promoter activity was determined as previously described.21
7. Intracellular peroxide production
Generation of intracellular reactive oxygen species (ROS) was measured using a specific fluorescent dye, H2DCF-DA. Cells were treated with acrolein, 10 mM N-acetyl-cysteine (NAC; Sigma Co.), or a combination of acrolein and 10 mM NAC, and were then stained with 20 µM H2DCF-DA in a 37°C incubator for 30 minutes; fluorescence was observed using a FACScan instrument (Becton–Dickinson, Mountain View, CA, USA). For image analysis, cells were analyzed for fluorescence intensity using a lysis cell analysis system.
8. Statistical analysis
Data were analyzed by the Student's t-test and the results were expressed as mean±standard deviation (GraphPad Prism version 4; GraphPad Software, San Diego, CA, USA).
RESULTS
1. Curcumin suppresses acrolein-induced COX-2 and prostaglandin production
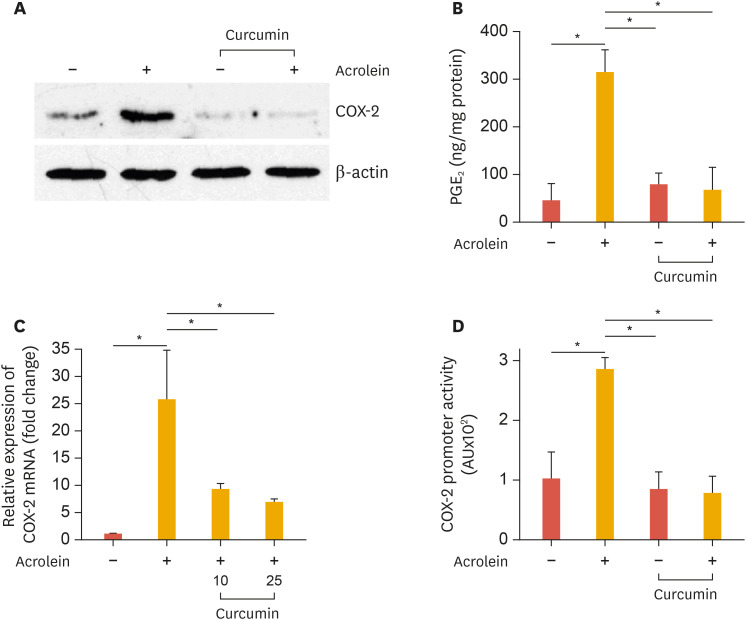
To determine the effect of curcumin on the expression COX-2 protein and mRNA, cells were pretreated with curcumin for 30 minutes, followed by addition of 25 µM acrolein for 16 hours and 1 hour, respectively. COX-2 levels were analyzed by western blotting and real-time PCR. COX-2 protein and mRNA was completely abolished by curcumin in HUVECs (Fig. 1A and B). We tested whether curcumin affects PGE2 production, the product of COX-2, which was increased by acrolein. As shown in Fig. 1C, curcumin decreased PGE2 secretion to basal levels at 16 hours. These results indicate that curcumin could suppress acrolein-induced COX-2 protein expression and subsequently PGE2 biosynthesis in HUVECs.
Fig. 1. Curcumin suppresses acrolein-induced COX-2 and prostaglandin production in HUVECs.
(A) HUVECs were preincubated with 25 µM curcumin before treatment with 10 µM acrolein and COX-2 protein levels were assessed by western blot. (B) Relative expression of COX-2 was measured by real-time quantitative PCR as described in the methods section. (C) The production of PGE2 was measured in the supernatants as described in the methods section. (D) Inhibitory effect of curcumin on acrolein-induced activation of COX-2 promoter. HUVECs were transfected with COX-2 promoter construct (−1,432 to +59) for 48 hours. After transfection, cells were preincubated with 25 µM curcumin for 30 minutes before exposure to 10 µM acrolein for 6 hours. Data represents luciferase activity that has been normalized to co-transfected β-galactosidase activity. Data were analyzed by the Student's t-test. Data represent the mean±standard deviation of results from three independent experiments.
HUVEC, human umbilical vein endothelial cell; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2.
*p<0.05 vs control group.
2. Inhibitory effect of curcumin on acrolein-induced activation of COX-2 promoter
Cells were transiently transfected with a human COX-2 promoter-luciferase reporter construct (−1,432 to +59) and treated with 10 µM acrolein for 6 hours, and luciferase activity was determined. As expected, treatment of cells with low concentration (10 µM) of acrolein increased COX-2 promoter activity by approximately 8-fold and decreased it to basal levels when treated with curcumin (Fig. 1D).
3. Curcumin inhibits ROS production induced by acrolein
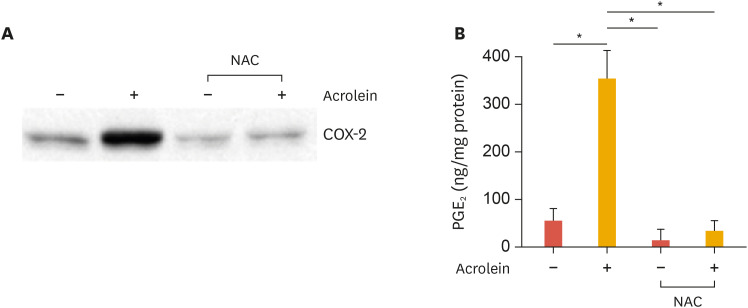
To assess the role of ROS during the induction of COX-2 by acrolein, the effect of NAC, an antioxidant, was examined on acrolein-induced COX-2 induction in HUVECs. The NAC pretreatment completely abolished acrolein-induced COX-2 expression (Fig. 2). This data strongly suggests that the COX-2 induction by acrolein depends on ROS, which are produced as a result of acrolein treatment. Further, we examined the effect of curcumin on acrolein-induced PGE2 production. As shown in Fig. 3, NAC prevented acrolein-induced PGE2 production.
Fig. 2. Effect of NAC on acrolein-induced COX-2 and PGE2 production in HUVECs.
(A) HUVECs were preincubated with 10 mM NAC for 12 hours. The cells were treated with 10 µM acrolein for 16 hours and western blotting was performed for COX-2 expression. (B) HUVECs were preincubated with 10 mM NAC for 12 hours. The cells were then treated with 10 µM acrolein for 16 hours and then release of PGE2 was measured from supernatants as described in the methods section. The values shown for PGE2 production are the mean±standard deviation of 3 independent experiments. Data were analyzed by the Student's t-test. Results are from 3 independent experiments.
NAC, N-acetyl-cysteine; COX-2, cyclooxygenase-2; HUVEC, human umbilical vein endothelial cell; PGE2, prostaglandin E2.
*p<0.05 compared to untreated control cells.
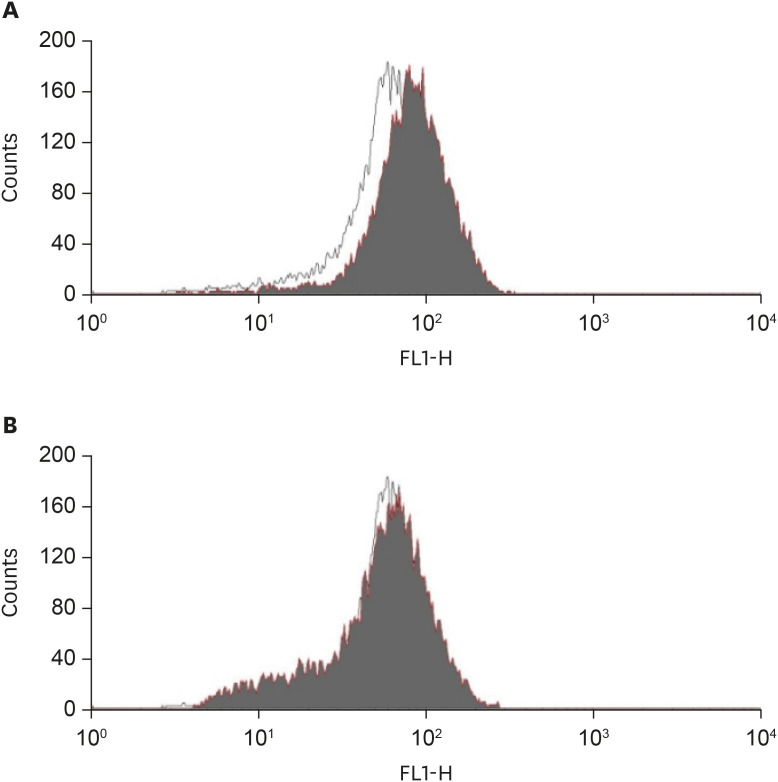
Fig. 3. Effect of curcumin on acrolein-induced intracellular peroxide production.
(A) Cells were incubated with (black area) or without (white area) 10 µM acrolein for 30 minutes, and then treated with a peroxide sensitive dye, H2DCF-DA (20 µM) during the final 30 minutes of each treatment. The relative peroxide concentrations in cells were then measured by flow cytometry. (B) After preincubation with 10 mM NAC for 4 hours, the cells were treated with (black area) or without (white area) 10 µM acrolein and flow cytometric analysis was performed. X-axis represents intensity of fluorescence.
NAC, N-acetyl-cysteine.
4. Curcumin inhibits phosphorylation of p38 and CREB in acrolein-induced HUVECs
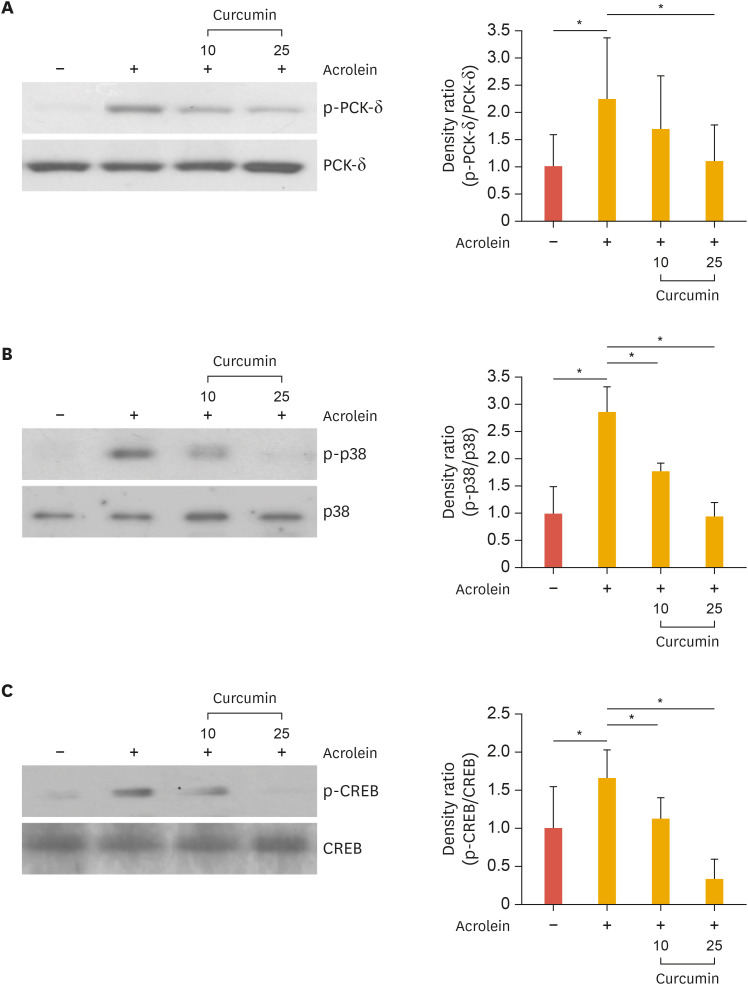
In our previous study we found that activation of PKC, p38 MAPK, and CREB were required for COX-2 activation by acrolein. To test whether curcumin inhibits signaling pathways including PKC, CREB, and p38 MAPK in HUVECs, cells were pretreated with curcumin for 0.5 hours before adding acrolein to the culture medium. We found that activation of these kinases by acrolein was completely abolished by curcumin treatment in HUVECs (Fig. 4). This result implies that the PKCδ-p38 MAPK-CREB pathway is involved in the inhibition of COX-2 expression.
Fig. 4. Effects of curcumin on acrolein-induced phosphorylation of PKCδ, p38, and CREB in HUVECs.
Cells were pre-treated with curcumin (10 and 25 µM) for 30 minutes prior to acrolein treatment (10 µM). After 30 minutes of incubation, the cell lysates (20 µg) were prepared and western blot analysis was performed with Abs against phosphorylated PKCδ, p38, and CREB or total PKCδ, p38, and CREB. Quantitative data were obtained using an imaging densitometer (ImageJ version 1.52a software, NIH, Bethesda, MD, USA). Data were analyzed by the Student's t-test. Data represent the mean±standard deviation of results from 3 independent experiments.
PKC, protein kinase C; CREB, cAMP response element-binding protein; HUVEC, human umbilical vein endothelial cell; Ab, antibody; p, phosphorylated.
*p<0.05 vs. control group.
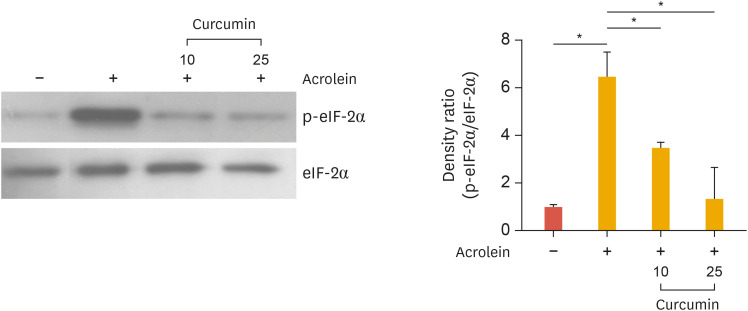
5. Curcumin prevents the phosphorylation of eukaryotic initiation factor (eIF)-2α induced by acrolein
Recently Haberzettl et al.22 reported that acrolein is a potent inducer of ER stress via phosphorylation of eIF-2α. To examine whether curcumin abolished ER stress induced by acrolein, cells were treated with curcumin in HUVECs. Curcumin completely abolished acrolein-induced phosphorylation of eIF-2α (Fig. 5). This finding supports that decreasing ER stress by curcumin treatment could inhibit acrolein-induced inflammatory responses in endothelial cells.
Fig. 5. Effects of curcumin on acrolein-induced phosphorylation of eIF-2α in HUVECs.
HUVECs were treated with curcumin (10 and 25 µM) for 30 minutes before acrolein (10 µM) treatment. After 30 minutes, cell lysates were prepared, and 20 µg of protein was used to perform western blotting using the anti-phosphorylated eIF-2α, and total eIF-2α antibody was used as a loading control. Quantitative data were obtained using an imaging densitometer (ImageJ version 1.52a software, NIH). Data were analyzed by the Student's t-test. Data represent the mean±standard deviation of results from three independent experiments.
eIF, eukaryotic initiation factor; HUVEC, human umbilical vein endothelial cell; p, phosphorylated.
*p<0.05 vs. control group.
Discussion
In this study, we show that curcumin suppresses acrolein-induced COX-2 expression at the protein level, and this inhibition is involved in the abolition of oxidative stress and ER stress in HUVECs. We also found that acrolein-induced activation of PKCδ, p38 MAPK, and CREB pathway is blocked by curcumin.
The expression of COX-2 enzyme is known to rapidly increase during inflammatory reactions, and many studies have reported that toxic components present in tobacco induce this enzyme.20 Increased COX-2 expression promotes and exacerbates atherosclerotic inflammation. Therefore, chronic inflammation with increased COX-2 might play an important role in vascular diseases such as atherosclerosis.23
Curcumin is a chemical substance that is present in large amounts (0.5%–6.5%), in turmeric root.24 Turmeric, also known as yellow ginger, is used as a dietary spice and a natural dye. It contains “curcumin” as its main ingredient and “glucuronide” as a secondary plant phenolic substance. It has been used for thousands of years in Asia, including India, as a natural remedy. As a traditional medicine, curcumin has been widely used for centuries to treat inflammatory disorders such as arthritis, colitis, hepatitis, and cancer.25,26 It is known that curcumin exhibits better anti-inflammatory effects compared to ibuprofen and naproxen, which are general anti-inflammatory analgesics. In traditional Indian medicine, turmeric has long been used as a medicine to relieve inflammation.27
The effect of curcumin has been investigated in various inflammatory chronic diseases such as arthritis, Crohn's disease, diabetes, obesity and atherosclerosis.28 Several epidemiological studies have reported that curcumin has a good effect on vascular diseases.29,30 The anti-inflammatory effects of curcumin have been attributed to the suppression of PG synthesis.17,31 In experiments using animal models of arteriosclerosis, curcumin was effective in inhibiting arteriosclerosis.32 Therefore, inhibition of COX-2 expression by curcumin could be a protective mechanism in vascular diseases such as arteriosclerosis. Anti-atherosclerotic action of curcumin is not fully understood but, it is possible that curcumin might suppress the anti-inflammatory component in atherosclerosis.
Atherosclerosis is caused due to impaired function of the vascular endothelium.33,34 Endothelial cells are primary targets of oxidative stress. Upon exposure to ROS, endothelial cells increase cell permeability, produce inflammatory mediators, and increase the level of adhesion molecules. Increased endothelial cell damage by ROS is thought to be the cause of many cardiovascular diseases. Therefore, removal of ROS could be an effective therapeutic target. The extent of COX-2 induction by acrolein in HUVECs depends upon the antioxidant potential of HUVECs, which is strongly diminished by treatment with NAC, a precursor of glutathione (Fig. 2). These data suggest that the oxidative effects of acrolein and its metabolites mediate the induction of COX-2 in HUVECs.
In conclusion, the present study demonstrates that curcumin attenuates inflammatory response via inhibition of COX-2 and prostaglandin production in acrolein-induced human endothelial cells suggesting that the inhibitory effect of curcumin on acrolein-induced COX-2 expression could be associated with inhibition of the activation of p38, CREB, and ROS. Our finding suggests that curcumin might be a useful agent in the treatment of endothelial dysfunction against acrolein-induced inflammatory response.
Footnotes
Funding: This research was funded by National Research Foundation of Korea (NRF), grant number NRF-2017M3A9E4044650 and NRF-2017R1D1A1B03033148.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Lee SE, Park YS.
- Data curation: Lee SE.
- Formal analysis: Lee SE, Park HR, Park YS.
- Investigation: Lee SE, Park HR, Jeon S, Han D.
- Methodology: Jeon S.
- Supervision: Park YS.
- Writing - original draft: Lee SE.
- Writing - review & editing: Park YS.
References
- 1.Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond) 2018;132:1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thweatt WD, Harward CN, Sr, Parrish ME. Measurement of acrolein and 1,3-butadiene in a single puff of cigarette smoke using lead-salt tunable diode laser infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2007;67:16–24. doi: 10.1016/j.saa.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Zirak MR, Mehri S, Karimani A, Zeinali M, Hayes AW, Karimi G. Mechanisms behind the atherothrombotic effects of acrolein, a review. Food Chem Toxicol. 2019;129:38–53. doi: 10.1016/j.fct.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Park YS, Kim J, Misonou Y, Takamiya R, Takahashi M, Freeman MR, et al. Acrolein induces cyclooxygenase-2 and prostaglandin production in human umbilical vein endothelial cells: roles of p38 MAP kinase. Arterioscler Thromb Vasc Biol. 2007;27:1319–1325. doi: 10.1161/ATVBAHA.106.132837. [DOI] [PubMed] [Google Scholar]
- 8.Park YS, Taniguchi N. Acrolein induces inflammatory response underlying endothelial dysfunction: a risk factor for atherosclerosis. Ann N Y Acad Sci. 2008;1126:185–189. doi: 10.1196/annals.1433.034. [DOI] [PubMed] [Google Scholar]
- 9.Rumzhum NN, Ammit AJ. Cyclooxygenase 2: its regulation, role and impact in airway inflammation. Clin Exp Allergy. 2016;46:397–410. doi: 10.1111/cea.12697. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut. 2008;57:1509–1517. doi: 10.1136/gut.2008.152496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev. 2015;73:155–165. doi: 10.1093/nutrit/nuu064. [DOI] [PubMed] [Google Scholar]
- 14.Gardener SL, Rainey-Smith SR, Martins RN. Diet and inflammation in Alzheimer's disease and related chronic diseases: a review. J Alzheimers Dis. 2016;50:301–334. doi: 10.3233/JAD-150765. [DOI] [PubMed] [Google Scholar]
- 15.Karimian MS, Pirro M, Johnston TP, Majeed M, Sahebkar A. Curcumin and endothelial function: evidence and mechanisms of protective effects. Curr Pharm Des. 2017;23:2462–2473. doi: 10.2174/1381612823666170222122822. [DOI] [PubMed] [Google Scholar]
- 16.Midura-Kiela MT, Radhakrishnan VM, Larmonier CB, Laubitz D, Ghishan FK, Kiela PR. Curcumin inhibits interferon-γ signaling in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G85–G96. doi: 10.1152/ajpgi.00275.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho JW, Park K, Kweon GR, Jang BC, Baek WK, Suh MH, et al. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp Mol Med. 2005;37:186–192. doi: 10.1038/emm.2005.25. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SE, Park HR, Park CS, Ahn HJ, Cho JJ, Lee J, et al. Autophagy in crotonaldehyde-induced endothelial toxicity. Molecules. 2019;24:E1137. doi: 10.3390/molecules24061137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SE, Park HR, Kim H, Choi Y, Jin YH, Park CS, et al. Effect of crotonaldehyde on the induction of COX-2 expression in human endothelial cells. Mol Cell Toxicol. 2017;13:345–350. [Google Scholar]
- 21.Lee SE, Park YS. Korean Red Ginseng water extract inhibits COX-2 expression by suppressing p38 in acrolein-treated human endothelial cells. J Ginseng Res. 2014;38:34–39. doi: 10.1016/j.jgr.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haberzettl P, Vladykovskaya E, Srivastava S, Bhatnagar A. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol Appl Pharmacol. 2009;234:14–24. doi: 10.1016/j.taap.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez-Hernández A, Martín-Ventura JL, Sánchez-Galán E, Vidal C, Ortego M, Blanco-Colio LM, et al. Overexpression of COX-2, Prostaglandin E synthase-1 and prostaglandin E receptors in blood mononuclear cells and plaque of patients with carotid atherosclerosis: regulation by nuclear factor-κB. Atherosclerosis. 2006;187:139–149. doi: 10.1016/j.atherosclerosis.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burge K, Gunasekaran A, Eckert J, Chaaban H. Curcumin and intestinal inflammatory diseases: molecular mechanisms of protection. Int J Mol Sci. 2019;20:E1912. doi: 10.3390/ijms20081912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma C, Kaur J, Shishodia S, Aggarwal BB, Ralhan R. Curcumin down regulates smokeless tobacco-induced NF-κB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology. 2006;228:1–15. doi: 10.1016/j.tox.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Lelli D, Sahebkar A, Johnston TP, Pedone C. Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol Res. 2017;115:133–148. doi: 10.1016/j.phrs.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Shehzad A, Ha T, Subhan F, Lee YS. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr. 2011;50:151–161. doi: 10.1007/s00394-011-0188-1. [DOI] [PubMed] [Google Scholar]
- 30.Sikora E, Scapagnini G, Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun Ageing. 2010;7:1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V, et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007;7:333–342. doi: 10.1016/j.intimp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Ramírez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baró L, Ramirez-Tortosa CL, et al. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/s0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 33.Haybar H, Shahrabi S, Rezaeeyan H, Shirzad R, Saki N. Endothelial cells: from dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovasc Toxicol. 2019;19:13–22. doi: 10.1007/s12012-018-9493-8. [DOI] [PubMed] [Google Scholar]
- 34.Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]