Abstract
Ceramides are minor components of the hepatic lipidome that have major effects on liver function. These products of lipid and protein metabolism accumulate when the energy needs of the hepatocyte have been met and its storage capacity is full, such that free fatty acids start to couple to the sphingoid backbone rather than the glycerol moiety that is the scaffold for glycerolipids (e.g., triglycerides) or the carnitine moiety that shunts them into mitochondria. As ceramides accrue, they initiate actions that protect cells from acute increases in detergent-like fatty acids; for example, they alter cellular substrate preference from glucose to lipids and they enhance triglyceride storage. When prolonged, these ceramide actions cause insulin resistance and hepatic steatosis, 2 of the underlying drivers of cardiometabolic diseases. Herein the author discusses the mechanisms linking ceramides to the development of insulin resistance, hepatosteatosis and resultant cardiometabolic disorders.
Keywords: Ceramides, Steatohepatitis, Insulin resistance, Non-alcoholic fatty liver disease, Diabetes
INTRODUCTION
Cells and organisms undergo highly-coordinated responses to the energy status of their environment. Nutrient abundance up-regulates anabolic signaling pathways while inhibiting catabolic ones. Nutrient deprivation opposes all of these activities to liberate energy stores. Despite the relevance of these responses to virtually all physiological processes, the molecular events accounting for cell autonomous nutrient sensing are poorly understood.
The preferential energy store in living species is the triglyceride molecule, which has a remarkable capacity to pack energy into a distinct organelle known as the lipid droplet. For example, a typical, well-nourished 70 kg person has fuel reserves totaling 135,000 kcal as triglycerides, as opposed to only 1,600 kcal as glycogen or 24,000 kcal as modifiable protein.1 Triglycerides comprise 3 fatty acid molecules coupled to a glycerol backbone. The glycerol moiety derives from carbohydrate molecules during the course of glycolysis. The fatty acids can come from dietary intake or through the metabolism of carbohydrates or proteins. Every macronutrient can be converted into acetyl-coenzyme A (CoA) through the citric acid cycle, which can in turn be used to synthesize new fatty acids. The body is thus highly tuned to store excess fuel as triglyceride.
Unlike proteins and carbohydrates, fatty acids are not anapleurotic; they cannot be converted back into intermediates for the citric acid cycle and thus cannot be used to produce glucose or amino acids. After being coupled to CoA, they enter one of 3 metabolic pathways:
1) When energy needs are high, acyl-CoAs couple to carnitine, which allows them to be imported into mitochondria for beta oxidation and production of heat or adenosine triphosphate.
2) When energy needs are low, acyl-CoAs couple to glycerol, which produces predominant membrane glycerolipids (e.g., phosphatidylcholine, phosphatidylethanolamine, etc.) as well as the triglyceride stores described above.
3) When the energy needs have been met and the storage capacity is full, they can be coupled to a sphingoid backbone derived from the condensation of amino acids and palmitoyl-CoA, producing the sphingolipids.
We hypothesize that the sphingolipids serve as nutritional signals of lipid overload, accumulating when the fatty acid burden of a cell is particularly high.2 The resultant sphingolipids, particularly ceramides, alter the metabolic properties of cells and organisms to help them adapt to the fuel surplus.2 Herein we will discuss how ceramides alter tissue metabolism and will evaluate how these actions contribute to the development of hepatosteatosis and cardiometabolic disease.
FUNDAMENTALS OF SPHINGOLIPID BIOSYNTHESIS
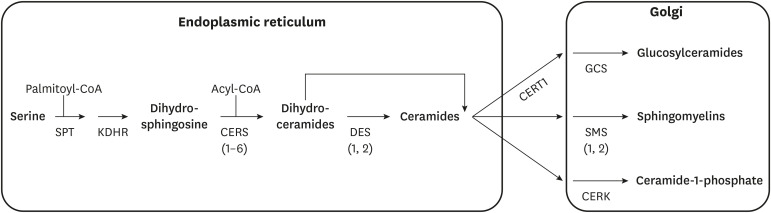
The chief site of sphingolipid biosynthesis is the endoplasmic reticulum, where fatty acids and amino acids are converted into the dihydroceramides and ceramides that are the building blocks of other sphingolipids (e.g., sphingomyelins (SMs) and dihydro-SMs, gangliosides and dihydro-gangliosides, etc.) (Fig. 1).3 The enzymes that convert ceramides and dihydroceramides into complex sphingolipid species are localized in the Golgi apparatus. The early steps of the biosynthesis pathway involve the following enzymes:
Fig. 1. Schematic depicting the key reactions in the ceramide biosynthesis pathway.
CoA, coenzyme A; SPT, serine palmitoyltransferase; KDHR, 3-ketodihydrosphingosine reductase; CERS, (dihydro)ceramide synthases (isoforms in parentheses); DES, dihydroceramide desaturase (isoforms in parentheses); GCS, glucosyceramide synthase; SMS, sphingomyelin synthase (isoforms in parentheses); CERK, ceramide kinase.
• Serine palmitoyltransferase (SPT) condenses palmitoyl-CoA and serine to produce a transient intermediate termed 3-ketosphinganine.3 Occasionally, this enzyme can also use alternative amino acids (i.e., alanine and glycine) to produce deoxysphingolipids.4 The enzyme is multimeric, comprising 2 essential subunits (SPTLC1 and 2) which are requisite for enzyme function, as well as a third (SPTLC3) that can allow myristoyl-CoA to be used in place of palmitoyl-CoA. A family of 3 ORMDL proteins modulate SPT activity; their simultaneous knockdown increases ceramide biosynthesis while their overexpression lowers ceramides.5 The broad spectrum of substrates and components of this enzyme complex suggests that the enzyme complex may serve as a highly regulated gateway for nutrients entering the sphingolipid pool.
• 3-ketosphinganine reductase converts the 3-ketosphinganine produced by SPT into dihydrosphingosine. This reaction proceeds rapidly, such that 3-ketosphinganine levels are exceptionally low. The resultant dihydrosphingosine serves as the sphingoid scaffold that acquires additional, variable fatty acids through the reaction that follows.
• (Dihydro)Ceramide synthases add a variable acyl-CoA to dihydrosphingosine to produce dihydroceramides. The family includes 6 isoforms (CERS1–6) that differ by substrate preference and tissue distribution.6,7 Though CERS2 is the major isoform in the liver, its products, which are very long chain ceramides that include C24 or C24:1 acyl-chains, do not elicit deleterious actions on tissue metabolism.8 The species that most influence anabolic and catabolic processes are the C16 ceramides produced by CERS6, which is upregulated in obesity.9,10 CERS5 also produces C16 ceramides and has been implicated in cardiovascular complications of obesity,11 but does not influence glucose and lipid homeostasis.12 CERS1, which produces C18 ceramides and is the major isoform in skeletal muscle, also influences tissue metabolism and insulin sensitivity.13 CERS3, which also produces really long ceramides, is the major isoform in skin and sperm. As we will discuss below, a recent study suggests that these enzymes have the ability to migrate to the nucleus under conditions of high activity, where they serve as transcriptional repressors of lipase gene expression.14
• Dihydroceramide desaturases insert a conserved double bond into the d4 position of the sphingoid backbone to convert the dihydroceramides into ceramides. This structural feature imparts many of the sphingolipids unique biophysical properties and enables ceramides to serve as regulators of cellular metabolism.2 Two desaturases are present in mammalian tissues, including the ubiquitous isoform DES1 and the tissue-restricted DES2 which is present in the skin and gut. The products of DES2 can contain an additional hydroxyl-group in the D4 position and are termed phytoceramides.
Sporadic reports have also suggested that the process may also occur in mitochondria, but the quantitative or biological importance of this as a site of ceramide synthesis is still unclear.
Both the dihydroceramides and ceramides can then be transported to the Golgi, where they serve as substrates for enzymes that add various headgroups. For example, the SM synthases add phosphocholine, producing the SMs which represent the most abundant sphingolipid class. Glucosyceramide synthase adds a glucose moiety to initiate the enzymatic cascade that produces the gangliosides. And ceramide kinase can phosphorylate ceramides to produce the bioactive signaling molecule ceramide-1-phosphate.
For the purposes of this review, we will concentrate on the ceramides, which have evolved important roles as metabolic regulators during conditions of nutrient surplus.
HYPOTHESIS: CERAMIDES ARE EVOLUTIONARILY CONSERVED SIGNALS OF FREE FATTY ACID OVERLOAD
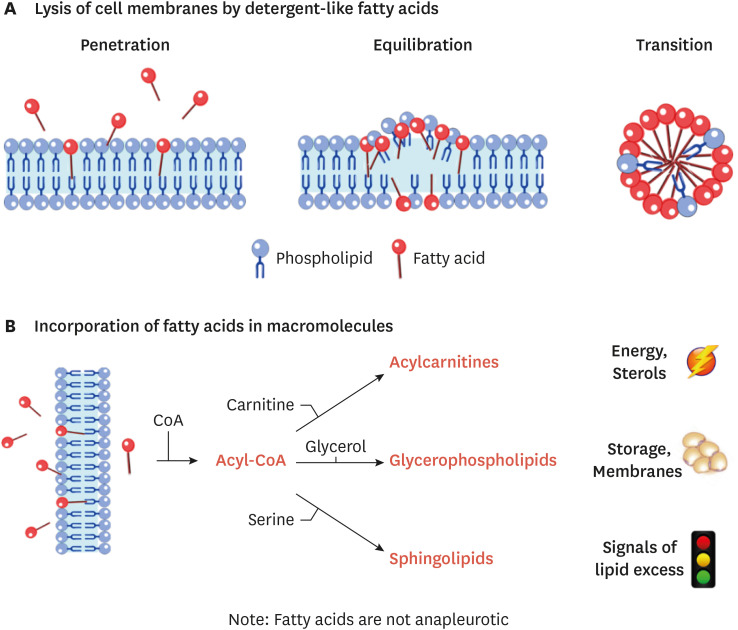
Free fatty acids (FFAs) are amphipathic, detergent-like molecules. Since FFAs would otherwise perturb bilayer structures (Fig. 2A), cells go to great lengths to quickly esterify them, coupling them to CoA and subsequently to carnitine, glycerol or sphingoid bases as we described above (Fig. 2B). As ceramides start to accrue, they initiate a series of changes to cell metabolism that effectively lower levels of FFAs (Figs. 3 and 4). We hypothesize that they thus play an evolutionarily conserved role to protect cellular bilayers in times of lipid excess. We will detail these cellular actions below.
Fig. 2. Schematic depicting the pathways that incorporate fatty acids entering the cell. (A) When detergent-like fatty acid levels get too high, they emulsify cellular membranes. The lysis occurs over 3 stages: fatty acid penetration into the bilayer; their equilibration into both faces of the bilayer; and the transition of the bilayer into a micelle. (B) To prevent lysis, fatty acids entering the cell are quickly coupled to CoA and then incorporated into backbones to produce acylcarnitines, glycerolipids, and sphingolipids. Acylcarnitines are formed as fatty acids translocate into mitochondria. Glycerolipids that are produced make up the bulk of membrane bilayers and create the triglyceride stores that form the majority of the lipid droplet. Sphingolipids are signals of lipid excess that signal to upregulate the other 2 pathways under conditions of lipid excess.
CoA, coenzyme A.
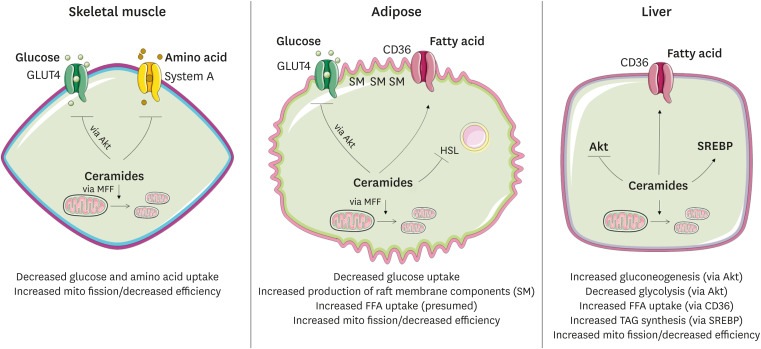
Fig. 3. Schematic depicting the means by which ceramides influence cellular metabolism. Mechanisms described in the text and depicted in greater detail in Fig. 4.
SM, sphingomyelin; GLUT4, the insulin-responsive glucose transporter; Akt, the serine threonine kinase Akt/PKB; HSL, hormone-sensitive lipase; SREBP, sterol response element binding protein; MFF, mitochondrial fission factor; BAX, the pro-apoptotic BCL2 family member BAX; FFA, free fatty acid. Green denotes a process that is increased and red denotes decreased.
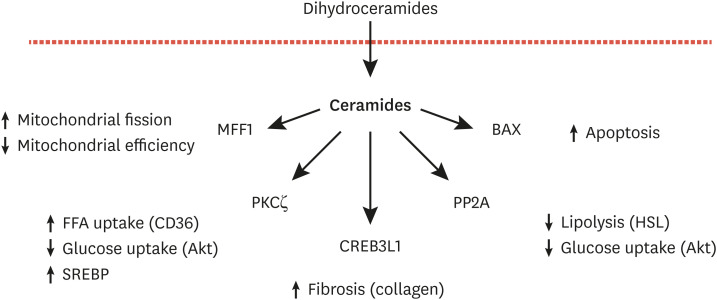
Fig. 4. Schematic depicting the mechanisms of ceramide action.
MFF, mitochondrial fission factor; PKCζ, protein kinase C-zeta; CREB3L1, cAMP responsive element binding protein 3 like 1; PP2A, protein phosphatase 2A; BAX, the pro-apoptotic BCL2 family member BAX; Akt, the serine threonine kinase Akt/PKB; SREBP, sterol response element binding protein; HSL, hormone sensitive lipase.
Ceramides (and SM) facilitate FFA uptake and esterification
The means by which fatty acids traverse the plasma membrane has been a source of active discussion and occasional debate. They are fully capable of diffusing through the bilayer,15-19 owing to their amphipathic nature. Nonetheless, families of translocases (e.g., CD36) that increase the rate of fatty acid uptake have been identified.20 Many researchers have argued that the role of these translocases is to direct fatty acids to acyl-CoA synthetases, thus enhancing their esterification and thus their rate of retention within the cell.21,22
The Scherer lab demonstrated an interesting relationship between ceramides and CD36.23 The work stemmed from an animal model that overexpressed acid ceramidase in either adipose tissue or the liver. Overexpression of the enzyme in either locale led to substantial decreases in CD36. It also decreased the activity of the atypical protein kinase C (PKC) isoform PKCζ, which is ceramide effector that had been shown previously to play a permissive role in lipid uptake. Using cultured H4IIe hepatocytes, the authors determined that short-chain ceramide analogues or cell treatments that increased endogenous ceramides stimulated palmitate uptake and markedly enhanced PKCζ activity. Overexpression of dominant-negative PKCζ isoforms negated the ceramide actions on lipid uptake, while constitutively active constructs enhanced lipid uptake. Lastly, they determined that ceramide analogs, via a PKCζ-dependent mechanism, enhanced translocation of CD36 to plasma membranes. This study identified an unanticipated role for ceramides to enhance lipid uptake and esterification.
In 2019, my group confirmed this mechanism using mice lacking DES1, where the livers predominantly contained dihydroceramides in place of ceramides.2 In those animals, lipid uptake into the liver was greatly reduced, such that they were protected from hepatic steatosis. In cultured hepatocytes, ceramides, but not dihydroceramides, stimulated CD36 translocation.
These actions of ceramides effectively lower the concentration of FFA in the lipid bilayer, by enhancing their transport and facilitating their esterification. These actions protect cells from these detergent-like structures.
Beyond these ceramide actions on CD36, we suspect that other sphingolipids also serve an evolutionarily conserved role to facilitate safe diffusion of FFA across lipid bilayers. SMs, with its binding partner cholesterol, is a key component of caveolae and lipid rafts. Studies in adipocytes, where caveolae are particularly abundant, indicate that these SM and cholesterol-rich microdomains are important scaffolds that support fatty acid diffusion and uptake.24-26 Thus, sphingolipids may also have a role in producing membrane structures that support the safe diffusion of the detergent-like FFA across cellular membranes.
Ceramides induce sterol response element binding protein (SREBP) to drive triglyceride formation
SREBPs are master regulators of triglyceride and cholesterol synthesis. Gonzalez and colleagues determined that exogenous administration of C16-ceramides to mice was sufficient to induce hepatic expression of the Srebf1 gene and several of its downstream targets that enable either triglyceride production (e.g., Dgat1, Dgat2, etc.) or fatty acid elongation (Elovl1).27 Similarly, our studies demonstrated that the Srebf1 gene that encodes SREBPs was the most dramatically downregulated gene in liver-specific ceramide-depleted, Degs1 knockout mice.2 Our data confirmed that ceramide reduction reduced levels of the Dgat enzymes and Elovl1. These findings are consistent with work by Worgall and colleagues28 in cell lines, where ceramide depletion lowered SRE-dependent gene expression in vitro. Collectively, these data suggest that ceramides modulate gene expression profiles to enhance incorporation of the excessive fatty acids into triglycerides.
The likely mechanism for these ceramide actions again involves the atypical PKCs (e.g., PKCλ or PKCζ) that are ceramide effectors and established inducers of hepatic steatosis.29,30 Prior studies revealed a ceramide-PKCζ axis that contributes to hepatic steatosis and hypertriglyceridemia in mice.30
Ceramides inhibit glucose and amino acid uptake and utilization
While ceramides are facilitating the entry of fatty acids into the cell, they are simultaneously inhibiting the uptake of glucose31,32 and amino acids.33-35 This series of ceramide actions enables the cell to adapt to use the abundant fatty acids as the preferred fuel source.
Most of the studies that evaluated ceramide effects on glucose or amino acid uptake have been done in cultured adipocytes or myotubes, typically looking at the insulin-responsive changes in transport rates that occur in the postprandial condition. Nonetheless, this attribute of sphingolipids to modulate nutrient uptake also appears to be present in single cell organisms, including yeast.35-38
In mammals, the ceramide antagonism of glucose uptake results from the regulation of the insulin-responsive GLUT4 glucose transporter. The first studies utilized exogenous short-chain ceramide analogs, which potently inhibited GLUT4 translocation to the plasma membrane in adipocytes.31,32,39 These analogues altered GLUT4 trafficking by blocking insulin-stimulation of Akt/PKB, a serine/threonine kinase that is obligate for insulin and growth-factor activation of glucose uptake, anabolic signaling events (e.g., protein, glycogen, and fatty acid synthesis), and cell survival.40-46 Ceramide inhibition of Akt/PKB is accomplished by one of 2 mechanisms.
1. Ceramides inhibit insulin and growth-factor stimulated translocation of the anabolic serine/threonine kinase Akt/PKB to the plasma membrane.40 This step precedes the phosphorylation of Akt/PKB phosphorylation on 2 activating residues. The ceramide effector PKCζ is an obligate intermediate in this ceramide action, as it phosphorylates Akt/PKB on a third, inhibitory site in the enzyme's PH-domain.42 The phosphorylation lowers the kinase's affinity for phosphoinositides, thus preventing its PI3-kinase-dependent activation.42 Expression of an Akt/PKB isoform with the S34 site converted to an alanine confers resistance to ceramide in cultured myotubes and vascular smooth muscle.47,48 Ceramides stabilize interactions between Akt/PKB and PKCζ by recruiting the enzymes to membrane rafts or caveolae.48,49
2. Ceramides enhance dephosphorylation of Akt/PKB by activating protein phosphatase 2A.43-46 Inhibition of PP2A, which could be accomplished in cells by either treating with okadaic acid or by overexpressing the SV40 small T antigen, prevents the ceramide inhibition of Akt/PKB in a number of cell types.43-46
The relative importance of either pathway varies by cell type.41,43,47,48,50 In cells with abundant caveolae, the PKCζ-dependent mechanism predominates.51
Ceramides and ceramide synthases inhibit TAG lipolysis
Since ceramides appeared to be enacting cellular actions that lowered the FFA burden of cells, we predicted that they might inhibit release of fatty acids from the lipid droplet. Indeed, ceramide analogues inhibited isoproterenol-stimulated phosphorylation of hormone sensitive lipase.11 Simultaneous addition of the PP2A inhibitor microcystin-LR negated these ceramide actions.
Ceramide synthases, via actions unrelated to their ability to make ceramides, may also regulate lipolysis. The Drosophila, and most mammalian, CERS isoforms contain a nuclear localization sequence and a homeodomain, suggesting that they may regulate gene expression. Sociale and colleagues14 determined that CERS enzymes respond to fatty acids by translocating to the nucleus and repressing expression of lipases. This observation identifies an additional means by which the entry of FFA into the ceramide synthesis pathway may alter metabolism to alleviate the FFA burden of cells during conditions of lipid oversupply.
Ceramides decrease mitochondrial efficiency
Obesity is often associated with mitochondrial “dysfunction” evidenced by decreased activity (i.e., oxygen consumption) of the complexes in the electron transport chain (ETC). Review of the literature indicates that ceramides can account for this obesity-induced change in mitochondrial efficiency.
Short-chain ceramide analogs,52,53 as well as interventions that increase endogenous ceramide levels, inhibit the activity of several ETC complexes.8,54,55 For example, treating mitochondria with C16-ceramide, sphingosine or sphinganine inhibits complex IV activity in vitro.54 Similarly, overexpressing CERS6 in hepatocytes increases C16-ceramide levels and inhibits complex II activity.8 Lastly, pharmacological inhibition or knockdown of sphingomyelinase synthase-2 induces accumulation of ceramides while inhibiting mitochondrial respiration.55
Studies in rodents also suggest that ceramides impair mitochondrial function. 1) Cert1 knockout mice, which lack the ceramide transport protein that carries ceramides from the ER to the Golgi for ganglioside production, display elevated mitochondrial ceramide content and impaired complex IV activity.56 2) Cers2 null mice display compensatory increases in C16-ceramides and decreased complex II and IV activity. 3) Cers2 heterozygous mice, when fed a high fat diet, display decreased complex I, II and IV activity in the liver and increased susceptibility to hepatosteatosis.8 For this latter study, treatment with the SPT inhibitor myriocin negates the effect of high fat feeding or haploinsufficiency for Cers2 on mitochondrial activity in adipose tissue and/or the liver. 4) Convincingly, depletion of ceramides (i.e., knockout of Cers6, Cers1, or Degs1) from the whole body or specific tissues reduces ceramide levels and increases mitochondrial oxygen consumption in liver, muscle, and brown and white adipose tissue.2,10,12,13,57
The mechanism linking ceramides to impaired complex activity are unresolved. Hammerschmidt and colleagues12 determined that sphingolipids produced by CERS6 bound to mitochondrial fission factor, which led to enhanced mitochondrial fission. Indeed, the Cers6 knockout mice contain fused, very large, and highly efficient mitochondria.12 Bikman and colleagues58 also reported that ceramides induced fission, which they found responsible for ceramide actions on oxygen consumption. Ceramides likely initiate other actions on mitochondria, whoever, as the fission effects cannot explain the acute, direct effects of sphingolipids on the purified organelle.
We surmise that the decrease in mitochondrial efficiency serves a biological purpose. By doing so, ceramides limit the effect of a given substrate on the mitochondrial membrane potential, which may enable the cell to burn a larger number of fatty acid substrates.
The end is nigh: ceramides in apoptosis and fibrosis
As ceramides continue to accrue, they signal the cell to undergo apoptosis and to initiate the processes of fibrosis. This likely evolved as an extension of the aforementioned actions, as they trigger the organism to rid itself of cells that have a compromised lipid bilayer.
Ceramides increase mitochondrial outer membrane permeability (MOMP) to increase cytochrome C release and subsequent caspase activation.59 Several mechanisms underlie these effects. Ceramides recruit the pro-apoptotic protein BAX to mitochondrial membranes.60 They also bind to voltage-dependent anion channel 2, another protein implicated in the induction of MOMP.61 Certain ceramides are also reported to form channels that allow passage of cytochrome C.62-65 The anti-apoptotic protein BCLXL1 disrupts formation of these ceramide channels.66-68
Ceramides may also trigger fibrosis, which will protect the tissue from a lysing cell. Ceramides may modulate expression of collagen genes via a mechanism termed “regulated intramembrane proteolysis.” The mechanism was identified from research CREB3L1,69 an ER-bound protein. Site-1 or site-2 proteases (S1P/S2P) cleave CREB3L1, releasing a fragment that enters the nucleus and binds Smad4, subsequently inducing transcription of genes required for assembly of collagen-containing extracellular matrix.69,70 Ceramides activate this process by altering the orientation of TM4SF20, a protein that otherwise blocks access of S1P and S2P to CREB3L1.69,71
Summary of evolutionary basis of these ceramide mechanisms
We thus surmise that ceramides are evolutionarily-conserved signals of lipid excess. The ceramide actions described above prevent the aberrant lysis of cell membranes caused by the accumulation of detergent-like FFA. The ceramide effects on apoptosis and fibrosis likely evolved as an extension of this response, helping the organism limit the damage resulting from the spilling of cytosolic components following aberrant FFA-driven membrane lysis. This hypothesis provides a unifying framework for understanding ceramide actions in cellular stress and generates several testable hypotheses. Moreover, it provides a plausible mechanism for the role of ceramides in “selective” insulin resistance and cardiometabolic diseases (discussed below).
CERAMIDES DRIVE SELECTIVE INSULIN RESISTANCE, HEPATOSTEATOSIS, AND OTHER CARDIOMETABOLIC DISORDERS
Hepatosteatosis and other cardiometabolic disorders result from a common disease progression that includes 2 stages: selective insulin resistance followed by lipotoxicity (e.g., lipo-apoptosis and fibrosis). The mechanisms described earlier explain how ceramides contribute to both stages of disease progression.
Stage 1: selective insulin resistance
The peptide hormone insulin has 2 major actions. First, insulin lowers blood glucose levels by enhancing its uptake and storage in skeletal muscle and adipose tissue and blocking its production by the liver. Second, insulin promotes the storage of nutrients as triglycerides. Individuals with pre-diabetes or the metabolic syndrome typically display impaired insulin-stimulated glucose disposal, as tissues adjust to utilize fatty acids as the primary insulin source. Simultaneously, they display enhanced triglyceride storage. Brown and Goldstein72 termed this “selective” insulin resistance in order to highlight the fact that insulin actions towards lipid storage are actually enhanced in the pre-diabetic condition.
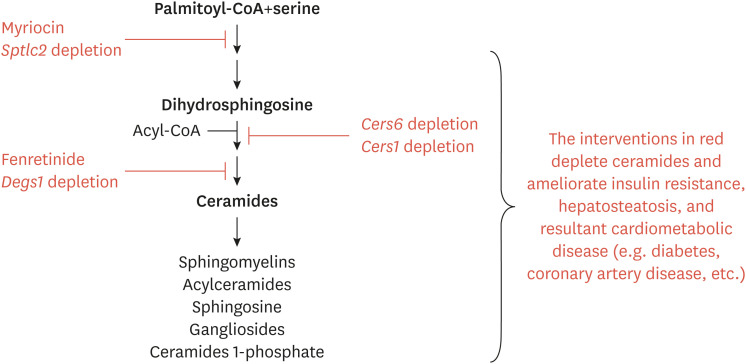
Ceramides, as signals of acute lipid overload, manage to accomplish most of the elements of selective insulin resistance. Ceramides inhibit insulin-stimulated glucose disposal by blocking activation of Akt/PKB, which leads to decreased translocation of the GLUT4 glucose transporter in adipose tissue and skeletal muscle. Ceramide's inhibition of Akt/PKB also releases the insulin-applied brake on gluconeogenesis, leading to enhanced hepatic glucose output. Ceramides induce steatosis by enhancing lipid uptake and triglyceride production into the liver. It does this by enhancing CD36 translocation to the membrane and upregulating SREBP and its target genes. Indeed, inhibition of ceramide biosynthesis improves glucose homeostasis and resolves hepatic steatosis in a number of rodent models (Fig. 5).
Fig. 5. Schematic depicting the interventions that have been shown to ameliorate cardiometabolic disorders. Pharmaceutical interventions or genetic knockout/knockdown of ceramide-synthesizing genes improves insulin sensitivity, resolves hepatic steatosis, and prevents the development of resultant metabolic disorders including diabetes, cardiomyopathy, coronary artery disease, hypertension, and heart failure.
Sptlc2, Serine palmitoyltransferase subunit 2; Cers, ceramide synthase genes; Degs1, dihydroceramide desaturase-1 gene; CoA, coenzyme A.
1. Myriocin, the aforementioned inhibitor of SPT, prevents or reverses atherosclerosis,73-78 insulin resistance (i.e., glucose lowering),8,79-83 and hepatic steatosis8,30,79,84-86 in mice, rats, and/or hamsters.
2. Deletion of an essential SPT subunit from adipose tissue of mice resolves glucose intolerance and hepatic steatosis.87
3. Excision of CERS6 from the whole body, liver, or brown adipose tissue of mice improves insulin sensitivity and resolves hepatic steatosis.9,10,12
4. Excision of CERS1 from skeletal muscle of mice resolves insulin resistance.13
5. Excision of DES1 from whole body, adipose tissue, or the liver of mice resolves insulin resistance and hepatic steatosis.2
6. Fenretinide, an inhibitor of DES1, resolves insulin resistance and hepatic steatosis.88
These data strongly suggest that ceramides, through the mechanisms described earlier, drive the key features of metabolic syndrome and pre-diabetes.
Stage 2: lipoaptosis and fibrosis
This perfect storm of insulin resistance and hepatic steatosis exacerbates the hyperlipidemia that is associated with obesity, leading to additional delivery of fatty acids to tissues not suited for fat storage. As ceramides continue to accrue, they cause irreversible damage resulting from the initiation of apoptosis and fibrosis. This leads to hepatosteatosis, type 2 diabetes, and renal and heart failure. Indeed, inhibition of SPT has been shown to reverse type 2 diabetes70,89 and heart failure.90,91 Though additional work is needed, the induction of apoptosis and fibrosis by ceramides appears to be a key component of cardiometabolic disease.
THERAPEUTIC TARGETS WITHIN THE CERAMIDE BIOSYNTHESIS PATHWAY
Inhibition of ceramide synthesis thus holds enormous potential to combat the metabolic diseases associated with obesity including non-alcoholic fatty liver disease/non-alcoholic steatohepatitis, type 2 diabetes, coronary artery disease, and heart failure. Two enzymes have emerged as particularly intriguing therapeutic targets that show an intriguing combination of efficacy and potential safety. 1) Knockout or knockdown of CERS6 ameliorates most metabolic complications of obesity, as it depletes the C16-ceramides that drive the insulin resistance and hepatic steatosis. Researchers at Evotec (Hamburg, Germany) and Sanofi-Aventis (Paris, France) used anti-sense oligonucleotides to target the gene in the livers of mice, finding that it recapitulated the effects of gene ablation.9 The authors of this manuscript recommended that such an isoform selective targeting approach should be sufficient to ameliorate insulin resistance and hepatic steatosis. Similarly, 2) we collaborated with Merck Research Laboratories (Kenilworth, NJ, USA) to interrogate the therapeutic efficacy of inhibiting DES1 activity, and thus producing a higher percentage of ceramides that lack the double bond that is critical ceramide action. Delivery of short hairpins targeting the Degs1 transcript recapitulated the beneficial effects of gene depletion, restoring insulin sensitivity and resolving fatty liver disease.2 Therapeutics targeting these enzymes hold enormous potential as a means of combating a broad spectrum of cardiometabolic disorders.
SUMMARY
We surmise that ceramides initiate actions that relieve the cell of an acute FFA burden, inhibiting glucose utilization and enhance triglyceride deposition. This theory provides a unifying framework for understanding the role of ceramides in the development of cardiometabolic disease. While these ceramide actions at some point served a protective role for organism survival, they now present an incredible burden to humans, as they drive the “selective” insulin resistance that is a hallmark of cardiometabolic disease. Therapeutic approaches targeting ceramide production thus holds great promise for treating a broad spectrum of diseases associated with obesity.
Footnotes
Funding: The author receives research support from the National Institutes of Health (DK115824 and DK116450), the Juvenile Diabetes Research Foundation (JDRF 3-SRA-2019-768-A-B), the American Diabetes Association, the American Heart Association, and the Margolis Foundation.
Conflict of Interest: Scott Summers is a co-founder and consultant with Centaurus Therapeutics (San Francisco, CA, USA).
References
- 1.Cahill GF., Jr Starvation in man. Clin Endocrinol Metab. 1976;5:397–415. doi: 10.1016/s0300-595x(76)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365:386–392. doi: 10.1126/science.aav3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 4.Lone MA, Santos T, Alecu I, Silva LC, Hornemann T. 1-Deoxysphingolipids. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:512–521. doi: 10.1016/j.bbalip.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Davis D, Kannan M, Wattenberg B. Orm/ORMDL proteins: gate guardians and master regulators. Adv Biol Regul. 2018;70:3–18. doi: 10.1016/j.jbior.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cingolani F, Futerman AH, Casas J. Ceramide synthases in biomedical research. Chem Phys Lipids. 2016;197:25–32. doi: 10.1016/j.chemphyslip.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841:671–681. doi: 10.1016/j.bbalip.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, et al. Cers2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Raichur S, Brunner B, Bielohuby M, Hansen G, Pfenninger A, Wang B, et al. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: Cers6 inhibition as a novel therapeutic approach. Mol Metab. 2019;21:36–50. doi: 10.1016/j.molmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, et al. Obesity-induced Cers6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerschmidt P, Ostkotte D, Nolte H, Gerl MJ, Jais A, Brunner HL, et al. Cers6-derived sphingolipids interact with MFF and promote mitochondrial fragmentation in obesity. Cell. 2019;177:1536–1552.e23. doi: 10.1016/j.cell.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Turpin-Nolan SM, Hammerschmidt P, Chen W, Jais A, Timper K, Awazawa M, et al. Cers1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 2019;26:1–10.e7. doi: 10.1016/j.celrep.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Sociale M, Wulf AL, Breiden B, Klee K, Thielisch M, Eckardt F, et al. Ceramide synthase schlank is a transcriptional regulator adapting gene expression to energy requirements. Cell Reports. 2018;22:967–978. doi: 10.1016/j.celrep.2017.12.090. [DOI] [PubMed] [Google Scholar]
- 15.Pillai BK, Jasuja R, Simard JR, Hamilton JA. Fast diffusion of very long chain saturated fatty acids across a bilayer membrane and their rapid extraction by cyclodextrins: implications for adrenoleukodystrophy. J Biol Chem. 2009;284:33296–33304. doi: 10.1074/jbc.M109.043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MA, Bishop RE. Molecular mechanism for lateral lipid diffusion between the outer membrane external leaflet and a beta-barrel hydrocarbon ruler. Biochemistry. 2009;48:9745–9756. doi: 10.1021/bi9013566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunaldi K, Huang N, Hamilton JA. Fatty acids are rapidly delivered to and extracted from membranes by methyl-beta-cyclodextrin. J Lipid Res. 2010;51:120–131. doi: 10.1194/jlr.M900200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Huang N, Cai J, Xie W, Hamilton JA. Fatty acid transport and metabolism in HepG2 cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G528–G534. doi: 10.1152/ajpgi.00386.2005. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton JA, Johnson RA, Corkey B, Kamp F. Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci. 2001;16:99–108. doi: 10.1385/JMN:16:2-3:99. [DOI] [PubMed] [Google Scholar]
- 20.Glatz JF. Lipids and lipid binding proteins: a perfect match. Prostaglandins Leukot Essent Fatty Acids. 2015;93:45–49. doi: 10.1016/j.plefa.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Jay AG, Hamilton JA. The enigmatic membrane fatty acid transporter CD36: new insights into fatty acid binding and their effects on uptake of oxidized LDL. Prostaglandins Leukot Essent Fatty Acids. 2018;138:64–70. doi: 10.1016/j.plefa.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Xu S, Jay A, Brunaldi K, Huang N, Hamilton JA. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 2013;52:7254–7261. doi: 10.1021/bi400914c. [DOI] [PubMed] [Google Scholar]
- 23.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015;22:266–278. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehehalt R, Sparla R, Kulaksiz H, Herrmann T, Füllekrug J, Stremmel W. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts) BMC Cell Biol. 2008;9:45. doi: 10.1186/1471-2121-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohl J, Ring A, Ehehalt R, Schulze-Bergkamen H, Schad A, Verkade P, et al. Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry. 2004;43:4179–4187. doi: 10.1021/bi035743m. [DOI] [PubMed] [Google Scholar]
- 26.Covey SD, Brunet RH, Gandhi SG, McFarlane N, Boreham DR, Gerber GE, et al. Cholesterol depletion inhibits fatty acid uptake without affecting CD36 or caveolin-1 distribution in adipocytes. Biochem Biophys Res Commun. 2007;355:67–71. doi: 10.1016/j.bbrc.2007.01.135. [DOI] [PubMed] [Google Scholar]
- 27.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worgall TS, Juliano RA, Seo T, Deckelbaum RJ. Ceramide synthesis correlates with the posttranscriptional regulation of the sterol-regulatory element-binding protein. Arterioscler Thromb Vasc Biol. 2004;24:943–948. doi: 10.1161/01.atv.0000125703.20434.4d. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Chen TC, Lee RA, Tsai SL, Kanamaluru D, Gray NE, Yiv N, et al. An ANGPTL4-ceramide-protein kinase Cζ axis mediates chronic glucocorticoid exposure-induced hepatic steatosis and hypertriglyceridemia in mice. J Biol Chem. 2019;294:9213–9224. doi: 10.1074/jbc.RA118.006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang CN, O'Brien L, Brindley DN. Effects of cell-permeable ceramides and tumor necrosis factor-alpha on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes. 1998;47:24–31. doi: 10.2337/diab.47.1.24. [DOI] [PubMed] [Google Scholar]
- 33.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 34.Finicle BT, Ramirez MU, Liu G, Selwan EM, McCracken AN, Yu J, et al. Sphingolipids inhibit endosomal recycling of nutrient transporters by inactivating ARF6. J Cell Sci. 2018;131:jcs213314. doi: 10.1242/jcs.213314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci U S A. 2008;105:17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edinger AL. Starvation in the midst of plenty: making sense of ceramide-induced autophagy by analysing nutrient transporter expression. Biochem Soc Trans. 2009;37:253–258. doi: 10.1042/BST0370253. [DOI] [PubMed] [Google Scholar]
- 37.Cowart LA, Obeid LM. Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim Biophys Acta. 2007;1771:421–431. doi: 10.1016/j.bbalip.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung N, Mao C, Heitman J, Hannun YA, Obeid LM. Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae . J Biol Chem. 2001;276:35614–35621. doi: 10.1074/jbc.M105653200. [DOI] [PubMed] [Google Scholar]
- 39.Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, et al. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- 40.Stratford S, DeWald DB, Summers SA. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem J. 2001;354:359–368. doi: 10.1042/0264-6021:3540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 42.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 44.Salinas M, López-Valdaliso R, Martín D, Alvarez A, Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol Cell Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 45.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 46.Zinda MJ, Vlahos CJ, Lai MT. Ceramide induces the dephosphorylation and inhibition of constitutively activated Akt in PTEN negative U87mg cells. Biochem Biophys Res Commun. 2001;280:1107–1115. doi: 10.1006/bbrc.2000.4248. [DOI] [PubMed] [Google Scholar]
- 47.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Cζ: implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 48.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, et al. Ceramide recruits and activates protein kinase C ζ (PKCζ) within structured membrane microdomains. J Biol Chem. 2007;282:12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 49.Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, et al. Targeting of PKCζ and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J. 2008;410:369–379. doi: 10.1042/BJ20070936. [DOI] [PubMed] [Google Scholar]
- 50.Dey D, Basu D, Roy SS, Bandyopadhyay A, Bhattacharya S. Involvement of novel PKC isoforms in FFA induced defects in insulin signaling. Mol Cell Endocrinol. 2006;246:60–64. doi: 10.1016/j.mce.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Blouin CM, Prado C, Takane KK, Lasnier F, Garcia-Ocana A, Ferré P, et al. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes. 2010;59:600–610. doi: 10.2337/db09-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 53.Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 54.Zigdon H, Kogot-Levin A, Park JW, Goldschmidt R, Kelly S, Merrill AH, Jr, et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J Biol Chem. 2013;288:4947–4956. doi: 10.1074/jbc.M112.402719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park M, Kaddai V, Ching J, Fridianto KT, Sieli RJ, Sugii S, et al. A role for ceramides, but not sphingomyelins, as antagonists of insulin signaling and mitochondrial metabolism in C2C12 myotubes. J Biol Chem. 2016;291:23978–23988. doi: 10.1074/jbc.M116.737684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Rao RP, Kosakowska-Cholody T, Masood MA, Southon E, Zhang H, et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol. 2009;184:143–158. doi: 10.1083/jcb.200807176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner N, Lim XY, Toop HD, Osborne B, Brandon AE, Taylor EN, et al. A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat Commun. 2018;9:3165. doi: 10.1038/s41467-018-05613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith ME, Tippetts TS, Brassfield ES, Tucker BJ, Ockey A, Swensen AC, et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem J. 2013;456:427–439. doi: 10.1042/BJ20130807. [DOI] [PubMed] [Google Scholar]
- 59.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 60.Ganesan V, Perera MN, Colombini D, Datskovskiy D, Chadha K, Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- 61.Dadsena S, Bockelmann S, Mina JG, Hassan DG, Korneev S, Razzera G, et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun. 2019;10:1832. doi: 10.1038/s41467-019-09654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siskind LJ, Davoody A, Lewin N, Marshall S, Colombini M. Enlargement and contracture of C2-ceramide channels. Biophys J. 2003;85:1560–1575. doi: 10.1016/S0006-3495(03)74588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perera MN, Lin SH, Peterson YK, Bielawska A, Szulc ZM, Bittman R, et al. Bax and Bcl-xL exert their regulation on different sites of the ceramide channel. Biochem J. 2012;445:81–91. doi: 10.1042/BJ20112103. [DOI] [PubMed] [Google Scholar]
- 67.Ganesan V, Colombini M. Regulation of ceramide channels by Bcl-2 family proteins. FEBS Lett. 2010;584:2128–2134. doi: 10.1016/j.febslet.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 68.Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, et al. Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J Biol Chem. 2008;283:6622–6630. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Q, Denard B, Lee CE, Han S, Ye JS, Ye J. Inverting the topology of a transmembrane protein by regulating the translocation of the first transmembrane helix. Mol Cell. 2016;63:567–578. doi: 10.1016/j.molcel.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Q, Lee CE, Denard B, Ye J. Sustained induction of collagen synthesis by TGF-β requires regulated intramembrane proteolysis of CREB3L1. PLoS One. 2014;9:e108528. doi: 10.1371/journal.pone.0108528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Denard B, Lee C, Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. eLife. 2012;1:e00090. doi: 10.7554/eLife.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 73.Park TS, Rosebury W, Kindt EK, Kowala MC, Panek RL. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol Res. 2008;58:45–51. doi: 10.1016/j.phrs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Glaros EN, Kim WS, Quinn CM, Jessup W, Rye KA, Garner B. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J Lipid Res. 2008;49:324–331. doi: 10.1194/jlr.M700261-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Glaros EN, Kim WS, Wu BJ, Suarna C, Quinn CM, Rye KA, et al. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem Pharmacol. 2007;73:1340–1346. doi: 10.1016/j.bcp.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 76.Park TS, Panek RL, Rekhter MD, Mueller SB, Rosebury WS, Robertson A, et al. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis. 2006;189:264–272. doi: 10.1016/j.atherosclerosis.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 77.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 78.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 79.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Dekker MJ, Baker C, Naples M, Samsoondar J, Zhang R, Qiu W, et al. Inhibition of sphingolipid synthesis improves dyslipidemia in the diet-induced hamster model of insulin resistance: evidence for the role of sphingosine and sphinganine in hepatic VLDL-apoB100 overproduction. Atherosclerosis. 2013;228:98–109. doi: 10.1016/j.atherosclerosis.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 81.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blachnio-Zabielska AU, Hady HR, Markowski AR, Kurianiuk A, Karwowska A, Górski J, et al. Inhibition of ceramide de novo synthesis affects adipocytokine secretion and improves systemic and adipose tissue insulin sensitivity. Int J Mol Sci. 2018;19:E3995. doi: 10.3390/ijms19123995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurek K, Piotrowska DM, Wiesiołek-Kurek P, Łukaszuk B, Chabowski A, Górski J, et al. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 2014;34:1074–1083. doi: 10.1111/liv.12331. [DOI] [PubMed] [Google Scholar]
- 85.Correnti JM, Juskeviciute E, Swarup A, Hoek JB. Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G959–G973. doi: 10.1152/ajpgi.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kasumov T, Li L, Li M, Gulshan K, Kirwan JP, Liu X, et al. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One. 2015;10:e0126910. doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, Galam DL, et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 2016;24:820–834. doi: 10.1016/j.cmet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Bikman BT, Guan Y, Shui G, Siddique MM, Holland WL, Kim JY, et al. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J Biol Chem. 2012;287:17426–17437. doi: 10.1074/jbc.M112.359950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 90.Ji R, Akashi H, Drosatos K, Liao X, Jiang H, Kennel PJ, et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight. 2017;2:96203. doi: 10.1172/jci.insight.96203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]