Graphical Abstract
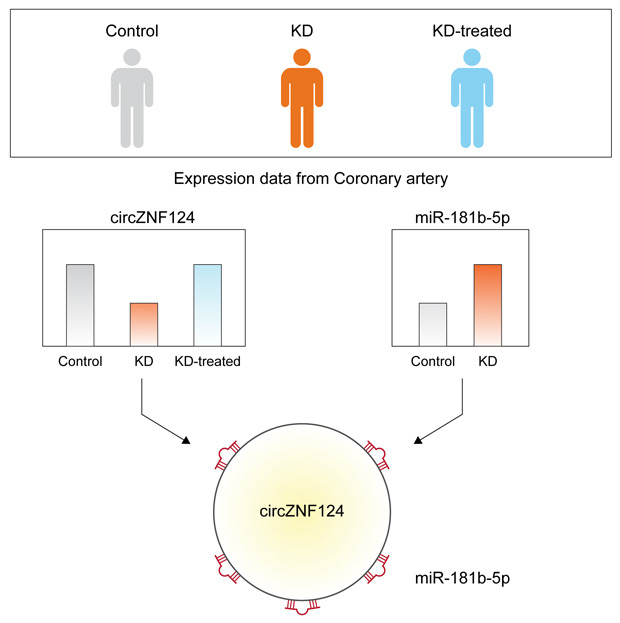
The expression profiles of circular RNAs (circRNAs) from coronary artery tissue of patients with Kawasaki disease were analyzed using public sequencing datasets.
Several circRNAs including circZNF124 were significantly down-regulated in the untreated patients but returned to normal level after the treatment.
For circWHAMMP1 and circZNF124, the microRNAs that may be regulated by these circRNAs were also identified.
Keywords: Kawasaki disease; Coronary artery; RNA, circular; microRNAs
Abstract
Objectives
Kawasaki disease is a disease in children that presents with diverse symptoms including acute fever, conjunctivitis, body rash, swollen lymph nodes of the neck, and peeling of the skin on the hands and feet. Although patients with Kawasaki disease are continually observed and diagnosed, there are no established molecular markers to diagnose this disease quickly and accurately. Moreover, there have been very few studies on the molecular mechanism underlying Kawasaki disease.
Methods
The expression profiles of circular RNAs (circRNAs) from coronary artery tissue of patients with Kawasaki disease were analyzed using public sequencing datasets. After selecting reliable sequencing libraries and high-quality reads, bioinformatics pipelines were applied to quantify the expression of back-splicing reads of host genes.
Results
Many circRNAs were identified to be differentially expressed between the controls and patients with Kawasaki disease. Among them, circRNAs originating from host genes including homeodomain interacting protein kinase 3 (circHIPK3), zinc finger protein 124 (circZNF124), WAS protein homolog associated with actin, Golgi membranes, and microtubules pseudogene 1 (circWHAMMP1), SLAIN motif family, member 2 (circSLAIN2), and ataxia telangiectasia mutated (circATM) were down-regulated significantly in untreated patients with Kawasaki disease. Importantly, the level of these circRNAs returned to normal in the coronary arteries of treated patients, suggesting these circRNAs are possible molecular markers for Kawasaki disease. For circWHAMMP1 and circZNF124, the microRNAs that may be regulated by these circRNAs were also identified.
Conclusions
This study will contribute to future research seeking to determine the regulatory pathways involved in the pathogenesis of Kawasaki disease.
INTRODUCTION
Kawasaki disease is a disease in children with acute vasculitis whereby the blood vessels throughout the body are inflamed.1,2 The incidence of Kawasaki disease varies by race and country, but the highest incidence is observed in Asian countries, especially in Japan and Korea.3,4 The cause and pathological mechanism of Kawasaki disease remain largely unknown. Moreover, a molecular marker for this disease has not been established. Current criteria to diagnose Kawasaki disease are mainly clinical symptoms including polymorphous rash, conjunctival injection, strawberry tongue, cervical lymphadenopathy, and desquamation.5 Because of the high probability of coronary artery aneurysms and other serious conditions without prompt treatment, establishing a molecular marker to quickly diagnose Kawasaki disease is essential.1 Therapy for Kawasaki disease includes anti-inflammatory treatment using intravenous immunoglobulin (IVIG) with aspirin and/or steroids such as prednisone, although the detailed mechanisms for these treatments are not well understood.2,5 Thus, more studies are needed to determine the pathology and identify specific biomarkers for Kawasaki disease.
Circular RNA (circRNA) is a class of single-stranded RNA with a closed circular structure. In humans, over ten thousand circRNAs have been discovered, and most do not yet have annotated functions.6 These circRNAs originate from diverse sources, but most are produced from the exons of host genes through back-splicing.7,8 During the back-splicing reaction, the 3ʹ end of a downstream exon is connected into the 5ʹ end of an upstream exon, producing the closed circular structure. The working mechanism of several circRNAs has been reported to date, where it has been shown that many circular RNAs have a microRNA (miRNA)-binding site in their sequences.9 Because miRNAs primarily suppress the translation of their target mRNAs, circRNAs can function to indirectly increase the translation of protein-coding mRNAs through absorbing miRNAs. Other studies have shown that circRNAs repress the function of proteins by binding them.10 In addition, some circRNAs have an open reading frame that is able to produce small proteins.11,12 Therefore, circRNAs can behave as important regulators in cells.
Although the function of circRNAs has been reported in human pathogenesis, the role of circRNAs in Kawasaki disease has not yet been reported. To understand how differential circRNA expression may affect the regulatory mechanism of this disease, circRNA expression profiles were analyzed using publicly available datasets from studies that performed RNA sequencing on coronary artery tissues from patients with Kawasaki disease. Several circRNAs were identified to be differentially expressed in untreated Kawasaki disease tissues, but their level returned to normal after treatment. The data obtained from this study will be useful for future studies focusing on the underlying molecular mechanism of Kawasaki disease progression and for determining molecular markers for this disease.
MATERIALS AND METHODS
1. Samples used for analysis
RNA sequencing data from coronary artery tissue samples from controls and patients with Kawasaki disease were obtained from the Gene Expression Omnibus (GEO) dataset GSE64486. The information for patients with Kawasaki disease and controls was described previously.13 Briefly, the control group had normal coronary artery histology. Among the two groups of patients with Kawasaki disease, the untreated group did not receive IVIG therapy after the onset of fever while the treated group received treatment with IVIG, aspirin, steroids, and/or infliximab.13
2. Analysis of circRNA expression from RNA sequencing data
The raw data produced from RNA sequencing was filtered using the Trimmomatic trimmer tool to trim the 5ʹ and 3ʹ ends of sequences and to remove reads with overall low quality (option: LEADING: 5 TRAILING: 5 SLIDINGWINDOW: 4:20 MINLEN: 36).14 The remaining adaptor sequences were also trimmed during this procedure. The filtered sequences were aligned to the human genome using the STAR aligner with paired reads or single reads, respectively.15 Using the alignment information, circRNA expression was calculated using the DCC algorithm, which resulted in the identification of 9,134 circRNAs.16 Among them, only 311 circRNAs with average counts greater than 2 among whole samples were selected for further analysis. For the selected circRNAs, their expression levels were normalized by dividing the counts with the entire circRNA counts in each sample, and then the fold change of circRNA expression was calculated among the groups. The host gene and exon structures where the circRNAs originated were inspected manually using the UCSC Genome Browser server, and only those circRNAs whose exon structures fully overlapped with the exon structure of the host genes were selected.17
3. Analysis of circRNA regulation on miRNAs
To identify potential miRNAs that may be regulated by the selected circRNAs, the miRNA list reported to be differentially expressed between artery tissues from patients with Kawasaki disease and those from control patients was obtained.18 There were 26 miRNAs on this list, including miR-92a, miR-93, miR-127-3p, miR-146b-5p, miR-150, miR-181b, miR-185, miR-210, miR-223, miR-320a, miR-320b, miR-423-5p, miR-548c-5p, miR-566, miR-638, miR-650, miR-654-5p, miR-663, miR-663b, miR-675, miR-1246, miR-1274b, miR-1285, miR-1291, miR-1300, and miR-1307. The binding sites for these miRNAs in the circRNA sequences were analyzed using the TargetScan algorithm.19
RESULTS
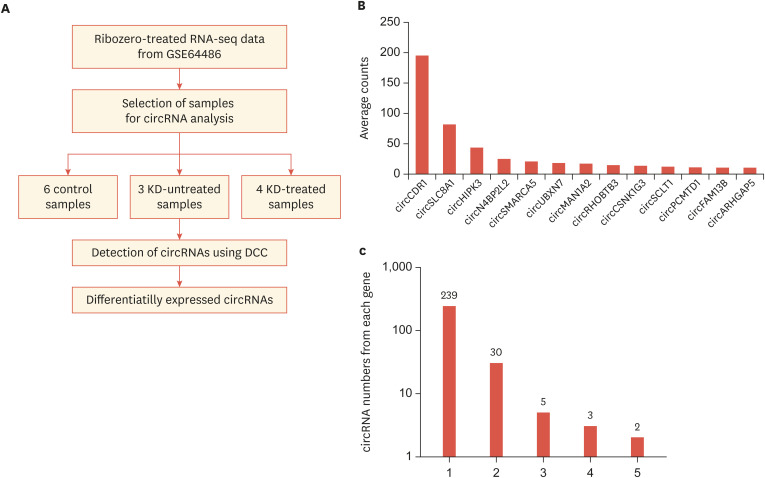
To identify circRNAs that may be involved in the pathogenesis of Kawasaki disease, RNA sequencing data from coronary artery tissue from two groups of patients with Kawasaki disease and a group of control patients was utilized (Fig. 1A).13 The control group contained seven samples with normal coronary artery histology. The two patient groups were classified into untreated Kawasaki patients and Kawasaki patients treated with IVIG, with each group containing four samples. After trimming low-quality reads from the sequencing results, circRNA expression levels were calculated using the DCC algorithm, which counts the chimeric reads produced by linking the 3ʹ end of a downstream exon and the 5ʹ end of an upstream exon. Because one control sample and one sample from the untreated Kawasaki disease group had a small number of circRNA counts, these samples were removed from the subsequent analysis (Fig. 1A).
Fig. 1. Identification of circRNAs expressed in coronary artery tissue from control and Kawasaki disease groups. (A) The procedure used to identify circRNAs in the control and Kawasaki disease samples. Based on data from the GSE64486 dataset, the expression level of circRNAs in each group was calculated using the DCC algorithm and differentially expressed circRNAs were selected for further study.13,16 (B) Average expression counts of highly expressed circRNAs. circRNAs with normalized average counts greater than 10 are shown. (C) The number of circRNAs produced from each gene was calculated.
circRNA, circular RNA.
Overall, 311 circRNAs with reliable expression counts were identified in the coronary artery tissues (Supplementary Table 1, see Methods). Among these circRNAs, circCDR1 exhibited the highest expression in the samples (Fig. 1B). This circRNA was previously reported to be a potent miRNA suppressor with over 60 binding sites for miR-7.20,21 In most cases, only a single circRNA type was produced from each host gene, but in some cases, diverse circRNAs were produced from a different combination of host gene exons (Fig. 1C).
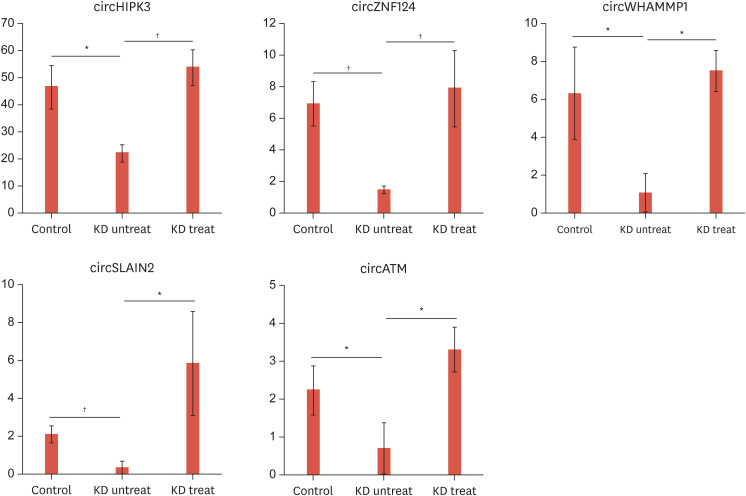
To identify circRNAs that may have a role in the progression of Kawasaki disease or may be used as a potential biomarker, the fold change in expression of each circRNA was calculated and circRNAs with differential expression between any two groups were selected. This analysis resulted in 32 circRNAs (Supplementary Table 2). Among the circRNAs that were differentially expressed between the control group and the untreated Kawasaki disease group, five circRNAs including circHIPK3, circZNF124, circWHAMMP1, circSLAIN2, and circATM exhibited recovered expression after treatment (Fig. 2).
Fig. 2. Selected circRNAs with probable roles in Kawasaki disease. Among the circRNAs listed in Supplementary Table 2, only circRNAs that showed differential expression between control and untreated Kawasaki disease groups (p<0.05), and between untreated and treated Kawasaki disease groups (p<0.05), but no significant difference between the control and treated Kawasaki disease groups (p>0.05), were selected. p values were calculated by a t-test between samples from 2 groups, respectively.
circRNA, circular RNA.
*p<0.05; †p<0.01.
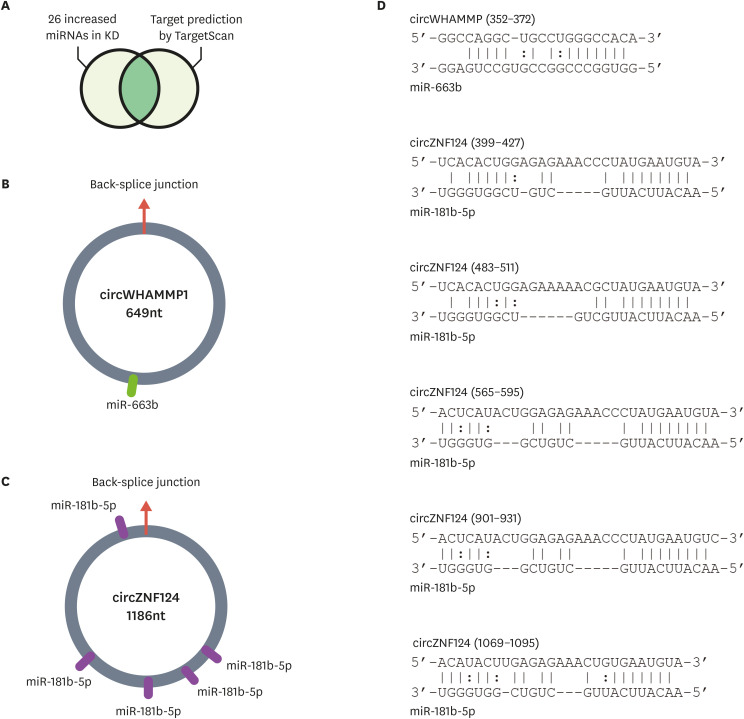
CircRNAs are generally involved in gene regulatory networks by suppressing the function of miRNAs. In a previous study, miRNA expression levels were compared between coronary artery samples from patients with Kawasaki disease and control patients, and 26 miRNAs were reported to show significantly increased expression in the Kawasaki disease group.18 Because expression of the selected circRNAs was decreased in the untreated Kawasaki disease group compared to that in the control group (Fig. 2), it was predicted that the target miRNAs of these circRNAs would be increased in the same group. Using TargetScan, a miRNA target prediction algorithm, the target sites of the increased miRNAs in the selected circRNA sequences were predicted (Fig. 3A).19 This analysis identified that circWHAMMP1 and circZNF124 are most likely regulated by miR-663b and miR-181b-5p, respectively (Fig. 3B and C). Strikingly, there were five binding sites for miR-181b-5p in the sequence of circZNF124, suggesting that circZNF124 may be an efficient suppressor of miR-181b-5p.
Fig. 3. Possible working mechanism of circRNAs as miRNA suppressors. (A) Analysis of circRNAs as miRNA suppressors. Among the increased miRNAs in patients with Kawasaki disease, miRNAs with predicted binding sites in circRNA sequences were selected using TargetScan.19 (B-C) The miRNA binding sites in circRNAs are depicted for (B) circWHAMMP1 and (C) circZNF124. (D) Base pairing between the predicted miRNA binding sites and circRNAs is shown. The nucleotide position of the back-splice junction depicted in (B, C) was set as zero, and the relative position of the nucleotide sequences was calculated.
circRNA, circular RNA; miRNA, microRNA.
DISCUSSION
In this study, the expression profile of circRNAs in the artery tissue of patients with Kawasaki disease was analyzed using RNA sequencing data from the GEO database.13,22 By comparing the expression level of circRNAs among control patients, patients with untreated Kawasaki disease, and treated patients with Kawasaki disease, five circRNAs were identified that could be used as molecular biomarkers to reflect the status of Kawasaki disease (Fig. 2). Among those circRNAs, only the function of circHIPK3 has been studied previously. This circRNA was previously reported to be involved in vascular dysfunction of the retina in patients with diabetes.23 Moreover, another study showed that circHIPK3 is involved in regulating human cell growth by suppressing multiple miRNAs, suggesting that circHIPK3 is an important regulator in human diseases.24
To date, many studies have identified the function of miR-663b and miR-181b-5p, which may be suppressed by circWHAMMP1 and circZNF124, respectively. One previous study found that miR-663b increases cell proliferation and migration in nasopharyngeal carcinoma.25 Another study showed that miR-181b expression decreases during the aging process and is involved in regulating vascular stiffness.26 Although there is still no direct evidence that these miRNAs have any role in the progression of Kawasaki disease, misregulation in miRNA activity due to altered circRNA expression may affect cellular pathways in arteries. Thus, it will be interesting to identify the role of these miRNAs in the progression of Kawasaki disease in future studies.
Footnotes
Funding: This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) (NRF-2018R1A2B6001104).
Conflict of Interest: The author has no conflicts of interest to declare.
SUPPLEMENTARY MATERIALS
CircRNA expression level in controls and the treated and untreated Kawasaki disease patient groups
CircRNAs with differential expression among controls and patients with treated or untreated Kawasaki disease
References
- 1.Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67:1738–1749. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 2.Cohen E, Sundel R. Kawasaki disease at 50 years. JAMA Pediatr. 2016;170:1093–1099. doi: 10.1001/jamapediatrics.2016.1446. [DOI] [PubMed] [Google Scholar]
- 3.Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22:79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha S, Seo GH, Kim KY, Kim DS. Epidemiologic study on Kawasaki disease in Korea, 2007–2014: based on Health Insurance Review & Assessment Service Claims. J Korean Med Sci. 2016;31:1445–1449. doi: 10.3346/jkms.2016.31.9.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 6.Glažar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta. 2016;1859:163–168. doi: 10.1016/j.bbagrm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Wilusz JE. Circular RNAs: unexpected outputs of many protein-coding genes. RNA Biol. 2017;14:1007–1017. doi: 10.1080/15476286.2016.1227905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol. 2016;238:42–51. doi: 10.1016/j.jbiotec.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Du WW, Zhang C, Yang W, Yong T, Awan FM, Yang BB. Identifying and characterizing circRNA-protein interaction. Theranostics. 2017;7:4183–4191. doi: 10.7150/thno.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, et al. Translation of CircRNAs. Mol Cell. 2017;66:9–21.e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37.e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowley AH, Wylie KM, Kim KY, Pink AJ, Yang A, Reindel R, et al. The transcriptional profile of coronary arteritis in Kawasaki disease. BMC Genomics. 2015;16:1076. doi: 10.1186/s12864-015-2323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng J, Metge F, Dieterich C. Specific identification and quantification of circular RNAs from sequencing data. Bioinformatics. 2016;32:1094–1096. doi: 10.1093/bioinformatics/btv656. [DOI] [PubMed] [Google Scholar]
- 17.Tyner C, Barber GP, Casper J, Clawson H, Diekhans M, Eisenhart C, et al. The UCSC Genome Browser database: 2017 update. Nucleic Acids Res. 2017;45:D626–D634. doi: 10.1093/nar/gkw1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowley AH, Pink AJ, Reindel R, Innocentini N, Baker SC, Shulman ST, et al. A study of cardiovascular miRNA biomarkers for Kawasaki disease. Pediatr Infect Dis J. 2014;33:1296–1299. doi: 10.1097/INF.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 21.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 22.Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol. 2016;1418:93–110. doi: 10.1007/978-1-4939-3578-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, et al. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136:1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang S, Zhang N, Deng Y, Chen L, Zhang Y, Zheng Z, et al. miR-663b promotes tumor cell proliferation, migration and invasion in nasopharyngeal carcinoma through targeting TUSC2. Exp Ther Med. 2017;14:1095–1103. doi: 10.3892/etm.2017.4608. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Hori D, Dunkerly-Eyring B, Nomura Y, Biswas D, Steppan J, Henao-Mejia J, et al. miR-181b regulates vascular stiffness age dependently in part by regulating TGF-β signaling. PLoS One. 2017;12:e0174108. doi: 10.1371/journal.pone.0174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CircRNA expression level in controls and the treated and untreated Kawasaki disease patient groups
CircRNAs with differential expression among controls and patients with treated or untreated Kawasaki disease