Abstract
Vascular complications from uncontrolled hyperglycemia are the leading cause of death in patients with diabetes mellitus. Previous reports have shown a strong correlation between hyperglycemia and vascular calcification, which increases mortality and morbidity in individuals with diabetes. However, the precise underlying molecular mechanisms of hyperglycemia-induced vascular calcification remain largely unknown. Transdifferentiation of vascular smooth muscle cells (VSMC) into osteoblast-like cells is a known culprit underlying the development of vascular calcification in the diabetic vasculature. Pathological conditions such as high glucose levels and oxidative stress are linked to enhanced osteogenic differentiation of VSMC both in vivo and in vitro. It has been demonstrated that increased expression of runt-related transcription factor 2 (Runx2), a bone-related transcription factor, in VSMC is necessary and sufficient for the induction of VSMC calcification. Addition of a single O-linked β-N-acetylglucosamine (O-GlcNAc) moiety to the serine/threonine residues of target proteins (O-GlcNAcylation) has been observed in the arteries of diabetic patients, as well as in animal models in association with the enhanced expression of Runx2 and aggravated vascular calcification. O-GlcNAcylation is a dynamic and tightly regulated process, that is mediated by 2 enzymes, O-GlcNAc transferase and O-GlcNAcase. Glucose is metabolized into UDP-β-D-N-acetylglucosamine, an active sugar donor of O-GlcNAcylation via the hexosamine biosynthetic pathway. Overall increases in the O-GlcNAcylation of cellular proteins have been closely associated with cardiovascular complications of diabetes. In this review, the authors provide molecular insights into cardiovascular complications, including diabetic vasculopathy, that feature increased O-GlcNAcylation in people with diabetes.
Keywords: O-GlcNAc modification, Hyperglycemia, Vascular calcification, Vascular smooth muscle cells, Runx2
INTRODUCTION
According to the International Diabetes Federation, approximately 463 million adults aged 20–79 years are living with diabetes, and the number of people with diabetes will rise to 700 million by 2045.1 People with diabetes are at an elevated risk of multiple complications, such as cardiovascular disease (CVD), diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy. In all Westernized countries, CVD is the most common cause of death in people with diabetes. Therefore, the therapeutic and prognostic value of diabetic complications, such as vascular calcification, in patients with diabetes cannot be underestimated.
As epidemiological evidence of the prevalence of vascular calcification in patients with diabetes mellitus and resulting cardiovascular complications, reports have repeatedly described an increased incidence of arterial stiffness and thrombosis, which lead to elevated rates of morbidity and mortality.2,3 Vascular calcification, previously considered to be a passive deposition of calcium phosphate crystals in the vasculature, has now been recognized as an active process regulated by multiple relevant cell types, similar to bone mineralization.4 A key cell type involved in the development of vascular calcification, vascular smooth muscle cells (VSMC) undergo transdifferentiation into osteoblast-like cells and deposit calcium as hydroxyapatite crystals in the arteries of patients with diabetes.5,6
Hyperglycemia in individuals with diabetes and animal models of diabetes is closely linked to increased vascular calcification.6,7 According to previous studies, a high rate of modification of target proteins by the addition of O-linked β-N-acetylglucosamine (O-GlcNAc) was observed in the vasculature of patients with diabetes and in diabetic mouse models.8,9,10 Through the hexosamine biosynthetic pathway, extracellular glucose is metabolized into UDP-β-D-N-acetylglucosamine (UDP-GlcNAc), an active sugar donor for O-GlcNAcylation of cellular proteins.11 Hence, hyperglycemia—defined as excessive blood levels of glucose, a substrate for the production of UDP-GlcNAc—may result in enhanced O-GlcNAcylation in patients with diabetes, as well as cardiovascular events.6,8,12
More recently, it was shown that aggravated vascular calcification is accompanied by increased O-GlcNAcylation in a low-dose streptozotocin (STZ)-induced diabetes model via O-GlcNAc-mediated activation of the AKT signaling pathway.10 Since vascular calcification is a major contributor to increased morbidity and mortality in patients with diabetes, we review the molecular mechanisms underlying O-GlcNAcylation-mediated vascular calcification and, most importantly, provide crucial molecular insights into the function of O-GlcNAcylation in regulating diabetic vasculopathy.
ROLE OF O-GlcNAcylation IN DIABETIC VASCULOPATHY
1. O-GlcNAcylation: a double-edged sword in cardiovascular pathologies
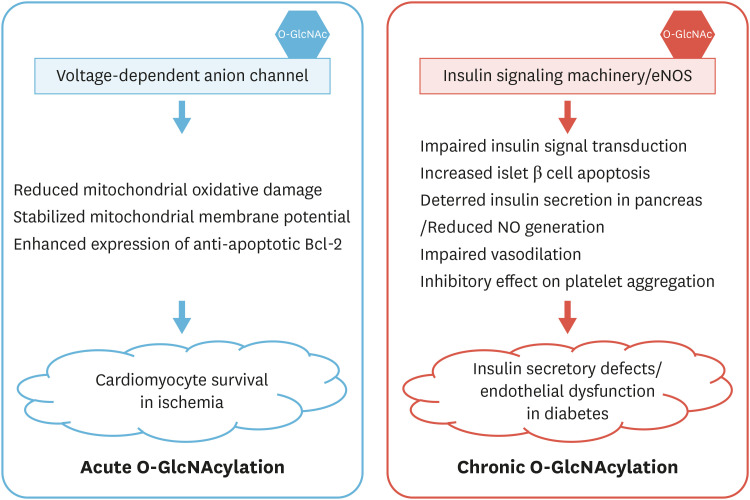
It has been reported that acute increments of O-GlcNAcylation confer protection from oxidative stress—induced calcium overload and structural damage in ischemia-reperfusion models of heart failure.13,14,15 The cardioprotective effects of O-GlcNAcylation have been attributed to improved tolerance of mitochondrial oxidative damage through enhanced O-GlcNAc modifications of voltage-dependent anion channels, thereby attenuating the loss of mitochondrial membrane potential and ultimately leading to the survival of cardiomyocytes.16,17 In an in vitro model of ischemia-reperfusion injury, upregulated protein O-GlcNAcylation and subsequent increases in the expression and translocation of members of the Bcl-2 protein family ameliorated the mitochondrial dysfunction and apoptotic cell death induced by ischemic injuries.18 More directly, cardiomyocyte-specific deletion of O-GlcNAc transferase (OGT) aggravated heart failure by reducing the heart's compensatory capacity in mice with myocardial infarction, clearly demonstrating the necessity of cardiac OGT expression in the failing heart.13
Chronic hyperglycemia exerts devastating impact on vascular function, thereby leading to cardiovascular complications including diabetic cardiomyopathy, nephropathy, retinopathy, neuropathy, and atherosclerosis secondary to diabetes mellitus.19 Diabetic vascular dysfunction manifests in the early stages of this complex disease and continuously accompanies the progression of pathology in diabetes, ultimately leading to morbidity and mortality from cardiovascular complications.20 In this process, chronic elevation of O-GlcNAcylation—possibly through chronic hyperglycemia, as occurs in diabetes—exerts adverse effects on the cardiovascular system.21 Hyperglycemia led to significant increases in both OGT expression and O-GlcNAcylation of cellular proteins, reducing insulin secretion in the pancreas of diabetic Goto-Kakizaki rats and in isolated islets through enhanced O-GlcNAcylation of pancreatic and duodenal homeobox-1 (PDX-1), the pancreatic/duodenal homeobox-1 protein, a critical regulator of β-cell survival and a transcription factor for insulin production in the pancreas.22,23 It was also demonstrated that enhanced O-GlcNAcylation of insulin signaling machinery resulted in impaired insulin signal transduction, leading to insulin resistance and dyslipidemia in the hepatic system.24
In patients with diabetes, traditionally defined as metabolic vascular syndrome, the increased incidence of cardiovascular complications has been linked to the adverse effect of hyperglycemic milieu on macrovascular and microvascular beds.25 The cardiovascular complications caused by diabetes mellitus can be largely characterized as endothelial dysfunction, which involves functional impairment of the vascular endothelium via reduced nitric oxide (NO) bioavailability.26 In the vasculature, NO is mainly synthesized by endothelial NO synthase (eNOS) through a series of redox reactions in the endothelium.27 As it diffuses into VSMC, NO activates guanylate cyclase, yielding a concomitant increase in cyclic guanosine monophosphate. Through this mechanism, it induces relaxation of VSMC, and the resulting vasodilation by basal NO has a major impact on the regulation of blood pressure.28 Impairment of NO signaling may, therefore, be linked to several pathological states in the vasculature, including impaired fibrinolytic activity,29 over-expressed inflammatory molecules,30 and increased oxidative stress,31 resulting in the aggravation of cardiovascular risk. The vascular endothelium constitutes a single layer of cells encompassing the vascular lumen and is involved in modulating vascular tone and structure. Until recently, the endothelial layer has been regarded as a benign barrier between the circulating blood and the underlying vascular tissues. Endothelial cells, however, are now recognized as critical regulators of vascular homeostasis through the secretion of a wide range of factors affecting endocrine, paracrine, and autocrine functions of blood vessels under normal conditions.32 Hence, the endothelial layer performs a variety of functions that are closely involved in the maintenance of homeostasis; for instance, it controls immune cell adhesion, smooth muscle cell proliferation, angiogenic migration, vascular integrity, and vessel wall inflammation. Endothelial dysfunction is closely associated with multiple pathological conditions such as diabetes, atherosclerosis, and hypertension.33 Therefore, in early works demonstrating the role of O-GlcNAcylation in vascular function, many studies focused on the identification of molecular mechanisms underlying hyperglycemia-induced endothelial dysfunction; in one of these mechanisms, hyperglycemia in diabetes leads to the chronic impairment of eNOS activity in bovine aortic endothelial cells through O-GlcNAcylation of the activation domain within the enzyme.34 Hyperglycemic conditions in diabetes result in the downregulation and inactivation of eNOS, with a concomitant increase in the O-GlcNAcylation of the protein.34,35 Hyperglycemia-induced O-GlcNAcylation of eNOS at Ser1177, a primary phosphorylation-dependent activation site, contributes to erectile dysfunction in diabetes patients through reduced NO generation.35 Moreover, reduced NO production due to enhanced O-GlcNAcylation of eNOS results in endothelial cell dysfunction via the regulatory role of NO in vasodilation and the inhibitory effect of the diffusible gas on platelet aggregation,36 thereby promoting the development of cardiovascular complications in patients with diabetes. Beleznai and Bagi37 also reported that hyperglycemia-induced O-GlcNAcylation contributed to the impaired NO-mediated vasodilation of skeletal muscle arterioles isolated from male Wistar rats, further confirming the role of O-GlcNAcylation as a contributor to microvascular complications in patients with diabetes. In rats chronically fed a high-sugar diet in an animal model of metabolic syndrome, interference with the vasorelaxant function of perivascular adipose tissue (PVAT) was closely linked to decreased generation of NO, reduced expression of eNOS, and increased O-GlcNAcylation of eNOS,38 implying that O-GlcNAcylation of eNOS contributes to hyperglycemia-induced PVAT dysfunction and suggesting a therapeutic target for diabetes-associated vascular dysfunction (Fig. 1).
Fig. 1. Protective and adverse effects of O-GlcNAcylation on cardiovascular diseases.
NO, nitric oxide; eNOS, endothelial nitric oxide synthase; O-GlcNAc, O-linked β-N-acetylglucosamine.
2. Vascular calcification: implications for diabetic vasculopathy
In the patients with diabetes, cardiovascular complications are the leading cause of increased mortality and morbidity.39 Efforts have been made to reduce the high death rate among adults with diabetes; however, mortality still remains high in people with CVDs caused by the diabetic milieu. Since early detection of CVD is highly valuable from a diagnostic standpoint, strategies for developing reliable and non-invasive medical tests are urgently necessary to reduce the need for intensive therapies and to minimize the economic burden faced by people with diabetes. Since all major risk factors for CVD, including diabetes, have shown close associations with the development of vascular calcification, the medical assessment of arterial calcification in individuals with diabetes might be highly useful as a marker for risk stratification of patients with cardiovascular complications.40
Vascular calcification can be classified into 2 distinct but overlapping types, intimal and medial calcification, which correspond to different etiologies.41 Calcifications of the intimal layer are mainly dependent upon the classic risk factors for CVD such as high blood pressure, high cholesterol, and smoking, and are evoked by oxidative stress or inflammatory responses in atherosclerosis, which could result from lipid accumulation, inflammation, fibrosis, and development of focal plaques.42,43 In contrast, medial vascular calcification involves the nucleation and deposition of calcium hydroxyapatite nanocrystals along the elastic lamina and extracellular matrix, in close association with diabetes and chronic kidney disease.44 However, both forms of vascular calcification often coincide and frequently overlap with each other.
There is ample evidence that patients with diabetes have a higher propensity for developing vascular calcification than people without diabetes, as highlighted by elevated expression of osteogenic markers such as osteopontin, osteocalcin, and alkaline phosphatase in the medial layer of the vasculature.5 In an 18-year longitudinal study of 833 individuals with type 2 diabetes and 1,292 individuals without diabetes, arterial calcification was shown to be a strong predictor of cardiovascular and all-cause mortality in patients with diabetes, establishing the prognostic value of aortic calcification in symptomatic subjects.45
Aortic stiffness, a hallmark of the aging process, can be described as elastic resistance to deformation, culminating in reduced vascular compliance.46 Expressed as aortic pulse wave velocity (PWV), arterial wall stiffening represents a strong predictor of cardiovascular and all-cause mortality.47 In a meta-analysis of 17 longitudinal studies evaluating the aortic PWV with 15,877 participants and a mean follow-up of 7.7 years, it was shown that adjusted rates of cardiovascular events, cardiovascular mortality, and all-cause mortality increased by 14%, 15%, and 15%, respectively, for every 1 m/s increment in the aortic PWV.47 Arterial wall stiffening occurs in arteries frayed by mechanical stress caused by several disrupting factors, including vascular calcification. A well-established marker of bone metabolism, osteoprotegerin (OPG), was linked to the incidence of aortic stiffness and the extent of coronary artery disease.48 In a 15-year follow-up study of cardiovascular complications in patients with type 1 diabetes, the authors observed that OPG was closely associated with arterial calcification, leading to the onset of aortic stiffness and accompanying cardiovascular events.49 Therefore, diabetic medial calcification could be directly linked to the onset of arterial stiffening, which is a prelude to multiple CVDs, such as elevated blood pressure, increased cardiac afterload, and impaired vascular reactivity.46,49,50
3. O-GlcNAcylation: a strong inducer of vascular calcification
It has been shown that an overall increase in O-GlcNAcylation promoted osteoblastic differentiation of MC3T3-E1 cells with enhanced expression of bone-related markers such as alkaline phosphatase, osteocalcin, and bone sialoprotein via transcriptional activation of runt-related transcription factor 2 (Runx2), an osteogenic transcription factor.51 Moreover, high glucose levels and PUGNAC, an inhibitor of O-GlcNAcase, induced osteogenic differentiation of human cartilage endplate stem cells and MC3T3-E1 cells via O-GlcNAcylation of Runx2, demonstrating the ability of O-GlcNAcylation to stimulate mineralization of extracellular matrix.52,53
In a diabetic mouse model, STZ treatment combined with an atherogenic diet induced accelerated atherosclerosis in response to hyperglycemia, as shown by exaggerated fatty streaks and atherosclerotic plaques resembling those of human type II lesions.54 Recently, Heath et al.10 demonstrated that STZ-induced hyperglycemia was associated with elevated vascular O-GlcNAcylation and aortic calcification in a murine diabetic model involving multiple low-dose STZ injections, suggesting a positive correlation between O-GlcNAcylation and calcification in diabetes. With impaired vascular compliance, as found in mice, increased O-GlcNAcylation in diabetic arteries is directly associated with AKT activation and upregulated expression of Runx2. O-GlcNAcylation of AKT at T430 and T479 promotes AKT phosphorylation and activation, which in turn enhances the expression of Runx2 and calcification of VSMC in vitro.10 In summary, O-GlcNAcylation, which is enhanced by hyperglycemia in diabetic vasculature, may promote the osteogenic differentiation of VSMC, thereby leading to increased aortic calcification and reduced vascular compliance in patients with diabetes.
4. Mechanistic perspectives on O-GlcNAcylation in VSMC
Dysfunction of VSMC in the vasculature significantly contributes to the development of vascular pathologies such as atherosclerosis, hypertension, and restenosis, which are commonly associated with diabetes, via phenotypic switching of VSMC from the contractile state to the synthetic state.55,56,57 Diabetes-mediated VSMC dysfunction, broadly defined as a transition into pro-inflammatory phenotype or dedifferentiated status, is accelerated by multiple pathological factors associated with diabetes such as high glucose levels, heightened oxidative stress, and altered lipid metabolism.58,59,60,61 Therefore, the pathophysiological manifestations of VSMC dysfunction could involve enhanced inflammatory responses, migration, proliferation, and dedifferentiation via multiple downstream signaling pathways and transcriptional activators.59,61,62,63
In rat aortic smooth muscle cells cultured in high-glucose media, increased expression and activity of OGT were mainly observed in the nucleus, and altered patterns of O-GlcNAc-modified nuclear proteins were clearly demonstrated compared to control rats.64 It was also found that the elevated production of matrix hyaluronan (HA) observed in diabetic arteries was triggered by O-GlcNAcylation of HA synthase 2, the main HA synthase in aortic smooth muscle cells, through stabilization of the enzyme.65 This finding provides further confirmation that increased HA synthesis through this process could mediate VSMC dedifferentiation, which is critical for vascular pathologies in diabetes.66
It was previously shown that O-GlcNAcylation of transcription factor specificity protein 1 (Sp1) in VSMC confers protection against proteasomal degradation, providing a causative link between this versatile transcriptional regulator and a nutritional checkpoint.67 The hyperglycemic milieu often enhances the migration, proliferation, and inflammation of VSMC by modulating the signaling molecules and growth factors involved in each process, thereby providing putative therapeutic targets to protect the vascular system in people with diabetes.68,69,70 In rat aortic smooth muscle cells, plasminogen activator inhibitor-1 (PAI-1), a well-known stimulator of VSMC migration (especially in the setting of chronic transforming growth factor [TGF]-β1 activation), is upregulated via Sp1-binding to the PAI-1 promoter region through the release of a transcriptional repressor from Sp1 complexes under high-glucose conditions.71 Barnes et al.72 demonstrated that higher O-GlcNAcylation of Sp1 in pulmonary arterial smooth muscle cells from idiopathic pulmonary arterial hypertension facilitated cell migration via increased expression of vascular endothelial growth factor. It was also shown that the increased expression of TGF-β1 in endothelial cells cultured under high-glucose conditions was mediated by elevated O-GlcNAcylation of Sp1,73 which may be implicated in VSMC proliferation through the synthesis of proteoglycans and extracellular matrix proteins.74
Chen et al.75 observed that increased expression of PAI-1 promoted apoptotic resistance of VSMC in association with enhanced FLIP activity in the vascular wall through a signaling pathway mediated by nuclear factor (NF)-κB and extracellular signal-regulated kinases (ERK), demonstrating a causative link between PAI-1-induced VSMC proliferation and cardiovascular pathologies, including restenosis after coronary intervention. It has been reported that upregulation of vascular cell adhesion molecule-1 (VCAM-1) in rat glomerulus cells is prompted by enhanced NF-κB binding to the VCAM-1 promoter in hyperglycemia.76 Furthermore, high glucose-mediated augmentation of the O-GlcNAcylation of NF-κB has been found to result in production of tumor necrosis factor alpha and interleukin-6 in rat placenta.77 This data led to the hypothesis that O-GlcNAcylation of NF-κB may participate in inflammatory responses of VSMC. Under hyperglycemic conditions, increased expression of VCAM-1 is induced by the O-GlcNAcylation of NF-κB through the elevated translocation of NF-κB to the nucleus via the separation of NF-κB and IκB, an inhibitor of NF-κB in rat vascular smooth muscle tissue. This indicates that O-GlcNAcylation of NF-κB may contribute to exaggerated inflammatory responses in VSMC during the progression of diabetes.78 Recently, aldose reductase, a well-known obligatory mediator of the inflammatory changes in diabetic hyperglycemia, was shown to be essential for protein kinase C and NF-κB–induced expression of intercellular adhesion molecule-1 and VCAM-1, further suggesting that under high-glucose conditions, NF-κB could be a critical determinant of the inflammatory response of injured diabetic vasculature.79
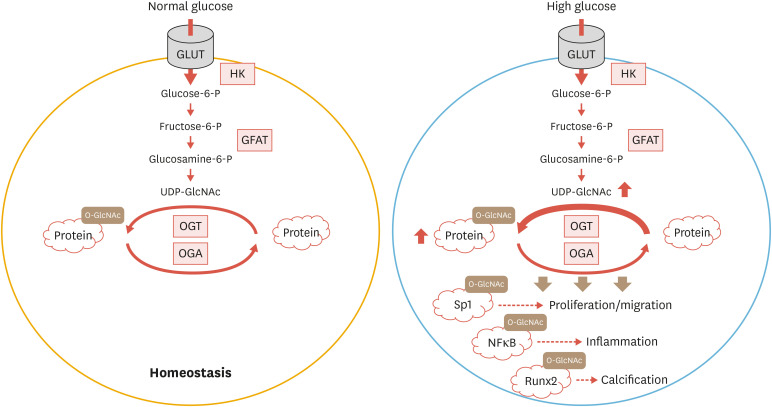
As mentioned earlier, Runx2 is a master regulator of vascular calcification in both physiological and pathological conditions. Post-translational modifications of Runx2 by phosphorylation, ubiquitination, and acetylation exert a variety of significant influences on its activity, stability, and interactions with other signaling co-factors downstream of key osteogenic cues.80 In a previous report, O-GlcNAcylation of Runx2 at multiple sites was reported to be closely linked to increased activation of the transcription factor in MC3T3-E1 pre-osteoblasts.81 More interestingly, overall increases in O-GlcNAcylation of cellular proteins, including Runx2, were detected in bone marrow-derived mesenchymal stem cells cultured in an osteogenic medium in the presence of bone morphogenetic protein 2/7 (BMP2/7), further confirming a strong correlation between O-GlcNAc cycling and the Runx2-dependent regulation of osteogenic differentiation (Fig. 2).81
Fig. 2. Pathological effects of O-GlcNAcylation in VSMC under diabetic conditions. The hexosamine biosynthetic pathway in VSMC. After its entry into VSMC, glucose is phosphorylated to glucose-6-P by HK and further metabolized into fructose-6-p. Conversion of fructose-6-p to glucosamine-6-p is carried out by the rate-limiting enzyme, GFAT. The end-product of the hexosamine biosynthetic pathway, UDP-GlcNAc serves as an obligatory substrate for OGT, generating O-GlcNAc-modified proteins, while OGA catalyzes the removal of O-GlcNAc from the targets. O-GlcNAc modification of transcription factors such as Sp1, NFκB, and Runx2 mediates hyperglycemia-induced pathological responses in VSMC.
VSMC, vascular smooth muscle cell; O-GlcNAc, O-linked β-N-acetylglucosamine; GLUT, glucose transporter; HK, hexokinase; GFAT, glutamine:fructose 6-phosphate amidotransferase; UDP-GlcNAc, UDP-β-D-N-acetylglucosamine; OGT, O-GlcNAc transferase; OGA, O-GlcNAcase; NF-κB, nuclear factor κB; Runx2, runt-related transcription factor 2; glucose-6-P, glucose-6-phosphate; fructose-6-p, fructose-6-phosphate; glucosamine-6-p, glucosamine-6-phosphate.
CONCLUSION
In conclusion, we reviewed the role of O-GlcNAcylation in vascular pathology associated with diabetes mellitus. Although the spatial and temporal regulation of protein O-GlcNAc modification during the development of cardiovascular pathologies should be carefully considered in future studies, we found strong evidences of the association between the O-GlcNAcylation of cellular proteins such as Sp1, NF-κB, and Runx2 and diabetic vasculopathy. We speculate that modulation of O-GlcNAcylation of target proteins could be an attractive therapeutic target to alleviate vascular pathologies in patients with diabetes.
Footnotes
Funding: This work was supported by Ministry of Science and ICT of Korea (No. 2019R1F1A1057219).
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Byon CH.
- Funding acquisition: Byon CH.
- Investigation: Byon CH.
- Supervision: Kim SW.
- Visualization: Byon CH.
- Writing - original draft: Byon CH.
- Writing - review & editing: Byon CH.
References
- 1.International Diabetes Federation. IDF diabetes atlas. 9th ed. Brussels: International Diabetes Federation; 2019. [Google Scholar]
- 2.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276:618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 3.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. 2017;97:1555–1617. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 4.Boström KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen NX, Moe SM. Arterial calcification in diabetes. Curr Diab Rep. 2003;3:28–32. doi: 10.1007/s11892-003-0049-2. [DOI] [PubMed] [Google Scholar]
- 6.Carr JJ, Register TC, Hsu FC, Lohman K, Lenchik L, Bowden DW, et al. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: the diabetes heart study. Bone. 2008;42:43–52. doi: 10.1016/j.bone.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boström KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CE, Johnsen VL, Shearer J, Belke DD. Exercise training mitigates aberrant cardiac protein O-GlcNAcylation in streptozotocin-induced diabetic mice. Life Sci. 2013;92:657–663. doi: 10.1016/j.lfs.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell'Italia LJ, et al. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res. 2014;114:1094–1102. doi: 10.1161/CIRCRESAHA.114.302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 12.Lunde IG, Aronsen JM, Kvaløy H, Qvigstad E, Sjaastad I, Tønnessen T, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics. 2012;44:162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 13.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, et al. O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laczy B, Marsh SA, Brocks CA, Wittmann I, Chatham JC. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am J Physiol Heart Circ Physiol. 2010;299:H1715–H1727. doi: 10.1152/ajpheart.00337.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 17.Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function. J Mol Cell Cardiol. 2012;52:538–549. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol. 2008;294:C1509–C1520. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Hart GW. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev Proteomics. 2013;10:365–380. doi: 10.1586/14789450.2013.820536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Y, Wu J, Zhang H, Wang Y, Zhang C. Vascular dysfunction in type 2 diabetes: emerging targets for therapy. Expert Rev Cardiovasc Ther. 2009;7:209–213. doi: 10.1586/14779072.7.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medford HM, Chatham JC, Marsh SA. Chronic ingestion of a Western diet increases O-linked-β-N-acetylglucosamine (O-GlcNAc) protein modification in the rat heart. Life Sci. 2012;90:883–888. doi: 10.1016/j.lfs.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, et al. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2007;17:127–140. doi: 10.1093/glycob/cwl067. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci U S A. 2006;103:19575–19580. doi: 10.1073/pnas.0604208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 25.Nesto RW. Correlation between cardiovascular disease and diabetes mellitus: current concepts. Am J Med. 2004;116(Suppl 5A):11S–22S. doi: 10.1016/j.amjmed.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–1198. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo . Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama H, Kimura Y, Mitsuhashi H, Kinouchi T, Yoshida H, Kushiro T, et al. Relationship between endothelial function and fibrinolysis in early hypertension. Hypertension. 1998;31:321–327. doi: 10.1161/01.hyp.31.1.321. [DOI] [PubMed] [Google Scholar]
- 30.Adams MR, Jessup W, Hailstones D, Celermajer DS. L-arginine reduces human monocyte adhesion to vascular endothelium and endothelial expression of cell adhesion molecules. Circulation. 1997;95:662–668. doi: 10.1161/01.cir.95.3.662. [DOI] [PubMed] [Google Scholar]
- 31.Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003;79:195–199. doi: 10.1136/pmj.79.930.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 33.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005;102:11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20:255–268. doi: 10.1046/j.1464-5491.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 37.Beleznai T, Bagi Z. Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation. Vascul Pharmacol. 2012;56:115–121. doi: 10.1016/j.vph.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Costa RM, da Silva JF, Alves JV, Dias TB, Rassi DM, Garcia LV, et al. Increased O-GlcNAcylation of endothelial nitric oxide synthase compromises the anti-contractile properties of perivascular adipose tissue in metabolic syndrome. Front Physiol. 2018;9:341–341. doi: 10.3389/fphys.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avogaro A, Fadini GP. Mechanisms of ectopic calcification: implications for diabetic vasculopathy. Cardiovasc Diagn Ther. 2015;5:343–352. doi: 10.3978/j.issn.2223-3652.2015.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 42.Virmani R, Joner M, Sakakura K. Recent highlights of ATVB: calcification. Arterioscler Thromb Vasc Biol. 2014;34:1329–1332. doi: 10.1161/ATVBAHA.114.304000. [DOI] [PubMed] [Google Scholar]
- 43.Mackey RH, Venkitachalam L, Sutton-Tyrrell K. Calcifications, arterial stiffness and atherosclerosis. Adv Cardiol. 2007;44:234–244. doi: 10.1159/000096744. [DOI] [PubMed] [Google Scholar]
- 44.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515–1525. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juutilainen A, Lehto S, Suhonen M, Rönnemaa T, Laakso M. Thoracoabdominal calcifications predict cardiovascular disease mortality in type 2 diabetic and nondiabetic subjects: 18-year follow-up study. Diabetes Care. 2010;33:583–585. doi: 10.2337/dc09-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sethi S, Rivera O, Oliveros R, Chilton R. Aortic stiffness: pathophysiology, clinical implications, and approach to treatment. Integr Blood Press Control. 2014;7:29–34. doi: 10.2147/IBPC.S59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 48.Tousoulis D, Siasos G, Maniatis K, Oikonomou E, Kioufis S, Zaromitidou M, et al. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int J Cardiol. 2013;167:1924–1928. doi: 10.1016/j.ijcard.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Gordin D, Groop PH. Aspects of hyperglycemia contribution to arterial stiffness and cardiovascular complications in patients with type 1 diabetes. J Diabetes Sci Technol. 2016;10:1059–1064. doi: 10.1177/1932296816636894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theilade S, Lajer M, Persson F, Joergensen C, Rossing P. Arterial stiffness is associated with cardiovascular, renal, retinal, and autonomic disease in type 1 diabetes. Diabetes Care. 2013;36:715–721. doi: 10.2337/dc12-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koyama T, Kamemura K. Global increase in O-linked N-acetylglucosamine modification promotes osteoblast differentiation. Exp Cell Res. 2015;338:194–202. doi: 10.1016/j.yexcr.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Sun C, Lan W, Li B, Zuo R, Xing H, Liu M, et al. Glucose regulates tissue-specific chondro-osteogenic differentiation of human cartilage endplate stem cells via O-GlcNAcylation of Sox9 and Runx2. Stem Cell Res Ther. 2019;10:357. doi: 10.1186/s13287-019-1440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SH, Kim YH, Song M, An SH, Byun HY, Heo K, et al. O-GlcNAc modification modulates the expression of osteocalcin via OSE2 and Runx2. Biochem Biophys Res Commun. 2007;362:325–329. doi: 10.1016/j.bbrc.2007.07.149. [DOI] [PubMed] [Google Scholar]
- 54.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikhail N, Fukuda N, Tremblay J, Hamet P. Platelets, growth factors, and vascular smooth-muscle cells in hypertension and diabetes. J Cardiovasc Pharmacol. 1993;22(Suppl 6):S64–S74. [PubMed] [Google Scholar]
- 57.Wall VZ, Bornfeldt KE. Arterial smooth muscle. Arterioscler Thromb Vasc Biol. 2014;34:2175–2179. doi: 10.1161/ATVBAHA.114.304441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li SL, Reddy MA, Cai Q, Meng L, Yuan H, Lanting L, et al. Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes. 2006;55:2611–2619. doi: 10.2337/db06-0164. [DOI] [PubMed] [Google Scholar]
- 59.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 60.Meng L, Park J, Cai Q, Lanting L, Reddy MA, Natarajan R. Diabetic conditions promote binding of monocytes to vascular smooth muscle cells and their subsequent differentiation. Am J Physiol Heart Circ Physiol. 2010;298:H736–H745. doi: 10.1152/ajpheart.00935.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR. Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology. 2007;148:2435–2443. doi: 10.1210/en.2006-1440. [DOI] [PubMed] [Google Scholar]
- 62.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 64.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Hyperglycemia and the O-GlcNAc transferase in rat aortic smooth muscle cells: elevated expression and altered patterns of O-GlcNAcylation. Arch Biochem Biophys. 2001;389:166–175. doi: 10.1006/abbi.2001.2331. [DOI] [PubMed] [Google Scholar]
- 65.Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, et al. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem. 2012;287:35544–35555. doi: 10.1074/jbc.M112.402347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moretto P, Karousou E, Viola M, Caon I, D'Angelo ML, De Luca G, et al. Regulation of hyaluronan synthesis in vascular diseases and diabetes. J Diabetes Res. 2015;2015:167283–167283. doi: 10.1155/2015/167283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faries PL, Rohan DI, Takahara H, Wyers MC, Contreras MA, Quist WC, et al. Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. J Vasc Surg. 2001;33:601–607. doi: 10.1067/mva.2001.111806. [DOI] [PubMed] [Google Scholar]
- 69.Hsueh WA, Jackson S, Law RE. Control of vascular cell proliferation and migration by PPAR-gamma: a new approach to the macrovascular complications of diabetes. Diabetes Care. 2001;24:392–397. doi: 10.2337/diacare.24.2.392. [DOI] [PubMed] [Google Scholar]
- 70.Natarajan R, Reddy MA, Malik KU, Fatima S, Khan BV. Signaling mechanisms of nuclear factor-kappab-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:1408–1413. doi: 10.1161/hq0901.095278. [DOI] [PubMed] [Google Scholar]
- 71.Chen YQ, Su M, Walia RR, Hao Q, Covington JW, Vaughan DE. Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem. 1998;273:8225–8231. doi: 10.1074/jbc.273.14.8225. [DOI] [PubMed] [Google Scholar]
- 72.Barnes JW, Tian L, Krick S, Helton ES, Denson RS, Comhair SA, et al. O-GlcNAc transferase regulates angiogenesis in idiopathic pulmonary arterial hypertension. Int J Mol Sci. 2019;20:6299. doi: 10.3390/ijms20246299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suwanabol PA, Seedial SM, Shi X, Zhang F, Yamanouchi D, Roenneburg D, et al. Transforming growth factor-β increases vascular smooth muscle cell proliferation through the Smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways. J Vasc Surg. 2012;56:446–454. doi: 10.1016/j.jvs.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 76.James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, et al. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes. 2002;51:1146–1156. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 77.Dela Justina V, Gonçalves JS, de Freitas RA, Fonseca AD, Volpato GT, Tostes RC, et al. Increased O-linked N-acetylglucosamine modification of NF-κB and augmented cytokine production in the placentas from hyperglycemic rats. Inflammation. 2017;40:1773–1781. doi: 10.1007/s10753-017-0620-7. [DOI] [PubMed] [Google Scholar]
- 78.Yang WH, Park SY, Nam HW, Kim DH, Kang JG, Kang ES, et al. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 80.Jonason JH, Xiao G, Zhang M, Xing L, Chen D. Post-translational regulation of Runx2 in bone and cartilage. J Dent Res. 2009;88:693–703. doi: 10.1177/0022034509341629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagel AK, Ball LE. O-GlcNAc modification of the runt-related transcription factor 2 (Runx2) links osteogenesis and nutrient metabolism in bone marrow mesenchymal stem cells. Mol Cell Proteomics. 2014;13:3381–3395. doi: 10.1074/mcp.M114.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]