Abstract
Lithium–sulfur (Li–S) batteries have long been expected to be a promising high-energy-density secondary battery system since their first prototype in the 1960s. During the past decade, great progress has been achieved in promoting the performances of Li–S batteries by addressing the challenges at the laboratory-level model systems. With growing attention paid to the application of Li–S batteries, new challenges at practical cell scales emerge as the bottleneck. In this Outlook, the key parameters for practical Li–S batteries to achieve practical high energy density are emphasized regarding high-sulfur-loading cathodes, lean electrolytes, and limited excess anodes. Subsequently, the key scientific problems are redefined in practical Li–S batteries beyond the previous ones under ideal conditions. Finally, viable strategies are proposed to address the above challenges as future research directions.
Short abstract
This Outlook emphasizes the key parameters and redefines the fundamental scientific problems of practical Li−S batteries, and further proposes viable strategies to address the emerging challenges as future research directions.
Introduction
The pursuit of high-energy-density secondary battery systems has never been stopped. In the 1990s, lithium–ion batteries (LIBs) were successfully commercialized and rapidly occupied the worldwide energy market until today.1 However, with the increasing demand of device duration especially under new applied situations such as unmanned aerial vehicles, the current LIB technology has gradually reached its limit.2 Meanwhile, lithium–sulfur (Li–S) batteries have long been expected as a promising high-energy-density secondary battery system since their first prototype in the 1960s.3−5 The ultrahigh theoretical energy density up to 2600 Wh kg–1 and the advantages of cathode material including low cost, Earth abundance, and environmental friendliness together endow Li–S batteries as a viable scheme to realize energy density beyond 500 Wh kg–1.6,7
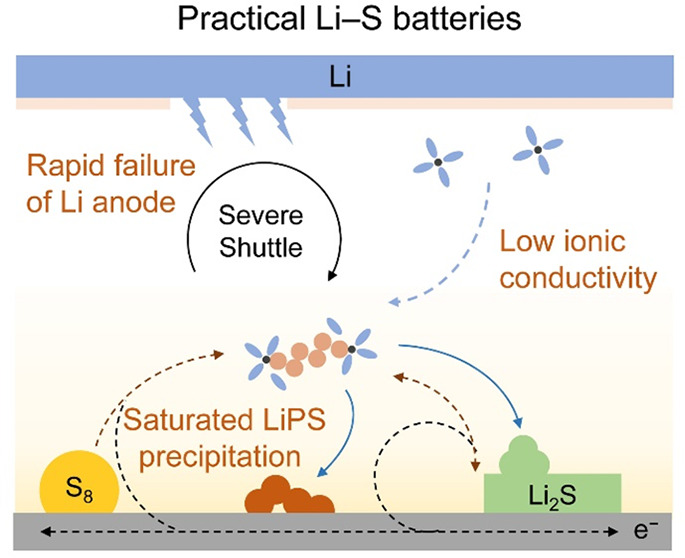
However, the development of the Li–S battery technology has never been smooth sailing.8,9 First, the end products of discharging and charging are intrinsically electronic/ionic insulating to render severe kinetic difficulty.10 Second, volume changes up to 80% during cycling exerts heavy pressure on the cathode integrity with rapid loss of active materials.11 Furthermore, the lithium polysulfide (LiPS) intermediates dissolve in the electrolyte and shuttle between the cathode and the anode, reducing the Coulombic efficiency (CE) and cycling stability.12,13 Overall, the above three aspects constitute the main challenges of Li–S batteries since their first proposal.
In the past decade, numerous efforts have been devoted to overcome these above-mentioned challenges.14−18 In 2009, Nazar and co-workers proposed a thermal-melt treatment method to incorporate sulfur within conductive carbon matrix that significantly alleviates the insulating nature of sulfur.19 Moreover, the hierarchical porous carbon matrix helps to mitigate the volume change and facilitate ionic conduction.20,21 Since then, the carbon/sulfur composite cathode has been rapidly developed with an advanced structural design and composition method to achieve progressive performances of Li–S batteries.22,23 To address the shuttle issue, Aurbach and co-workers studied the lithium nitrate (LiNO3) additive that forms a protective layer on the lithium metal anode to significantly increase the CE to 98%.24 Other advanced electrolyte formulas including localized high-concentration electrolyte25 and ionic liquid26,27 are also proposed to reduce the dissolution of polysulfides. Meanwhile, cathodes that eliminate the generation of dissolved polysulfides are designed such as atomic sulfur cathode (sulfurized polyacrylonitrile)28−30 and small molecular sulfur (microporous carbon/S2–4 composites),31 providing an alternative path for stable Li–S batteries. In addition, constructing an interlayer also assists to confine the dissolved polysulfides within the cathode side via physical blocking, chemical adsorption, or electrostatic repulsion.32−34
To particularly address the kinetic issue of the sulfur cathode, heterogeneous polysulfide electrocatalysis and homogeneous polysulfide redox mediations have been recently developed.35−37 Polysulfide electrocatalysis aims to accelerate the cathode redox kinetics by reducing the activation energy barriers.38 Various polysulfide electrocatalysts including metals,39 metal compounds,40,41 heteroatom-doped carbon,42 and other organic composites43,44 demonstrate performance advantages in reducing polarization, improving the rate response, and extending the cycling lifespan. On the other hand, polysulfide redox mediation regulates a targeted sulfur redox reaction by introducing an extrinsic kinetically favorable redox couple that participates in the intrinsic sulfur redox reaction.45 Typical redox mediators can increase the deposition dimension and specific capacity of lithium sulfide (Li2S),46 reduce the Li2S oxidation overpotential during charge,47 or promote the reduction of S8 depending on the electrochemical potential of the redox mediators.48 All of the above efforts have contributed to greatly enhanced Li–S performances, including an ultrastable cycle of over 1500 cycles,49,50 a high specific capacity of over 1500 mAh g–1,51,52 or an excellent rate performance of more than 40 C.53,54
However, it is worth noticing that most of the performance promotion is realized at the laboratory level under ideal conditions such as low sulfur loading around 1.0 mgS cm–2, high electrolyte volume with the electrolyte to sulfur ratio (E/S ratio) over 10 μL mgS–1, and excessive lithium anode with the negative to positive electrode ratio (N/P ratio) over 150 (Figure 1a).55−59 Although the above conditions are conducive to the improvement of specific capacity and cycling stability under coin cell evaluation, high-energy-density Li–S batteries are utterly impossible to be realized under such conditions. Moreover, the performance improvements may mislead the research direction to ignore the huge gap between laboratory coin cells and practical pouch cells under working conditions. Bearing in mind the original and intrinsic advantage of Li–S batteries being high energy density, electrochemical evaluation in pouch cells with high-sulfur-loading cathodes, low E/S ratio, and ultrathin lithium metal anode is important to provide solid guidance for practical Li–S batteries (Figure 1b).
Figure 1.
Estimation of the actual energy density of Li–S batteries. Schematic illustration of (a) a Li–S coin cell and (b) a Li–S pouch cell. (c) Calculated gravimetric energy densities of a Li–S pouch cell depending on areal sulfur loading and E/S ratio. (d) Boundary condition of a Li–S pouch cell with a targeted specific energy of 400 and 500 Wh kg–1.
To achieve a target energy density of 400 or 500 Wh kg–1, herein we evaluate the key parameters of a practical Li–S pouch cell using a routine carbon/sulfur composite cathode and ether electrolyte as a model. The mass of the involved components is based on previous reports.55 Among them, the mass of separators, current collectors, and packing is fixed, while the remaining components including binder, lithium anode, conductive carbon, and electrolyte change with the sulfur loading. It is assumed that the carbon/sulfur composite contains 83 wt % sulfur and further mixed with a binder in a weight ratio of 9:1, and the N/P ratio is fixed to be 2.0. In addition, an increase in areal sulfur loading will inevitably impair the actual discharge specific capacity. Therefore, each additional 1.0 mg cm–2 sulfur loading is assumed to result in a specific capacity reduction of 30 mAh g–1 and a voltage drop of 10 mV. On the basis of the above assumptions, the actual energy density of the Li–S pouch cell is estimated using the following equation.
where E is the average discharge voltage, Q is the specific discharge capacity based on sulfur, MS is the areal sulfur loading, and Mtotal is the total weight of the Li–S pouch cell.
The relationship between the estimated actual energy density of the Li–S pouch cell with areal sulfur loading and E/S ratio is established (Figure 1c). As the sulfur loading increases, the energy density first increases sharply and then decreases gradually, implying an optimal range of sulfur loading in cell design. On the other hand, the energy density increases significantly as the E/S ratio decreases for a fixed sulfur loading, especially for the cells with high sulfur loading. Nevertheless, the E/S ratio cannot be reduced infinitely considering the specific capacity of sulfur and the cycling lifespan to be met. On the basis of the above simulations, the optimal parameters for a 400 Wh kg–1 Li–S pouch cell include an areal sulfur loading of 5.0 mgS cm–2 and an E/S ratio of 4.0 μL mgS–1 (Figure 1d). When the energy density target increases to 500 Wh kg–1, the two parameters are supposed to be 6.0 mgS cm–2 and 2.5 μL mgS–1, respectively. Besides, the excess of metal lithium anode under practical conditions must be strictly controlled due to the economic costs. Therefore, practical high-energy-density Li–S batteries can be achieved with an areal sulfur loading higher than 5.0 mgS cm–2, an E/S ratio lower than 4.0 μL mgS–1, and a N/P ratio less than 2.0. These restrictions are of vital importance for further development of practical Li–S batteries in terms of high energy density.
Considering the key parameters of areal sulfur loading, E/S ratio, and N/P ratio are very different between ideal and practical conditions, it is rational to understand the huge gap between Li–S coin cells and pouch cells that the excellent Li–S performances in coin cells can hardly be reproduced in pouch cells by simple amplification. Besides, changes such as the tap introduction, internal pressure, and electrolyte flowability also lead to spatially uneven electrochemical reactions in pouch cells and premature failure of the overall battery performance. On one hand, pouch cell-level evaluation is therefore emphasized for the development of practical Li–S batteries. On the other hand, coin-cell level evaluation is also important to afford comprehensive information as a guidance for fundamental understanding of basic Li–S chemistry and design strategies of practical Li–S batteries.
Challenges Redefined in Practical Lithium–Sulfur Batteries
The proposed restrictions to achieve actual high energy density define the boundaries of practical Li–S batteries. With the traditional challenges of insulating active materials, volume change, and polysulfide shuttle being addressed, new challenges emerge in practical Li–S batteries and become the bottleneck to realize actual high energy density. Therefore, these emerging challenges shall be redefined in the first place to guide further research in understanding the Li–S chemistry different from that under ideal conditions and developing regulation strategies for the development of practical Li–S batteries, which are discussed as follows:
-
(1)
Low ionic conductivity. For a commonly used ether-based electrolyte of 1,3-dioxolane (DOL)/1,2-dimethoxyethane (DME) (v/v = 1/1) with 1.0 mol L–1 lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and 2 wt % LiNO3, the ionic conductivity is sufficient for initial electrochemical reactions to take place. However, the sulfur redox reactions go through a dissolution–precipitation pathway that massive LiPSs dissolve in the electrolyte. Especially with high-sulfur-loading cathodes and under lean electrolyte conditions, the polysulfide concentration can be as high as 7 mol[S] L–1. With such a high LiPS concentration, the viscosity increases to significantly reduce the electrolyte ionic conductivity according to the Stokes–Einstein relationship (Figure 2a).60 In addition, the dissolved LiPSs interact with free solvents through solvation and even with lithium salts by forming clusters to reduce ionic conductivity.61 Consequently, low ionic conductivity caused by concentrated LiPS affords additional kinetic obstructs on the sulfur redox reactions manifested by large polarization of the second discharge plateau and poor rate performances compared with ideal conditions (Figure 2b). Enlarged ohmic polarization can therefore be a possible reason for battery failure especially in practical Li–S pouch cells, which shall be taken seriously as an emerging main challenge.
-
(2)
Saturated and premature precipitated LiPSs. Generally, LiPSs from long-chain Li2S8 to short-chain Li2S4 are supposed to be soluble in ether-based electrolyte, although the solubility of Li2S4 is relatively lower.62 Such an argument is valid when the E/S ratio is high under ideal conditions in coin cells. However, with the E/S ratio as low as 3.0 μL mg–1 in practical Li–S pouch cells, high LiPS concentration can be over 10 mol[S] L–1 and consequently exceed the dissolution limit and result in saturation and premature precipitation of LiPSs (Figure 3a).63 The premature precipitation of LiPSs on conductive matrix blocks the electronic/ionic conduction pathway and forces kinetically sluggish solid–solid conversion to take place. Previous research studies have verified the above argument that the polarization of the second discharge plateau referring solid precipitation becomes large enough at relative low E/S ratios to reach the voltage cutoff and significantly reduce specific capacity (Figure 3b).64 Therefore, the saturation and premature precipitation of LiPSs exert great pressure on the electrochemical kinetics of sulfur conversion in practical Li–S pouch cells that induce a dramatically rapid battery failure. In addition, the chemical comproportionation and disproportionation of polysulfides become more dominant with higher LiPS concentration, affording increased deposition dimensions and large solid aggregates that tend to lose electrical contact during cycling. Consequently, the Li–S chemistry is very different from that under ideal conditions with distinct electrochemical processes and a new balance between the electrochemical pathway and the chemical pathway. The emerging challenge of LiPS saturation and premature precipitation requires new insights regarding the fundamental understanding and effective regulation strategies as the second.
-
(3)
Rapid failure of lithium metal anode. Electrochemical evaluation in Li–S coin cells usually employs thick lithium metal anodes with the N/P ratio higher than 150. Under such ideal conditions, the failure of the lithium metal anode is covered, performing as a gradual specific capacity decrease within several hundreds of cycles. However, Li–S pouch cells usually go through a rapid failure with dramatic capacity loss in only several cycles (Figure 4a). The cycled lithium anode displays a highly uneven morphology where several regions are severely corroded in a failed pouch cell (Figure 4b), and further analyses identify anode failure as the main reason. The cause of battery failure from gradual cathode capacity loss in coin cells to rapid capacity decrease in pouch cells mainly results from the following three aspects: (i) Higher actual current density and cycling capacity applied on the Li metal anode using high-sulfur-loading cathodes that aggravate uneven lithium deposition and anode volume change;65 (ii) higher LiPS concentration and a more severe shuttle effect that render serious lithium corrosion by LiPSs;66 (iii) low N/P ratio with less lithium excess which cannot support the continuous irreversible loss of active lithium by reacting with LiPS or electrolyte (Figure 4c). Thereby, the failure of lithium metal anode becomes the main reason for practical Li–S pouch cells. In addition, the growth of dendrites, pulverization, and gas-generated reactions at the surface of lithium anode also pose a threat to battery safety, which shall be seriously considered under working conditions. When addressing the above issue, the existence of massive LiPSs in the electrolyte is essential as the precondition to evaluate the protection strategies of lithium metal anodes in Li–S pouch cells, together with other rigorous conditions including high cathode capacity and low N/P ratio. The lithium metal chemistry under such rigorous conditions needs more attention.
Figure 2.
Low ionic conductivity of practical Li–S batteries. (a) The relationship between the ionic conductivity and the polysulfide concentration in DME solvent.60 (b) Schematic illustration of sulfur redox reactions with high LiPS concentration. The LiPS kinetics on electrode surface deteriorates due to the slow lithium ion transportation.
Figure 3.
Saturated and prematurely precipitated LiPSs in practical Li–S batteries. (a) Polysulfide concentration in correlation with the E/S ratio supposing all sulfur is dissolved. (b) Galvanostatic discharge curves for Li–S cells at three different E/S ratios.63 (c) Schematic illustration of spatially uneven electrochemical reactions on the cathode surface in Li–S batteries with lean electrolyte.
Figure 4.
Rapid lithium metal anode failure in practical Li–S batteries. (a) Cycling performance of a Li–S pouch cell.65 (b) Optical images of lithium metal anode with corresponding scanning electron microscopy images before (inset) and after 40 cycles at different regions including the bottom (B), top (T), and central (T) regions.66 (c) Schematic illustration of lithium corrosion due to severe concentrated LiPS shuttle in practical Li–S batteries.
Guidelines of Practical Lithium–Sulfur Batteries
In order to ensure the intrinsic advantage of Li–S batteries being high energy density, the application of practical Li–S batteries must employ a high-sulfur-loading cathode, lean electrolyte, and limit anode excess. New challenges are redefined under these boundaries regarding low ionic conductivity of high-concentrated LiPS electrolyte, saturation and premature precipitation of LiPSs, and rapid failure of lithium metal anode. These new challenges indicate distinct Li–S chemistry from that under ideal conditions with different key processes and failure mechanisms. To address these emerging challenges in practical Li–S batteries, the following four directions require extensive efforts and are expected with essential advances in future.
(1) Next-Generation Sulfur Cathode
The design of carbon/sulfur composite cathode has been proven to be a great success to propel the development of secondary Li–S batteries with potential high energy density.67−69 In consideration of the restriction of high sulfur loading and lean electrolyte, the conductive carbon matrix is expected with further functions to address the new challenges beyond the existing requirements (Figure 5a). Polar carbon hosts (either heteroatom-doped carbon or hybrids with transition metal compounds) demonstrate favorable interactions with LiPSs in many reports,37 and therefore polarity besides conductivity shall be recognized as an additional necessary factor for the design of the carbon matrix. Second, electrolyte wettability needs to be taken seriously under lean electrolyte conditions. Improved electrolyte wettability guarantees sufficient ionic conduction pathways and high utilization of the cathode sulfur, while it leaves more electrolyte for ion conduction and anode consumption during cycling. To this end, the structure of the composite cathode regarding carbon porosity, sulfur distribution, and binder selection requires careful reconsideration. Third, mechanical and chemical stabilities of the carbon matrix are again emphasized under high-sulfur-loading conditions that a dramatic phase change takes place continuously within the cathode. A robust conductive scaffold is a prerequisite. Besides, material cost and manufacturability should also be considered.
Figure 5.
Guidelines of advanced practical Li–S batteries. (a) Design of next-generation sulfur cathode. (b) Introduction of kinetic promoters. (c) Design of specific ion–solvent complex in the electrolyte. (d) Protection of the lithium metal anode.
Despite the previous reports of high-performance sulfur cathodes under ideal conditions, rational design principles of the cathode remain controversial especially for high-sulfur-loading cathodes with lean electrolyte. Instead of more trial-and-error efforts, insights are needed to understand the evolution of practical sulfur cathodes under working conditions. Information such as sulfur spatial distribution and electrolyte penetration can be very helpful, where in situ or operando observations are very helpful yet require more attentions to develop applicable methods. Overall, the success of the carbon/sulfur composite cathode has come to a consensus, and the next-generation sulfur cathode for practical Li–S batteries is on its way.
(2) Introduction of Kinetic Promoter
Poor redox kinetics has always been an intrinsic difficulty for the sulfur redox reactions that take place at multiphase boundaries along with severe phase migration. Various kinetic promoters such as oxide,11 sulfide,70 nitride,71,72 polymer,73 and framework materials74 have demonstrated performance advantages in many aspects from rate performance to long lifespan.75,76 Kinetic promoters can be generally divided into heterogeneous electrocatalysts and homogeneous redox mediators as discussed above, both of which have been investigated regarding the mechanism and the performance.
For a practical Li–S pouch cell with high-concentrated LiPS electrolyte and premature precipitated LiPSs, kinetic promotion is particularly essential to facilitate the sulfur redox reactions (Figure 5b). However, simple introduction of the reported kinetic promoters may not definitely result in performance promotion considering the huge gap between coin cells and pouch cells. For a polysulfide electrocatalyst, its mass ratio shall be limited not to influence the overall energy density, and therefore how to make the most utilization of polysulfide electrocatalysis requires careful considerations. In addition to the selection of high-performance electrocatalysts, their configuration also matters. Constructing an electrocatalytic interlayer between the cathode and the separator affords a promising scheme, where more investigation is required. For a redox mediator, its own shuttle can reduce the CE and even corrode the lithium metal anode.77 Fixation of homogeneous redox mediators within the cathode side can address the above side effect; yet, the full reservation of the redox mediation capability requires delicate design. Meanwhile, a redox mediator generally regulates a target process with a narrow voltage range. Therefore, multirange redox mediators are potentially needed but will improve the complexity of practical Li–S batteries. Nevertheless, kinetic promoters have proven themselves to be highly effective in facilitating the sulfur redox reactions, and the introduction of kinetic promoters is an emerging direction for overcoming the challenges in practical Li–S batteries.
(3) Design of Specific Ion–Solvent Complex in Electrolyte
The development of electrolyte drives the revolution of Li–S batteries from LiPS-dissolved tetrahydrofuran and dimethyl sulfoxide to LiPS-insoluble ionic liquid and fluorinated ether, where the currently employed DOL/DME ether-based electrolyte affords a moderate LiPS solubility.78−80 Each electrolyte formula defines a unique Li–S conversion pathway and diversifies the Li–S chemistry. The current widely employed ether-based electrolyte supports the dissolution–precipitation pathway with a certain degree of dissolved LiPS for smooth sulfur redox.81 Unfortunately, more and more evidence indicates the current ether-based electrolyte is incapable to endow long-cycling Li–S batteries under practical conditions.82,83 To address the new challenges in practical Li–S batteries, innovation of the Li–S electrolyte is of great significance, and the following discussion can help to provide a general guideline for future research (Figure 5c).
(i) High ionic conductivity with high-concentrated LiPSs. The concentration of LiPSs in the electrolyte is always changing during discharge/charge, and high-concentrated LiPSs have a great influence on the overall properties of the electrolyte under a low E/S ratio. Therefore, besides the evaluation of the original electrolyte without LiPSs, high-concentrated LiPS electrolytes especially LiPS saturated solutions need more attention. Physicochemical properties, electrochemical reactive features, and compatibility with the cathode and the anode need to be clarified. High ionic conductivity should be realized with the existence of LiPSs.
(ii) Sparing LiPS dissolution. For a carbon/sulfur composite cathode undergoing the dissolution–precipitation pathway, dissolved LiPS is essential to bridge the solid end products. However, under lean electrolyte conditions and with limited lithium excess, concentrated LiPSs result in cathode kinetic sluggishness, low electrolyte ionic conductivity, and anode corrosion as discussed above. To this end, a sparing LiPS dissolution electrolyte may settle the dilemma with the dissolved LiPSs sufficient to render smooth sulfur conversion and not to significantly influence the electrolyte and anode. The sparing LiPS dissolution electrolyte herein refers to a relative low LiPS concentration compared with that in common ether-based electrolytes rather than complete insolubility of the LiPSs that the dissolution–precipitation pathway is expected to be maintained. Nevertheless, it requires further comprehensive investigations to define an optimal LiPS concentration range and corresponding electrolyte formula. The sparingly LiPS dissolved electrolyte shall balance the requirements from both the cathode and the anode.
To realize high ionic conductivity and sparing LiPS dissolution, a single-component and single-function solvent that renders a similar ion–solvent complex for lithium ions and LiPSs is less competitive. Designing specific ion–solvent complexes for lithium ions and LiPSs is more rational for them to contribute distinct functions separately. The ion–solvent complex for lithium ions needs to provide sufficient ionic conductivity and reversible lithium stripping/plating, while the ion–solvent complex for LiPSs is expected to protect the LiPSs from interacting with lithium ions or lithium metal and support the cathode redox reactions at the same time. Therefore, at least two solvents targeting lithium ions and LiPSs are needed. Also, a diluent is possibly needed to reduce the viscosity caused by concentrated LiPSs as a third component. It is worth noticing that anions can participate in the ion–solvent complex, and their influence also requires research attention. In addition, the sparing LiPS dissolution electrolyte design may reduce the sulfur redox kinetics to some extent. Therefore, the employment of a next-generation sulfur cathode and the introduction of kinetic promoters are of greater significance in Li–S pouch cells with the sparing LiPS dissolution electrolyte.
(4) Protection of Lithium Metal Anode
Strategies of lithium metal anode protection have been massively reported in lithium metal batteries.84−92 However, the existence of concentrated LiPSs renders additional problems of continuous LiPS shuttle and anode corrosion. The solid electrolyte interface (SEI) formed on the lithium metal surface plays a decisive role in determining the lithium stripping/plating behaviors,93−95 and the SEI in Li–S batteries is expected with an additional function to selectively block LiPSs from interacting with the lithium metal anode beyond the protective functions in lithium metal batteries (Figure 5d). However, the generation of such an advanced SEI is highly challenging. Electrolyte regulation, artificial coating, and pretreatment are potential feasible methods.
Furthermore, the as-generated SEI cannot be considered to be absolutely stable because the surface of lithium metal varies significantly during cycling. Therefore, in spite of the original well-designed protective SEI, the newly deposited lithium metal reacts with the electrolyte to form subsequent SEI, and the subsequent SEI can even dominate the lithium stripping/plating behaviors as the main component within prolonged cycles.96 Hence, sustainable SEI is highly considered besides the originally designed SEI to afford a continuous protective layer on Li metal. The combination of artificial SEI and electrolyte-derived sustainable SEI is expected to synergistically extend the lifespan of the lithium anode. The role of concentrated LiPSs on forming the subsequent SEI shall also be taken seriously, which is supposed to contribute to the subsequent SEI distinct from that in lithium metal batteries. Besides, the design of sustainable SEI should avoid harmful side reactions such as gas production and other hazards to pouch cells. On the basis of the premise of a favorable SEI that fulfills the above requirements, the introduction of a lithium host to construct a composite lithium metal anode can further effectively extend the lifespan of practical batteries, benefiting from relieved volume change, reduced local current density, and controllable lithium affinity modification to reduce the nucleation barrier. Otherwise, without the desired SEI, the introduction of the lithium host may result in more severe consumption of the electrolyte and side reactions with LiPSs.
Conclusion
Remarkable advances have been made on developing high-performance Li–S batteries in the past decade with in-depth understanding of fundamental Li–S chemistry and regulation strategies from electrode design to electrolyte modulation. To fulfill the original goal and realize the intrinsic advantage of actual high energy density, more attention should be paid to practical Li–S batteries with high-sulfur-loading cathodes, lean electrolyte, and limited anode excess. Such restrictions redefine the research boundaries, and new challenges emerge as the bottleneck of practical Li–S batteries regarding low ionic conductivity to render large ohmic polarization, saturation and premature precipitation of LiPSs to cause kinetic difficulty, and rapid lithium metal anode failure in Li–S pouch cells. On one hand, these new challenges raise higher requests for targeted and sufficient regulation strategies under complex practical conditions. On the other hand, new opportunities exist with distinctive phenomenon and mechanisms to be revealed while exploring exciting Li–S chemistry under practical conditions. Furthermore, in-depth investigations on the novel Li–S chemistry will afford fresh insights and enlighten other essential energy-related processes based on multielectron and multiphase electrochemical reactions.
Acknowledgments
This work was supported by National Natural Science Foundation of China (21776019, 21825001, and U1801257), National Key Research and Development Program (2016YFA0202500 and 2016YFA0200102), and the Tsinghua University Initiative Scientific Research Program. We thank Jin Xie, Li-Peng Hou, Hong-Jie Peng, and Hong Yuan for helpful discussion.
The authors declare no competing financial interest.
References
- Dunn B.; Kamath H.; Tarascon J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334 (6058), 928–935. 10.1126/science.1212741. [DOI] [PubMed] [Google Scholar]
- Pang Q.; Liang X.; Kwok C. Y.; Nazar L. F. Advances in lithium–sulfur batteries based on multifunctional cathodes andelectrolytes. Nat. Energy 2016, 1 (9), 16132. 10.1038/nenergy.2016.132. [DOI] [Google Scholar]
- Van Noorden R. Sulphur back in vogue for batteries. Nature 2013, 498 (7455), 416–417. 10.1038/498416a. [DOI] [PubMed] [Google Scholar]
- Xia C.; Kwok C. Y.; Nazar L. F. A high energy density lithium-oxygen battery based on a reversible four electron conversion to lithium oxide. Science 2018, 361 (6404), 777–781. 10.1126/science.aas9343. [DOI] [PubMed] [Google Scholar]
- Liu J.; Bao Z. N.; Cui Y.; Dufek E. J.; Goodenough J. B.; Khalifah P.; Li Q.; Liaw B. Y.; Liu P.; Manthiram A.; Meng Y. S.; Subramanian V. R.; Toney M. F.; Viswanathan V. V.; Whittingham M. S.; Xiao J.; Xu W.; Yang J. H.; Yang X. Q.; Zhang J. G. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4 (3), 180–186. 10.1038/s41560-019-0338-x. [DOI] [Google Scholar]
- Li G. R.; Wang S.; Zhang Y. N.; Li M.; Chen Z. W.; Lu J. Revisiting the role of polysulfides in lithium–sulfur batteries. Adv. Mater. 2018, 30 (22), 1705590. 10.1002/adma.201705590. [DOI] [PubMed] [Google Scholar]
- Lei D.; Shi K.; Ye H.; Wan Z.; Wang Y.; Shen L.; Li B.; Yang Q. H.; Kang F.; He Y.-B. Progress and perspective of solid-state lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28 (38), 1707570. 10.1002/adfm.201707570. [DOI] [Google Scholar]
- Peng H. J.; Huang J. Q.; Cheng X. B.; Zhang Q. Review on high-loading and high-energy lithium–sulfur batteries. Adv. Energy Mater. 2017, 7 (24), 1700260. 10.1002/aenm.201700260. [DOI] [Google Scholar]
- Xu Z. L.; Kim S. J.; Chang D.; Park K. Y.; Dae K. S.; Dao K. P.; Yuk J. M.; Kang K. Visualization of regulated nucleation and growth of lithium sulfides for high energy lithium sulfur batteries. Energy Environ. Sci. 2019, 12 (10), 3144–3155. 10.1039/C9EE01338E. [DOI] [Google Scholar]
- Qie L.; Manthiram A. High-energy-density lithium–sulfur batteries based on blade-cast pure sulfur electrodes. ACS Energy Lett. 2016, 1 (1), 46–51. 10.1021/acsenergylett.6b00033. [DOI] [Google Scholar]
- Wei Seh Z.; Li W.; Cha J. J.; Zheng G. Y.; Yang Y.; McDowell M. T.; Hsu P. C.; Cui Y. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4 (1), 1331. 10.1038/ncomms2327. [DOI] [PubMed] [Google Scholar]
- Peled E.; Shekhtman I.; Mukra T.; Goor M.; Belenkaya I.; Golodnitsky D. Improving the durability and minimizing the polysulfide shuttle in the Li/S battery. J. Electrochem. Soc. 2018, 165 (1), A6051–A6057. 10.1149/2.0101801jes. [DOI] [Google Scholar]
- Lei T. Y.; Chen W.; Lv W. Q.; Huang J. W.; Zhu J.; Chu J. W.; Yan C. Y.; Wu C. Y.; Yan Y. C.; He W. D.; Xiong J.; Li Y. R.; Yan C. L.; Goodenough J. B.; Duan X. F. Inhibiting polysulfide shuttling with a graphene composite separator for highly robust lithium–sulfur batteries. Joule 2018, 2 (10), 2091–2104. 10.1016/j.joule.2018.07.022. [DOI] [Google Scholar]
- Zheng D.; Wang G. W.; Liu D.; Si J. Y.; Ding T. Y.; Qu D. Y.; Yang X. Q.; Qu D. Y. The progress of Li–S batteries-understanding of the sulfur redox mechanism: Dissolved polysulfide ions in the electrolytes. Adv. Mater. Technol. 2018, 3 (9), 1700233. 10.1002/admt.201700233. [DOI] [Google Scholar]
- Hu Y.; Chen W.; Lei T. Y.; Jiao Y.; Huang J. W.; Hu A. J.; Gong C. H.; Yan C. Y.; Wang X. F.; Xiong J. Strategies toward high-loading lithium–sulfur battery. Adv. Energy Mater. 2020, 10 (17), 2000082. 10.1002/aenm.202000082. [DOI] [Google Scholar]
- Li T.; Bai X.; Gulzar U.; Bai Y. J.; Capiglia C.; Deng W.; Zhou X.; Liu Z.; Feng Z.; Proietti Zaccaria R. A comprehensive understanding of lithium–sulfur battery technology. Adv. Funct. Mater. 2019, 29 (32), 1901730. 10.1002/adfm.201901730. [DOI] [Google Scholar]
- Qi Q.; Lv X. H.; Lv W.; Yang Q. H. Multifunctional binder designs for lithium–sulfur batteries. J. Energy Chem. 2019, 39, 88–100. 10.1016/j.jechem.2019.02.001. [DOI] [Google Scholar]
- Rana M.; Ahad S. A.; Li M.; Luo B.; Wang L.; Gentle I.; Knibbe R. Review on areal capacities and long-term cycling performances of lithium sulfur battery at high sulfur loading. Energy Storage Mater. 2019, 18, 289–310. 10.1016/j.ensm.2018.12.024. [DOI] [Google Scholar]
- Ji X. L.; Lee K. T.; Nazar L. F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8 (6), 500–506. 10.1038/nmat2460. [DOI] [PubMed] [Google Scholar]
- Tang C.; Li B. Q.; Zhang Q.; Zhu L.; Wang H. F.; Shi J. L.; Wei F. CaO-templated growth of hierarchical porous graphene for high-power lithium–sulfur battery applications. Adv. Funct. Mater. 2016, 26 (4), 577–585. 10.1002/adfm.201503726. [DOI] [Google Scholar]
- Schuster J.; He G.; Mandlmeier B.; Yim T.; Lee K. T.; Bein T.; Nazar L. F. Spherical ordered mesoporous carbon nanoparticles with high porosity for lithium–sulfur batteries. Angew. Chem., Int. Ed. 2012, 51 (15), 3591–3595. 10.1002/anie.201107817. [DOI] [PubMed] [Google Scholar]
- Liang J.; Sun Z. H.; Li F.; Cheng H. M. Carbon materials for Li–S batteries: Functional evolution and performance improvement. Energy Storage Mater. 2016, 2, 76–106. 10.1016/j.ensm.2015.09.007. [DOI] [Google Scholar]
- Yeon J. S.; Yun S.; Park J. M.; Park H. S. Surface-Modified Sulfur Nanorods Immobilized on Radially Assembled Open-Porous Graphene Microspheres for Lithium–Sulfur Batteries. ACS Nano 2019, 13 (5), 5163–5171. 10.1021/acsnano.8b08822. [DOI] [PubMed] [Google Scholar]
- Aurbach D.; Pollak E.; Elazari R.; Salitra G.; Kelley C. S.; Affinito J. On the surface chemical aspects of very high energy density, rechargeable Li–sulfur batteries. J. Electrochem. Soc. 2009, 156 (8), A694–A702. 10.1149/1.3148721. [DOI] [Google Scholar]
- Weller C.; Thieme S.; Hartel P.; Althues H.; Kaskel S. Intrinsic shuttle suppression in lithium–sulfur batteries for pouch cell application. J. Electrochem. Soc. 2017, 164 (14), A3766–A3771. 10.1149/2.0981714jes. [DOI] [Google Scholar]
- Cuisinier M.; Cabelguen P. E.; Adams B. D.; Garsuch A.; Balasubramanian M.; Nazar L. F. Unique behaviour of nonsolvents for polysulphides in lithium–sulphur batteries. Energy Environ. Sci. 2014, 7 (8), 2697–2705. 10.1039/C4EE00372A. [DOI] [Google Scholar]
- Dokko K.; Tachikawa N.; Yamauchi K.; Tsuchiya M.; Yamazaki A.; Takashima E.; Park J. W.; Ueno K.; Seki S.; Serizawa N.; Watanabe M. Solvate ionic liquid electrolyte for Li–S batteries. J. Electrochem. Soc. 2013, 160 (8), A1304–A1310. 10.1149/2.111308jes. [DOI] [Google Scholar]
- Li Q. Y.; Yang H. J.; Naveed A.; Guo C.; Yang J.; Nuli Y. N.; Wang J. L. Duplex component additive of tris(trimethylsilyl) phosphite-vinylene carbonate for lithium sulfur batteries. Energy Storage Mater. 2018, 14, 75–81. 10.1016/j.ensm.2018.02.004. [DOI] [Google Scholar]
- Chen W. J.; Li B. Q.; Zhao C. X.; Zhao M.; Yuan T. Q.; Sun R. C.; Huang J. Q.; Zhang Q. Electrolyte regulation towards stable lithium metal anode in lithium–sulfur batteries with sulfurized polyacrylonitrile cathode. Angew. Chem. 2019, 59 (19), 7306–7318. 10.1002/ange.201912701. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wang M. F.; Xu N.; Qian T.; Yan C. L. Progress and perspective of organosulfur polymers as cathode materials for advanced lithium–sulfur batteries. Energy Storage Mater. 2018, 15, 53–64. 10.1016/j.ensm.2018.03.017. [DOI] [Google Scholar]
- Fu C. Y.; Oviedo M. B.; Zhu Y. H.; von Wald Cresce A.; Xu K.; Li G. H.; Itkis M. E.; Haddon R. C.; Chi M. F.; Han Y.; Wong B. M.; Guo J. C. Confined lithium–sulfur reactions in narrow-diameter carbon nanotubes reveal enhanced electrochemical reactivity. ACS Nano 2018, 12 (10), 9775–9784. 10.1021/acsnano.7b08778. [DOI] [PubMed] [Google Scholar]
- Lin H.; Zhang S.; Zhang T.; Cao S.; Ye H.; Yao Q.; Zheng G. W.; Lee J. Y. A cathode-integrated sulfur-deficient Co9S8 Catalytic interlayer for the reutilization of ″lost″ polysulfides in lithium–sulfur batteries. ACS Nano 2019, 13 (6), 7073–7082. 10.1021/acsnano.9b02374. [DOI] [PubMed] [Google Scholar]
- Qin J. L.; Zhao H. Y.; Huang J. Q. A metal nitride interlayer for long life lithium sulfur batteries. J. Energy Chem. 2019, 29 (1), 1–2. 10.1016/j.jechem.2018.07.001. [DOI] [Google Scholar]
- Kim S. H.; Yeon J. S.; Kim R.; Choi K. M.; Park H. S. Functional separator coated by sulfonated metal organic framework nafion hybrids for Li–S batteries. J. Mater. Chem. A 2018, 6, 24971–24978. 10.1039/C8TA08843H. [DOI] [Google Scholar]
- Zhang Z. W.; Peng H. J.; Zhao M.; Huang J. Q. Heterogeneous/homogeneous mediators for high-energy-density lithium–sulfur batteries: Progress and prospects. Adv. Funct. Mater. 2018, 28 (38), 1707536. 10.1002/adfm.201707536. [DOI] [Google Scholar]
- Lim W. G.; Kim S.; Jo C.; Lee J. A comprehensive review of materials with catalytic effects in Li–S batteries: Enhanced redox kinetics. Angew. Chem., Int. Ed. 2019, 58 (52), 18746–18757. 10.1002/anie.201902413. [DOI] [PubMed] [Google Scholar]
- Wang H. Q.; Zhang W. C.; Xu J. Z.; Guo Z. P. Advances in polar materials for lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28 (38), 1707520. 10.1002/adfm.201707520. [DOI] [Google Scholar]
- Thangavel N. K.; Gopalakrishnan D.; Arava L. M. R. Understanding heterogeneous electrocatalysis of lithium polysulfide redox on Pt and WS2 surfaces. J. Phys. Chem. C 2017, 121 (23), 12718–12725. 10.1021/acs.jpcc.7b01514. [DOI] [Google Scholar]
- Al Salem H.; Babu G.; Rao C. V.; Arava L. M. Electrocatalytic polysulfide traps for controlling redox shuttle process of Li–S batteries. J. Am. Chem. Soc. 2015, 137 (36), 11542–11545. 10.1021/jacs.5b04472. [DOI] [PubMed] [Google Scholar]
- Wang H. Q.; Zhang W. C.; Xu J. Z.; Guo Z. P. Advances in polar materials for lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28 (38), 1707520. 10.1002/adfm.201707520. [DOI] [Google Scholar]
- Pang Q.; Kwok C. Y.; Kundu D.; Liang X.; Nazar L. F. Lightweight metallic MgB2 mediates polysulfide redox and promises high-energy-density lithium–sulfur batteries. Joule 2019, 3 (1), 136–148. 10.1016/j.joule.2018.09.024. [DOI] [Google Scholar]
- Wang J.; Jia L.; Duan S.; Liu H.; Xiao Q.; Li T.; Fan H.; Feng K.; Yang J.; Wang Q.; Liu M.; Zhong J.; Duan W.; Lin H.; Zhang Y. Single atomic cobalt catalyst significantly accelerates lithium ion diffusion in high mass loading Li2S cathode. Energy Storage Mater. 2020, 28, 375–382. 10.1016/j.ensm.2020.03.023. [DOI] [Google Scholar]
- Li G.; Wang X. L.; Seo M. H.; Li M.; Ma L.; Yuan Y. F.; Wu T. P.; Yu A. P.; Wang S.; Lu J.; Chen Z. W. Chemisorption of polysulfides through redox reactions with organic molecules for lithium–sulfur batteries. Nat. Commun. 2018, 9 (1), 705. 10.1038/s41467-018-03116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.; Li B. Q.; Peng H. J.; Zhang R.; Xie J.; Huang J. Q.; Zhang Q. Porphyrin-derived graphene-based nanosheets enabling strong polysulfide chemisorption and rapid kinetics in lithium–sulfur batteries. Adv. Energy Mater. 2018, 8 (20), 1800849. 10.1002/aenm.201800849. [DOI] [Google Scholar]
- Meini S.; Elazari R.; Rosenman A.; Garsuch A.; Aurbach D. The use of redox mediators for enhancing utilization of Li2S cathodes for advanced Li–S battery systems. J. Phys. Chem. Lett. 2014, 5 (5), 915–918. 10.1021/jz500222f. [DOI] [PubMed] [Google Scholar]
- Gerber L. C.; Frischmann P. D.; Fan F. Y.; Doris S. E.; Qu X.; Scheuermann A. M.; Persson K.; Chiang Y. M.; Helms B. A. Three-dimensional growth of Li2S in lithium–sulfur batteries promoted by a redox mediator. Nano Lett. 2016, 16 (1), 549–554. 10.1021/acs.nanolett.5b04189. [DOI] [PubMed] [Google Scholar]
- Tsao Y.; Lee M.; Miller E. C.; Gao G. P.; Park J.; Chen S. C.; Katsumata T.; Tran H.; Wang L. W.; Toney M. F.; Cui Y.; Bao Z. N. Designing a quinone-based redox mediator to facilitate Li2S oxidation in Li–S batteries. Joule 2019, 3 (3), 872–884. 10.1016/j.joule.2018.12.018. [DOI] [Google Scholar]
- Frischmann P. D.; Gerber L. C. H.; Doris S. E.; Tsai E. Y.; Fan F. Y.; Qu X. H.; Jain A.; Persson K. A.; Chiang Y. M.; Helms B. A. Supramolecular perylene bisimide-polysulfide gel networks as nanostructured redox mediators in dissolved polysulfide lithium–sulfur batteries. Chem. Mater. 2015, 27 (19), 6765–6770. 10.1021/acs.chemmater.5b02955. [DOI] [Google Scholar]
- Pang Q.; Nazar L. F. Long-life and high-areal-capacity Li–S batteries enabled by a light-weight polar host with intrinsic polysulfide adsorption. ACS Nano 2016, 10 (4), 4111–4118. 10.1021/acsnano.5b07347. [DOI] [PubMed] [Google Scholar]
- Cai W. L.; Li G. R.; Zhang K. L.; Xiao G. N.; Wang C.; Ye K. F.; Chen Z. W.; Zhu Y. C.; Qian Y. T. Conductive nanocrystalline niobium carbide as high-efficiency polysulfides tamer for lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28 (2), 1704865. 10.1002/adfm.201704865. [DOI] [Google Scholar]
- Han P.; Chung S. H.; Manthiram A. Pyrrolic-type nitrogen-doped hierarchical macro/mesoporous carbon as a bifunctional host for high-performance thick cathodes for lithium–sulfur batteries. Small 2019, 15 (16), 1900690. 10.1002/smll.201900690. [DOI] [PubMed] [Google Scholar]
- Deng D. R.; Xue F.; Bai C. D.; Lei J.; Yuan R. M.; Zheng M. S.; Dong Q. F. Enhanced adsorptions to polysulfides on graphene-supported bn nanosheets with excellent Li–S battery performance in a wide temperature range. ACS Nano 2018, 12 (11), 11120–11129. 10.1021/acsnano.8b05534. [DOI] [PubMed] [Google Scholar]
- Zhou J. B.; Liu X. J.; Zhu L. Q.; Zhou J.; Guan Y.; Chen L.; Niu S. W.; Cai J. Y.; Sun D.; Zhu Y. C.; Du J.; Wang G. M.; Qian Y. T. Deciphering the modulation essence of p bands in Co-based compounds on Li–S chemistry. Joule 2018, 2 (12), 2681–2693. 10.1016/j.joule.2018.08.010. [DOI] [Google Scholar]
- Moon S.; Jung Y. H.; Jung W. K.; Jung D. S.; Choi J. W.; Kim D. K. Encapsulated monoclinic sulfur for stable cycling of Li–S rechargeable batteries. Adv. Mater. 2013, 25 (45), 6547–6553. 10.1002/adma.201303166. [DOI] [PubMed] [Google Scholar]
- Zhao M.; Li B. Q.; Peng H. J.; Yuan H.; Wei J. Y.; Huang J. Q. Challenges and opportunities towards practical lithium–sulfur batteries under lean electrolyte conditions. Angew. Chem., Int. Ed. 2020, 10.1002/anie.201909339. [DOI] [PubMed] [Google Scholar]
- Hagen M.; Hanselmann D.; Ahlbrecht K.; Maca R.; Gerber D.; Tubke J. Lithium–sulfur cells: The gap between the state-of-the-art and the requirements for high energy battery cells. Adv. Energy Mater. 2015, 5 (16), 1401986. 10.1002/aenm.201401986. [DOI] [Google Scholar]
- Kong L.; Jin Q.; Zhang X. T.; Li B. Q.; Chen J. X.; Zhu W. C.; Huang J. Q.; Zhang Q. Towards full demonstration of high areal loading sulfur cathode in lithium–sulfur batteries. J. Energy Chem. 2019, 39, 17–22. 10.1016/j.jechem.2018.12.012. [DOI] [Google Scholar]
- Bhargav A.; He J. R.; Gupta A.; Manthiram A. Sulfur batteries: Attaining the critical metrics. Joule 2020, 4 (2), 285–291. 10.1016/j.joule.2020.01.001. [DOI] [Google Scholar]
- Chung S. H.; Chang C. H.; Manthiram A. Progress on the critical parameters for lithium–sulfur batteries to be practically viable. Adv. Funct. Mater. 2018, 28 (28), 1801188. 10.1002/adfm.201801188. [DOI] [Google Scholar]
- Xu R.; Belharouak I.; Li J. C. M.; Zhang X. F.; Bloom I.; Bareño J. Role of polysulfides in self-healing lithium–sulfur batteries. Adv. Energy Mater. 2013, 3 (7), 833–838. 10.1002/aenm.201200990. [DOI] [Google Scholar]
- Fan F. Y.; Pan M. S.; Lau K. C.; Assary R. S.; Woodford W. H.; Curtiss L. A.; Carter W. C.; Chiang Y.-M. Solvent effects on polysulfide redox kinetics and ionic conductivity in lithium–sulfur batteries. J. Electrochem. Soc. 2016, 163 (14), A3111–A3116. 10.1149/2.1181614jes. [DOI] [Google Scholar]
- Fu Y. S.; Wu Z.; Yuan Y. F.; Chen P.; Yu L.; Yuan L.; Han Q. R.; Lan Y. J.; Bai W. X.; Kan E.; Huang C. X.; Ouyang X.; Wang X.; Zhu J. W.; Lu J. Switchable encapsulation of polysulfides in the transition between sulfur and lithium sulfide. Nat. Commun. 2020, 11 (1), 845. 10.1038/s41467-020-14686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F. Y.; Chiang Y. M. Electrodeposition kinetics in Li–S batteries: Effects of low electrolyte/sulfur ratios and deposition surface composition. J. Electrochem. Soc. 2017, 164 (4), A917–A922. 10.1149/2.0051706jes. [DOI] [Google Scholar]
- Fan F. Y.; Carter W. C.; Chiang Y. M. Mechanism and kinetics of Li2S precipitation in lithium–sulfur batteries. Adv. Mater. 2015, 27 (35), 5203–5209. 10.1002/adma.201501559. [DOI] [PubMed] [Google Scholar]
- Cheng X. B.; Yan C.; Huang J. Q.; Li P.; Zhu L.; Zhao L. D.; Zhang Y. Y.; Zhu W. C.; Yang S. T.; Zhang Q. The gap between long lifespan Li–S coin and pouch cells: The importance of lithium metal anode protection. Energy Storage Mater. 2017, 6, 18–25. 10.1016/j.ensm.2016.09.003. [DOI] [Google Scholar]
- Kong L.; Jin Q.; Huang J. Q.; Zhao L. D.; Li P.; Li B. Q.; Peng H. J.; Zhang X. T.; Zhang Q. Nonuniform redistribution of sulfur and lithium upon cycling: Probing the origin of capacity fading in lithium–sulfur pouch cells. Energy Technol. 2019, 7 (12), 1900111. 10.1002/ente.201900111. [DOI] [Google Scholar]
- Fang R. P.; Zhao S. Y.; Hou P. X.; Cheng M.; Wang S. G.; Cheng H. M.; Liu C.; Li F. 3D interconnected electrode materials with ultrahigh areal sulfur loading for Li–S batteries. Adv. Mater. 2016, 28 (17), 3374–3382. 10.1002/adma.201506014. [DOI] [PubMed] [Google Scholar]
- Agostini M.; Hwang J. Y.; Kim H. M.; Bruni P.; Brutti S.; Croce F.; Matic A.; Sun Y. K. Minimizing the electrolyte volume in Li–S batteries: A step forward to high gravimetric energy density. Adv. Energy Mater. 2018, 8 (26), 1801560. 10.1002/aenm.201801560. [DOI] [Google Scholar]
- Chung S. H.; Chang C. H.; Manthiram A. A carbon-cotton cathode with ultrahigh-loading capability for statically and dynamically stable lithium–sulfur batteries. ACS Nano 2016, 10 (11), 10462–10470. 10.1021/acsnano.6b06369. [DOI] [PubMed] [Google Scholar]
- Yuan Z.; Peng H. J.; Hou T. Z.; Huang J. Q.; Chen C. M.; Wang D. W.; Cheng X. B.; Wei F.; Zhang Q. Powering lithium-sulfur battery performance by propelling polysulfide redox at sulfiphilic hosts. Nano Lett. 2016, 16 (1), 519–527. 10.1021/acs.nanolett.5b04166. [DOI] [PubMed] [Google Scholar]
- Qi B.; Zhao X. S.; Wang S. G.; Chen K.; Wei Y. J.; Chen G.; Gao Y.; Zhang D.; Sun Z. H.; Li F. Mesoporous TiN microspheres as efficient polysulfides barrier for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 14359–14366. 10.1039/C8TA04920C. [DOI] [Google Scholar]
- Zhao M.; Peng H. J.; Li B. Q.; Chen X.; Xie J.; Liu X.; Zhang Q.; Huang J. Q. Electrochemical phase evolution of metal-based pre-catalysts for high-rate polysulfide conversion. Angew. Chem., Int. Ed. 2020, 10.1002/anie.202003136. [DOI] [PubMed] [Google Scholar]
- Frischmann P. D.; Hwa Y.; Cairns E. J.; Helms B. A. Redox-active supramolecular polymer binders for lithium–sulfur batteries that adapt their transport properties in operando. Chem. Mater. 2016, 28 (20), 7414–7421. 10.1021/acs.chemmater.6b03013. [DOI] [Google Scholar]
- Wang H. Q.; Zhang W. C.; Liu H. K.; Guo Z. P. A strategy for configuration of an integrated flexible sulfur cathode for high-performance lithium–sulfur batteries. Angew. Chem., Int. Ed. 2016, 55 (12), 3992–3996. 10.1002/anie.201511673. [DOI] [PubMed] [Google Scholar]
- Li B. Q.; Kong L.; Zhao C. X.; Jin Q.; Chen X.; Peng H. J.; Qin J. L.; Chen J. X.; Yuan H.; Zhang Q.; Huang J. Q. Expediting redox kinetics of sulfur species by atomic-scale electrocatalysts in lithium–sulfur batteries. InfoMat 2019, 1 (4), 533–541. 10.1002/inf2.12056. [DOI] [Google Scholar]
- Li B. Q.; Peng H. J.; Chen X.; Zhang S. Y.; Xie J.; Zhao C. X.; Zhang Q. Polysulfide electrocatalysis on framework porphyrin in high-capacity and high-stable lithium–sulfur batteries. CCS Chemistry 2019, 1 (1), 128–137. 10.31635/ccschem.019.20180016. [DOI] [Google Scholar]
- Zhao M.; Peng H. J.; Wei J. Y.; Huang J. Q.; Li B. Q.; Yuan H.; Zhang Q. Dictating high-capacity lithium–sulfur batteries through redox-mediated lithium sulfde growth. Small Methods 2020, 4, 1900344. 10.1002/smtd.201900344. [DOI] [Google Scholar]
- Rauh R. D.; Shuker F. S.; Marston J. M.; Brummer S. B. Formation of lithium polysulfides in aprotic media. J. Inorg. Nucl. Chem. 1977, 39 (10), 1761–1766. 10.1016/0022-1902(77)80198-X. [DOI] [Google Scholar]
- Weller C.; Pampel J.; Dörfler S.; Althues H.; Kaskel S. Polysulfide shuttle suppression by electrolytes with low-density for high-energy lithium–sulfur batteries. Energy Technol. 2019, 7 (12), 1900625. 10.1002/ente.201900625. [DOI] [Google Scholar]
- Park J. W.; Ueno K.; Tachikawa N.; Dokko K.; Watanabe M. Ionic liquid electrolytes for lithium–sulfur batteries. J. Phys. Chem. C 2013, 117 (40), 20531–20541. 10.1021/jp408037e. [DOI] [Google Scholar]
- Wang L. L.; Ye Y. S.; Chen N.; Huang Y. X.; Li L.; Wu F.; Chen R. J. Development and challenges of functional electrolytes for high-performance lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28 (38), 1800919. 10.1002/adfm.201800919. [DOI] [Google Scholar]
- Zhang S. G.; Ueno K.; Dokko K.; Watanabe M. Recent advances in electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 2015, 5 (16), 1500117. 10.1002/aenm.201500117. [DOI] [Google Scholar]
- Zhang G.; Peng H. J.; Zhao C. Z.; Chen X.; Zhao L. D.; Li P.; Huang J. Q.; Zhang Q. The Radical pathway based on a lithium-metal-compatible high-dielectric electrolyte for lithium–sulfur batteries. Angew. Chem., Int. Ed. 2018, 57 (51), 16732–16736. 10.1002/anie.201810132. [DOI] [PubMed] [Google Scholar]
- Yoon S. Sulfolane/1,3-dioxolane electrolyte systems for Li–S batteries. Int. J. Appl. Eng. Res. 2018, 13 (18), 13547–13551. [Google Scholar]
- Miao R. R.; Yang J.; Feng X. J.; Jia H.; Wang J. L.; Nuli Y. Novel dual-salts electrolyte solution for dendrite-free lithium-metal based rechargeable batteries with high cycle reversibility. J. Power Sources 2014, 271 (20), 291–297. 10.1016/j.jpowsour.2014.08.011. [DOI] [Google Scholar]
- Xiong S.; Kai X.; Hong X.; Diao Y. Effect of LiBOB as additive on electrochemical properties of lithium–sulfur batteries. Ionics 2012, 18, 249–254. 10.1007/s11581-011-0628-1. [DOI] [Google Scholar]
- Wu H.; Shin M.; Liu Y.; See K. A.; Gewirth A. A. Thiol-based electrolyte additives for high-performance lithium–sulfur batteries. Nano Energy 2017, 32, 50–58. 10.1016/j.nanoen.2016.12.015. [DOI] [Google Scholar]
- Zhao Q.; Tu Z.; Wei S.; Zhang K.; Choudhury S.; Liu X.; Archer L. A. Building organic/inorganic hybrid interphases for fast interfacial transport in rechargeable metal batteries. Angew. Chem., Int. Ed. 2018, 57 (4), 992–996. 10.1002/anie.201711598. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Liu Z.; Fu W.; Dudney N. J.; Liang C. Phosphorous pentasulfide as a novel additive for high-performance lithium–sulfur batteries. Adv. Funct. Mater. 2013, 23 (8), 1064–1069. 10.1002/adfm.201200696. [DOI] [Google Scholar]
- Zhao C. Z.; Zhang Q. A leap towards stable Li-metal anode interphases. Trends Chem. 2019, 1 (8), 709–710. 10.1016/j.trechm.2019.10.002. [DOI] [Google Scholar]
- Xu R.; Cheng X. B.; Yan C.; Zhang X. Q.; Xiao Y.; Zhao C. Z.; Huang J. Q.; Zhang Q. Artificial Interphases for Highly Stable Lithium Metal Anode. Matter 2019, 1 (2), 317–344. 10.1016/j.matt.2019.05.016. [DOI] [Google Scholar]
- Shen X.; Cheng X.; Shi P.; Huang J. Q.; Zhang X.; Yan C.; Li T.; Zhang Q. Lithium–matrix composite anode protected by a solid electrolyte layer for stable lithium metal batteries. J. Energy Chem. 2019, 37, 29–34. 10.1016/j.jechem.2018.11.016. [DOI] [Google Scholar]
- Pang Q.; Liang X.; Kochetkov I. R.; Hartmann P.; Nazar L. F. Stabilizing lithium plating by a biphasic surface layer formed in situ. Angew. Chem., Int. Ed. 2018, 57 (31), 9795–9798. 10.1002/anie.201805456. [DOI] [PubMed] [Google Scholar]
- Tan S. J.; Yue J.; Hu X. C.; Shen Z. Z.; Wang W. P.; Li J. Y.; Zuo T. T.; Duan H.; Xiao Y.; Yin Y. X.; Wen R.; Guo Y. G. Nitriding-interface-regulated lithium plating enables flame-retardant electrolytes for high-voltage lithium metal batteries. Angew. Chem., Int. Ed. 2019, 58 (23), 7802–7807. 10.1002/anie.201903466. [DOI] [PubMed] [Google Scholar]
- Ghazi Z. A.; Sun Z.; Sun C.; Qi F.; An B.; Li F.; Cheng H. M. Key aspects of lithium metal anodes for lithium metal batteries. Small 2019, 15 (32), 1900687. 10.1002/smll.201900687. [DOI] [PubMed] [Google Scholar]
- Zhang X. Q.; Li T.; Li B. Q.; Zhang R.; Shi P.; Yan C.; Huang J. Q.; Zhang Q. A sustainable solid electrolyte interphase for high-energy-density lithium metal batteries under practical conditions. Angew. Chem., Int. Ed. 2020, 59 (8), 3252–3257. 10.1002/anie.201911724. [DOI] [PubMed] [Google Scholar]







