Abstract

Nano-CL-20/TNT cocrystal explosive was successfully prepared by mechanical ball milling with 0.38 mm grinding beads. The micromorphology and particle size of cocrystal explosive were characterized by scanning electron microscopy. The average particle size of nano-CL-20/TNT cocrystal explosive was 119.5 nm and showed a spherical-like micromorphology. The crystal structure of cocrystal explosive was characterized by powder X-ray diffraction, infrared spectroscopy, and Raman spectroscopy. The results show that mechanical ball milling does not change the molecular structure of the raw material, but the sample after ball milling has a new crystal phase, rather than a simple mixing of raw materials. Differential scanning calorimetry tests show that nano-CL-20/TNT cocrystal explosive has a higher decomposition temperature; impact sensitivity tests show that the properties of cocrystal explosive are 26 and 21.7 cm higher than those of CL-20 and CL-20/TNT mixture, respectively, which indicates that nano-CL-20/TNT cocrystal explosive has better thermal stability and safety.
1. Introduction
The contradiction of energy and sensitivity of energetic materials has always been an important topic. High-energy explosives such as CL-20 and HMX can cause effective and efficient damage as they have relatively high energy levels. However, high-energy explosives can readily explode upon external stimulations, resulting in severe losses.1−3 High energy and insensitivity have become the requirements of novel energetic materials and future research direction.4,5 CL-20 is an elemental explosive with high levels of energy which has been widely applied in explosives and solid propellants. Nevertheless, the high energy level of CL-20 is accompanied by high sensitivity. Upon external stimulation, CL-20 can readily cause unexpected explosions. Previous studies have demonstrated that the characteristic drop height of elemental CL-20 is approximately 10 cm,6 which severely limits its application in energetic materials. Hence, great effort has been invested in reducing the sensitivity of CL-20.7−9 Surface coating, ultrafine treatment, recrystallization, and cocrystalum have been effectively utilized for sensitivity reduction and safety improvement of CL-20.10−12
It has been demonstrated that the sensitivity and critical size of detonation of elemental explosives are significantly reduced if its average particle size exhibits d50 < 400 nm. Also, it delivers high energy output during microscale charging. Recently, cocrystal techniques have been effective in modifying high-energy explosives, which include CL-20/TNT,13,14 CL-20/HMX,15,16 and CL-20/DNB.17 In this way, the sensitivity of CL-20 was effectively reduced without affecting the energy output, and cocrystal explosives are found to be promising substitutes to CL-20. However, the preparation of cocrystal explosives is still at the laboratory scale, and industrial production has not been achieved. This can be attributed to the solvent evaporation method, which is employed to obtain single-crystal data. As a result, further investigations and applications of cocrystal explosives have been limited.
The proposed mechanical ball-milling method is a green cocrystal preparation technique. Using this method, Zhao et al. prepared nanoscale CL-20/HMX cocrystal explosives16 and Song et al. prepared nanoscale CL-20/RDX cocrystal explosives.18 The mechanical ball-milling method involves the addition of a solvent during mechanical ball-milling to facilitate the generation of a cocrystal explosive. This method can prepare nanoscale cocrystal explosives by effectively combining nanocrystallization and cocrystalum preparation to reduce the sensitivity of high-energy explosives. Meanwhile, the mechanical ball-milling method can be applied in industrial production and provides a possible route for industrial applications of cocrystal explosives. In 2011, Bolton and Matzger successfully prepared CL-20/TNT cocrystal explosives and reported single-crystal data.13 The structural schematics of CL-20 and TNT are shown in Figure 1. However, further studies of CL-20/TNT cocrystal explosives do not exist. In this study, nano-CL-20/TNT cocrystal explosives were prepared by the mechanical ball-milling method and provide a baseline for industrialization and further studies of CL-20/TNT cocrystal explosives.
Figure 1.

Structural schematic diagram of CL-20 and TNT.
2. Results and Discussion
2.1. Scanning Electron Microscopy Observation
The morphology and granularity of samples after ball-milling were characterized by scanning electron microscopy (SEM), and the results are shown in Figure 2a,b. As observed, samples after ball-milling exhibited spherical morphology and homogeneous granularity. The sizes of 500 particles in the figure were measured and summarized using a NanoMeasurer 1.2. Figure 2c shows the frequency distribution curve. It is observed that the granularity of the sample follows a Gaussian distribution with an average particle size of 115.9 nm. Figure 2d shows the accumulated size distribution curve. It is shown that the obtained samples own the median diameter (d50) of 103.2 nm, indicating that 50% of all particles have sizes below 103.2 nm. Also, the samples have d10 of 66.9 nm and d90 of 147.4 nm. Hence, it is reasonable to assume that 90% of all particles have sizes below 147.4 nm. The results demonstrate successful preparation of nanoscale cocrystal samples with homogeneous granularity.
Figure 2.
Morphology of nano-CL-20/TNT cocrystal (a,b) and particle-size distribution of nano-CL-20/TNT cocrystal (c,d).
2.2. X-ray Diffraction Analysis
The crystal structures of the prepared nano-CL-20/TNT cocrystal as well as raw CL-20, raw TNT, and CL-20/TNT mixture were analyzed via X-ray diffraction (XRD), and the resulting XRD patterns are exhibited in Figure 3. As observed, the XRD spectrum of the CL-20/TNT mixture is a simple superimposition of those of CL-20 and TNT, indicating that the mixture contains no new compounds. The XRD spectra of nano-CL-20/TNT cocrystal explosives after 360 min ball-milling were significantly different from those of CL-20, TNT, and the mixture. In the XRD spectra of CL-20, diffraction peaks were observed at 12.6, 15.6, 16.3, and 30.3°, none of which were observed in the nano-CL-20/TNT cocrystal explosives. In the XRD spectra of TNT, diffraction peaks were observed at 12.5, 15.6, 27.6, 29.2, and 33.6°, none of which were observed in nano-CL-20/TNT cocrystal explosives. Meanwhile, diffractions peaks were observed at 11.2, 12.4, 13.1, 14.4, 18.6, 19.3, 25.4, and 27.0° in the XRD spectra of the cocrystal explosives. None of these peaks were observed in the XRD spectra of CL-20, TNT, or the mixture.
Figure 3.
XRD patterns of raw CL-20, raw TNT, CL-20/TNT mixture, and CL-20/TNT cocrystal.
Meanwhile, CL-20/TNT cocrystal explosives prepared by the ball-milling method and Bolton method were highly consistent,12 demonstrating successful preparation of CL-20/TNT cocrystal explosives by the mechanical ball-milling method.
2.3. Infrared Spectroscopy Analysis
CL-20, TNT, the CL-20/TNT mixture, and nano-CL-20/TNT cocrystal explosives were investigated by infrared spectroscopy (IR) spectroscopy (Figure 4). As observed, main absorption peaks in the IR spectra of CL-20 and TNT can be observed in those of the cocrystal explosives and the mixture, demonstrating that the cocrystal compound contains CL-20 and TNT. More specifically, some of the main absorption peaks in the IR spectra of CL-20 and TNT shifted toward high or low wave numbers in those of nano-CL-20/TNT cocrystal explosives. However, these shifts were not observed in the IR spectrum of the mixture. Instead, the IR spectrum of the mixture is more likely a superposition of those of CL-20 and TNT. For instance, the stretching vibration of C–H in the benzene ring at 3097 cm–1 in TNT shifted to 3113 cm–1 in the IR spectrum of the nano-CL-20/TNT cocrystal explosive; the stretching vibration of C–H at 3045 cm–1 in CL-20 shifted to 3030 cm–1 in the IR spectrum of the nano-CL-20/TNT cocrystal explosive; antisymmetric stretching vibrations of −NO2 at 1585 and 1531 cm–1 in CL-20 and TNT shifted to 1593 and 1537 cm–1, respectively, in the IR spectrum of the nano-CL-20/TNT cocrystal explosive. Likewise, the bending vibration of −CH3 at 1436 cm–1 and symmetric stretching vibration of −NO2 at 1280 cm–1 in CL-20 shifted to 1461 and 1285 cm–1, respectively, in the IR spectrum of the nano-CL-20/TNT cocrystal explosive. However, none of these shifts were observed in the IR spectrum of the mixture.
Figure 4.

IR spectra of raw CL-20, raw TNT, CL-20/TNT mixture, and CL-20/TNT cocrystal.
The results demonstrate that mechanical ball-milling can effectively facilitate noncovalent interactions between molecules without changing molecular structures of raw materials. Specifically, ball-milling facilitates generation of C–H···O hydrogen bonds between CL-20 and TNT.
2.4. Raman Spectroscopy Analysis
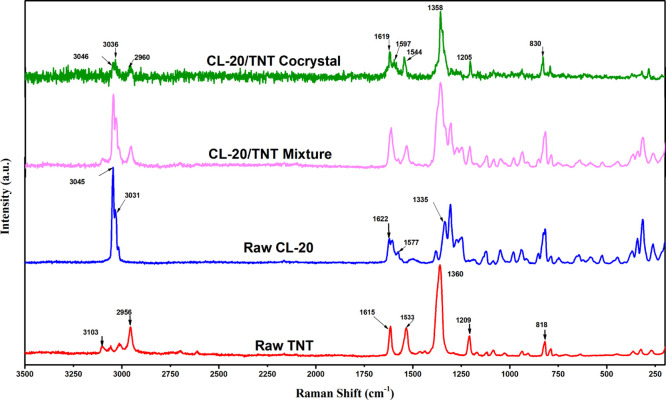
To better understand the development of cocrystal explosives, CL-20, TNT, the CL-20/TNT mixture, and the nano-CL-20/TNT cocrystal explosives were characterized using Raman spectroscopy, and the results are shown in Figure 5. As observed, the main peaks in the Raman spectra of cocrystal energetic materials shifted compared to those in the Raman spectra of CL-20 and TNT, whereas the Raman spectrum of the mixture is a superimposition of those of the raw materials. For instance, the asymmetric stretching vibration of C–H in −CH3 of TNT shifted from 2956 to 2960 cm–1 in the coni crystal compound and the antisymmetric stretching vibration of −NO2 on the benzene ring shifted from 1533 to 1544 cm–1. Similarly, the stretching vibration of C–H in CL-20 shifted from 3031 to 3036 cm–1, the symmetric stretching vibration of −NO2 shifted from 1335 to 1358 cm–1, and some peaks in the Raman spectra of the raw materials were not observed in that of the cocrystal explosive. This can be attributed to the C–H···O hydrogen bonds between −CH3 in TNT and −NO2 in CL-20. Hydrogen bonds in the cocrystal explosive lead to reduced intermolecular distances between CL-20 and TNT, thus damaging the cage structure of CL-20. As a result, some Raman peaks disappear.
Figure 5.
Raman spectra of raw CL-20, raw TNT, CL-20/TNT mixture, and CL-20/TNT cocrystal.
2.5. Differential Scanning Calorimetry Analysis
CL-20, TNT, the CL-20/TNT mixture, and the nano-CL-20/TNT cocrystal explosives were investigated by differential scanning calorimetry (DSC), and the results are shown in Figure 6. As observed, the thermal decompositions of nano-CL-20/TNT cocrystal explosives are significantly different from their raw materials and mixture. Nano-CL-20/TNT cocrystal explosives are characterized by a major endothermic peak at 132.0 °C, which corresponds to liquid conversion of TNT and conversion of ε-CL-20 to β-CL-20, and a broad exothermic decomposition peak at 235.5 °C (202.3–250.5 °C), which corresponds to exothermic decompositions of TNT and CL-20. The exothermic decomposition peak is broad instead of two individual ones. This can be attributed to the fact that ball-milling facilitates exothermic decomposition so that exothermic decompositions of TNT and CL-20 have no definite decomposition boundary. Overall, the nano-CL-20/TNT cocrystal explosives prepared by ball-milling and cocrystal explosives prepared by the Bolton method exhibit similar thermal decomposition performances. This also demonstrates successful preparation of nano-CL-20/TNT cocrystal explosives by the ball-milling method.
Figure 6.
DSC curves of raw CL-20, raw TNT, CL-20/TNT mixture, and CL-20/TNT cocrystal.
2.6. Sensitivity Analysis
Sensitivity is one of the key safety indicators of energetic materials. Table 1 summarizes impact sensitivities and friction sensitivities of CL-20, TNT, the CL-20/TNT mixture, and the nano-CL-20/TNT cocrystal explosives. According to the data shown in Table 1, it is obvious that the nano-CL-20/TNT cocrystal exhibits lower impact and friction sensitivity than the raw materials or CL-20/TNT mixture. Compared with CL-20 and the CL-20/TNT mixture, the characteristic drop height of nano-CL-20/TNT cocrystal explosives prepared by the mechanical ball-milling method increased by 26 and 21.7 cm, respectively, indicating that mechanical ball-milling can effectively reduce the sensitivity of CL-20 and formation of nano-CL-20/TNT cocrystal explosives. The friction sensitivity of the nano-CL-20/TNT cocrystal shows significant decrease compared with raw CL-20 and the CL-20/TNT mixture. The sensitivity reduction can be attributed to nanocrystallization, which can effectively reduce the sensitivity of high-energy materials. Meanwhile, the spherical structure of cocrystal explosives leads to reduced probability of internal hot spots. Additionally, noncovalent bonds, such as hydrogen bonds, facilitate the formation of cocrystal explosives, thus reducing the sensitivity of high-energy explosives. The results also demonstrate that cocrystal techniques can effectively enhance the safety of high-energy explosives.
Table 1. Sensitivity of Raw CL-20, Raw TNT, CL-20/TNT Mixture, and CL-20/TNT Cocrystal.
| sample | impact sensitivity (H50, cm) | friction sensitivity (P, %) |
|---|---|---|
| raw CL-20 | 13 | 100 |
| raw TNT | 92 | 4 |
| CL-20/TNT cocrystal | 39 | 68 |
| CL-20/TNT mixture | 18.2 | 92 |
3. Conclusions
Nano-CL-20/TNT cocrystal explosives were prepared by a mechanical ball-milling method. SEM characterization revealed that the average granularity of the prepared cocrystal explosives was 115.9 nm. Characterization by powder XRD, IR spectroscopy, and Raman spectroscopy revealed that the cocrystal explosives had a novel crystal structure, instead of a mixture of raw materials.
DSC tests revealed a broad exothermal decomposition peak (202.3–250.5 °C) and improved thermal decomposition performance for the nano-CL-20/TNT cocrystal explosives. Both impact and friction sensitivity of the nano-CL-20/TNT cocrystal are lower than those of the raw materials or CL-20/TNT mixture, demonstrating improved safety of the nano-CL-20/TNT cocrystal explosives.
By combining nanocrystallization and cocrystalum, this study provides an alternative route for industrial preparation of cocrystal explosives. Additionally, nano-CL-20/TNT cocrystal explosives are promising substitutes for CL-20.
4. Experimental Section
4.1. Reagents and Instruments
ε-CL-20 (industrial grade) was purchased from Qingyang Chemical Industry Corporation. TNT (industrial grade) was purchased from Gansu Yinguang Chemical Industry Group Co., Ltd. Absolute ethanol was purchased from Tianjin Guangfu Chemicals Co., Ltd, and milling balls (Φ = 0.38 mm, ZrO2 doped with 2–10% Y2O3) were purchased from Zibo Yubang Industrial Ceramics Co., Ltd.
SEM (S-4800II by Hitachi, Japan), XRD (ADVANCE D8 by Bruker, Germany), Fourier-transform infrared spectrometry (FTIR) (Nicolet 6700 by Thermo Fisher, USA), laser confocal micro Raman spectrometry (inVia by Renishaw, UK), DSC (DSC823E by Mettler Toledo Shanghai), and an impact sensitivity meter (HGZ-1 by Beijing Jinhengxiang Instruments Co., Ltd) were used for property measurements and characterization.
4.2. Sample Preparation
4.2.1. Pretreatment
Large particle TNT powders were further ground manually using an agate mortar and stored.
4.2.2. Preparation
CL-20 (8.76 g) and TNT (4.54 g) (molar ratio = 1:1) were weighed and added into an alumina jar. To it, 2 kg of 0.38 mm zirconium beads (2/3 of the cavity volume) and 200 mL of ethanol solution (volumetric ratio of ethanol and water = 1:1) were added. Then, the jar was fixed to a vertical ceramic membrane minimized ball miller. First, the mixture was ground at 200 rpm for several minutes. Upon homogeneous mixing of the raw materials and the ball, the mixture was ground at 1500 rpm for 360 min. Finally, the raw materials and ball were separated by an ultrasonic cleaner, and the product was collected and dried. In this way, nano-CL-20/TNT cocrystal explosives were obtained.
4.3. Characterization
The micromorphology and granularity of the cocrystal explosives were characterized by SEM with a voltage = 10–15 kV. The crystal structure of the cocrystal explosives was investigated by powder XRD (Cu target as the diffraction source, voltage = 40 kV, current = 30 mA, and scanning range = 5–60°). The cocrystal chemical bonds, functional groups, and molecular structures were characterized using FTIR and Raman spectroscopy. The data collection range and resolution of the IR spectroscopy were 500–4000 and 0.1 cm–1, respectively. The range of Raman spectroscopy was 4000–10 cm–1. The thermal decomposition of the cocrystal explosives was characterized by DSC. The temperature range was 50–350 °C, the heating rate was 8 °C/min, the flow rate of N2 was 50 mL/min, and an Al2O3 crucible was used with a sample mass ≤3.0 mg. The impact sensitivity was tested by the characteristic drop height method in GJB772A-97 601.2. The drop weight was 5 kg, the dosage was (35 ± 1) mg, the ambient temperature was (20 ± 2) °C, and the relative humidity was (60 ± 5)%. Each group consisted of 25 effective data sets, and the average of the three groups was defined as the final result. The friction sensitivity was tested by the explosion probability method in GJB772A-97 602.1 with a WM-1 friction instrument. In each test, whether an explosive event occurred or not was recorded when a weighted pendulum scraped across the sample (20 mg), and an explosion probability (P, %) was obtained. Each group consisted of 25 effective data sets, and the average of the three groups was defined as the final result.
Acknowledgments
The authors are thankful for the financial support from the Natural Science Foundation of Jiangsu Province (BK20180495, BK20180698) and the National Natural Science Foundation of China (21805139).
The authors declare no competing financial interest.
References
- Shu Y. J.; Huo J. C.. Introduction to Explosive Pharmacy; Chemical Industry Press: Beijing, 2011; pp 1–34. [Google Scholar]
- Wang Z. S.An Introduction to Energetic Materials; Harbin Institute of Technology Press: Harbin, 2006; pp 10–110. [Google Scholar]
- Ou Y. X.Pharmacy; Beijing University of Technology Press: Beijing, 2006; pp 1–50. [Google Scholar]
- Jia X. L.; Hou C. H.; Wang J. Y.; Tan Y.; Zhang Y.; Li C. Re search Progress on Sensitization Reduction Technology of Nitramine Explosives. Huozhayao Xuebao 2018, 41, 326–333. [Google Scholar]
- Badgujar D. M.; Talawar M. B.; Asthana S. N.; Mahulikar P. P. Advances in science and technology of modern energetic materials: an overview. J. Hazard. Mater. 2008, 151, 289–305. 10.1016/j.jhazmat.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Jia X.; Wang J. Preparation and characterization of spherical submicron ε-CL-20 via green mechanical demulsification. J. Energ. Mater. 2019, 37, 475–483. 10.1080/07370652.2019.1651421. [DOI] [Google Scholar]
- Jiang X.; Guo X.; Ren H.; Jiao Q. Preparation and Characterization of Desensitized-HNIW in Solvent-Antisolvent Recrystallizations. Cent. Eur. J. Energ. Mater. 2012, 9, 219–236. [Google Scholar]
- Ou Y. X.; Meng Z.; Liu J. Q. Advances in Application of High Energy Density Compound CL-20. Prog. Chem. Ind. 2007, 12, 1690–1694. [Google Scholar]
- Xu X.-J.; Zhu W.-H.; Xiao H.-M. DFT Studies on the Four Polymorphs of Crystalline CL-20 and the Influences of Hydrostatic Pressure on ε-CL-20 Crystal. J. Phys. Chem. B 2007, 111, 2090–2097. 10.1021/jp066833e. [DOI] [PubMed] [Google Scholar]
- Qu C.-X.; Ge Z. X.; Zhang M.; Xu C.; Bi F. Q.; Ding K.-W. Preparation, Characterization and Properties of CL-20/HATO Composite. Chin. J. Energ. Mater. 2018, 26, 850–855. 10.11943/CJEM2018104. [DOI] [Google Scholar]
- Bayat Y.; Zarandi M.; Zarei M. A.; Soleyman R.; zeynali V. A novel approach for preparation of CL-20 nanoparticles by microemulsion method. J. Mol. Liq. 2014, 193, 83–86. 10.1016/j.molliq.2013.12.028. [DOI] [Google Scholar]
- Hudson R. J.; Moniruzzaman M.; Gill P. P. Investigation of crystal morphology and shock sensitivity of cyclotrimethylenetrinitramine suspension by rheology. Propellants, Explos., Pyrotech. 2015, 40, 233–237. 10.1002/prep.201400129. [DOI] [Google Scholar]
- Bolton O.; Matzger A. J. Improved Stability and Smart-Ma terial Functionality Realized in an Energetic Cocrystal. Angew. Chem., Int. Ed. 2011, 50, 8960–8963. 10.1002/anie.201104164. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Li H.; Huang H.; Zhou X.; Li J.; Nie F. Preparation and Perfomance of a HNIW/TNT Cocrystal Explosive. Propellants, Explos., Pyrotech. 2013, 38, 495–501. 10.1002/prep.201200093. [DOI] [Google Scholar]
- Bolton O.; Simke L. R.; Pagoria P. F.; Matzger A. J. High Power Explosive with Good Sensitivity: A 2:1 Cocrystal of CL-20:HMX. Cryst. Growth Des. 2012, 12, 4311–4314. 10.1021/cg3010882. [DOI] [Google Scholar]
- Zhao S. S.; Song X. L.; Wang Y.; Liu S.; Zhang J.; Zhang S. M.; Feng K. Characterization of nano-CL-20/HMX cocrystal prepared by mechanical milling method. Guti Huojian Jishu 2018, 41, 479–482. [Google Scholar]
- Wang Y.; Yang Z.; Li H.; Zhou X.; Zhang Q.; Wang J.; Liu Y. A Novel Cocrystal Explosive of HNIW with Good Comprehensive Properties. Propellants, Explos., Pyrotech. 2014, 39, 590–596. 10.1002/prep.201300146. [DOI] [Google Scholar]
- Song X.; Wang Y.; Zhao S.; Li F. Mechanochemical fabri cation and properties of CL-20/RDX nano co/mixed crystals. RSC Adv. 2018, 8, 34126–34135. 10.1039/c8ra04122a. [DOI] [PMC free article] [PubMed] [Google Scholar]