Abstract
Dysregulated lipid metabolism, characterized by higher levels of circulating triglycerides, higher levels of small, low density lipoprotein, and accumulation of intracellular lipids, is linked to insulin resistance and related complications such as type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVD). Considering that various metabolic, genetic, and environmental factors are involved in the development of T2DM and CVD, the causalities of these diseases are often confounded. In recent years, Mendelian randomization (MR) studies coupling genetic data in population studies have revealed new insights into the risk factors influencing the development of CVD and T2DM. This review briefly conceptualizes MR and summarizes the genetic traits related to lipid metabolism by evaluating their effects on the indicators of insulin resistance based on the results of recent MR studies. The data from the MR study cases referred to in this review indicate that the causal associations between lipid status and insulin resistance in MR studies are not conclusive. Furthermore, available data on Asian ethnicities, including Korean, are very limited. More genome-wide association studies and MR studies on Asian populations should be conducted to identify Asian- or Korean-specific lipid traits in the development of insulin resistance and T2DM. The present review discusses certain studies that investigated genetic variants related to nutrient intake that can modify lipid metabolism outcomes. Up-to-date inferences on the causal association between lipids and insulin resistance using MR should be interpreted with caution because of several limitations, including pleiotropic effects and lack of information on genotype and ethnicity.
Keywords: Lipids, Insulin resistance, Mendelian randomization analysis
INTRODUCTION
Previous studies provided evidence that abnormal levels of circulating lipids and intracellular lipids in peripheral tissues (e.g., liver, adipose tissue, and skeletal muscle) are related to insulin resistance and related complications, including type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVD).1,2,3,4,5 Because these metabolic diseases appear to be influenced by various metabolic, genetic, and environmental factors, the causality between lipid status and metabolic complication is confounded. Moreover, targeting the risk factors for diseases (e.g., CVD) showed no remarkable advantage in improving treatment outcomes for patients.6 The randomized controlled trial design overcomes the limitations of observational and epidemiological studies by random assignment of study subjects to the control or treatment group, avoiding influential bias through blinding of researchers and subjects.7,8,9,10 Recently, Mendelian randomization (MR) studies coupling genetic data from population studies have revealed novel insights into the risk factors influencing the development of CVD and T2DM. Considering that the causality between risk factors and disease can help identify suitable targets for treatment, the MR approach can potentially advance our knowledge of causation or reverse causation between lipid metabolism and T2DM and can significantly improve treatment strategies, such as diet or behavioral modifications. To date, a limited number of MR study cases have investigated the relationship between lipid status and risk of T2DM, whereas most MR studies investigating CVD are relatively well reported and additionally analyze the indicators for insulin resistance (increased fasting glucose, insulin, and homeostatic model assessment for insulin resistance [HOMA-IR]) as the primary outcome of lipid status.11,12,13 Hence, this review incorporates data obtained from insulin resistance indicators from MR studies for both CVD and T2DM.
This review briefly conceptualizes MR and introduces the genetic traits related to lipid metabolism based on their consequences on insulin resistance in recent MR studies. The causality between lipid metabolism and risk of insulin resistance is discussed regarding 2 phases of lipid metabolism, namely, circulation of lipids and accumulation of lipids in peripheral tissues.
MR
MR studies share similar study design elements with randomized controlled trial (RCT) studies.2,14 An equal distribution of general features (e.g., age, sex, social factors, and disease history) in RCTs can reduce the probability of these variables affecting study results. Likewise, independent assortment of alleles according to Mendel's second law theoretically results in equal genetic background of individuals carrying the alleles of interest. Additionally, social and environmental factors should be equally distributed in the respective genotype groups if the cases and controls are selected from the same population. The biomarker modulated by the genetic variant can be extensively explored by analyzing its association with outcome or disease risk.9,10 For the MR study design, once genotypes in the population are identified on a genome-wide level, genetic modulation of the biomarker can be simulated by statistical exploration of the dataset.
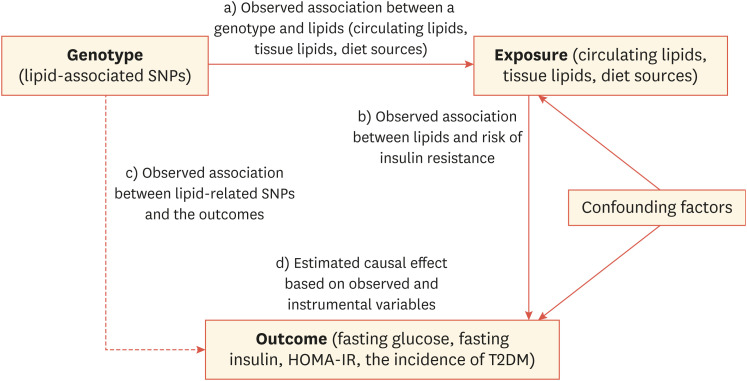
Enhanced genotyping technologies for analyzing single nucleotide polymorphisms (SNPs) in large population studies have enabled the accumulation of sufficient genetic data and the detection of weak genetic effects that contribute to various phenotypes with improved statistical power.7,8 Consequently, genome-wide association (GWA) studies have successfully identified several variants affecting the incidence of diseases (e.g., CVD and T2DM) and related biochemical indicators. In GWA studies, most SNPs that show a significant signal quantitatively influence biomarker levels based on the inherited copy numbers of variants in an individual. MR design studies can then be performed to investigate the association between the exposure and the outcome, the association between genetic variants and the exposure, and the association between genetic variants and outcome measurements. Thus, recently obtained large-scale genetic data have laid the foundation for MR studies for T2DM and CVD and merge genetic information on both biomarkers and disease phenotypes (Fig. 1). The goal of MR is to determine whether a risk factor is causally involved in the disease pathogenesis.
Fig. 1. Schematic diagram of MR analysis to estimate expected association for genotypes (SNPs) with the trait of insulin resistance. The underlying assumption of MR analysis is that the genotype and phenotype association is independent of confounding factors.
MR, Mendelian randomization; SNP, single nucleotide polymorphism; HOMA-IR, homeostatic model assessment for insulin resistance; T2DM, type 2 diabetes mellitus.
However, MR studies have certain assumptions. First, the genetic variant of interest only affects the exposure and does not affect other variables that confound the association between the exposure and the outcome. The second assumption is that there are no unmeasured confounders of the associations between genetic variants and the outcome. Last, MR studies assume that genetic variants do not directly affect the outcome, other than mediating the risk factor of interest. To evaluate underlying assumptions, most MR studies investigating multiple genetic variants perform sensitivity analysis.8,9,10,11
GENETICALLY ASSOCIATED SYSTEMIC LIPIDS AND RISK OF INSULIN RESISTANCE
1. Low-density lipoprotein cholesterol (LDL-C)
Several observational studies showed that higher levels of circulating lipids are associated with higher risk of insulin resistance and T2DM, although a causal association was not clear. Out of several types of circulating lipids, lipoprotein(a) is relatively well supported to be associated with T2DM risk.3,4,5 Lipoprotein(a) is a type of low-density lipoprotein (LDL) that contains an apolipoprotein B100 molecule.13,15 Observational studies reported an inverse relationship between lipoprotein(a) levels and T2DM incidence. While excess lipoprotein(a) levels are believed to cause coronary heart disease (CHD),11,13 the causal association between lipoprotein(a) levels and diabetes risk is complicated6,16 for several reasons, including the sizes of lipoproteins,16 and interaction with storage lipids in peripheral tissues (e.g., triglyceride [TG]). Ye et al.17 reported an inverse association between lipoprotein(a) levels and T2DM risk in the European Prospective Investigation of Cancer (EPIC)-Norfolk cohort combining both sexes. Although the hazard ratios from the bottom to top quintiles of lipoprotein(a) reached 0.56 (p<0.01), the SNP rs10455872, which causes elevation in circulating lipoprotein(a) levels, was not significantly associated with increased risk of diabetes (odds ratio [OR], 1.02; 95% confidence interval [CI], 0.86–1.20). An observational study that investigated the genetic risk scores of 140 SNPs 18 from the Meta Analyses of Glucose and Insulin-related Traits Consortium (MAGIC) and Diabetes Genetics Replication and Meta-analysis (DIAGRAM) consortia revealed that LDL-C levels were associated with insulin secretion and fasting glucose levels.19 However, this association was not detected when the same regression model was evaluated in the absence of an individual SNP, which significantly affected the primary analysis model. A study conducted by Buchmann et al.20 also reported no causal effect of rs10455872 on fasting insulin levels (p=0.29) based on data obtained from three independent cohorts, namely the Berlin Aging Study (BASE-II), Leipzig Research Centre for Civilization Diseases (LIFE)-Adult, and LIFE-Heart cohorts.
An MR analysis investigating the role of genetic variants encoding HMG-CoA reductase (HMGCR) showed that the HMGCR variant associated with lower LDL-C levels (0.06 mmol/L reduction) caused higher plasma insulin and fasting glucose levels.21 Risk analysis with a HMGCR variant as proxy for statin treatment increased the risk of T2DM (OR, 1.12; 95% CI, 1.06–1.18). Results indicated that the effect of LDL-lowering statins on T2DM risk was partially mediated by the HMGCR variant and other genetic variants targeting other cholesterol-lowering drugs. For instance, treatment with inhibitors of protein convertase subtilisin/kexin type 9 (PCSK9) can lead to on-target adverse effects of lipoprotein(a) on insulin resistance or glycemic control.22
2. TG
Multiple observational studies have shown that higher circulating TG levels are strongly correlated with insulin resistance, elevated plasma glucose levels, and T2DM,23,24,25 however, the causal relationships remain unclear because both circulating TG levels and the index of insulin resistance mediate the complex interactions among body fat, muscle function, dyslipidemia, and insulin secretion.23,24,25,26 On one hand, circulating TG has been thought to be secondary to insulin resistance or diabetes.25,26,27 Recent MR studies investigating the causality between circulating TGs and insulin resistance suggested the possibility of both hypotheses. De Silva et al.28 tested genetic variants related to circulating TGs against T2DM-related metabolic traits based on an MR design and found no association between circulating TG levels and insulin resistance. However, the phenotypic variations related to TG levels accounted for by ten SNPs utilized in this study ranged from 3% to 5%, indicating that a large proportion of the observational changes in TG levels were not captured. Another study that used allele scores from 140 SNPs enriched for TG or indicators of insulin resistance reported an association between higher circulating TG levels and higher fasting glucose (β=0.02, p=0.02) or fasting insulin levels (β=0.03, p=0.04) after adjusting for other systemic lipids and adiposity.19 Taken together, the effects of circulating TGs on insulin resistance in MR studies remain to be verified. Additional MR studies based on the segregated form of TG, free fatty acids, and genetic variants (e.g., FADS1) could help disentangle the effects of circulating TGs on insulin resistance.
3. High-density lipoprotein cholesterol (HDL-C)
Low HDL-C levels are associated with increased risk of insulin resistance25,29,30 and lowering the HDL-C levels has been suggested as a therapeutic alternative to reduce T2DM risk. Results from experimental studies also support the hypothesis that HDL-C directly causes T2DM by stimulating insulin secretion by pancreatic β-cells and modulating glucose uptake in skeletal muscles.31,32 However, these reported associations appeared to be dependent on the type of HDL-C (e.g., HDL2 vs. HDL3),33 and recent MR studies revealed that HDL-C levels influenced by genetic variations are not associated with glycemic control or insulin resistance.19,34,35
HDL2 genotype scores for three SNPs (ABCA1, LIPC, and CETP) were inversely associated with insulin resistance (β=−0.163, p<0.0001) and T2DM risk (β=0.98, p<0.0006). However, HDL2 levels influenced by genetic variation were significantly associated with insulin resistance.35 An MR analysis using allele scores from nine variants affecting HDL-C metabolism tested a similar hypothesis.34 The allele score was found to be strongly associated with HDL-C levels and generated up to 20% differences in circulating concentration of HDL-C in subjects from the Copenhagen City Heart Study (CCHS). However, no causal association was identified between allele score and T2DM risk. In addition, the analysis showed no causal association between the HDL-C allele scores of 140 SNPs from the large GWA study of lipids (The Global Lipid Genetics Consortium; GLGC) and risk of insulin resistance (for inulin sensitivity β=−0.08, p=0.72; for fasting insulin β=−0.02, p=0.27).19
Recent MR studies investigating circulating lipids and risk of insulin resistance indicate that although the results remain inconclusive, it is likely that genetically induced LDL-C levels are inversely associated with insulin resistance and T2DM, while genetic variation in TG or HDL-C show no or weak positive association with indicators of insulin resistance (Table 1).
Table 1. The association of systemic lipids-related genotypes with risk of insulin resistance or T2DM.
| Instrumental variables | Genes or related traits | Study subjects | Outcomes | OR (95% CI) or the effect per SD change (β or z score) | p-value | References | |
|---|---|---|---|---|---|---|---|
| LDL-C | |||||||
| rs10455872 | LPA/↑Lipoprotein(a) | EPIC-Norfolk cohort (n=15,044) | T2DM | OR, 1.03; 95% CI, 0.96–1.10 | 0.41 | Ye et al.17 | |
| DIAGRAM consortium (n=63,390) | |||||||
| Genetic risk score from 26 SNPs | ↑Lipoprotein(a) | DIAGRAM consortium (n=149,821) | T2DM | β=−0.21 (−0.29, −0.12) | 5.0×10−6 | Fall et al.37 | |
| MAGIC (n=42,854) | Fasting insulin | β=−0.01 (−0.03, 0.00) | 0.13 | ||||
| Fasting glucose | β=−0.02 (−0.04, −0.01) | 5.6×10−3 | |||||
| rs10455872 | LPA/↑Lipoprotein(a) | Berlin Aging Study (n=2,012) | Fasting insulin | β=0.026 (−0.02, 0.07) | 0.285 | Buchmann et al.20 | |
| LIFE-Adult (n=3,281) and LIFE-Heart (n=2,816) | |||||||
| rs17238484 | ↑HMGCR/Statin treatment | Whitehall II study (n=4,678) | T2DM | β=1.12 (1.06, 1.18) | 0.253 | Swerdlow et al.21 | |
| Meta-analysis from 43 studies (n=223,463) | |||||||
| Triglyceride | |||||||
| Weighted score from rs2954029, rs714052, rs7557067, rs17216525, rs10889353, rs7679, rs7819412, rs328, rs3135506, rs662799 | TRIBI, MLXIPL, APOB, NCAN, CLIP2, PBX4, ANGPTL3, PLTP, XKR6-AMAC1L2, LPL, APOA5/↑Circulating triglycerides | Go-DARTs study (n=12,497) | Fasting insulin or Fasting glucose | z=0.04 (−0.03, 0.11) | 0.31 | De Silva et al.28 | |
| z=0.01 (−0.06, 0.08) | 0.74 | ||||||
| Genetic risk score from 23 SNPs | ↑Circulating triglycerides | DIAGRAM consortium (n=149,821) | T2DM | β=0.13 (0.01, 0.25) | 0.04 | Fall et al.37 | |
| MAGIC (n=42,854) | Fasting insulin | β=0.03 (0.00, 0.05) | 0.03 | ||||
| Fasting glucose | β=0.02 (0.00, 0.04) | 0.04 | |||||
| HDL-C | |||||||
| Genotype score from rs2575876, rs6494005, and rs3764261 | ABCA1, LIPC, and CETP/↑High-density lipoprotein | Nagahama Prospective Cohort for Comprehensive Human Bioscience (n=8,365) | HOMA-IR | Decreased HOMA-IR by quintile of HDL-C | 0.056 | Tabara et al.35 | |
| Genetic risk score from 5 SNPs | ↑High-density lipoprotein | DIAGRAM consortium (n=149,821) | T2DM | β=−0.12 (−0.24, −0.01) | 0.03 | Fall et al.37 | |
| MAGIC (n=42,854) | Fasting insulin | β=−0.01 (−0.03, 0.01) | 0.25 | ||||
| Fasting glucose | β=−0.01 (−0.05, −0.01) | 3.4×10−3 | |||||
| HDL-C lowering allele score from rs146292819, rs1800775, rs708272, rs4986970, rs1800588, rs199759119, and rs138407155 | ABCA1, CETP, LCAT, LIPC, and APOA1/↓High-density lipoprotein | CCHS, CGPS (n=47,627) | No association with T2DM | β=0.93 (0.78, 1.11) | 0.42 | Haase et al.34 | |
T2DM, type 2 diabetes mellitus; OR, odds ratio; CI, confidence interval; SD, standard deviation; LDL-C, low-density lipoprotein cholesterol; EPIC, European Prospective Investigation of Cancer; DIAGRAM, Diabetes Genetics Replication and Meta-analysis; SNP, single nucleotide polymorphism; MAGIC, Meta Analyses of Glucose and Insulin-related Traits Consortium; LIFE, Leipzig Research Centre for Civilization Diseases; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance.
PERIPHERAL LIPID ACCUMULATION CAUSED BY GENETIC VARIANTS AND ITS CONSEQUENCES ON INSULIN RESISTANCE
1. Obesity
Among the risk factors of T2DM, higher body mass index (BMI), a surrogate measurement of adiposity, has been well-described in several large-scale MR studies (Table 2).12,36,37,38 BMI is a complex phenotype influenced by various determinants, including variants at several genetic loci.1,2 Most early MR studies investigated the FTO gene to determine the role of BMI in disease risk.
Table 2. The association of tissue lipids-related genotypes with risk of insulin resistance or T2DM.
| Instrumental variables | Genes or related traits | Study subjects | Outcomes | OR (95% CI) or the effect per SD change (β or z score) | p-value | Reference | |
|---|---|---|---|---|---|---|---|
| Adiposity (visceral/subcutaneous fat and BMI) | |||||||
| rs9939609 or Corresponding alleles (r2≥0.93 in LD) | FTO | ENGAGE (n=198,502) | Fasting insulin | β=0.26 (for <55 years)/0.32 (≥55 years) | 0.001/0.002 | Fall et al.37 | |
| rs9939609 | FTO | Meta-analysis from 7 studies (n=16,639) | Fasting insulin | z=0.039 (0.013, 0.064) | 0.003 | Freathy et al.36 | |
| Fasting glucose | z=0.024 (0.001, 0.048) | 0.044 | |||||
| (z score per allele) | |||||||
| Genetic score from 14 SNPs | MAP2K5, SREBF2, COL4A3BP/HMGCR, TNN13K1, MC4R, FTO, BDNF, TFAP2B, TOMM40, MC4R, SH2B1, NTRK2, BDNF, FANCL/FLJ30838, MAP2K, SREBF2, COL4A3BP/HMGCR, TNN13K1/↑BMI | 8 cohorts including | Fasting insulin | β=8.47 (5.94, 11.06) | N.A. | Holmes et al.12 | |
| rs2229616, rs1421085, rs10767664, rs2272903, rs2075650, rs17066846, rs4788099, rs1211166, rs1401635, rs12617233 | ARIC, CHS, CARDIA, EPIC-NL, FHS, MEDAL, MESA, and WHI (n=34,538) | Fasting glucose | β=0.18 (0.12, 0.24) | ||||
| rs997295, rs5996074 | T2DM | OR, 1.27; 95% CI, 1.18–1.36 | |||||
| rs4704220, rs7753158 | |||||||
| Weighted allele score from 77 SNPs | From the MAGIC consortium (n=46,368) | Fasting insulin | β=0.07 (0.03, 0.11) | <0.0001 | Xu et al.38 | ||
| Fasting glucose | β=0.18 (0.14, 0.22) | <0.0001 | |||||
| rs9939609 | FTO | Malay ethnicity in Singapore residents (n=3,000) | Nuclear cataract | OR, 1.30; 95% CI, 1.08–1.55 | 0.004 | Lim et al.42 | |
| Hepatic fat accumulation | |||||||
| rs738409 | PNPLA3 | Liver Biopsy Cohort (n=1,515) | HOMA-IR | β=0.27 (0.07, 0.48) | 0.089 | Dongiovanni et al.43 | |
| rs58542926 | TM6SF2 | Obese Subject Study (n=3,329) | β=0.21 (0.06, 0.35) | 0.006 | |||
| rs1260326 | GCKR | ||||||
| Genetic risk score from 7 SNPs | LYPLAL1, TM6SF2, PNPLA3, GCKR, SAMM50, PARVB, COL13A1 | SPECT-China study (n=9,182) | Fasting glucose, HbA1C | No interaction with NAFLD-GRS | 0.53 | Wang et al.44 | |
| rs12137855, rs58542926, rs738409, rs780094, rs738491, rs5764455, and rs1227756 | 0.95 | ||||||
T2DM, type 2 diabetes mellitus; OR, odds ratio; CI, confidence interval; SD, standard deviation; LD, linkage disequilibrium; BMI, body mass index; ENGAGE, European Network for Genetic and Genomic Epidemiology; SNP, single nucleotide polymorphism; ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; CARDIA, Coronary Artery Risk Development in Young Adults study; EPIC-NL, European Prospective Investigation into Cancer and Nutrition–Netherlands; FHS, Framingham Heart Study; MEDAL, Multinational Etoricoxib and Diclofenac Arthritis Long-term; MESA, Multi-Ethnic Study of Atherosclerosis; WHI, Women's Health Initiative; MAGIC, Meta Analyses of Glucose and Insulin-related Traits Consortium; HOMA-IR, homeostatic model assessment for insulin resistance; HbA1C, hemoglobin A1C; NAFLD, non-alcoholic fatty liver disease; GRS, genetic risk score.
Early analysis of a single variant at the FTO locus demonstrated its causal role for higher BMI.39 In one MR study, a SNP for FTO (rs9939609) and corresponding alleles in linkage disequilibrium (r2≥0.93) were used as instrumental variables; the analysis revealed significantly increased fasting insulin levels (β=0.26 for <55 years, β=0.32 for ≥55 years) in individuals with this variant regardless of age.37 A meta-analysis using data obtained from seven studies demonstrated causal associations between higher BMI and higher fasting plasma glucose (z=0.024, p=0.003) and fasting insulin (z=0.039, p=0.044).36 Similar to studies investigating circulating lipids, many BMI-associated variants were pooled into a single genetic score and incorporated into MR analysis as an instrumental variable. This score-based method could increase the effect size of the genetic variants on the exposure and has the advantage of reducing bias caused by single genetic variants with low explanatory power.7,8 A study that used a genetic score based on 14 SNPs showed that the allele score that corresponds to increased BMI (95% CI, 0.95–1.21) was causally associated with higher fasting insulin (β=8.47; 95% CI, 5.94–11.06 per 1.0 kg/m2 BMI) and fasting glucose (β=0.18; 95% CI, 0.12–0.24).12 Results from the MAGIC study also demonstrated causal associations between a higher weighted allele score from 77 SNPs40 and higher fasting insulin (β=0.07; 95% CI, 0.03–0.11) or fasting glucose (β=0.18; 95% CI, 0.14–0.22).38
The above findings suggest a strong causal role of BMI in the development of insulin resistance, consistent with the findings reported in traditional observational studies, and indicate a relationship between adiposity and insulin resistance.12,36,41 So far, very few MR studies have investigated the relationship between FTO genotype and risk of insulin resistance for Asian populations. One MR study on Malay ethnicity in Singapore residents (n=3,000) demonstrated a causal association between rs9939609 and the incidence of nuclear cataract (OR, 1.30; 95% CI, 1.08–1.55) but did not explore the association of this SNP with glycemic control or T2DM risk.42
2. Hepatic lipid accumulation
The causality of genetic variations related to hepatic fat accumulation with insulin resistance has been explored in a few MR studies (Table 2).43,44 One performed genotyping for PNPLA3, TM6SF2, and GCKR alleles using three complementary cohorts (liver biopsy cohort, Swedish Obese Subjects, and Dallas Heart Study).43 The GRS score was then determined using these alleles, and risk loci for hepatic fat accumulation were established and subsequently used as instrumental variables in MR analysis for hepatic fat accumulation. Results of this study indicated that hepatic fat accumulation was associated with insulin resistance in participants with high risk of liver cirrhosis in the liver biopsy cohort and Swedish Obese Subjects.43 However, other MR studies from the Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China) study showed no significant association between the risk scores from seven SNPs for fatty liver and fasting glucose or with HbA1c levels.44
GENETICALLY MEDIATED FAT AND NUTRIENT INTAKE AND THEIR POTENTIAL ROLES IN INSULIN RESISTANCE
In addition to the endogenous lipid status discussed above, lipids derived from the diet (calories, carbohydrates, and fats) could act as risk factors for insulin resistance and related complications. Some GWA studies have been conducted to understand a genetic role in diet, including macronutrient intake45,46 or alcohol consumption47; however, considering that diet is an exogenous factor, it can be difficult to use as an instrumental variable in the analysis. To date, few studies have attempted to use MR analyses for identifying genetic variants that mediate nutrient intake and their corresponding effects on the risk of developing insulin resistance or diabetes (Table 3).44,48,49
Table 3. Genotypes related to diet lipids or potential modulators for diet lipids and their effects on risk of insulin resistance or T2DM.
| Instrumental variables | Genes or related traits | Study subjects | Outcomes | OR (95% CI) or the effect per SD change (β or z score) | p-value | Reference | |
|---|---|---|---|---|---|---|---|
| Source diet for body fat | |||||||
| rs10163409, rs197273, and rs838145 | FTO, TANK, IZUMO/↑Carbohydrate intake | DIAGRAM consortium (n=10,895) and MAGIC (n=46,368) | HOMA-IR | β=0.000 (±0.011) | 0.981 | Ding et al.48 | |
| rs838145 | IZUMO/↑Fat intake | DIAGRAM consortium (n=10,895) and MAGIC (n=46,368) | HOMA-IR | β=0.007 (±0.002) | 0.722 | Ding et al.48 | |
| Genetic risk score from 4 SNPs | DHCR7, CYP2R1, GC, CYP24A1/↑[25(OH)D] | SPECT-China study (n=9,182) | Fasting glucose | No change by GRS quartile | 0.17 | Wang et al.44 | |
| rs12785878, rs10741657, rs2282679, rs6013897 | |||||||
| Possible modulating factors for diet lipids | |||||||
| rs17238484-G | LDL-lowering allele | Meta-analysis from 43 genetic studies (n=223,463) | T2DM | β=1.02 (1.00, 1.05) | N.A. | Swerdlow et al.21 | |
| rs12916-T | β=1.06 (1.03, 1.09) | ||||||
| Other nutrient factors affecting diet lipids absorption or metabolism | |||||||
| rs602662-A | ↑Serum concentration of Vitamin B12 | MAGIC (n=45,576) | Fasting glucose | β=0.032 (±0.017) | 0.06 | Moen et al.49 | |
| HOMA-beta | β=−0.033 (±0.011) | 1.81×10−3 | |||||
T2DM, type 2 diabetes mellitus; OR, odds ratio; CI, confidence interval; SD, standard deviation; LD, linkage disequilibrium; DIAGRAM, Diabetes Genetics Replication and Meta-analysis; MAGIC, Meta Analyses of Glucose and Insulin-related Traits Consortium; HOMA-IR, homeostatic model assessment for insulin resistance; SNP, single nucleotide polymorphism; SPECT-China, Survey on Prevalence in East China for Metabolic Diseases and Risk Factors; GRS, genetic risk score; LDL, low-density lipoprotein.
A recent study reported multiple SNPs that are strongly associated with nutrient intake and conducted MR analysis using those SNPs as instrumental variables.48 The study utilized data from the DIAGRAM (n=10,895) and MAGIC consortia (n=46,368) and found no significant association between SNPs related to increased carbohydrate intake (rs10163409, rs197273, and rs838145) and HOMA-IR or T2DM risk. In addition, genetically mediated increase in fat intake (rs838145) showed no significant effects on HOMA-IR. However, increased protein intake was causally associated with increased risk of insulin resistance (β=0.067, p=0.046) and T2DM incidence (β=0.806, p=0.002). In an MR study on Chinese participants (n=9,182), the genetic risk score for levels of 25-hydroxyvitamin D, a fat soluble vitamin, was not associated with fasting blood glucose or hemoglobin glycation levels.44
Although the study did not directly evaluate the effects of lipid intake on the risk of insulin resistance, results from other MR studies suggest the possibility of diet modification through genetic variation.21,49 One of the MR studies that analyzed data from the MAGIC consortium49 reported that elevated serum concentrations of vitamin B12 based on the presence of genotype variant (FUT2) were inversely associated with HOMA-B (β=−0.033, p=1.81×10−3) without affecting circulating lipid levels or body fat accumulation. As noted above, a genetic variant for HMGCR, the target gene for statin, increased the risk of higher plasma insulin and fasting glucose levels in randomized CVD prevention trials.21 These results indicate that genotypes targeted by the intake of foods or nutrients with LDL-lowering properties or those that affect body fat warrant future MR studies.
LIMITATIONS OF MR STUDIES
Many MR studies show discrepancies between the observational analysis and the secondary instrumental analysis. In particular, circulating lipids show positive association with the indicators of insulin resistance, but this association was not detected in the analysis with instrumental variables.17,20,34 Such discrepancies could be partially attributed to bias from residual pleiotropic effects in the MR study, in which a genetic variant exerts effects beyond its specific effects on the biomarker of interest. In addition, it is possible that associations between lipid genotypes and indicators of insulin resistance were biased. First, carrying a larger number of LDL-C-raising alleles28 will increase the likelihood of an individual requiring medical therapy such as statins, which will reduce the effect of LDL-C. As a result, the effects of LDL-C-related SNPs on the outcomes of insulin resistance could be underestimated. Second, the effects of a SNP on patient outcomes (e.g., fasting glucose, fasting insulin, and T2DM incidence) can be diluted by the variability of biomarkers, which may only exert a small effect on an individual SNP and any risk factor acting in concert with multiple other factors; as a result, only a fraction of the inherited element could contribute to the disease. Investigating individual SNPs can limit the likelihood for unexpected pleiotropic effects. In particular, a study by Fall et al.19 showed that using more LDL-C-specific SNPs weakened the association between LDL-C levels and indicators of insulin resistance, suggesting that other SNPs can exert unknown pleiotropic effects. However, because of limited information on the function of SNPs, direct selection of the genes that play crucial roles in lipid metabolism is not practical. In addition, study populations should be sufficiently large to facilitate the detection of small effects to circumvent the dilution effect.
There are extremely limited data from Asian populations, including Koreans, in recent MR studies investigating T2DM and CVD. One Chinese study and one Japanese study were the only sources that could be referred to in this review. In most MR studies, a genetic variant explains only a small proportion of the total variance (usually less than 10%) in the biomarker levels. Therefore, if an SNP that could serve as a proxy for a certain type of lipids and a variant is highly ethnicity-specific (e.g., ADH1B), this will limit cross-comparisons of the same genetic variable across different ethnicities. Results should be interpreted with caution to estimate disease susceptibility and treatment response for Koreans or Asians. This current review also cites some studies that investigated the genetic variants related to nutrient intake that can modify lipid metabolism. However, genetic variants related to diet intake exert their effects indirectly and are highly pleiotropic, so that inference of the causal effect and key assumptions of MR may be biased. Considering that very limited data are available for the discovery of diet-related genetic variants, the association between diet-related genetic variants and insulin resistance in MR studies are challenging, and genome-wide and sequencing-based studies should be continued.
CONCLUSION
Identification of genetic variants of lipid-associated genes has provided useful information for stratification of disease risk as well as prediction of disease. Moreover, investigations into the genetic variants for metabolic diseases help to find novel therapeutic targets (e.g., PCSK9 and ApoB) and the development of modulatory factors for those targets. Hence many GWA studies have identified a number of loci as potential therapeutic targets for T2DM, and MR studies could provide validation of these loci. MR studies have revealed a strong association between lipid metabolism and insulin resistance and provided insights into the underlying disease mechanisms. MR studies also have confirmed the causal role of genetically altered lipid metabolism, particularly adiposity (e.g., FTO), in the loss of glycemic control and could support the utility of preventive interventions for public health. However, the relationship between circulating lipid levels and insulin resistance is not conclusive based on recent MR studies. Non-communicable diseases, such as T2DM and CVD, involve multiple genetic variants, and the complex nature of the biomarkers for these diseases raises methodological and practical challenges in conducting MR studies. The causal association between lipid levels and insulin resistance using MR should be interpreted with caution because of several limitations, including pleiotropic effects, lack of information on genotypes, and ethnic differences. Nevertheless, considering that metabolomics and proteomic technologies are more widely applied in population studies, MR will help advance knowledge on metabolic diseases and the development of suitable treatment strategies, including diet or behavioral modifications.
Footnotes
Funding: None.
Conflict of Interest: The author has no conflicts of interest to declare.
References
- 1.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hingorani A, Humphries S. Nature's randomised trials. Lancet. 2005;366:1906–1908. doi: 10.1016/S0140-6736(05)67767-7. [DOI] [PubMed] [Google Scholar]
- 3.Rainwater DL, MacCluer JW, Stern MP, VandeBerg JL, Haffner SM. Effects of NIDDM on lipoprotein(a) concentration and apolipoprotein(a) size. Diabetes. 1994;43:942–946. doi: 10.2337/diab.43.7.942. [DOI] [PubMed] [Google Scholar]
- 4.Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010;56:1252–1260. doi: 10.1373/clinchem.2010.146779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koschinsky ML, Marcovina SM. The relationship between lipoprotein(a) and the complications of diabetes mellitus. Acta Diabetol. 2003;40:65–76. doi: 10.1007/s005920300007. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow DI, Sattar N. Blood lipids and type 2 diabetes risk: can genetics help untangle the web? Diabetes. 2015;64:2344–2345. doi: 10.2337/db15-0458. [DOI] [PubMed] [Google Scholar]
- 7.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251–260. doi: 10.1093/aje/kwu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol. 2013;42:1134–1144. doi: 10.1093/ije/dyt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahim S, Davey Smith G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet. 2008;123:15–33. doi: 10.1007/s00439-007-0448-6. [DOI] [PubMed] [Google Scholar]
- 11.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102:1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 12.Holmes MV, Lange LA, Palmer T, Lanktree MB, North KE, Almoguera B, et al. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet. 2014;94:198–208. doi: 10.1016/j.ajhg.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig WY, Neveux LM, Palomaki GE, Cleveland MM, Haddow JE. Lipoprotein(a) as a risk factor for ischemic heart disease: metaanalysis of prospective studies. Clin Chem. 1998;44:2301–2306. [PubMed] [Google Scholar]
- 14.Burgess S, Timpson NJ, Ebrahim S, Davey Smith G. Mendelian randomization: where are we now and where are we going? Int J Epidemiol. 2015;44:379–388. doi: 10.1093/ije/dyv108. [DOI] [PubMed] [Google Scholar]
- 15.Dubé JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr Opin Lipidol. 2012;23:133–140. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 16.Kamstrup PR, Nordestgaard BG. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2013;1:220–227. doi: 10.1016/S2213-8587(13)70064-0. [DOI] [PubMed] [Google Scholar]
- 17.Ye Z, Haycock PC, Gurdasani D, Pomilla C, Boekholdt SM, Tsimikas S, et al. The association between circulating lipoprotein(a) and type 2 diabetes: is it causal? Diabetes. 2014;63:332–342. doi: 10.2337/db13-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fall T, Xie W, Poon W, Yaghootkar H, Mägi R, Knowles JW, et al. Using genetic variants to assess the relationship between circulating lipids and type 2 diabetes. Diabetes. 2015;64:2676–2684. doi: 10.2337/db14-1710. [DOI] [PubMed] [Google Scholar]
- 20.Buchmann N, Scholz M, Lill CM, Burkhardt R, Eckardt R, Norman K, et al. Association between lipoprotein(a) level and type 2 diabetes: no evidence for a causal role of lipoprotein(a) and insulin. Acta Diabetol. 2017;54:1031–1038. doi: 10.1007/s00592-017-1036-4. [DOI] [PubMed] [Google Scholar]
- 21.Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–361. doi: 10.1016/S0140-6736(14)61183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst-Hunter Z, Lyall DM, et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2017;5:97–105. doi: 10.1016/S2213-8587(16)30396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage DB, Semple RK. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr Opin Lipidol. 2010;21:329–336. doi: 10.1097/MOL.0b013e32833b7782. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR, et al. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial--Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982–988. doi: 10.2337/dc07-1768. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, et al. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 26.Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 28.De Silva NM, Freathy RM, Palmer TM, Donnelly LA, Luan J, Gaunt T, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes. 2011;60:1008–1018. doi: 10.2337/db10-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8:237–245. doi: 10.1038/nrendo.2011.235. [DOI] [PubMed] [Google Scholar]
- 30.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 31.Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 32.Fryirs MA, Barter PJ, Appavoo M, Tuch BE, Tabet F, Heather AK, et al. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler Thromb Vasc Biol. 2010;30:1642–1648. doi: 10.1161/ATVBAHA.110.207373. [DOI] [PubMed] [Google Scholar]
- 33.Moriyama K, Negami M, Takahashi E. HDL2-cholesterol/HDL3-cholesterol ratio was associated with insulin resistance, high-molecular-weight adiponectin, and components for metabolic syndrome in Japanese. Diabetes Res Clin Pract. 2014;106:360–365. doi: 10.1016/j.diabres.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Haase CL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. HDL Cholesterol and risk of type 2 diabetes: a Mendelian randomization study. Diabetes. 2015;64:3328–3333. doi: 10.2337/db14-1603. [DOI] [PubMed] [Google Scholar]
- 35.Tabara Y, Arai H, Hirao Y, Takahashi Y, Setoh K, Kawaguchi T, et al. Different inverse association of large high-density lipoprotein subclasses with exacerbation of insulin resistance and incidence of type 2 diabetes: the Nagahama study. Diabetes Res Clin Pract. 2017;127:123–131. doi: 10.1016/j.diabres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Freathy RM, Timpson NJ, Lawlor DA, Pouta A, Ben-Shlomo Y, Ruokonen A, et al. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes. 2008;57:1419–1426. doi: 10.2337/db07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fall T, Hägg S, Ploner A, Mägi R, Fischer K, Draisma HH, et al. Age- and sex-specific causal effects of adiposity on cardiovascular risk factors. Diabetes. 2015;64:1841–1852. doi: 10.2337/db14-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L, Borges MC, Hemani G, Lawlor DA. The role of glycaemic and lipid risk factors in mediating the effect of BMI on coronary heart disease: a two-step, two-sample Mendelian randomisation study. Diabetologia. 2017;60:2210–2220. doi: 10.1007/s00125-017-4396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, Chasman DI, et al. FTO genotype is associated with phenotypic variability of body mass index. Nature. 2012;490:267–272. doi: 10.1038/nature11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fall T, Hägg S, Mägi R, Ploner A, Fischer K, Horikoshi M, et al. The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med. 2013;10:e1001474. doi: 10.1371/journal.pmed.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim LS, Tai ES, Aung T, Tay WT, Saw SM, Seielstad M, et al. Relation of age-related cataract with obesity and obesity genes in an Asian population. Am J Epidemiol. 2009;169:1267–1274. doi: 10.1093/aje/kwp045. [DOI] [PubMed] [Google Scholar]
- 43.Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283:356–370. doi: 10.1111/joim.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang N, Chen C, Zhao L, Chen Y, Han B, Xia F, et al. Vitamin D and nonalcoholic fatty liver disease: bi-directional Mendelian randomization analysis. EBioMedicine. 2018;28:187–193. doi: 10.1016/j.ebiom.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr. 2013;97:1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet. 2013;22:1895–1902. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding R, Huang T, Han J. Diet/lifestyle and risk of diabetes and glycemic traits: a Mendelian randomization study. Lipids Health Dis. 2018;17:18. doi: 10.1186/s12944-018-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moen GH, Qvigstad E, Birkeland KI, Evans DM, Sommer C. Are serum concentrations of vitamin B-12 causally related to cardiometabolic risk factors and disease? A Mendelian randomization study. Am J Clin Nutr. 2018;108:398–404. doi: 10.1093/ajcn/nqy101. [DOI] [PubMed] [Google Scholar]