Abstract
Wolbachia can profoundly influence the survival, reproduction, and defenses of insect hosts. These interactions could potentially be harnessed for managing pests or insect‐transmitted diseases. Diaphorina citri Kuwayama is a phloem‐feeding pest capable of transmitting the putative causal agent of citrus greening, Candidatus Liberibacter asiaticus (CLas). Like many insects, D. citri is also infected with Wolbachia (wDi). Recent studies indicate that the relative abundance of wDi could be associated with the abundance of CLas, and that wDi may contribute to regulating expression of phage lytic cycle genes in CLas, suggesting the need for better understanding of wDi biology in general. This study investigated the genetic diversity of wDi among D. citri in populations spanning eleven countries and two U.S. territories. Six Wolbachia genes, wsp, coxA, fbpA, ftsZ, gatB, and hcpA, were sequenced and compared across samples. Two prevalent wDi strains were identified across the samples, and screening of clone libraries revealed possible coinfection of wDi strains in specific populations. D. citri mitochondrial cytochrome oxidase subunit I gene (mtCOI) were more divergent between D. citri populations that were infected with different wDi strains or had different infection statuses (single infection vs. coinfection). While we could not eliminate the possibility that maternal transmission may contribute to such patterns, it is also possible that wDi may induce cytoplasmic incompatibility in their host. These findings should contribute to the understanding of wDi population ecology, which may facilitate manipulation of this endosymbiont for management of citrus greening disease worldwide.
Keywords: bacteria, genetic diversity, Huanglongbing, plant disease, superinfection, vertically transmitted endosymbionts
Introduction
Bacterial endosymbionts of insects have a diverse and profound influence on host biology (Moran & Telang, 1998; Werren et al., 2008; Engelstädter & Hurst, 2009; Feldhaar, 2011). The effects these bacteria have on their host include nutrient provision or recycling (Douglas, 1998; Sabree et al., 2009; Hosokawa et al., 2010), protecting the host against infective agents or natural enemies (Oliver et al., 2002; Scarborough et al., 2005; Kambris et al., 2009; Moreira et al., 2009), manipulation of host reproduction or sexuality (Rousset et al., 1992a,b; Hurst et al., 1999; Engelstädter & Hurst, 2009), and stress tolerance (Montllor et al., 2002; Feldhaar, 2011). These effects may be exploited to achieve applicative purposes, such as controlling pest population sizes or interfering with transmission of insect‐borne diseases (Brelsfoard et al., 2009; Hancock et al., 2011; Iturbe‐Ormaetxe et al., 2011).
Diaphorina citri Kuwayama (Hemiptera: Liviidae) is a phloem‐feeding pest of citrus. This hemipteran is considered one of the most economically important citrus pests worldwide due to its capability to transmit the putative bacterial causal agent of citrus greening, Candidatus Liberibacter asiaticus (CLas) (Halbert & Manjunath, 2004). CLas is acquired by D. citri during feeding. The bacterial cells can enter the digestive tract, invade the hemolymph and salivary gland, and are then transmitted to a healthy plant, thereby completing a propagative, persistent transmission process (Hogenhout et al., 2008; Hall et al., 2013). D. citri was initially identified in Taiwan, China in 1908 (Kuwayama, 1908), and is found throughout most citrus growing regions of Asia and the Americas. The first description of D. citri in the United States occurred in Palm Beach County Florida in 1998. Analyses of the cytochrome oxidase gene indicates that D. citri in Florida originated from southwestern Asia (Boykin et al., 2012). Current control strategies, including tree removal, and insecticide and antimicrobial applications have limited or variable efficacy (Michaud, 2004; Qureshi et al., 2009; Tiwari et al., 2011; Hall et al., 2013; Stansly et al., 2014). These facts and the continued spread of citrus greening suggest the need for additional methods to supplement existing integrated pest management strategies.
In recent years, mounting evidence suggests that insect endosymbionts could hold keys to new approaches for managing insect‐transmitted diseases. Of particular interest are several strains of the alpha‐proteobacteria Wolbachia, which provide their host protection against different types of infective agents and potentially reduce the transmission of insect‐borne pathogens/parasites under controlled conditions (Kambris et al., 2009; Moreira et al., 2009; Hancock et al., 2011). Moreover, in some insects, Wolbachia induce cytoplasmic incompatibility (CI), a phenomenon where mating between same‐species individuals with different Wolbachia strains or infection status fails to produce viable offspring (Yen & Barr, 1973; Engelstadter & Telschow, 2009); these CI‐inducing strains may also facilitate disease management by manipulating vector populations (Sinkins & Gould, 2006; Bourtzis, 2008; Brelsfoard et al., 2009). Like many insect species, D. citri is also infected with Wolbachia (designated wDi) (Subandiyah et al., 2000; Guidolin & Consoli, 2013; Dossi et al., 2014; Hoffmann et al., 2014). Although the direct influence of wDi on D. citri biology remains to be determined, recent studies indicated that the relative abundance of wDi may be associated with the abundance of CLas within hosts (Fagen et al., 2012), and that wDi may contribute to the regulation of phage lytic cycle genes in CLas (Jain et al., 2017). These findings highlight the potential importance of wDi in the citrus greening disease system, and call for better characterization of wDi biology in general.
A fundamental step for understanding endosymbionts like wDi is to determine the distribution and diversity of the endosymbiont across different host populations. In this regard, several studies have investigated the genetic diversity of wDi across different populations (Saha et al., 2012; Guidolin & Consoli, 2013; Lashkari et al., 2014). Lashkari et al. (2014) found that the wDi wsp sequence was associated with host genetic diversity and hypothesized that wDi may induce CI in their host (Lashkari et al., 2014). In general, previous studies were based on sequences of only one or two wDi genes (wsp and ftsZ) (Saha et al., 2012; Lashkari et al., 2014), or restricted to populations in specific regions of the world (Guidolin & Consoli, 2013). Considering D. citri’s impact on citrus production worldwide and the mosaic nature of some Wolbachia genes, including wsp (Baldo et al., 2005), a broader and more robust assessment of wDi genetic diversity and its association with D. citri is necessary towards understanding the global diversity of D. citri populations.
In this study, we investigated genetic variation of wDi among 24 D. citri populations located across multiple countries. Six Wolbachia genes, including the surface protein gene (wsp) and genes used in the multilocus sequence typing (MLST) procedure (Baldo et al., 2006), coxA, fbpA, ftsZ, gatB, and hcpA, were sequenced for every sample to allow sensitive and robust analyses of wDi diversity. The MLST procedure recognizes different sequences of the same gene as separate alleles and unambiguously characterizes each bacterial strain based on the allele profiles of the genes analyzed (Baldo et al., 2006). Sequencing of wDi genes and subsequent clone library screening allowed identification of prevalent wDi profiles and the possible occurrence of Wolbachia coinfection among the D. citri populations tested. We also tested whether there is an association between D. citri and wDi’s genetic diversity by sequencing a mitochondrial cytochrome oxidase subunit I gene (mtCOI) fragment of D. citri. Obtaining the wDi and mtCOI from the exact same samples (instead of using data from other sources/individuals) helped assure that the associations between wDi and D. citri sequences drawn from the data were reliable. Results from this work could improve our understanding of wDi and D. citri ecology.
Materials and methods
Diaphorina citri DNA samples
Adult D. citri were sampled from Homestead and Fort Pierce, FL. Insects were also collected from two laboratory colonies; one derived from a Weslaco, TX population and the other from a (Oahu, HI) population (Table 1). Samples were collected from commercial and unmanaged citrus groves during 2015–2016. Whole‐body genomic DNA was extracted from individual insects using the DNeasy Blood & Tissue kit (Qiagen, Inc. Valencia, CA, USA) following the manufacturer's instructions. In addition, D. citri DNA was obtained from specimens stored at the United States Department of Agriculture, Animal and Plant Health Inspection Service, Center for Plant Health Science and Technology, Mission Laboratory; these included DNA isolates of D. citri collected from eleven countries and two U.S. territories during 2015 and 2016 (Table 1). DNA samples from a previous study of Florida D. citri populations (Chu et al., 2016) were also included in the analyses. Overall, 61 samples collected from 24 populations were used in the analyses.
Table 1.
Details of Diaphorina citri samples used in this study
| Sampling location | Number of samples | Source of sample |
|---|---|---|
| USA, FL, Clermont | 4 | Chu et al., 2016 |
| USA, FL, Lake Alfred | 5 | Chu et al., 2016 |
| USA, FL, LaBelle | 5 | Chu et al., 2016 |
| USA, FL, Fort Pierce | 6 | This work (2016) |
| USA, FL, Homestead | 6 | This work (2016) |
| USA, HI, Oahu | 1 | Laboratory colony (2016) |
| USA, TX, Weslaco | 1 | Laboratory colony (2016) |
| USA, TX, Edinburg | 1 | The Mission Laboratory (2016) |
| American Samoa, Tafuna | 1 | The Mission Laboratory (2015) |
| Puerto Rico, Santa Isabel | 2 | The Mission Laboratory (2015) |
| Mexico, Tamaulipas, Cuidad Victoria | 2 | The Mission Laboratory (2015) |
| Mexico, Veracruz, Cazones | 2 | The Mission Laboratory (2015) |
| Trinidad and Tobago, Trinidad | 2 | The Mission Laboratory (2015) |
| Barbados, Golden Grove | 3 | The Mission Laboratory (2015) |
| Belize, Stann Creek | 3 | The Mission Laboratory (2015) |
| Colombia, Tolima, Armero‐Guayabal | 2 | The Mission Laboratory (2015) |
| Pakistan, Punjab, Multan | 1 | The Mission Laboratory(2015) |
| Pakistan, Punjab, Lalian | 2 | The Mission Laboratory (2015) |
| Thailand, Nakhon Si Thammarat, Meuang Nakhon Si Thammarat | 2 | The Mission Laboratory (2015) |
| China, Yunnan, Ruili | 1 | The Mission Laboratory (2015) |
| China, Fujian, Fuzhou | 3 | The Mission Laboratory (2015) |
| Singapore, Chinese Garden | 2 | The Mission Laboratory (2015) |
| Argentina, Salta, Yuchán, | 2 | The Mission Laboratory (2015) |
| Argentina, Jujuy, Fraile Pintado | 2 | The Mission Laboratory (2015) |
Amplification and sequencing of Wolbachia and D. citri mitochondrial genes
Fragments of seven genes were sequenced for all of the DNA isolates. These include D. citri mtCOI and six Wolbachia genes (wsp, coxA, fbpA, ftsZ, gatB, and hcpA). Amplifications of the Wolbachia genes and the D. citri mtCOI gene were conducted using primer pairs and PCR conditions described in previous studies (Baldo et al., 2006; Boykin et al., 2012) with slight modification (Table 2). GoTaq Colorless Master Mix (Promega Inc., Madison, WI, USA) was used for these assays. The final concentrations of the MLST and wsp primer pairs were 1 μmmol/L and the final volume was 40 μL for all of the reactions. The PCR products were purified using ExoSAP‐IT (USP Corp., Cleveland, OH, USA) and sequenced (from both forward and reverse ends) at the Interdisciplinary Center for Biotechnology Research at the University of Florida (ICBR).
Table 2.
Details of primers used in this study
| Target gene | Primer sequences (5′→3′) | Amplicon size | Annealing temperature | Reference |
|---|---|---|---|---|
| gatB |
|
471 bp | 54 °C | Baldo et al., 2006 |
| coxA |
|
487 bp | 54 °C | Baldo et al., 2006 |
| hcpA |
|
515 bp | 54 °C | Baldo et al., 2006 |
| ftsZ |
|
524 bp | 52 °C | Baldo et al., 2006 |
| fbpA |
|
509 bp | 58 °C | Baldo et al., 2006 |
| wsp |
|
603 bp | 58 °C | Baldo et al., 2006 |
| D. citri’s mtCOI |
|
821 bp | 53 °C | Boykin et al., 2012 |
Data processing and analyses
All Sanger sequencing data were analyzed using Geneious 8.1.6 (Kearse et al., 2012). For each gene, sequence chromatograms were screened for errors or artifacts affecting sequence calls. Forward and reverse sequences were then assembled and the resulting sequences were compared among the samples in Geneious using MUSCLE (8 iterations) (Edgar, 2004). For wDi genes, unique sequences were searched against the Wolbachia MLST database (http://www.pubmlst.org/wolbachia/) (Baldo et al., 2006). The sequencing chromatograms of some amplicons in some samples exhibited dual peaks at several sequence positions. Dual chromatogram peaks at individual sequence positions were consistent among sequencing reactions with multiple alleles among the amplicon pool. As this is suggestive of cooccurrence of more than one wDi profiles within the same D. citri individual, forward and reverse chromatograms were compared to evaluate the consistency of these patterns. Individuals with strong evidence of coinfection in both forward and reverse chromatograms were grouped into “coinfection groups.” That is, “coinfection groups” were composed of those individuals that exhibited dual peaks of the same nucleotides at the same sequence locations for the same genes. PCR amplicons from genes of representative individuals of different coinfection groups were cloned and libraries were sequenced to verify coinfection and identify haplotypes.
Construction and sequencing of clone libraries
To determine whether the coinfection patterns observed within some samples (described later in detail) were due to the infection of more than one wDi strains, representative DNA samples exhibiting the observed patterns were cloned and sequenced for each “coinfection group.” For each locus that exhibited strong signs of coinfection, two replicate libraries derived from independent PCR reactions of the same representative DNA sample were screened; between 42 and 47 clones were sequenced per library. A total of eight libraries (357 clones) were included in the analyses. Briefly, PCR reactions were conducted using the FideliTaq PCR Master Mix (with proofreading; Affymetrix, Santa Clara, CA, USA) and the PCR conditions/protocols described above. The PCR products were purified with the QIAquick PCR Purification Kit (Qiagen, Inc.) and cloned into the pGEM‐T Easy Vector (Promega, Inc.). Transformed clones (Escherichia coli JM109) carrying plasmids with the inserts were submitted for Sanger sequencing at ICBR. To differentiate putative wDi alleles from random errors introduced during PCR reactions, sequences that were present in two independent libraries (prepared using independent PCR reactions) and explained the original coinfection pattern were identified. These sequences represent alleles that were sufficiently abundant to be repeatedly detected within the sequencing depth used in this work, which should also be free of random PCR or sequencing errors.
Bayesian analysis of D. citri mtCOI sequences
Assembly of mtCOI sequences was conducted using Geneious; low‐quality ends were trimmed (Error Probability Limit = 0.05). The sequences were then curated and aligned. A 752 bp fragment available for all 61 samples was then compared among each other. Unique mtCOI sequences were subjected to Bayesian analyses (Huelsenbeck & Ronquist, 2001), using a Cacopsylla coccinea mtCOI sequence (Que et al., 2016) as an outgroup. Model selection and data analyses were conducted using MEGA 7 (Kumar et al., 2016) and the MrBayes plugin for Geneious, respectively. The available model that had the lowest Bayesian Information Criterion (BIC) score was selected (HYK85) (Hasegawa et al., 1985). The Markov chain Monte Carlo settings were: four heated chains, heated chain temperature = 0.2, chain length = 1 100 000, subsampling frequency = 200, burn‐in length = 100 000. The priors were set as the default parameters. The average standard deviation of split frequencies was below 0.01 at the end of the analysis.
Results
Identification of two prevalent wDi profiles
Wolbachia was detected in all 61 DNA samples tested. Compilation of the sequencing data revealed that in 20 of the 24 populations surveyed, all individuals exhibited one of two wDi profiles. Psyllids with each profile appear to be infected by a single dominant wDi strain. One wDi profile includes all five MLST alleles completely matching ST‐173 in the MLST database (the database assigns different strains with different strain numbers); this profile was detected in all individuals sampled from China, Singapore, and Argentina. A different profile was detected in all samples originated from the United States (Florida, Hawaii, Texas), American Samoa, Belize, Mexico, Pakistan, and Colombia; the alleles of this profile did not completely match to any known profiles in the MLST database, but were identical to sequences of a published Wolbachia genomic assembly obtained from Florida‐reared D. citri (Saha et al., 2012); this profile is hereafter referred to as ST‐FL (Table 3; Fig. 1). Overall, only the Thailand, Trinidad, Barbados, and Puerto Rico populations had samples exhibiting wDi profiles different from ST‐173 and ST‐FL.
Table 3.
wDi allelic profiles identified in this study
| Wolbachia genes | ||||||||
|---|---|---|---|---|---|---|---|---|
| wDi profile | gatB | coxA | hcpA | ftsZ | fbpA | wsp | Location | Number of samples |
| ST‐173 | 109† | 86 | 29 | 81 | 27 | 160 | China, Singapore, Argentina | 10 |
| Co‐1 | 109/106/Co‐1a ‡ | 86 | 29 | 81/7/208 | 27 | 160 | Thailand | 2 |
| ST‐FL | 246 | 11 | 101 | 209 | 4 | 308 | USA, Mexico, Belize, American Samoa, Pakistan, Colombia | 42 |
| Co‐2 | 246 | 11 | 101/106/Co‐2a ‡ | 209/208 | 4 | 308 | Puerto Rico, Trinidad, Barbados | 7 |
†For sequences matching (100%) the alleles in the Multilocus Sequence Typing (MLST) database, the allele identifiers (IDs/numbers) are shown.
‡Alleles detected via clone library analyses are italicized. Among them, Co‐1a and Co‐2a did not have exact matches in the MLST database.
Figure 1.
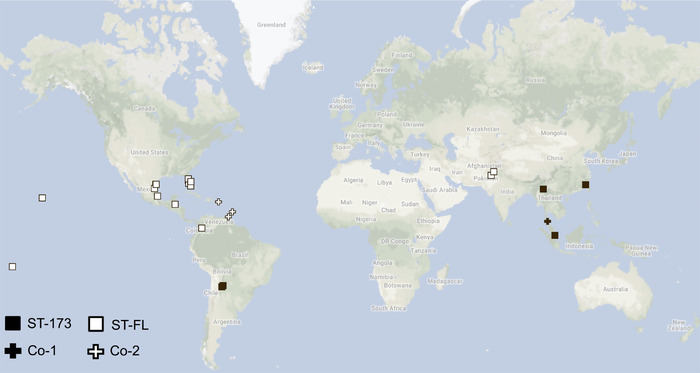
Distribution of Diaphorina citri populations with different Wolbachia (wDi) infection profiles. Symbols representing each infection profile are illustrated on the lower‐left. Created using Google Maps.
While the MLST method examines genetic diversity by looking at unique allelic profiles, comparing allele sequences from this work with those of previous studies could help understand how ST‐173 and ST‐FL fit within the known Wolbachia diversity. In an earlier work (Saha et al., 2012), phylogenetic analyses were conducted using Wolbachia sequences of various insect species and D. citri populations from Florida, USA and China. There, it was shown that all wDi tested belonged to Wolbachia supergroup B, and that based on the wsp sequences, the Floridian wDi isolate belonged to a sub‐clade of supergroup B, which is distinct from Chinese isolates (samples from Beihai, Liuzhou, Fuzhou, and Shenzhen, China). Analysis of the wsp sequences from the present work indicated that sequences of ST‐173 and ST‐FL matched 100% to sequences of D. citri from Beihai, China (Accession number: GQ385974.1) and Florida, USA (Genome assembly accession number: PRJNA29451), respectively.
Coinfection patterns detected in specific D. citri populations
Variability in the gatB and ftsZ sequences was detected between the two Thailand samples tested. One sample's gatB and ftsZ sequences matched completely to the alleles of ST‐173, while those of the other do not. Further inspection of their chromatograms revealed that such inconsistencies were due to additional “peaks” (i.e., signals of florescence from alternative nucleotides) present at several nucleotide sites, which resulted in different sequence calls. These peaks were found in both Thailand samples at the same nucleotide sites. Similar intrapopulation variations in the wDi profile were also detected in sequencing data of D. citri originated from the West Indies (Trinidad, Barbados, and Puerto Rico); the ftsZ sequences varied within the Puerto Rican and Trinidadian populations, while variation in the hcpA sequence was only detected within the Puerto Rican population. A comparison of the ftsZ and hcpA chromatograms from these samples and the Barbados samples revealed that the presence of additional peaks in several nucleotide sites led to inconsistent or different sequence calls; these peaks, albeit differed in their relative sizes, were detectable in the ftsZ and hcpA chromatograms of all samples collected from the West Indies.
An example illustrating strong “coinfection patterns” described above is shown in Figure 2. The substantial sizes of the additional peaks and their presence in multiple samples/populations indicated that they are not merely artifacts of PCR or experimental errors. Based on these patterns, the wDi profiles of samples collected from the Thailand and West Indies were sorted into two coinfection profiles/groups, designated as “Co‐1” and “Co‐2,” respectively (Fig. 1).
Figure 2.
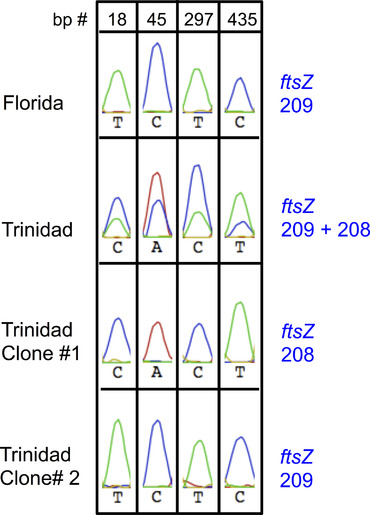
Examples of sequencing chromatograms illustrating strong Wolbachia “coinfection patterns” detected in this study and how clone library sequencing was used to validate these observations. The origins of the samples and the corresponding allele numbers (based on the multilocus sequence typing [MLST] database) are shown on the left and right, respectively. Strong coinfection patterns can be detected at various nucleotides of specific gene sequences (in this case at bp 18, 45, 297, and 435 of a ftsZ fragment). A sample originated from Lake Alfred, Florida only carries allele 209, while a coinfected sample collected from Trinidad contains more than one allele. Screening of clone libraries constructed from the Trinidad sample identified clones carrying different alleles (alleles 208 and 209; lower two panels) and explained the patterns seen in the original chromatogram. The peaks shown are parts of the actual chromatograms of the sequencing data (in the Geneious software). Peaks with different colors represent different nucleotides. Red: A; Green: T; Blue: C.
Notably, although presence of additional peak signals in Co‐1 and Co‐2 samples resulted in sequence calls different from those of the ST‐173 and ST‐FL alleles, signals matching the ST‐173 or ST‐FL sequences were detected in the Co‐1 and Co‐2 samples, respectively; these observations were further validated using clone library screening (described later in detail). To determine whether the additional peaks in Co‐1 and Co‐2 chromatograms (peaks different from those of ST‐173 or ST‐FL alleles) were unique to each coinfection group, signals identified in Co‐1 (gatB and ftsZ) and Co‐2 (ftsZ and hcpA) samples were manually searched against ST‐173 and ST‐FL individuals/chromatograms, respectively. The additional peaks visible in the West Indies samples were not found in ST‐FL populations (Fig. S1). There were minor signals in some ST‐173 samples, particularly those collected from China and Singapore, that resembled the additional peaks found in the gatB and ftsZ data of Co‐1 samples. Nevertheless, the relative abundance of such signals was weaker than those found in the Co‐1 samples and in no cases did they result in sequence calls different from those of the ST‐173 profile. Thus, although discrepancy between Co‐1 and ST‐173 populations should be interpreted with caution, this study grouped them as different wDi profiles.
Sequencing and analyses of clone libraries
Using representative DNA samples of the Co‐1 and Co‐2 groups, we constructed and screened clone libraries of genes whose chromatogram exhibited strong patterns of coinfection (one representative DNA sample for each “gene × group”). For the Co‐1 group, libraries of ftsZ and gatB were screened (using a Thailand sample); for Co‐2, we analyzed libraries of ftsZ and hcpA (using a Trinidad sample and a Barbados sample, respectively). An example illustrating how clone screening was used to validate strong coinfection patterns detected in the sequencing data is shown in Figure 2. When aligning clone sequences to the original chromatographs of their respective D. citri DNA sample, the combinations of sequences from different alleles (of the same gene) reflected the coinfection patterns observed in the original data. Data from the clone library analyses showed that Co‐1 and Co‐2 samples indeed harbor more than one wDi strains; Co‐1 and Co‐2 populations not only have alleles completely matching the ST‐173 and ST‐FL profiles, respectively, but also carry additional alleles not found in the two prevalent wDi strains (Table 3). Most of the additional alleles have exact matches in the MLST database; the sequences of two alleles that did not have matches in the MLST and GenBank databases have been deposited into GenBank (accession numbers: KX990271 and KX990272). Comparisons of these sequences with other wDi alleles detected are shown in Figures S2–S4. The fact that these additional alleles do not match to any of the ST‐173 and ST‐FL alleles shows that the coinfection patterns observed did not result from cooccurrence between ST‐173 and ST‐FL samples.
Association between mtCOI sequences and wDi profiles
Collections with the ST‐173 profile all shared the same mtCOI sequence (Fig. 3). With the exception of the Pakistani and Colombian samples, D. citri carrying ST‐FL all shared an mtCOI sequence different from that of ST‐173 samples (Fig. 3). The Pakistani and Colombian samples shared another mtCOI sequence, which had one nucleotide different from that of the other ST‐FL populations (Table S1).
Figure 3.
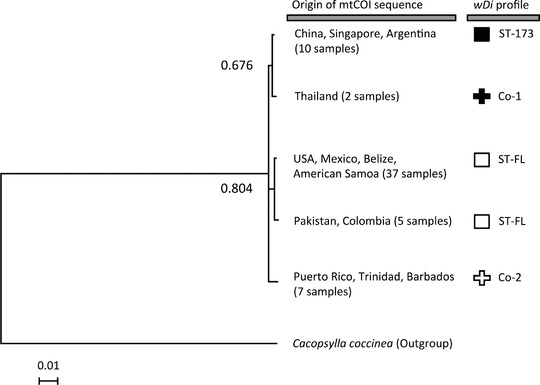
Bayesian analysis of mtCOI sequences of D. citri samples with different Wolbachia (wDi) infection profiles. The wDi profiles for the samples are shown on the right. The sequence of Cacopsylla coccinea’s mtCOI was used as an outgroup. The posterior probabilities are shown next to the branches.
The Co‐1 and Co‐2 populations’ mtCOI sequences were different from those of the ST‐173 and ST‐FL populations; the Co‐1 and Co‐2 mtCOI sequences were also different from each other (Fig. 3). Among all D. citri that carried the ST‐FL alleles (ST‐FL and Co‐1 populations), D. citri from the West Indies had the most different mtCOI sequences compared to the other D. citri (five to six nucleotide differences), while the other ST‐FL D. citri only had up to one nucleotide difference among each other (Table S1). Most of the additional alleles have exact matches in the MLST database, and their sequences are available at https://pubmlst.org; the sequences of two alleles that did not have matches in the MLST and GenBank databases were deposited into GenBank (accession numbers: KX990271 and KX990272).
Discussion
Two prevalent wDi strains, ST‐173 and ST‐FL, were identified in this study. The distribution of each wDi strain was not entirely restricted to specific geographic regions, suggesting that factors such as human transportation may have played a major role in distributing D. citri (along with their wDi strains) to various parts of the world. Overall, the distribution patterns of profiles ST‐173 and ST‐FL also corroborated findings from previous works. For example, ST‐173 was reported as the most prevalent strain infecting D. citri in many populations of Brazil (Guidolin & Consoli, 2013), an area located closely to the ST‐173‐carrying Argentinean populations studied in this work. Previously, Saha et al. (2012) showed that wDi in Floridian D. citri populations belonged to a supergroup B sub‐clade different from Chinese isolates, and suggested that D. citri in Florida did not originate from China. Here, the data also showed clear distinction between the Florida (ST‐FL) and Chinese (ST‐173) wDi allelic profiles, and that the ST‐173 and ST‐FL sequences were identical to those of the Florida, USA and a Chinese samples (from Beihai, China), respectively. Therefore, the data obtained from analysis of six wDi genes not only supported previous findings, but also provided more robust support for the global distribution, diversity, and prevalence of major wDi strains as compared with data from analysis of wsp and ftsZ alone.
Coinfection of more than one dominant wDi strains was detected in populations located in Thailand (Co‐1) and the West Indies (Co‐2). Interestingly, the Wolbachia strains that the Co‐1 and Co‐2 populations carry included ST‐173 and ST‐FL, respectively. Since Thailand is located near China and Singapore (ST‐173 areas), and the Co‐2 populations are distributed near ST‐FL populations, it is plausible that D. citri in Co‐1 and Co‐2 populations may have originated from their surrounding areas. Psyllids in the coinfected populations may have acquired additional wDi strains as they moved into their current locations, or have experienced changes in host‐microbe interactions as a result of altered environments. Arthropods are known to acquire different Wolbachia strains horizontally via brief blood/wound contacts with insects sharing same ecological niches (Rigaud & Juchault, 1995) or obtain exogenous strains during attack by predators and parasitoids (Werren et al., 1995). Environmental factors, host genetics, or infection by other microbial species may also contribute to the infection densities of Wolbachia strains (Goto et al., 2006; Mouton et al., 2006; Chu et al., 2016). It is possible that such interactions or factors may have occurred in some of the coinfected populations. Further investigation is needed to determine the sources and cause of the coinfection patterns observed in this work.
Examination of mtCOI sequences revealed geographic patterns of D. citri and wDi populations that are in agreement with those of other studies. Comparison among mtCOI genes suggests two separate introductions of D. citri into the Americas, one in North America and the other in South America (de León et al., 2011), while our data showed that the mtCOI sequences in these two areas are relatively different. The difference between mtCOI sequences from North America and Southeast Asia identified in our study was also concordant with patterns shown in other studies (Lashkari et al., 2014).
Similar to a previous study focusing on mtCOI and wDi wsp alone (Lashkari et al., 2014), the current analyses including multiple wDi genes showed that populations carrying different wDi strains have different mtCOI, and that samples with different haplotypes do not cooccur within the same population. Moreover, our data showed that the Co‐2 populations are coinfected with strains that were undetected in all of the ST‐FL populations, and that these D. citri had the most different mtCOI sequence compared to the haplotypes of ST‐FL populations (Table S1). Specifically, the geographically distant North American, Hawaiian and Belizean populations share the same ST‐FL profile, yet Co‐2 (West Indies) populations have the most divergent mtCOI sequence as compared with those from all other ST‐FL‐carrying populations (including populations located in nearby countries such as the United States, Belize, Mexico, and Colombia; Table S1). In some insect species, infection with different wDi strains in males and females could induce bidirectional CI, a phenomenon that may facilitate genetic divergence (Teschlow et al., 2002), while coinfection with additional Wolbachia strains could have an additional effect, such that crosses between coinfected and singly infected insects may result in unidirectional CI (Sinkins et al., 1995; Dobson et al., 2001). Therefore, while we could not rule out the possibility that maternal transmission may contribute to patterns detected in this work, it is also possible that infection with different wDi strains or the difference in infection status may have induced CI and facilitated genetic divergence (Sinkins et al., 1995; Dobson et al., 2001; Telschow et al., 2002). And thus, although difficulties in obtaining larger sample numbers, DNA quantities, and live insects across different geographic locations restricted this study from fully validating the potential links between CI and the patterns observed, the data presented here does warrant further investigation of wDi's effect on D. citri reproduction.
In the present work, samples within same populations all shared identical wDi profiles. However, it is likely that other less prevalent wDi profiles (infection status) or mtCOI haplotypes may also exist within populations. A previous study of Brazilian D. citri populations found that, although the majority of individuals tested carried the same wDi strain (ST‐173), some individuals within the same populations could harbor different wDi strains (Guidolin & Consoli, 2013). Our previous study across Florida D. citri populations also indicated that a very small proportion of D. citri individuals could be free of wDi infection (Chu et al., 2016), suggesting that intrapopulation variation in wDi profiles could exist in other populations as well. Nevertheless, given our repeated detection of identical wDi profiles among multiple samples of the same or closely located populations, the data was able to provide a broad assessment of the distribution patterns of prominent wDi strains across continents. It is also important to note that the approaches used in this work were not intended for detection of Wolbachia strains/alleles that have lower infection densities/abundances. Previous findings indicate that Wolbachia functions are dependent on infection levels (Breeuwer & Werren, 1993; Hurst et al., 2000; Unckless et al., 2009); therefore, wDi strains with higher within‐host density may be more likely to have biologically relevant effects on the host. Thus, the methods used in this work could facilitate detection of additional strains that may have substantial influence on D. citri biology, such as CI induction.
Characterizing the diversity and ecology of wDi could help elucidate the population structures of D. citri and wDi as well as their interactions in the field. In strategies used to control insect pests or insect‐borne pathogens, Wolbachia‐induced CI could also play an important role in reducing insect population size or acting as a drive system for disseminating desirable genes/alleles (Sinkins & Gould, 2006; Bourtzis, 2008). This study identified prevalent wDi strains infecting D. citri across widespread populations, wDi coinfection in field populations, and interesting associations between wDi infection status and D. citri mtCOI sequences. Findings from this work may facilitate the understanding of wDi–D. citri interactions that could benefit the development of control strategies for D. citri and citrus greening. These data also show that transportation of an insect pest could enable dissemination of different endosymbiont strains across the globe, potentially resulting in complex and diverse host–microbe associations in the field.
Disclosure
The authors declare that they have no conflict of interest.
Supporting information
Table S1. Differences among mtCOI sequences of D. citri with different wDi profiles.
Fig. S1. Examples of minor signals in ST‐173 samples that resembled the additional peaks found in Co‐1 samples. The peaks shown are parts of the chromatograms of the sequencing data (in the Geneious software). Peaks with different colors represent different nucleotides. Red: A; Green: T; Blue: C; Yellow: G.
Fig. S2. Alignment of gatB sequences detected in this study.
Fig. S3. Alignment of ftsZ sequences detected in this study.
Fig. S4. Alignment of hcpA sequences detected in this study.
Acknowledgment
The authors would like to acknowledge A. G. McGowan, L. D. Nguyen, and I. P. Foote for technical and experimental assistance, B. Falk, K. Godfrey, C. Higashi, P. A. Stansly, B. McLean II, B. McLean III and W. Meyer for their assistance in insect sampling, and A. F. Sandoval Mojica, T. A. Gill for their comments on the study and manuscript. This research was supported by a United States Department of Agriculture, National Institute for Food and Agriculture Specialty Crop Research Initiative grant (#2012‐51181‐20086).
References
- Baldo, L. , Dunning Hotopp, J.C. , Jolley, K.A. , Bordenstein, S.R. , Biber, S.A. , Choudhury, R.R. et al (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis . Applied and Environmental Microbiology, 72, 7098–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo, L. , Lo, N. and Werren, J.H. (2005) Mosaic nature of the Wolbachia surface protein. Journal of Bacteriology, 187, 5406–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtzis, K. (2008) Wolbachia‐based technologies for insect pest population control. Advances in Experimental Medicine and Biology, 627, 104–113. [DOI] [PubMed] [Google Scholar]
- Boykin, L.M. , De Barro, P. , Hall, D.G. , Hunter, W.B. , McKenzie, C.L. , Powell, C.A. et al (2012) Overview of worldwide diversity of Diaphorina citri Kuwayama mitochondrial cytochrome oxidase 1 haplotypes: two Old World lineages and a New World invasion. Bulletin of Entomological Research, 102, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer, J.A. and Werren, J.H. (1993) Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis . Genetics, 135, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsfoard, C.L. , St. Clair, W. and Dobson, S.L. (2009) Integration of irradiation with cytoplasmic incompatibility to facilitate a lymphatic filariasis vector elimination approach. Parasites & Vectors, 2, 38 10.1186/1756-3305-2-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, C.C. , Gill, T.A. , Hoffmann, M. and Pelz‐Stelinski, K.S. (2016) Inter‐population variability of endosymbiont densities in the Asian citrus psyllid (Diaphorina citri Kuwayama). Microbial Ecology, 71, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de León, J.H. , Sétamou, M. , Gastaminza, G.A. , Buenahora, J. , Cáceres, S. , Yamamoto, P.T. et al (2011) Two separate introductions of Asian citrus psyllid populations found in the American continents. Annals of the Entomological Society of America, 104, 1392–1398. [Google Scholar]
- Dobson, S.L. , Marsland, E.J. and Rattanadechakul, W. (2001) Wolbachia‐induced cytoplasmic incompatibility in single‐ and superinfected Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology, 38, 382–387. [DOI] [PubMed] [Google Scholar]
- Dossi, F.C. , da Silva, E.P. and Consoli, F.L. (2014) Population dynamics and growth rates of endosymbionts during Diaphorina citri (Hemiptera, Liviidae) ontogeny. Microbial Ecology, 68, 881–889. [DOI] [PubMed] [Google Scholar]
- Douglas, A.E. (1998) Nutritional interactions in insect‐microbial symbioses: aphids and their symbiotic bacteria Buchnera . Annunal Review of Entomology, 43, 17–37. [DOI] [PubMed] [Google Scholar]
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstädter, J. and Hurst, G.D.D. (2009) The ecology and evolution of microbes that manipulate host reproduction. Annual Review of Ecology, Evolution, and Systematics, 40, 127–149. [Google Scholar]
- Engelstadter, J. and Telschow, A. (2009) Cytoplasmic incompatibility and host population structure. Heredity, 103, 196–207. [DOI] [PubMed] [Google Scholar]
- Fagen, J.R. , Giongo, A. , Brown, C.T. , Davis‐Richardson, A.G. , Gano, K.A. and Triplett, E.W. (2012) Characterization of the relative abundance of the citrus pathogen Ca. Liberibacter asiaticus in the microbiome of its insect vector, Diaphorina citri, using high throughput 16S rRNA sequencing. The Open Microbiology Journal, 6, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaar, H. (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecological Entomology, 36, 533–543. [Google Scholar]
- Goto, S. , Anbutsu, H. and Fukatsu, T. (2006) Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Applied and Environmental Microbiology, 72, 4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidolin, A.S. and Consoli, F.L. (2013) Molecular characterization of Wolbachia strains associated with the invasive Asian citrus psyllid Diaphorina citri in Brazil. Microbial Ecology, 65, 475–486. [DOI] [PubMed] [Google Scholar]
- Halbert, S.E. and Manjunath, K.L. (2004) Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Florida Entomologist, 87, 330–353. [Google Scholar]
- Hall, D.G. , Richardson, M.L. , El‐Desouky, A. and Halbert, S.E. (2013) Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomologia Experimentalis et Applicata, 146, 207–223. [Google Scholar]
- Hancock, P.A. , Sinkins, S.P. and Godfray, H.C. (2011) Strategies for introducing Wolbachia to reduce transmission of mosquito‐borne diseases. PLoS Neglected Tropical Diseases, 5, e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, M. , Kishino, H. and Yano, T. (1985) Dating the human‐ape split by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 22, 160–174. [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Coy, M.R. , Kingdom Gibbard, H.N. and Pelz‐Stelinski, K.S. (2014) Wolbachia infection density in populations of the Asian citrus psyllid (Hemiptera: Liviidae). Environmental Entomology, 43, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Hogenhout, S.A. , Ammar el, D. , Whitfield, A.E. and Redinbaugh, M.G. (2008) Insect vector interactions with persistently transmitted viruses. Annual Review of Phytopathology, 46, 327–359. [DOI] [PubMed] [Google Scholar]
- Hosokawa, T. , Koga, R. , Kikuchi, Y. , Meng, X.Y. and Fukatsu, T. (2010) Wolbachia as a bacteriocyte‐associated nutritional mutualist. Proceedings of the National Academy of Sciences USA, 107, 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck, J.P. and Ronquist, F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755. [DOI] [PubMed] [Google Scholar]
- Hurst, G.D. , Johnson, A.P. , Schulenburg, J.H. and Fuyama, Y. (2000) Male‐killing Wolbachia in Drosophila: a temperature‐sensitive trait with a threshold bacterial density. Genetics, 156, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, G.D.D. , Jiggins, F.M. , von der Schulenburg, J.H.G. , Bertrand, D. , West, S.A. , Goriacheva, I.I. et al (1999) Male‐killing Wolbachia in two species of insect. Proceedings of the Royal Society B: Biological Sciences, 266, 735–740. [Google Scholar]
- Iturbe‐Ormaetxe, I. , Walker, T. and O'Neill, S.L. (2011) Wolbachia and the biological control of mosquito‐borne disease. EMBO Reports, 12, 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, M. , Fleites, L.A. and Gabriel, D.W. (2017) A small Wolbachia protein directly represses phage lytic cycle genes in “Candidatus Liberibacter asiaticus” within psyllids. mSphere, 2, e00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris, Z. , Cook, P.E. , Phuc, H.K. and Sinkins, S.P. (2009) Immune activation by life‐shortening Wolbachia and reduced filarial competence in mosquitoes. Science, 326, 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. et al (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. and Tamura, K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution, 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashkari, M. , Manzari, S. , Sahragard, A. , Malagnini, V. , Boykin, L.M. and Hosseini, R. (2014) Global genetic variation in the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae) and the endosymbiont Wolbachia: Links between Iran and the USA detected. Pest Management Science, 70, 1033–1040. [DOI] [PubMed] [Google Scholar]
- Michaud, J.P. (2004) Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biological Control, 29, 260–269. [Google Scholar]
- Montllor, C.B. , Maxmen, A. and Purcell, A.H. (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecological Entomology, 27, 189–195. [Google Scholar]
- Moran, N.A. and Telang, A. (1998) Bacteriocyte‐associated symbionts of insects. BioScience, 48, 295–304. [Google Scholar]
- Moreira, L.A. , Iturbe‐Ormaetxe, I. , Jeffery, J.A. , Lu, G. , Pyke, A.T. , Hedges, L.M. et al (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell, 139, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Mouton, L. , Henri, H. , Bouletreau, M. and Vavre, F. (2006) Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology, 132, 49–56. [DOI] [PubMed] [Google Scholar]
- Oliver, K.M. , Russell, J.A. , Moran, N.A. and Hunter, M.S. (2002) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proceedings of the National Academy of Sciences USA, 100, 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que, S.Q. , Yu, L.P. , Xin, T.R. , Zou, Z.W. , Hu, L.X. and Xia, B. (2016) Complete mitochondrial genome of Cacopsylla coccinae (Hemiptera: Psyllidae). Mitochondrial DNA. Part A, DNA Mapping, Sequencing, and Analysis, 27, 3169–3170. [DOI] [PubMed] [Google Scholar]
- Qureshi, J.A. , Rogers, M.E. , Hall, D.G. and Stansly, P.A. (2009) Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. Journal of Economic Entomology, 102, 247–256. [DOI] [PubMed] [Google Scholar]
- Rigaud, T. and Juchault, P. (1995) Success and failure of horizontal transfers of feminizing Wolbachia endosymbionts in woodlice. Journal of Evolutionary Biology, 8, 249–255. [Google Scholar]
- Rousset, F. , Bouchon, D. , Pintureau, B. , Juchault, P. and Solignac, M. (1992a) Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proceedings of the Royal Society B: Biological Sciences, 250, 91–98. [DOI] [PubMed] [Google Scholar]
- Rousset, F. , Vautrin, D. and Solignac, M. (1992b) Molecular identification of Wolbachia, the agent of cytoplasmic incompatibility in Drosophila simulans, and variability in relation with host mitochondrial types. Proceedings of the Royal Society B: Biological Sciences, 247, 163–168. [DOI] [PubMed] [Google Scholar]
- Sabree, Z.L. , Kambhampati, S. and Moran, N.A. (2009) Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proceedings of the National Academy of Sciences USA, 106, 19521–19526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, S. , Hunter, W.B. , Reese, J. , Morgan, J.K. , Marutani‐Hert, M. , Huang, H. et al (2012) Survey of endosymbionts in the Diaphorina citri metagenome and assembly of a Wolbachia wDi draft genome. PLoS ONE, 7, e50067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough, C.L. , Ferrari, J. and Godfray, H.C.J. (2005) Aphid protected from pathogen by endosymbiont. Science, 310, 1781 10.1126/science.1120180. [DOI] [PubMed] [Google Scholar]
- Sinkins, S.P. , Braig, H.R. and O'Neill, S.L. (1995) Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proceedings of the Royal Society B: Biological Sciences, 261, 325–330. [DOI] [PubMed] [Google Scholar]
- Sinkins, S.P. and Gould, F. (2006) Gene drive systems for insect disease vectors. Nature Reviews Genetics, 7, 427–435. [DOI] [PubMed] [Google Scholar]
- Stansly, P.A. , Arevalo, H.A. , Qureshi, J.A. , Jones, M.M. , Hendricks, K. , Roberts, P.D. et al (2014) Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest Management Science, 70, 415–426. [DOI] [PubMed] [Google Scholar]
- Subandiyah, S. , Nikoh, N. , Tsuyumu, S. , Somowiyarjo, S. and Fukatsu, T. (2000) Complex endosymbiotic microbiota of the citrus psyllid Diaphorina citri (Homoptera: Psylloidea). Zoological Science, 17, 983–989. [Google Scholar]
- Telschow, A. , Hammerstein, P. and Werren, J.H. (2002) The effect of Wolbachia on genetic divergence between populations: models with two‐way micration. The American Naturalist, 160, S54–S66. [DOI] [PubMed] [Google Scholar]
- Tiwari, S. , Mann, R.S. , Rogers, M.E. and Stelinski, L.L. (2011) Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Management Science, 67, 1258–1268. [DOI] [PubMed] [Google Scholar]
- Unckless, R.L. , Boelio, L.M. , Herren, J.K. and Jaenike, J. (2009) Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proceedings of the Royal Society B: Biological Sciences, 276, 2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J.H. , Baldo, L. and Clark, M.E. (2008) Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology, 6, 741–751. [DOI] [PubMed] [Google Scholar]
- Werren, J.H. , Zhang, W. and Guo, L.R. (1995) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proceedings of the Royal Society B: Biological Sciences, 261, 55–63. [DOI] [PubMed] [Google Scholar]
- Yen, J.H. and Barr, A.R. (1973) The etiological agent of cytoplasmic incompatibility in Culex pipiens . Journal of Invertebrate Pathology, 22, 242–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Differences among mtCOI sequences of D. citri with different wDi profiles.
Fig. S1. Examples of minor signals in ST‐173 samples that resembled the additional peaks found in Co‐1 samples. The peaks shown are parts of the chromatograms of the sequencing data (in the Geneious software). Peaks with different colors represent different nucleotides. Red: A; Green: T; Blue: C; Yellow: G.
Fig. S2. Alignment of gatB sequences detected in this study.
Fig. S3. Alignment of ftsZ sequences detected in this study.
Fig. S4. Alignment of hcpA sequences detected in this study.


