Abstract
We hypothesized that positive airway pressure treatment would induce nasal obstruction and decrease nasal cavity due to mucosal swelling. We further hypothesized that subjective and objective nasal obstruction at baseline would negatively affect positive airway pressure adherence. A total of 728 patients with sleep apnea were investigated in the Icelandic Sleep Apnea Cohort at baseline and 2 years after starting positive airway pressure. Patients underwent home sleep apnea testing at baseline. Questionnaires were answered and acoustic rhinometry was completed at baseline and follow‐up. The proportion of patients reporting subjective nocturnal nasal obstruction was reduced (baseline: 35% versus follow‐up: 24%; p < 0.001). Small interior nasal dimensions increased (p < 0.001) independent of adherence to treatment. Small nasal volume at baseline was a determinant for becoming a non‐user of positive airway pressure treatment (odds ratio 2.22, confidence interval 95% 1.35–3.67, p = 0.002). Subjective nasal obstruction decreased 2 years after initiating positive airway treatment in sleep apnea, and objectively small nasal dimensions increased. Small nasal volume at baseline was a negative predictor for positive airway pressure treatment adherence. Maybe most importantly, positive airway pressure treatment did not cause long‐term objective or subjective nasal obstruction.
Keywords: acoustic measurements, adherence, apnea, breathing, nasal anatomy, nose, survey
1. INTRODUCTION
Nasal obstruction in patients with obstructive sleep apnea (OSA) is common, and has an impact on quality of life and sleep quality (Värendh et al., 2018). Some patients with OSA experience daytime sleepiness and insomnia (Björnsdóttir et al., 2013). Patients with OSA commonly have co‐morbidities, such as arterial hypertension and cardiovascular disease (Ye et al., 2014).
Studies evaluating patients with OSA in the 1990s indicated that positive airway pressure (PAP) treatment induced nasal side‐effects in 25%–64% of patients (Brander, Soirinsuo, & Lohela, 1999; Engleman et al., 1996; Hoffstein, Viner, Mateika, & Conway, 1992; Pépin et al., 1995; Waldhorn et al., 1990), and most commonly increased nasal obstruction (Kribbs et al., 1993). Kreivi, Virkkula, Lehto, and Brander (2010) found a reduced proportion of patients reporting nasal stuffiness after 2 months of PAP use. However, another study, with the follow‐up time of 1 year, found that PAP induced nasal stuffiness if not treated with heated humidification (Kreivi, Maasilta, & Bachour, 2016).
Some studies have been investigating the histopathological impact of PAP treatment on the nasal mucosa. Concerning the mucosal structure, the ciliary transport does not seem to be changed after 3 months of PAP treatment (Saka et al., 2012). There are conflicting results concerning the impact of PAP treatment on the inflammatory response of the nasal mucosa. Saka et al. (2012) found an increase in inflammatory cells after 3 months of PAP use, but on the other hand Gelardi et al. (2012) showed that patients with OSA using PAP had a reduction in inflammatory cells after 8 weeks of treatment.
When focusing on the anatomical aspects, Iriz et al. (2017) demonstrated a decrease in nasal dimensions measured with acoustic rhinometry (AR) after 1 month of PAP use, and a return to baseline values after 3 months of PAP usage. Balsalobre et al. (2017) has described immediate subjective and objective nasal blockage after 2 hr of PAP use. To our knowledge, the long‐term effect of PAP on internal nasal dimensions has not been described, and no large, well‐defined, prospective study has investigated the nose, either objectively or subjectively. Our primary objective was to investigate the long‐term effects of PAP treatment on subjective nasal obstruction and objectively measured nasal dimensions. Moreover, we wanted to determine whether subjective and objective nasal obstruction at baseline influenced PAP adherence. We hypothesized that PAP treatment induces nasal obstruction and decreases nasal cavity due to mucosal swelling. We further hypothesized that subjective and objective nasal obstruction at baseline negatively affects PAP adherence.
2. MATERIALS AND METHODS
2.1. Study design and study subjects
The present study is a prospective cohort study, part of the Icelandic Sleep Apnea Cohort (ISAC), as previously described (Arnardottir, Janson, et al., 2013; Arnardottir, Maislin, et al., 2013). From September 2005 to December 2009, all patients with OSA in Iceland who were referred for PAP treatment were invited to participate. For financial reasons, patients underwent AR if follow‐up was performed before 1 September 2008. Hence, 419 patients underwent AR at follow‐up, which was 78% of the patients who had follow‐up before 1 September 2008 (see Figure 1 for an outline of the included patients). The ISAC study was approved by The National Bioethics Committee of Iceland, the Data Protection Authority of Iceland, and the Institutional Review Board of the University of Pennsylvania. All patients signed a written informed consent.
Figure 1.
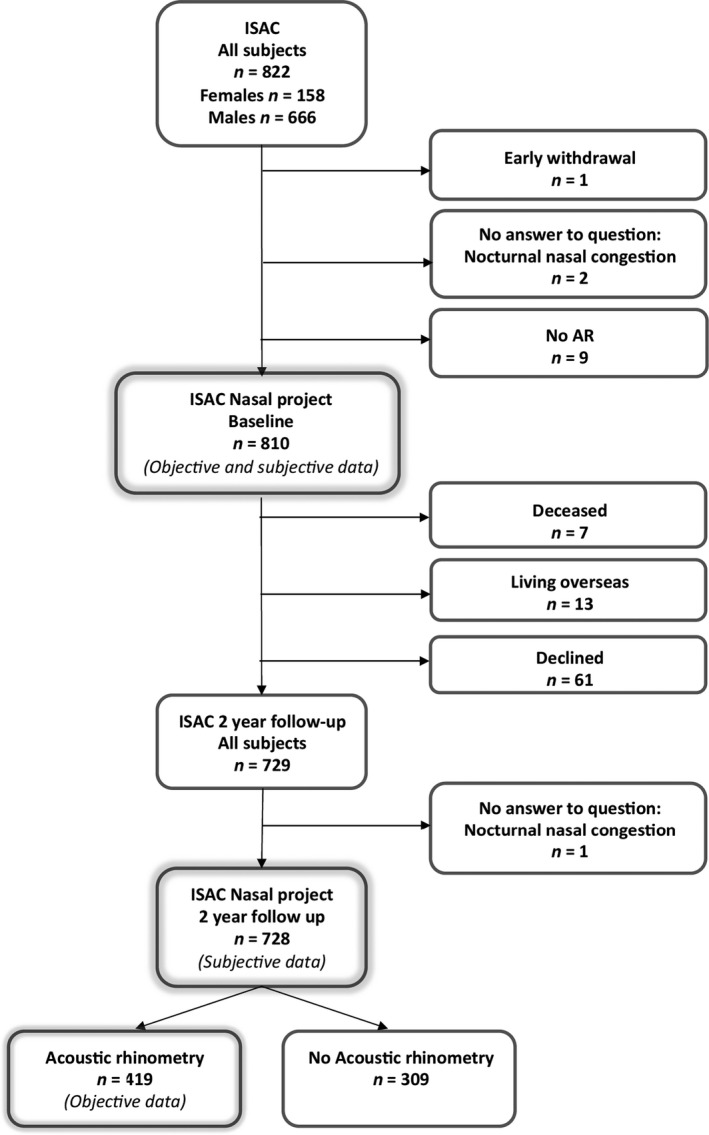
Flowchart showing the patients included in subjective data and objective data calculations respectively. A total of 728 patients have answered the question about nocturnal nasal obstruction at both baseline and follow‐up. Four‐hundred and nineteen patients have been examined with acoustic rhinometry (AR) both at baseline and at follow‐up
2.2. Measurements and questionnaires
At baseline, the patients answered the standardized questionnaires, Epworth Sleepiness Scale (ESS; Björnsdóttir et al., 2012; Johns, 1991), Basic Nordic Sleep Questionnaire (BNSQ; Partinen & Gislason, 1995) and the 12‐item Short‐Form Health Survey (SF‐12; Ware, Kosinski, & Keller, 1996). Subjective nasal obstruction was examined with the question: “Is your nose congested at night?” The responses were categorized from 1 to 5: 1 = never or very seldom; 2 = less than once a week; 3 = once–twice a week; 4 = three–five times a week; and 5 = every night or almost every night of the week. A score of 4 or 5 was defined as nocturnal nasal obstruction. The Basic Nordic Sleep Questionnaire was used to examine symptoms of poor sleep quality, including insomnia symptoms (Partinen & Gislason, 1995). Definitions of the insomnia subtypes – initial insomnia, middle insomnia, and late insomnia (≥ 3 × per week) – have been described previously (Björnsdóttir et al., 2012). The use of nasal and oral cortisone medication was also investigated (yes/no).
2.3. Acoustic rhinometry
In AR, an acoustic pulse is sent into the nasal valves from the nostrils to provide an anatomical description with three‐dimensional measurements of the nasal cavity (Clement & Gordts, 2005). A single‐impulse rhinometer (Rhinoscan SRE2000, Rhinometrics, Assens, Denmark) was used. The dimensions examined before and 15 min after administration of the nasal spray oxymetazoline (0.5 mg ml−1; two puffs per nostril) were: TMCA (total minimal cross‐section area in the left and right nasal cavity); MCA‐min (the minimal cross‐section area in either left or right nasal valve); TVOL (the total volume of left and right nasal cavity); and TMCA‐diff (the difference between TMCA before and after nasal decongestive spray). The equipment was calibrated every morning prior to use. On all days of examinations, some patients were examined in the morning (n = 2) and some (n = 1) in the afternoon. One of the authors (MV) re‐evaluated all AR results during 2–6 November 2015. Invalid measurements at baseline (n = 3) and follow‐up (n = 4) were excluded.
2.4. Sleep study
At baseline, patients underwent a home sleep apnea testing with an Embletta portable monitor, an Embla 12‐channel system (EMBLATM; Flaga, Reykjavik, Iceland) or a T3 device (Nox Medical, Reykjavik, Iceland; Arnardottir, Janson, et al., 2013; Arnardottir, Maislin, et al., 2013; Björnsdóttir et al., 2012).
More than 4 hr of a scorable oxygen saturation (SaO2) signal was required to include a sleep study. The apnea–hypopnea index (AHI) was determined as the mean number of apnea and hypopnea per hour of recording (upright time excluded). Apnea was defined as ≥ 80% decrease in flow for a period of ≥ 10 s. Hypopnea was defined as ≥ 30% decrease in the flow with ≥ 4% oxygen desaturation, or ≥ 50% decrease in flow for ≥ 10 s with an immediate increase in flow at the end of the episode.
2.5. Positive airway pressure
Patients received an auto‐adjusting PAP or continuous PAP device (ResMed, San Diego, CA, USA; Arnardottir, Janson, et al., 2013; Arnardottir, Maislin, et al., 2013; Björnsdóttir et al., 2012). In case of problems with treatment efficacy, the treatment was changed to bi‐level PAP (n = 17) or adaptive servo ventilation (n = 6). The definition for inadequate treatment efficacy was AHI ≥ 15 events per hour during PAP use. Patients had a choice of different masks and humidifiers. From 2009 onwards, all PAP devices were delivered with heated in‐line humidifiers.
Adherence of PAP at the 2‐year follow‐up was estimated from memory cards or from a questionnaire (Arnardottir, Janson, et al., 2013; Arnardottir, Maislin, et al., 2013. Full PAP usage was defined, in mean, ≥ 20 days and ≥ 4 hr per night for the previous 4 weeks of machine‐derived data, or ≥ 5 nights per week for ≥ 60% of the night by questionnaire. Subjects with less use were considered partial users. Early quitters returned their devices within 1 year after treatment initiation, and late quitters kept the equipment for 365–729 days (Eysteinsdottir et al., 2017). Adherence data were missing in three cases.
2.6. Nasal surgery
Data from patient files on the frequency of nasal surgery during the 2 years of study included septoplasty (n = 59), turbinectomy (n = 66), and endoscopic surgery (n = 4). Patients with nasal surgery prior to study start did not differ from the rest of the study sample with respect to subjective or objective nasal obstruction, and were therefore not included in the group with nasal surgery.
2.7. Statistical analysis
Nominal data are presented as frequencies and percentages without decimals. The chi‐squared test was used in comparisons between nominal data in independent groups. Differences over time were tested using McNemar’s test. Quantitative data and ordinal data were presented by means and standard deviations (± SD). Mann–Whitney U‐test was used when calculating group differences for two independent groups, and Kruskal–Wallis test for comparisons including more than two groups. Post hoc analyses were calculated using the Mann–Whitney U‐test. Paired samples were tested using the Wilcoxon signed rank test. The enter method was used in the multiple logistic regression analysis, and the method evaluates all the covariate variables regardless of whether significant or not. Covariates divided into more than two groups were categorized using the last category containing the highest values as contrast. The statistical software used was SPSS 23.0. A two‐sided p‐value < 0.05 was considered significant.
3. RESULTS
3.1. General information
A flowchart showing the study cohort is shown in Figure 1. Subjective data were examined and reported for 728 patients, and objective data for 419 patients. No differences in age, gender, or subjective nasal obstruction at baseline between the study sample and the dropout group were observed (p = 0.26–0.91). General information about the sample cohort is shown in Table 1. The women had on average smaller nasal dimensions, lower weight and lower physical quality of life than men. No sex differences in objective nasal dimension change or in subjective nocturnal nasal obstruction were found (Table S1). A few patients were on medications that can influence nasal obstruction, including systemic corticosteroids at baseline (1%) and at follow‐up (4%), nasal corticosteroids at baseline (1%) and at follow‐up (5%), antihistamines (1%) and at follow‐up (1%).
Table 1.
Background data from baseline and 2‐year follow‐up comparing patients with and without objective data
| All patients (n = 728) | Patients with objective data (n = 419) | |||||
|---|---|---|---|---|---|---|
| Baseline | 2‐year follow‐up | p‐value for group difference | Baseline | 2‐year follow‐up | p‐value for group difference | |
| Age (years; mean ± SD) | 54.7 ± 10.4 | 56.8 ± 10.4 | – | 54.1 ± 10.3 | 56.1 ± 10.3 | – |
| Female (%) | 24 | 24 | – | 18 | 18 | – |
| Never smoker (%) | 27 | 28 | 1.00 | 27 | 28 | 0.52 |
| Previous smoker (%) | 52 | 52 | 50 | 50 | ||
| Current smoker (%) | 20 | 20 | 0.80 | 23 | 22 | 0.74 |
| BMI (kg m−2) | 33.5 ± 5.6 | 34.0 ± 5.8 | <0.001 | 33.0 ± 5.6 | 33.5 ± 5.7 | <0.001 |
| Arterial hypertension (%) | 58 | 59 | 0.36 | 55 | 55 | 1.00 |
| Coronary heart disease, including coronary heart occlusion, heart failure, and/or stroke (%) | 18 | 19 | 0.23 | 17 | 19 | 0.17 |
| Diabetes (%) | 11 | 13 | 0.006 | 11 | 12 | 0.23 |
Significance given in bold. Numbers given as mean ± SD if not specified, and p‐values when comparing mean values were calculated using the Wilcoxon signed rank test. Independent groups shown with %, and when comparing nominal data the McNemar test was used.
BMI, body mass index.
3.2. Subjective and objective results at baseline and follow‐up
The baseline data were published previously (Värendh et al., 2018). The prevalence of subjective nocturnal nasal obstruction (≥ 3 × per week) was 35% in the patients with OSA. Patients with complaints of nasal obstruction had more daytime sleepiness (ESS: 12.5 ± 4.9 versus 10.8 ± 5.0; p < 0.001) and slightly lower mental quality of life (SF‐12: 46.4 ± 11.4 versus 49.8 ± 10.5, p < 0.001) than patients without nocturnal nasal obstruction. The minimum cross‐section area within the smaller nasal valve prior to decongestive spray was smaller in the patients with subjective nocturnal nasal obstruction (0.42 ± 0.17 versus 0.45 ± 0.16 cm2, p = 0.013). In the current study, the patients are divided into four groups depending on subjective nocturnal nasal obstruction (Table 2). Patients with nocturnal nasal obstruction at follow‐up were more likely to have symptoms of poor quality of sleep, such as nocturnal sweating, insomnia and reduced physical quality of life independent of their symptoms at baseline (p‐values < 0.02).
Table 2.
A larger proportion of the patients with nocturnal nasal obstruction at follow‐up had nocturnal sweating, nocturnal gastroesophageal reflux, insomnia and impaired physical quality of life
| Never (n = 418) | Only at baseline (n = 134) | Only at 2‐year follow‐up (n = 52) | Both at baseline and at 2‐year follow‐up (n = 124) | p‐value for group difference | |
|---|---|---|---|---|---|
| Underwent nasal surgery during the study | 12% | 25% | 10% | 15% | 0.01 |
| Nocturnal sweating, ≥ 3 × per week, 2 years | 13% | 14% | 62% | 25% | 0.007 |
| Nocturnal gastroesophageal reflux, ≥ 1 × per week, 2 years | 5% | 14% | 8% | 15% | 0.002 |
| Daytime sleepiness (ESS), 2 years | 8.4 ± 4.6 | 8.6 ± 4.7 | 9.7 ± 4.2 | 8.8 ± 4.9 | 0.17 |
| Initial insomnia, ≥ 3 × per week, 2‐year follow‐up | 9% | 16% | 10% | 18% | 0.01 |
| Middle insomnia, ≥ 3 × per week, 2 years | 29% | 39% | 57% | 44% | 0.001 |
| Late insomnia, ≥ 3 × per week, 2 years | 18% | 31% | 35% | 30% | <0.001 |
| SF‐12, Mental part, 2 years | 51.7 ± 9.9 | 50.2 ± 10.8 | 49.1 ± 10.0 | 50.2 ± 10.5 | 0.16 |
| SF‐12, Physical part, 2 years | 43.4 ± 11.3 | 44.2 ± 10.7 | 42.4 ± 11.9 | 39.9 ± 11.4 | 0.01 |
| Number of days on PAP | 595.0 ± 241.1 | 593.2 ± 235.3 | 592.7 ± 223.9 | 573.4 ± 251.7 | 0.10 |
| Hours of PAP use, last 28 days before 2 years (fu007; n = 203, missing 215) Objective data | 6.17 ± 2.22 | 6.49 ± 1.67 | 5.47 ± 2.42 | 6.60 ± 1.88 | 0.10 |
| Late quitters (n = 17) | 2% | 2% | 4% | 3% | 0.41 |
| Early quitters (n = 131) | 18% | 19% | 15% | 23% | |
| Partial users (n = 199) | 14% | 8% | 23% | 15% | |
| Full users (n = 480) | 66% | 71% | 58% | 63% |
ESS, Epworth Sleepiness Scale; PAP, positive airway pressure; SF‐12, the 12‐Item Short‐Form Health Survey, a smaller version of the SF‐36v2 Health Survey, measuring quality of life.
Significance given in bold. Mean values shown as mean ± SD and p‐values. Chi‐squared test when comparing proportions of groups here in %. Kruskal–Wallis ANOVA when comparing ordinal data. Post hoc test (Mann–Whitney U‐test): subgroup analysis comparing the groups SF‐12, Physical part, 2 years, only baseline with both baseline and follow‐up, p‐value 0.18.
3.3. Changes in subjective results over 2 years
Nocturnal nasal obstruction (≥ 3 × per week) decreased from 35% to 24% (p < 0.001) in the whole study group. When patients were divided into groups depending on PAP adherence, there was a decrease in the proportion of patients reporting subjective nocturnal nasal obstruction between baseline and follow‐up in full users (p < 0.001), as well as in early quitters (p = 0.005; Figure 2). There was no significant difference in reported nocturnal nasal obstruction between the groups full user, partial user, early quitter, and late quitter at baseline (p = 0.67) or at follow‐up (p = 0.20; Table S2). The late quitters were few in number (seven at baseline with nocturnal nasal obstruction and six at follow‐up).
Figure 2.
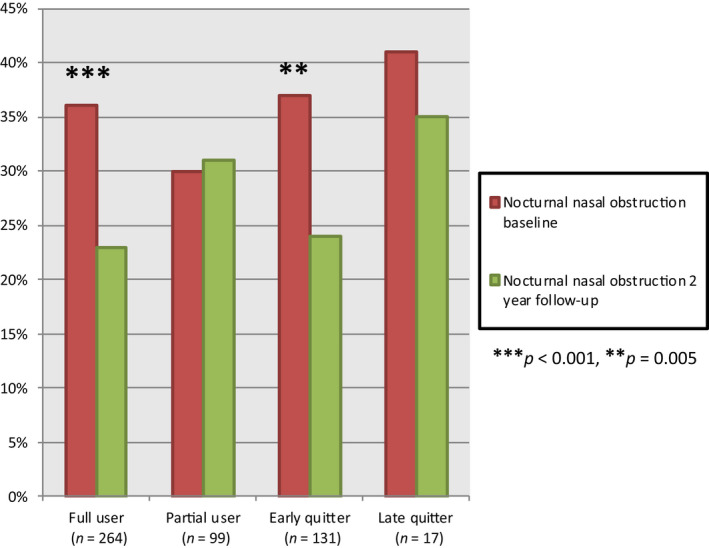
The proportion of patients reporting subjective nasal obstruction (≥ 3 × per week) at baseline compared with follow‐up. There was a significant (p ≤ 0.005) decrease in the proportion with nocturnal nasal obstruction in full users and early quitters. There was no significant difference between the groups at baseline (p = 0.67) or at follow‐up (p = 0.20). The early quitters used their equipment for less than 1 year. The late quitters used their treatment for more than 1 year and less than 2 years
3.4. Changes in objective results over 2 years
All objective nasal dimensions increased on average after 2 years (Tables 3 and S3). However, the reactivity of the nasal mucosa was unchanged. This indicates that the increase in nasal dimensions was not related to decongestion of the nasal mucosa. The reactivity did not differ at the 2‐year follow‐up between the different groups of PAP use (p = 0.94–0.97). Patients with a small total minimal cross‐section area at baseline had an increase in dimensions at follow‐up (p < 0.001), while no changes in dimensions were found in patients with large total minimum cross‐section area at baseline (p = 0.65; Figure 3). When comparing the group examined by AR with the rest of the samples, no differences in age or weight (p = 0.07 and 0.20) were found. However, the objectively examined patients had a slightly lower body mass index (BMI; 33.6 ± 5.7 versus 34.5 ± 5.8 kg m−2, p = 0.02).
Table 3.
All objective nasal dimensions increased on average after 2 years except the reactivity of the nasal mucosa, which was unchanged
| Baseline | 2‐year follow‐up | p‐value for group difference | |
|---|---|---|---|
| TMCA, total minimal cross‐section area in the nose, left and right nostril combined before nasal decongestant spray (cm2) | 1.06 ± 0.31 | 1.16 ± 0.33 | <0.001 |
| TMCA, total minimal cross‐section area in the nose, left and right nostril combined after decongestant spray, (cm2) | 1.24 ± 0.33 | 1.35 ± 0.32 | <0.001 |
| MCA‐min, smallest nasal valve of right and left, before decongestant spray (cm2) | 0.43 ± 0.16 | 0.48 ± 0.17 | <0.001 |
| MCA‐min, smallest nasal valve of right and left after decongestant spray (cm2) | 0.53 ± 0.17 | 0.58 ± 0.17 | <0.001 |
| TVOL, total volume of left and right nasal volume combined before nasal decongestant spray (cm3) | 4.10 ± 0.82 | 4.37 ± 0.88 | <0.001 |
| TVOL, Total volume of left and right nasal volume combined mean after decongestant spray (cm3) | 4.30 ± 0.84 | 4.61 ± 0.87 | <0.001 |
| Diff TMCA, difference between after and before decongestant spray (cm2), reactivity of the nasal mucosa | 0.19 ± 0.21 | 0.20 ± 0.24 | 0.61 |
| Diff MCA‐min, smallest nasal valve of right and left, difference between after and before decongestant spray (cm2), reactivity of the nasal mucosa | 0.10 ± 0.12 | 0.10 ± 0.13 | 0.31 |
| Diff TVOL, difference between after and before decongestant spray (cm3), reactivity of the nasal mucosa | 0.20 ± 0.34 | 0.24 ± 0.41 | 0.37 |
N = 419 at baseline and at follow‐up after 2 years.
Significance given in bold. Numbers given as mean ± SD if not specified, and p‐values when comparing mean values were calculated using the Wilcoxon signed rank test. Nominal data in independent groups shown with %. The chi‐squared test was used for comparisons between nominal data.
Figure 3.
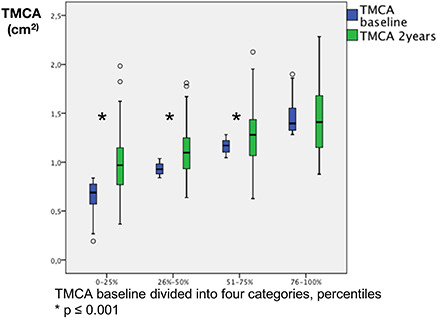
Patients with small total minimal cross‐section area (TMCA) of left and right nasal cavity before decongestant spray, at baseline increase in TMCA median (p ≤ 0.001) while patients with large TMCA do not change, median in boxplot (p = 0.65). x‐axis: 1 = smallest TMCA at baseline up to 25% percentile (n = 138); 2 = second smallest 26%–50% (n = 107); 3 = second largest percentile (n = 95); 4 = largest TMCA at baseline (n = 71). y‐axis: TMCA (cm2). Wilcoxon signed rank test was used when comparing change in median
3.5. Relationship between subjective and objective changes
The patients were divided into improved nocturnal nasal obstruction, no change, and worse nocturnal nasal obstruction (Figure 4). There were no significant differences in change in total minimal cross‐section area over the 2 years. There was no relationship between the groups with respect to changes in objective total minimal cross‐section area (r = −0.02; p = 0.66 and 0.98). The group of patients with more reported nocturnal nasal obstruction from baseline to follow‐up also had an increase in internal nasal dimensions during the 2‐years follow‐up (Table S4).
Figure 4.
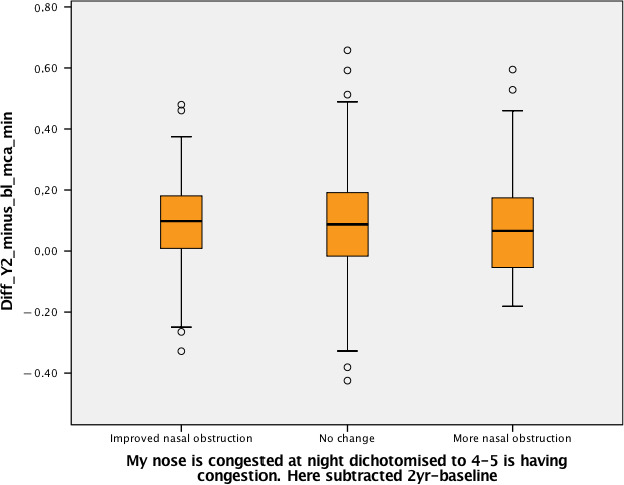
There was no difference between the groups of subjective changes (improved, no change, more nasal obstruction) in the objective changes in MCA‐min (p = 0.98, Kruskal–Wallis test)
3.6. The role of subjective and objective nasal measurements at baseline in PAP adherence
Having subjective nocturnal nasal obstruction at baseline was not a predictor of being a quitter within the first 2 years of use (Table 4). On the other hand, having a small volume at baseline was a determinant of becoming a non‐user of PAP after 2 years, compared with having a large volume at baseline.
Table 4.
Having a small volume at baseline was a predictor of becoming a non‐user of PAP after 2 years compared with having a large volume at baseline
| Unadjusted odds ratio (95% CI) | p‐value for group difference | Adjusted odds ratio (95% CI) | p‐value for group difference | ||
|---|---|---|---|---|---|
| Nocturnal nasal obstruction at baseline, ≥ 3 × per week | 1.11 (0.79–1.57) | 0.55 | 1.18 (0.80–1.74) | 0.42 | |
| TMCA percentiles at baseline, comparing the smallest quartile with the largest | 1.80 (1.11–2.93) | 0.02 | 0.35 (0.07–1.83) | 0.22 | |
| TVOL, percentiles at baseline, comparing the smallest quartile with the largest | 2.22 (1.35–3.67) | 0.002 | 3.31 (1.07–10.26) | 0.04 | |
| MCA‐min, percentiles at baseline, comparing the smallest quartile with the largest | 1.80 (1.08–3.00) | 0.02 | 2.01 (0.48–8.47) | 0.34 | |
The table shows the prediction of PAP non‐use (early and late quitters) depending on subjective and objective nasal obstruction at baseline. Included in this analysis: auto‐adjusting PAP, continuous PAP device and adaptive servo ventilation. Significance in bold. Multiple regression analysis was used.
Odds ratio adjusted for age, BMI and AHI at baseline.
CI, confidence interval; MCA‐min, minimal cross‐sectional area within the smallest nostril of either left or right before decongestant spray; TMCA, total minimal cross‐section area in the nose, left and right nostril combined before nasal decongestant spray; TVOL, total volume of left and right nasal volume combined before nasal decongestant spray.
3.7. Analysis of controller effects
3.7.1. Subjective data
An analysis was completed with the subjective data with the following groups excluded for each factor: nasal surgery, use of heated humidifier, full‐face mask, mandibular advancement, or a >10% weight decrease, respectively. None of the factors influenced the findings based on subjective reports.
3.7.2. Objective data
Analyses were also completed by assessing separately the objective data in subjects having had nasal surgery (14% of the patients with objective data), using a mandibular advancement device (8%), heated humidifier (yes 36%/no 64%) and different mask type (full face 49%, nasal 26% and pillow 7%). None of these factors influenced the objective results. It was not possible to perform adequate calculations on weight decrease (> 10%) due to the lower number of patients with objective measurements and weight decrease (eight patients).
4. DISCUSSION
This cohort study demonstrates that a significant number of patients improved in subjective and objective nasal obstruction 2 years after initiating PAP therapy. Patients with the smallest total minimal cross‐section area at baseline had the largest increase in total minimal cross‐section area. Subjective nasal obstruction at baseline did not affect PAP adherence, while objective nasal obstruction at baseline was a predictor for becoming a non‐user of PAP.
4.1. Subjective and objective results at baseline
The baseline data have been published previously. In that study, we reported that nasal obstruction is frequent and has an impact on quality of life in patients with OSA (Värendh et al., 2018).
4.2. Change in subjective nocturnal nasal obstruction
In the present study, a significant proportion of the population experienced an improved nocturnal nasal obstruction. There was a decrease in nocturnal nasal obstruction in the full users of PAP, but also in the early quitters. It is challenging to speculate why the early quitters also reported reduced nasal obstruction. However, it has been shown recently that there is a decrease in symptoms in general, even amongst non‐users to some extent (Pien et al., 2018).
Studies from the 1990s indicate an increase in nasal symptoms with the PAP treatment regime that was used at that time (Brander et al., 1999; Engleman et al., 1996; Hoffstein et al., 1992; Pépin et al., 1995; Waldhorn et al., 1990). With more modern treatment, including heated humidifiers, Kreivi et al. (2016) found that nasal symptoms decrease upon therapy. However, in our study, patients with heated humidification did not differ in nocturnal nasal obstruction to the patients without a humidifier.
It is possible that the new treatment regimens have been of importance in improving the nasal symptoms developed during PAP treatment. Another option is that the studies completed in the 1990s were not large follow‐up studies, and therefore did not observe the resolution of nasal dimensions and symptoms. It could be speculated that nasal symptoms are part of the OSA syndrome, and that a successful treatment improving OSA also will improve nasal symptoms associated with the disease itself.
4.3. Change in objective nocturnal nasal obstruction
The nasal dimensions did not decrease upon PAP therapy, as we had hypothesized; in contrast, nasal dimensions acutely increased. A small study by Iriz et al. (2017) showed a decrease in minimal cross‐section area after 1 month with PAP, while after 3 months on PAP the area was back to baseline values. Another study by Balsalobre et al. (2017) found more subjective and objective (AR) nasal obstruction immediately after 2 hr of PAP use in non‐OSA patients.
These observations indicate that there might be an initial swelling of the nasal mucosa that lasts for the first month of PAP use due to the pressure from the airflow. After 3 months, the mucosa adjusts, and after 2 years the nasal dimensions have improved. Two years after initiating PAP treatment, we found that patients with small dimensions have the biggest increases. It can be speculated that PAP treatment could affect any interaction between afferent/efferent nasal tracheobronchial and inspiratory muscle mechanoreceptors that participate in the coordination of inspiratory and expiratory efforts (McNicholas, Coffey, & Boyle, 1993). After interrupting the positive nasal air pressure ventilation treatment, the nasal airflow resistance increased (Fontanari, Burnet, Zattara‐Hartmann, Badier, & Jammes, 1999). Another option is a mechanical widening of the nose by the nasal PAP mask or the air pressure. The mechanisms are certainly of interest for further studies.
4.4. Relationship between subjective and objective changes
There was no relationship between improvements in subjective nocturnal obstruction and improvements in objective measurements other than the fact that both improved. It is not clear whether there is a relationship between subjective and objective nasal obstruction. One review by André, Vuyk, Ahmed, Graamans, and Nolst Trenité (2009) showed no strong evidence for a correlation between subjective and objective measurements or the opposite.
4.5. The role of subjective and objective nasal measurements at baseline in PAP adherence
Having a small volume at baseline was a predictor of becoming non‐adherent to PAP treatment. In contrast, having self‐reported nocturnal nasal obstruction at baseline was not predictive of becoming non‐adherent to PAP treatment. This is in line with previous studies. Li et al. (2005) found that in patients with small minimal cross‐section area at baseline, the PAP adherence was lower after 3 months compared with patients with large minimal cross‐section area. According to a study by Sugiura et al. (2006), higher nasal resistance indicated lower odds ratio for acceptance of PAP treatment. Our findings indicate that having a small volume at baseline indicates a higher risk that the patient will become non‐adherent. There are many factors involved when patients become non‐adherent to PAP treatment, and nasal obstruction can be one of the reasons, at least for the patients quitting early in the treatment. It is possible that subjective nasal obstruction can be improved by PAP adherence, while the objective nasal obstruction should be treated surgically.
4.6. Strength and limitations of the study
The main strength of the study is that the results are based on a well‐defined, large group of patients with OSA prior to and 2 years after initiating PAP treatment.
The main concern is that there is no control group to prove causality between PAP use and the improved subjective or objective nasal obstruction. There were only nine patients without any PAP use at all, and with such a small group it is not possible to draw any conclusions.
Other limitations include not having a full polysomnography, but rather home sleep apnea testing, which evaluates breathing rather than sleep.
Subjective data have limitations, and self‐reported PAP use was used in the early home sleep apnea testing studies when objective data were not available. However, self‐reported data have, in a previous study, demonstrated 98.6% sensitivity and 45.1% specificity in separating full users from partial users of PAP treatment (Björnsdottir et al., 2015). There is another concern on subjective data in regard to nasal obstruction in relation to PAP. However, when investigating the differences in nasal obstruction between groups with different levels of PAP adherence, subjective data were chosen as no relation was found between objective data and level of PAP use.
The nasal cycle has an influence on nasal dimensions with variations within hours, which could be argued to limit the objective AR results. The effect of the nasal cycle is limited by adding the two valves together in TMCA. In terms of the parameter MCA‐min, the results are consistent with the other parameters. When finding improvements in nasal dimensions in a large population over a time of 2 years apart with p‐values of < 0.001, we believe that the impact of the nasal cycle on the results in the current study is most likely restricted.
It would have been interesting to perform AR during sleep and in the supine position, but this is not possible to achieve. One limitation could also be regression to the mean in terms of the smaller minimal cross‐section areas becoming larger after 2 years.
4.7. What is new? Clinical implications
The findings that nasal subjective and objective obstruction improve 2 years after the treatment start are of clinical interest when informing patients at the beginning of the PAP treatment.
Subjective nasal obstruction can be improved by adherence to PAP treatment, while patients with objective nasal obstruction should possibly be offered surgical treatment prior to initiating PAP treatment.
5. CONCLUSIONS
Subjective nasal obstruction improved 2 years after initiating PAP treatment for sleep apnea. There was an objective improvement, especially in patients with small nasal dimensions. Objectively small nasal volume at baseline was a predictor for becoming a non‐user of PAP. And, primarily, PAP treatment did not cause long‐term objective or subjective nasal obstruction.
CONFLICT OF INTERESTS
MV, MA, EB, AJ, TG and SJ: no conflict of interest. HH‐Strøm: received payments for lectures from the companies Nox Medical, Takeda, and ResMed outside the submitted work. ESA: part‐time consultant for Nox Medical, Reykjavik, Iceland, unrelated to manuscript, outside the submitted work.
AUTHOR CONTRIBUTIONS
MV: contributed to designing the calculations, did statistical calculations, took part in evaluating the results, and drafted the paper, and participated in all revisions of the paper with the co‐authors. MA: contributed to designing the calculations, took part in evaluating the results, and reviewed the manuscript, and participated in all revisions of the paper with the co‐authors. EB: contributed to designing the calculations, took part in evaluating the results, and reviewed the manuscript, and participated in all revisions of the paper with the co‐authors. HH‐S.: contributed to designing the calculations, took part in evaluating the results, and reviewed the manuscript, and participated in all revisions of the paper with the co‐authors. AJ: contributed to design of statistical analysis and final multiple logistic regression analysis. AIP: designed the study, took part in evaluating the results, and reviewed the manuscript, and participated in all revisions of the paper with the co‐authors. ESA: designed the study, contributed to designing the calculations, took part in evaluating the results, and reviewed the manuscript, and participated in all revisions of the paper with the co‐authors. TG: designed the study, contributed to designing the calculations, took part in evaluating the results, and reviewed the manuscript, and participated in all revisions of the paper with the co‐authors. SJ: designed the study, participated in data collection, contributed to designing the calculations, took part in evaluating the results, and reviewed the manuscript, and participated in all revisions of the paper with the co‐authors.
Supporting information
ACKNOWLEDGEMENTS
The authors are thankful to Sigrun Gudmundsdottir and Lovisa Gudmundsdottir, Sleep Department, Landspitali, Reykjavík, Iceland, for all their work with data collection; and Heidar Bergsson and Eyjolfur Sigurdsson for statistical preparation of the data. The authors would also like to show their gratitude to Greg Maislin, Bethany Staley, Brendan Keenan and the other staff at the Centre for Sleep and Circadian Neurobiology at the University of Pennsylvania, who helped analyse the sleep studies and other data of the cohort. Finally, the authors are thankful to Professor Robin L. Anderson, La Trobe University, and Olivia Newton‐John, Cancer Research Institute, for valuable review and English language proofreading.
Värendh M, Andersson M, Björnsdóttir E, et al. PAP treatment in patients with OSA does not induce long‐term nasal obstruction. J Sleep Res. 2019;28:e12768 10.1111/jsr.12768
Funding information
NIH‐supported project, grant number: R01HL072067. ACTA and FoU from Region Skåne.
REFERENCES
- André, R. F. , Vuyk, H. D. , Ahmed, A. , Graamans, K. , & Nolst Trenité, G. J. (2009). Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clinical Otolaryngology, 34, 518–525. 10.1111/j.1749-4486.2009.02042.x [DOI] [PubMed] [Google Scholar]
- Arnardottir, E. S. , Janson, C. , Bjornsdottir, E. , Benediktsdottir, B. , Juliusson, S. , Kuna, S. T. , …, Gislason, T. (2013). Nocturnal sweating – a common symptom of obstructive sleep apnoea: The Icelandic Sleep Apnoea Cohort. British Medical Journal Open, 3(5), e002795. 10.1136/bmjopen-2013-002795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir, E. S. , Maislin, G. , Jackson, N. , Schwab, R. J. , Benediktsdottir, B. , Teff, K. , …, Gislason, T. (2013). The role of obesity, different fat compartments and sleep apnea severity in circulating leptin levels: The Icelandic Sleep Apnea Cohort study. International Journal of Obesity, 37(6), 835–842. 10.1038/ijo.2012.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre, L. , Pezato, R. , Gasparini, H. , Haddad, F. , Gregório, L. C. , & Fujita, R. R. (2017). Acute impact of continuous positive airway pressure on nasal patency. International Forum of Allergy & Rhinology, 7, 712–717. 10.1002/alr.21948 [DOI] [PubMed] [Google Scholar]
- Björnsdóttir, E. , Janson, C. , Gíslason, T. , Sigurdsson, J. F. , Pack, A. I. , Gehrman, P. , & Benediktsdóttir, B. (2012). Insomnia in untreated sleep apnea patients compared to controls. Journal of Sleep Research, 21, 131–138. 10.1111/j.1365-2869.2011.00972.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsdóttir, E. , Janson, C. , Sigurdsson, J. F. , Gehrman, P. , Perlis, M. , Juliusson, S. , …, Benediktsdóttir, B. (2013). Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep, 36, 1901–1909. 10.5665/sleep.3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdottir, E. , Keenan, B. T. , Eysteinsdottir, B. , Arnardottir, E. S. , Janson, C. , Gislason, T. , … Benediktsdottir, B. (2015). Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. Journal of Sleep Research, 24, 328–338. 10.1111/jsr.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander, P. E. , Soirinsuo, M. , & Lohela, P. (1999). Nasopharyngeal symptoms in patients with obstructive sleep apnea syndrome. Effect of nasal CPAP treatment. Respiration, 66, 128–135. 10.1159/000029354 [DOI] [PubMed] [Google Scholar]
- Clement, P. A. , & Gordts, F. (2005). Consensus report on acoustic rhinometry and rhinomanometry. Rhinology, 43, 169–179. [PubMed] [Google Scholar]
- Engleman, H. M. , Asgari‐Jirhandeh, N. , McLeod, A. L. , Ramsay, C. F. , Deary, I. J. , & Douglas, N. J. (1996). Self‐reported use of CPAP and benefits of CPAP therapy: A patient survey. Chest, 109, 1470–1476. 10.1378/chest.109.6.1470 [DOI] [PubMed] [Google Scholar]
- Eysteinsdottir, B. , Gislason, T. , Pack, A. I. , Benediktsdottir, B. , Arnardottir, E. S. , Kuna, S. T. , & Björnsdottir, E. (2017). Insomnia complaints in lean patients with obstructive sleep apnea negatively affect positive airway pressure treatment adherence. Journal of Sleep Research, 26, 159–165. 10.1111/jsr.12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanari, P. , Burnet, H. , Zattara‐Hartmann, M. C. , Badier, M. , & Jammes, Y. (1999). Changes in airway resistance induced by nasal or oral intermittent positive pressure ventilation in normal individuals. European Respiratory Journal, 13, 867–872. 10.1034/j.1399-3003.1999.13d29.x [DOI] [PubMed] [Google Scholar]
- Gelardi, M. , Carbonara, G. , Maffezzoni, E. , Marvisi, M. , Quaranta, N. , & Ferri, R. (2012). Regular CPAP utilization reduces nasal inflammation assessed by nasal cytology in obstructive sleep apnea syndrome. Sleep Medicine, 13(7), 859–863. 10.1016/j.sleep.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Hoffstein, V. , Viner, S. , Mateika, S. , & Conway, J. (1992). Treatment of obstructive sleep apnea with nasal continuous positive airway pressure. Patient compliance, perception of benefits, and side effects. American Review of Respiratory Disease, 145, 841–845. 10.1164/ajrccm/145.4_Pt_1.841 [DOI] [PubMed] [Google Scholar]
- İriz, A. , Düzlü, M. , Köktürk, O. , Kemaloğlu, Y. K. , Eravcı, F. C. , Zorlu, M. E. , & Karamert, R. (2017). Does nasal congestion have a role in decreased resistance to regular CPAP usage? European Archives of Oto‐Rhino‐Laryngology, 274, 4031–4034. 10.1007/s00405-017-4744-z [DOI] [PubMed] [Google Scholar]
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep, 14, 540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- Kreivi, H.‐R.‐R. , Maasilta, P. , & Bachour, A. (2016). Persistence of upper‐airway symptoms during CPAP compromises adherence at 1 year. Respiratory Care, 61(5), 652–657. 10.4187/respcare.04113 [DOI] [PubMed] [Google Scholar]
- Kreivi, H.‐R.‐R. , Virkkula, P. , Lehto, J. , & Brander, P. (2010). Frequency of upper airway symptoms before and during continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Respiration, 80, 488–494. 10.1159/000321371 [DOI] [PubMed] [Google Scholar]
- Kribbs, N. B. , Pack, A. I. , Kline, L. R. , Smith, P. L. , Schwartz, A. R. , Schubert, N. M. , …, Dinges, D. F. (1993). Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. American Review of Respiratory Disease, 147, 887–895. 10.1164/ajrccm/147.4.887 [DOI] [PubMed] [Google Scholar]
- Li, H.‐Y. , Engleman, H. , Hsu, C.‐Y. , Izci, B. , Vennelle, M. , Cross, M. , & Douglas, N. J. (2005). Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea‐hypopnea syndrome. Sleep, 28, 1554–1559. 10.1093/sleep/28.12.1554 [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. , Coffey, M. , & Boyle, T. (1993). Effects of nasal airflow on breathing during sleep in normal humans. American Review of Respiratory Disease, 147, 620–623. 10.1164/ajrccm/147.3.620 [DOI] [PubMed] [Google Scholar]
- Partinen, M. , & Gislason, T. (1995). Basic Nordic Sleep Questionnaire (BNSQ): A quantitated measure of subjective sleep complaints. Journal of Sleep Research, 4, 150–155. 10.1111/j.1365-2869.1995.tb00205.x [DOI] [PubMed] [Google Scholar]
- Pépin, J. L. , Leger, P. , Veale, D. , Langevin, B. , Robert, D. , & Lévy, P. (1995). Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest, 107, 375–381. 10.1378/chest.107.2.375 [DOI] [PubMed] [Google Scholar]
- Pien, G. W. , Ye, L. , Keenan, B. T. , Maislin, G. , Björnsdóttir, E. , Arnardottir, E. S. , …, Pack, A. I. (2018). Changing faces of OSA: Treatment effects by cluster designation in the Icelandic Sleep Apnea Cohort. Sleep, 41 10.1093/sleep/zsx201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka, C. , Vuralkan, E. , Fırat, İ. H. , Alicura, S. , Hücümenoğlu, S. , Akın, İ. , …, Gökler, A. (2012). The effects of CPAP on nasal mucosa in patients with obstructive sleep apnea. European Archives of Oto‐Rhino‐Laryngology, 269(9), 2065–2067. [DOI] [PubMed] [Google Scholar]
- Sugiura, T. , Noda, A. , Nakata, S. , Yasuda, Y. , Soga, T. , Miyata, S. , …, Koike, Y. (2006). Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration, 74, 56–60. 10.1159/000089836 [DOI] [PubMed] [Google Scholar]
- Värendh, M. , Andersson, M. , Bjørnsdottir, E. , Hrubos‐Strøm, H. , Johannisson, A. , Arnardottir, E. S. , …, Juliusson, S. (2018). Nocturnal nasal obstruction is frequent and reduces sleep quality in patients with obstructive sleep apnea. Journal of Sleep Research, 27(4), e12631 10.1111/jsr.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhorn, R. E. , Herrick, T. W. , Nguyen, M. C. , O’Donnell, A. E. , Sodero, J. , & Potolicchio, S. J. (1990). Long‐term compliance with nasal continuous positive airway pressure therapy of obstructive sleep apnea. Chest, 97, 33–38. 10.1378/chest.97.1.33 [DOI] [PubMed] [Google Scholar]
- Ware, J. , Kosinski, M. , & Keller, S. D. (1996). A 12‐Item Short‐Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- Ye, L. , Pien, G. W. , Ratcliffe, S. J. , Björnsdottir, E. , Arnardottir, E. S. , Pack, A. I. , …, Gislason, T. (2014). The different clinical faces of obstructive sleep apnoea: A cluster analysis. European Respiratory Journal, 44, 1600–1607. 10.1183/09031936.00032314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


