Summary
Nonalcoholic fatty liver disease (NAFLD) is a major cause of chronic liver disease, characterized by hepatic fat accumulation and possible development of inflammation, fibrosis, and cancer. The ketogenic diet (KD), with its drastic carbohydrate reduction, is a now popular weight loss intervention, despite safety concerns on a possible association with fatty liver. However, KDs were also reported to be beneficial on hepatic pathology, with ketone bodies recently proposed as effective modulators of inflammation and fibrosis. If the beneficial impact of weight loss on NAFLD is established, less is known on the effect of macronutrient distribution on such outcome. In a hypocaloric regimen, the latter seems not to be crucial, whereas at higher calorie intake, macronutrient ratio and, theoretically, ketosis, may become important. KDs could positively impact NAFLD for their very low carbohydrate content, and whether ketosis plays an additional role is unknown. Indeed, several mechanisms may directly link ketosis and NAFLD improvement, and elucidating these aspects would pave the way for new therapeutic strategies. We herein aimed at providing an accurate revision of current literature on KDs and NAFLD, focusing on clinical evidence, metabolic pathways involved, and strict categorization of dietary interventions.
Keywords: liver steatosis, low carbohydrate diet, obesity, very low calorie diet
1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), defined as the spectrum of liver diseases not associated with significant alcohol intake or other known causes of hepatic fat accumulation, comprises “simple” fatty liver up to severe fibrosis and inflammation, and is nowadays the second cause of liver transplant in the United States. 1 Given the alarming increase in prevalence of this condition, research has lately focused on several possible interventions to tame the pandemic, with nutritional ketosis proposed as a possible treatment option.
Ketosis is achieved when less than 50 g of carbohydrates per day are introduced, with either restricted or unrestricted fat and calorie intake. 2 The ketogenic diet (KDs) was first introduced as an alternative treatment for patients with refractory epilepsy, and its adapted versions later became widely popular to treat obesity thanks to their excellent ability to induce satiety and weight loss. 3 Despite their success, some authors have raised safety concerns for KDs—especially those with a high fat content—for their reported effects on serum cholesterol levels 4 and liver function, 5 , 6 implying an influence in the onset/worsening of hepatic steatosis.
Conversely, the beneficial effects of KDs on NAFLD have also been reviewed, but there has been partial lack of diet categorization 7 , 8 and a predominant evaluation of basic science‐oriented evidence. 9 Because recent studies reported an interesting action of ketone bodies in reducing inflammation and metabolic complications involved in NAFLD pathogenesis, 10 , 11 , 12 , 13 , 14 , 15 we herein aimed at providing a revision of current literature focusing on clinical evidence relative to hepatic outcomes, metabolic pathways possibly involved, and strict categorization of dietary interventions, in order to aid the tailoring of dietary interventions to the patient's needs on the one hand and direct future research to better understand the underlying molecular mechanisms on the other.
1.1. What is ketosis?
Human metabolism is characterized by great flexibility, able as it is to utilize different metabolic substrates depending of their availability to obtain energy. Ketogenesis is a metabolic process leading to the production of ketone bodies—acetoacetate, beta‐hydroxybutyrate, acetone—an alternative fat‐derived metabolic fuel for vital organs in states of nutrient deprivation, such as fasting, glucose deprivation, and prolonged physical exercise.
Ketogenesis occurs in the hepatic mitochondrial matrix, where fatty acids (FAs), released during low glucose availability states from adipose tissue by adipokine signaling of high glucagon and epinephrine levels and low insulin levels, are broken down into acetyl‐CoA via β‐oxidation. Under normal conditions, acetyl‐CoA is further oxidized by the citric acid cycle (TCA/Krebs cycle) and then by the mitochondrial electron transport chain to release energy. However, if the amounts of acetyl‐CoA generated through FA β‐oxidation challenge the processing capacity of the TCA cycle, such as in case of prolonged fasting, glucose deprivation, and intense physical exercise, acetyl‐CoA is shifted to the biosynthesis of ketone bodies. In particular, acetoacetate and β‐hydroxybutyrate are the two ketones used by the body for energy. Once they reach extrahepatic tissues, they are converted back to acetyl‐CoA, which goes through the TCA cycle, and after oxidative phosphorylation, it produces 22 ATP molecules per unit. Acetone is not converted back to acetyl‐CoA, and it is either excreted through urine or exhaled. Physiologically, ketosis takes place in the human liver throughout the day, with a ketones production of up to 300 g/day. 16 Timing, rates, and speed of ketones production are orchestrated by a sophisticated and well‐integrated biochemical and hormonal complex and may vary based on how intense and prolonged the ketogenic stimulus is, sometimes leading to pathologic states such as diabetic ketoacidosis. 17
It is noteworthy to mention that prolonged fasting was ordinary during human evolution, especially in the Paleolithic, when social structure was based on small clusters of hunter‐gatherers. The discovery of agriculture in the Neolithic shifted the society dynamics towards large communities of farmers, leading to progressively larger abundance of food. The critical turning point came with the industrial revolution of the 1850s, which progressively led to easy accessibility to processed foods and to a diffusion of sedentary occupations requiring less energy to be expended. 18 Although evidences in this regard are mostly deductive, it is reasonable to assume that ketosis has been a crucial survival mechanism in humans before society became as it is now. These considerations become interesting if we think that the growing global widespread of obesity has followed an opposite trend to that of prolonged fasting behaviors.
1.2. What is a KD?
Considering the metabolic advantage of fat‐derived ketones utilization for energy purposes, in recent years, research has focused on the nutritional induction of ketosis. A first challenge that may occur in the study of nutritional ketosis is the definition of what is really ketogenic. There is common agreement on the assumption that a KD consists of a very low carbohydrate content, limited to 5–10% of total kcal daily intake, which consists in 50—and sometime 20—grams per day, 2 , 19 although the specific macronutrient composition may vary. Different types of nutritional ketosis have been defined in recent years, with or without calorie restriction, although a clear and commonly accepted classification does not exist yet (Table 1).
TABLE 1.
Classification of diets based on calorie, carbohydrate, fat daily intake and induced ketosis
| kcal/day | CHO/day | Fat/day | Ketosis | |
|---|---|---|---|---|
| High fat ketogenic diet (HFKD) | Usually unrestricted | <20–50 g | Unrestricted | Yes |
| Very low calorie ketogenic diet (VLCKD) | <800 kcal | <20–50 g | Low | Yes |
| Very low calorie diet (VLCD) | <800 kcal | >20–50 g | Low | Usually not |
| Low carbohydrate diet (LCD) | Variable | <130 g | Low | No |
High fat KDs (HFKD), commonly simply referred as KDs, are traditionally characterized by a restriction of carbohydrates (CHO) < 50 g per day with unrestricted intake of fat, a relative increase of protein (0.8–1.2 g per day), and ad libitum caloric intake, although the satiety effects tend to limit total calorie intake. 20 Despite having been introduced first in a neurological context, they are now the most widespread weight loss‐oriented KD type. Since then, moderate calorie and fat restriction variants have been introduced, together with an increased attention to the quality of fats.
A relatively new addition that enrichens the KD scenario is the very low calorie KD (VLCKD), a subtype of very low calorie diet (VLCD). This dietary pattern is far more popular in Europe than the United States, possibly due to different eating behavior and cultural buffering. VLCDs have extremely low daily food energy consumption (400–800 kcal/day). Apparently, such an extreme caloric restriction, if not accompanied by an appropriate carbohydrate intake reduction, is not sufficient to induce a metabolic shift towards ketosis. 21 The European Food Safety Authority (EFSA) stated that VLCDs are defined ketogenic when containing an amount of protein of 1.2–1.4 g/kg of ideal body weight (around 75 g/day), a very limited carbohydrate content (<30–50 g/day, <5–10%), a fixed amount of fats (20 g/day, 15–30%, mainly from olive oil), and micronutrients to fulfill the Dietary Reference Intake. 21 However, it should be acknowledged that the threshold under which one enters a ketogenic state is subjective, and only beta‐hydroxybutyrate testing would finally confirm this. It is therefore not possible to be conclusive on the possibility that VCLDs might cause ketosis in selected individuals, making the line between starvation and nutritional ketosis fine.
Often erroneously classified as KDs, low carbohydrate diets (LCDs) do not necessarily belong to this group, with a carbohydrate content that is typically less than 130 g/day. 22 , 23 LCD is not a new dietary concept, as it was first introduced in 1863 by William Banting, a formerly English undertaker and coffin maker with obesity, who published “Letter on Corpulence Addressed to the Public,” in which he described a diet for weight control giving up bread, butter, milk, sugar, beer, and potatoes. 24 In recent years, LCDs were shown to be effective in the treatment of obesity, and apart from significantly reducing weight, they can also effectively improve the blood lipid profile and insulin resistance. 23 Moreover, the American Diabetes Association has also confirmed the effectiveness of LCDs in reducing weight, improving blood glucose and lipids in patients with type 2 diabetes mellitus (T2DM). 25 For the purpose of a correct pathophysiological understanding of nutritional therapies, it appears necessary to clearly distinguish the beneficial effects of reduction in carbohydrate intake with and without induction of ketosis. We therefore classified KDs as those with a CHO content below 50 g and LCDs as those with one of 50–130 g/day. We did not include in this revision studies involving diets defined as LCD by the authors but with a content >130 g/day in order to limit heterogeneity.
Finally, an emerging nutritional approach capable of inducing a certain degree of ketosis is intermittent fasting (IF), which involves a great variety of eating patterns in which no or few calories are consumed for time periods that can range from 12 h to several days. For example, alternate‐day fasting is a form of IF where individuals alternate between not consuming any calories for 1 day and eating without restriction the next. 26 Similarly, alternate‐day modified fasting is a form of IF in which individuals alternate between consuming few calories 1 day (<25% energy needs) and eating without restriction the next. Lately, IF has become a popular alternative to continuous calorie restriction, and it may deliver similar benefits in terms of weight loss and cardiometabolic health. 26 , 27 , 28 , 29 , 30 One key mechanism responsible for many of these beneficial effects appears to be the body's preferential shift from glycogenolysis‐derived glucose to lipolysis‐derived ketones. 31
1.3. Part of the problem …
No matter the modality through which ketosis is achieved, several concerns have been raised regarding safety, especially long term, in view of the described detrimental effect of the high dietary fat characteristic of HFKDs possibly leading to NAFLD. 32 In fact, it is well known that in both wild type 33 , 34 , 35 and T2DM mice models, 36 KD may promote hepatic steatosis and, in some cases, inflammation. However, results described in rodent models consuming the KD are scarcely translational, as the one used in animals is significantly different to that consumed by human subjects. The first, in fact, is extremely high in saturated fats (lard and butter), markedly low in protein content (8.6%), and insufficient in choline, as opposed to the latter, that is moderately high in lipids with unsaturated fat consumption usually encouraged, it has a normal protein content, and it does not lack in choline. This substance is indeed crucial for VLDL production, and its lack exacerbates liver lipotoxicity, with the induction of inflammation and fibrosis. 37 Some clinical studies evaluating the effects of HFKD on epileptic children initially suggested a potential hepatotoxic role of these diets. Aminotransferase levels were screened and reported in many clinical studies, which included epileptic patients treated with HFKD. 38 , 39 , 40 , 41 In some of them, aminotransferase levels remained unchanged during KD treatment 40 , 41 ; in others, isolated cases of liver enzymes rise were described, 38 , 39 , 42 , 43 although liver injury was partially explained by the use of valproate. 42 , 43
1.4. … or part of the solution?
Nevertheless, significant evidence suggests a hepatoprotective role when applied to the fields of obesity and the metabolic syndrome. NAFLD, the hepatic reflex of excess body fat, has been linked to insulin resistance, increased oxidative stress, and inflammation, and it can comprise significant fibrosis. First of all, KDs, thanks to their very low carbohydrate content, decrease insulin levels, with subsequent reduction in lipogenesis and increase in FA oxidation rate. 44 Recent evidence also reports that such a drastic reduction of dietary carbohydrates leads to a gut microbiota shift that in turn induces folate production ameliorating lipid metabolism and limiting oxidative stress and inflammation. 45 Ketone bodies, produced in response to carbohydrate restriction, have also been proven to induce satiety, which leads to total calorie intake limitation and weight loss facilitation, although the molecular mechanisms underlying this observed effect have yet to be uncovered. 20 Moreover, researchers' attention has lately focused on “noncanonical” direct signaling roles of ketones, which may play a role in the protection against and treatment of metabolic syndrome, including NAFLD. Intriguingly, ketones have been proposed as direct and indirect epigenetic modifiers of key histones that serve as dynamic regulators of chromatin architecture and gene transcription. In particular, Shimazu et al. reported that βOHB increased global histone acetylation with subsequent transcriptional changes in mouse models, including genes encoding oxidative stress resistance factors, suggesting that βOHB expression may promote oxidative stress resistance. 12 Moreover, βOHB is an endogenous activator of GPR109A, abundantly expressed by many immune system cells, including macrophages or monocytes. 46 Anti‐inflammatory roles of GPR109A ligands in atherosclerosis, obesity, inflammatory bowel disease, neurological disease, and cancer have been reported. 11 βHB has also been linked to inhibition of inflammasomes, in particular NLRP3, 15 a key signaling platform that activates proinflammatory cytokines, in particular IL‐1β and IL‐18, strongly associated with obesity and the pathogenesis of IR and T2DM. 13 Intriguingly, growing evidence supports a central role of NLRP3‐induced pyroptosis in mouse models of liver fibrosis 10 , 14 (Figure 1). Taken together, this body of evidence may suggest a protective role of ketones in NAFLD pathogenesis that possibly goes beyond simple weight loss.
FIGURE 1.
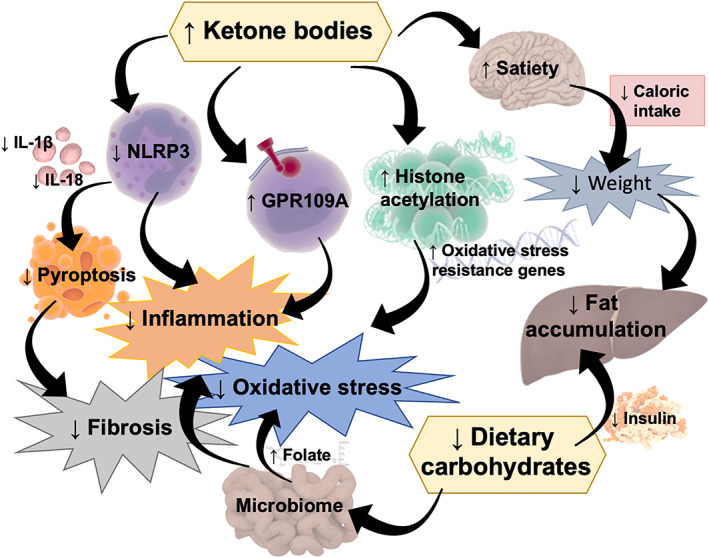
Mechanisms through which a ketogenic diet (KD) might protect against nonalcoholic fatty liver disease (NAFLD). A drastic decrease in carbohydrate intake leads to (1) decreased insulin levels with subsequent increase in fat oxidation and reduced lipogenesis and (2) a microbiome shift with increased folate production and subsequent inflammatory and oxidative stress limitation. Ketone bodies may also induce (1) satiety, leading to food intake limitation; (2) histone acetylation able to promote oxidative stress resistance; (3) activation of GPR109A, that is reported to have an anti‐inflammatory effect; and (4) inhibition of NLRP3, a key signaling platform that activates pro‐inflammatory cytokines, such as IL‐1β and IL‐18, and fibrosis inducing pyroptosis. ↓, decreased; ↑, increased; NLRP3, NLR family pyrin domain containing 3; IL, interleukin; GPR, G protein‐coupled receptor. Some illustrations adapted from Somersault18:24, CC BY‐NC‐SA 4.0
2. MATERIALS AND METHODS
Current literature was reviewed to assess the impact of different dietary patterns possibly leading to nutritional ketosis on NAFLD. The research was conducted on PubMed and Embase by using the following keywords: “ketogenic diet” or “Atkins” or “High fat low carbohydrate” or “very low calorie diet” or “very low calorie ketogenic diet” or “very low energy diet” or “low energy diet” or “very low carbohydrate diet” or “low carbohydrate diet” or “Intermittent fasting” and “Non‐alcoholic fatty liver disease” or “hepatic fat” or “fatty liver” or “obesity” and related acronyms. We included all studies meeting the following criteria: (1) observational prospective and retrospective studies, case–control studies, cohort studies, randomized clinical trials (RCTs); (2) reported biochemical, histological, or imaging signs relative to outcome measures of NAFLD; (3) female only, male only, and both genders enrolled; (4) no age limitation; (5) sufficient detail about nutritional intervention reported; and (6) studies written in English.
Three independent reviewers (R. T., M. W., and R. R.) evaluated the title, abstract, and keywords of selected papers, and full articles were retrieved when deemed necessary. References of retrieved articles were assessed for additional studies. All reports were evaluated by two reviewers (R. T. and R. R.) for inclusion, and a third reviewer resolved eventual disagreements (M. W.). One reviewer (R. T.) extracted data from selected studies, which were cross‐checked by two other reviewers (M. W. and L. G.). Data extraction included patient gender, mean age, inclusion and exclusion criteria, sample size, study design, dietary intervention total calorie intake and macronutrient composition, and outcomes (biochemistry, histology, imaging).
The authors of the included studies were contacted for missing values where required. Studies whose diet macronutrient composition was not obtained neither through study protocol/manuscript/direct contact with investigator were excluded.
A total of 1386 studies were identified through database search and reference lists of retrieved articles. After removal of 1232 studies based on title and abstract, 154 full text articles were assessed and 21 included in the present study (Figure 2).
FIGURE 2.
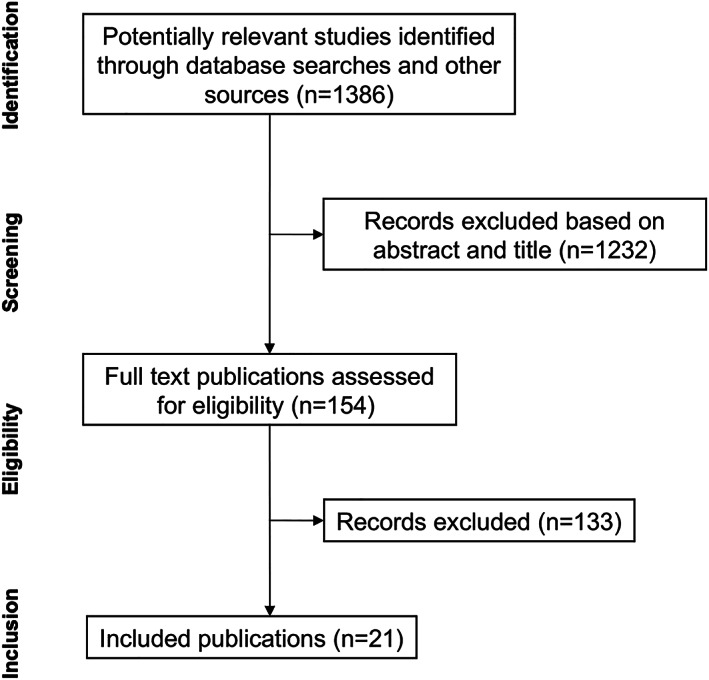
Flow chart of publications selection
3. RESULTS
3.1. High fat KDs
Commonly referred to simply as KDs, HFKD imply a carbohydrate (CHO) content of <20–50 g/day and may be mildly hypocaloric, normocaloric, or unrestricted, with a progressive increase in fat content. Tendler et al. recruited five patients with obesity and a histological diagnosis of NAFLD and subjected them to a calorie unrestricted HFKD for 6 months. Besides a general weight loss of 10.9%, improvements in steatosis, necroinflammatory grade, and fibrosis were described in four out of five patients at the end of the study. The documented histopathological worsening of Patient 5 was hypothesized to be a consequence of dietary nonadherence, as this patient did not lose weight and had only one biochemical evidence of ketosis during the 6‐month study. 47 In another prospective, 12‐week study, 14 overweight male patients diagnosed with metabolic syndrome and with ALT level > 40 UI were prescribed a Mediterranean HFKD, unrestricted in calorie intake and high in unsaturated fats such as omega 3 fish oil and extra virgin olive oil. At the end of the study, there was an extremely significant improvement in body weight, aminotransferase level, ultrasound (US)‐derived steatosis degree (complete fatty liver regression was observed in 21.4% of the patients, and an overall reduction was found in 92.86% of the patients). 48 Similar results were demonstrated in a cohort of 27 patients with morbid obesity (body mass index [BMI] of 45.2 kg/m2) in whom US‐measured hepatic lobe volume decreased by 19.8% after 4 weeks of prebariatric HFKD at ~1250 kcal/day (CHO 4%, fat 71%, protein 25%). 49 Mardinoglu et al. reported a 2‐week intervention with an HFKD (3115 kcal/day, CHO 20–30 g/day, fat 241 g/day) in 17 patients diagnosed with obesity and NAFLD. Despite the small weight loss, a significant liver fat reduction assessed by MRS was observed together with a concomitant decrease in hepatic de novo lipogenesis genes 45 (Table 2).
TABLE 2.
Observational studies evaluating the effect of diets with different daily calorie intake and macronutrient composition on hepatic outcomes
| Author | Country | n | Subjects | Duration | Intervention | kcal/day | CHO/day (g) | Fat/day (g) | Biochemistry | Imaging | Histology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tendler et al. 47 | USA | 5 | Obese, NAFLD | 6 months | HFKD | Unr | <20 | Unr | n/a | n/a | Improved |
| Perez‐Guisado et al. 48 | Spain | 14 | MS, NAFLD | 3 months | HFKD | Unr | <30 | n/a | Improved | Improved (US) | n/a |
| Schiavo et al 49 | Italy | 27 | Obese, NAFLD | 1 month | HFKD | 1200 | 12 | 91 | Improved | Improved (US) | n/a |
| Mardinoglu et al 45 | Sweden | 17 | MS, NAFLD | 2 weeks | HFKD | 3115 | 20–30 | 241* | Improved | Improved (MRS) | Improved |
| Paoli et al 50 | Italy | 106 | Obese | 6 weeks | VLCKD | 1098 | 34 | 63 | Unchanged | n/a | n/a |
| Paoli et al 44 | Italy | 81 | Obese | 20 days# | VLCKD | 976 | 30 | 51 | Unchanged | n/a | n/a |
| Basciani et al 51 | Italy | 24 | Obese | 6 months | VLCKD | 700 | 50 | 10^ | Improved | n/a | n/a |
| Leonetti et al 52 | Italy | 50 | Obese | 10 days# | VLCKD | 577 | 15 | 24 | Unchanged | Improved (US) | n/a |
| Colles et al 53 | USA | 32 | Obese | 12 weeks | VLCKD | 456 | 45 | 7 | Unchanged | Improved (CT, MRI) | n/a |
| Bruci et al 54 | Italy | 92 | Obese | 8 weeks#~ | VLCKD | 450–800 | 20–50 | 15–30 | Unchanged | n/a | n/a |
| Benjaminov et al 55 | Israel | 14 | Obese | 4 weeks | LCD | ~1520 | ~54 | ~94 | Unchanged | Trend improved (CT) | |
| Lewis et al 56 | Australia | 18 | Obese | 6 weeks | VLCD | 450–800 | n/a | n/a | n/a | Improved (MRS) | n/a |
| Schwenger et al 6 | Canada | 139 | Obese | 1 week | VLCD | 900 | 67 | 30 | n/a | n/a | Improved |
| Browning et al 57 | USA | 18 | Healthy | 48 hours | Fasting | 0 | 0 | 0 | Unchanged | Unchanged (MRS) | n/a |
| Wilhelmi de Toledo et al 58 | Germany | 1422 | Overweight | 4–21 days | Fasting | 200–250 | 25–35 | n/a | AST/ALT increase | n/a | n/a |
Note. Data are expressed as mean; in case of studies in which data on macronutrient compositions were published in percentages (*), the relative grams of carbohydrate and fat were calculated considering the following assumption: 1 g of CHO = 3.8 kcal; 1 g of fat = 9.3 kcal, 1 g of protein = 4 kcal. When possible, authors were contacted to apply for unpublished data relating to diets composition (^). In case of studies (#) using multistep diet regimens, only the duration of the intervention phase of interest is reported. (~): reported data have a significant standard deviation, especially relative to CHO intake (54 ± 22 g/day); the study has been therefore categorized as nonketogenic.
Abbreviations: HFKD: high fat ketogenic diet; VLCKD: very low calorie ketogenic diet; LCD: low carbohydrate diet; VLCD: very low calorie diet; NAFLD: nonalcoholic fatty liver disease; MS: metabolic syndrome; unr: unrestricted; n/a: not available data; US: ultrasound examination; MRS: magnetic resonance spectroscopy; CT: computerized tomography; kcal/day: daily kilocalorie intake; CHO/day (g): carbohydrate daily intake in grams; fat/day (g): fat daily intake in grams.
Kirk et al. conducted an RCT study on 22 subjects with obesity on an HFKD (75% fat; CHO <60 g/day, ≈10% of daily intake;) or low fat high CHO (LFHC) (20% fat; CHO >180 g/day, ≈65% of daily intake) hypocaloric diet (1000 kcal daily energy deficit, based on an estimated daily energy requirement). At 48 h, intrahepatic triglyceride content decreased more in the HFKD than the LFHC group (29.6 ± 4.8% vs. 8.9 ± 1.4%; p < 0.05) but was comparable after achieving 7% weight loss in 11 weeks (HFKD vs. LFHC diet, 38.0 ± 4.5% and 44.5 ± 13.5%, respectively). 59 Another RCT compared an HFKD (fat 105 ± 44 g/day, ≈60%) to a hypocaloric LFHC diet (1325 ± 180 kcal/day; CHO 165.62 ± 33 g/day, ≈50%; fat 49 ± 9 g/day, ≈35%,). Despite the slight difference in energy intake, weight loss in both diets was similar after 2 weeks, whereas the decrease in MRI measured hepatic triglyceride (TG) content was greater in the HFKD group, which indeed had a higher calorie content 57 (Table 3).
TABLE 3.
Randomized controlled trial evaluating the effect of diets with different daily calorie intake and macronutrient composition on liver outcome
| Author | Country | n | Subjects | Duration | Intervention | Kcal/d | CHO/day (g) | Fat/day (g) | Control | Kcal/d | CHO/day (g) | Fat/day (g) | Biochemistry | Imaging |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kirk et al 59 | USA | 22 | Obese, NAFLD | 11 weeks | HFKD | −1000 | ≤60 | 81* | HCD | −1000 | ≥180 | 12 | n/a | Unchanged (MRS) |
| Browning 57 | USA | 18 | Obese, NAFLD | 2 weeks | HFKD | 1553 | 26 | 105 | LCD | 1325 | 169 | 49 | n/a | Improved (MRS) |
| Ministrini et al 60 | Italy | 52 | Obese | 25 days | VLCKD | <800 | <50 | <30 | Nutritional advice | n/a | n/a | n/a | Unchanged | Improved (US) |
| Haufe et al 61 | Germany | 170 | Overweight | 6 months | LCD | 1544^ | ≤90 | 50* | LFD | 1700 | ≥90 | 37 | Improved | Improved (MRS) |
| Westerbacka et al 62 | Finland | 10 | Obese | 2 weeks | LCD | ~1260 | 97* | 78* | LFD | ~1260 | ~192 | ~22 | n/a | Worsened (MRS) |
| Lin et al 63 | Taiwan | 132 | Obese | 12 weeks | VLCD | 450 | n/a | n/a | VLCD | 800 | n/a | n/a | n/a | Both improved (US) |
Note. Data are expressed in means; in case of studies in which data on macronutrient compositions were published in percentages (*), the relative grams of carbohydrate and fat were calculated considering the following assumption: 1 g of CHO = 3.8 kcal; 1 g of fat = 9.3 kcal, 1 g of protein = 4 kcal. When possible, authors were contacted to apply for unpublished data relating to diets composition (^). In case of studies (#) using multistep diet regimens, only the duration of the intervention phase of interest is reported. ~: mean total daily calorie intake was calculated from the daily caloric intake for fat free mass, determined as: body weight (kg)/100 × (100‐body fat %).
Abbreviations: HFKD: high fat ketogenic diet; VLCKD: very low calorie ketogenic diet; LCD: low carbohydrate diet; VLCD: very low calorie diet; NAFLD: nonalcoholic fatty liver disease; IR: insulin resistance; n/a: not available data; US: ultrasound examination; MRS: magnetic resonance spectroscopy; kcal/day: daily kilocalorie intake; CHO/day (g): carbohydrate daily intake in grams; fat/day (g): fat daily intake in grams.
Altogether, these data suggest a positive effect of HFKDs on NAFLD both in the short and medium term, independent of calorie and fat intake. In particular, HFKDs appear to have greater and faster impact on liver fat content compared with isocaloric high CHO diets and to hypocaloric high CHO diets in the short term, with a possible flattening of the discrepancies as time progresses.
3.2. Very low calorie KDs
In a 6‐week study conducted by Paoli et al., 106 patients with obesity or overweight were sequentially placed on a 21‐day VLCKD step with significant CHO restriction (<34 g/day) and an equal duration LCD (CHO 74 g/day). At the end of the study, no significant changes in AST and ALT levels were detected despite weight loss and body composition improvement. 50 Another 12‐month multiphase dietetic protocol study carried out by the same group and including two VLCKD steps resulted in significant weight loss (BMI from 35.42 ± 4.11 to 30.27 ± 3.58 kg/m2) for the majority of subjects (88.25%) followed by successful maintenance. No significant changes in ALT, AST, or GGT were recorded. Similar results were confirmed in a real‐life setting, where 92 patients with obesity underwent a multistep dietary intervention including a VLCKD phase lasting approximately 8 weeks, and beyond the significant weight loss, no significant change in liver function was reported. 54 Conversely, we conducted a 6‐month study on 24 patients with obesity placed on a VLCKD with subsequent gradual CHO reintroduction, and a significant serum AST, ALT, and triglyceride decrease were detected at the end of the dietary intervention. 51 In another 30‐day prospective study conducted on 50 subjects with obesity candidates to bariatric surgery that were placed on a sequential diet including a 10‐day VLCKD step (CHO 15 g/day), liver steatosis improvement and a mean 30% reduction in hepatic volume were reported. 52 Similar results were shown in 32 patients with obesity placed on a VLCKD (CHO 45 g/day) for 12 weeks. At the end of the study period, CT‐ and MRI‐derived decrease in liver size was 28.7%, with 80% of it occurring in the first 2 weeks 53 (Table 2). Pointing towards the same direction, another study comparing VLCKD and simple dietary counselling showed better results with regard to US‐derived decrease in liver steatosis and GGT levels reduction 60 (Table 3).
The majority of the studies evaluating the effects of VLCKDs are prospective and short term, as VLCKDs are intrinsically hard to maintain for longer periods of time. Moreover, given the extreme caloric restriction, VLCKD steps only last 2–3 weeks, and most often, outcome measures are assessed at the end of the study rather than at the end of the most restricted first step, making overall results challenging to interpret. Paoli et al. and Bruci et al. do not report significant biochemical improvement in liver function, conversely to what reported by all other studies. This could be due to the relatively low baseline BMI and AST/ALT levels of the study cohort, suggesting that the enrolled patients might not have had NAFLD to start with.
3.3. Low‐carbohydrate non‐KDs
The evaluation of the hepatic effects of low‐carbohydrate nonketogenic (CHO content 50–130 g/day) diets, often defined as LCDs, is complicated by the great variety of dietary composition of this category. A 4‐week hypocaloric (1520 ± 285 kcal/day) LCD diet was reported to be effective in inducing weight loss and decreased US‐measured liver volume in patients with obesity affected by NAFLD and candidate to bariatric surgery. A trend improvement in fat content was also recorded 55 (Table 2). An RCT study comparing the effect of LCD and low‐fat diet (LFD) on liver fat content reported a greater decrease in MRI‐measured hepatic fat content in the LCD group. 61 On the contrary, Westerbacka et al. found that a 2‐week LFD diet was more effective in reducing proton spectroscopy‐measured liver fat content compared with an isocaloric LCD diet in 10 overweight premenopausal women, with no change in weight being described 62 (Table 3).
Evidence on hepatic outcomes in LCDs is extremely heterogeneous, most likely due to the great variety in dietary composition and study duration, together with different means of assessment. However, most studies suggest that, among LCD and LFD, calorie intake is primarily responsible in determining liver pathology improvement, and a relatively small difference in macronutrient distribution might not be sufficient to observe differential effects.
3.4. Very low calorie diets
Little evidence is available on the hepatic effect of VLCDs. A prospective study has shown a significant decrease in US‐derived liver volume and fat content after 6 weeks of VLCD. 56 Schwenger et al. retrospectively reported a lower prevalence of histologically confirmed NAFLD in a cohort of patients with obesity undergoing a 1‐week prebariatric VLCD compared with what reported in literature 6 (Table 2). A 12‐week study conducted on Taiwanese subjects with obesity placed on two different VLCDs (450 and 800 kcal) were equally effective in reducing weight and degree of steatosis as assessed by ultrasound 63 (Table 3).
Altogether, available evidence suggests that such an extreme caloric restriction is effective in improving liver conditions in subjects with obesity, although the studies conducted so far are only short term, and dietary interventions are incompletely described and potentially leading to ketosis, which was unassessed, making the interpretation of results potentially misleading.
3.5. Intermittent fasting
Fasting is a strong trigger to ketosis. IF regimens (IFRs) have gained the attention of researchers and nutritionists because they have been suggested to be a valid alternative to continuous calorie restriction. Anton et al. classified the variety of eating patterns involving restricted food intake or fasting in time‐restricted feeding (fasting for some hours a day), alternate days fasting (fasting every second day) or periodic fasting (fasting for some days every week), reviewing how the different regimens induce the switch to ketogenic metabolism. 31 The large majority of studies evaluating the effects of IFRs only focused on body composition and glucometabolic outcomes both short and long term, showing equal ability to induce weight loss and decrease insulin resistance in patients with obesity compared with simple calorie restriction. 31 A study conducted by Browning et al. investigating the MRI‐derived intrahepatic triglyceride content modification of 18 healthy subjects upon a 48‐hr fast showed a sexually dimorphic response of liver lipid accumulation, where men experienced a modest but significant increase (p = 0.043), as opposed to women who trended towards decrease (p = 0.092). 57 Wilhelmi de Toledo et al. evaluated 1422 subjects participating in a fasting program consisting of fasting periods of 4–21 days according to the Buchinger guidelines over 12 months. Daily caloric intake was 200–250 kcal during fasting periods, whose lengths were 5, 10, 15, or 20 ± 2 days. Besides overall significant reductions in weight, abdominal circumference, and blood pressure (p < 0.001), biochemical liver function indices showed a significant AST and ALT rise throughout the fast without difference between groups, although the values at baseline and at the end remained within normal ranges. Conversely, a more significant decrease of GGT and Alkaline phosphatase were observed in the longer fasting groups 58 (Table 2).
Evidence on the hepatic effects of IFRs are too few and controversial to draw definitive conclusions. However, recent evidence suggests that IFRs leads to ketosis and metabolic improvement likely to cause amelioration of NAFLD. 64 , 65 Targeted clinical studies are therefore needed to confirm whether the equal ability versus simple caloric restriction observed regarding body composition and glucometabolic outcomes is also true regarding liver function and pathology.
4. CONCLUSION
Whether it is acknowledged that weight loss is associated with amelioration of NAFLD, 66 , 67 less is known relative to the effect of macronutrient distribution on such outcome. 68 Virtually, all reviewed studies assessing liver fat content report positive results after VLCDs, VLCKDs, and HFKDs, with inconclusive results after LCDs diets and IFRs. It should be acknowledged that the high heterogeneity of the reports precluded a meta‐analytic approach. Moreover, many of the cited studies involved less than 50 individuals, limiting the generalization of results to a wider NAFLD population. Altogether, these data suggest that both caloric restriction and macronutrient distribution play a role that might be either additive or synergistic. The common belief that increasing dietary fat intake invariably leads to fatty liver and prevents fat mass loss has been recently proven wrong by an elegant experiment, showing that a normocaloric HFKD inhibits de novo lipogenesis and induces FA oxidation, leading to weight loss and reduced hepatic fat content. 69 On the contrary, a hypercaloric balanced diet decreases intrahepatic FA oxidation and increases de novo lipogenesis primarily from carbohydrate, and not lipid substrates, leading to NAFLD development. 70 At very low caloric intake, macronutrient distribution might not be crucial in obtaining liver pathology improvement that seems to be driven by weight loss itself, whereas at higher caloric contents, macronutrient ratio and, possibly, ketosis, seem to become more important, and the positive effect might be independent of weight modifications and calorie intake. Carbohydrate restriction, with its well‐established role in modulating insulin levels, and the newly proposed pathway involving the microbiome shift with increased folate production, surely plays a primary role in the reported effectiveness of KDs towards NAFLD. Apart from being the metabolic consequence of FA oxidation leading to liver fat reduction, ketosis may itself be involved in NAFLD pathogenesis through several mechanisms previously described (Figure 1) beyond carbohydrate restriction. Interestingly, ketone esters have been administered to athletes, reducing glycolysis and increasing muscle fat oxidation in the absence of a carbohydrate‐restricted diet, 71 but no evidence is available to date regarding subjects with NAFLD. This finding suggests that ketosis might exert beneficial effects independent of dietary composition and therefore warrants studies aiming at identifying the specific role played by ketone bodies in NAFLD pathophysiology, possibly paving the way for new therapeutic targets and strategies.
FUNDING
New Penta s.r.l. made funding available for publication charges. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
AUTHORS CONTRIBUTION
M. W., D. T., and L. G. conceived and designed the literature revision; R. R., R. T., S. B., and C. L. prepared the original draft; M. W. prepared the figure; R. R., R. T., and S. M. prepared the tables; M. W., D. T., S. B., S. M., L. G., G. S., and C. L. revised the manuscript.
CONFLICT OF INTEREST
The authors declare no competing interest.
Watanabe M, Tozzi R, Risi R, et al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obesity Reviews. 2020;21:e13024 10.1111/obr.13024
REFERENCES
- 1. Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113(11):1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phinney SD, Bistrian BR, Wolfe RR, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism. 1983;32(8):757‐768. [DOI] [PubMed] [Google Scholar]
- 3. Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70(3):309‐319. [DOI] [PubMed] [Google Scholar]
- 4. Saslow LR, Daubenmier JJ, Moskowitz JT, et al. Twelve‐month outcomes of a randomized trial of a moderate‐carbohydrate versus very low‐carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes. 2017;7(12):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colica C, Merra G, Gasbarrini A, et al. Efficacy and safety of very‐low‐calorie ketogenic diet: a double blind randomized crossover study. Eur Rev Med Pharmacol Sci. 2017;21(9):2274‐2289. [PubMed] [Google Scholar]
- 6. Schwenger KJP, Fischer SE, Jackson TD, Okrainec A, Allard JP. Non‐alcoholic fatty liver disease in morbidly obese individuals undergoing bariatric surgery: prevalence and effect of the pre‐bariatric very low calorie diet. Obes Surg. 2018;28(4):1109‐1116. [DOI] [PubMed] [Google Scholar]
- 7. Haghighatdoost F, Salehi‐Abargouei A, Surkan PJ, Azadbakht L. The effects of low carbohydrate diets on liver function tests in nonalcoholic fatty liver disease: a systematic review and meta‐analysis of clinical trials. J Res Med Sci. 2016;21:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yki‐Jarvinen H. Non‐alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901‐910. [DOI] [PubMed] [Google Scholar]
- 9. Schugar RC, Crawford PA. Low‐carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15(4):374‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alegre F, Pelegrin P, Feldstein AE. Inflammasomes in liver fibrosis. Semin Liver Dis. 2017;37(2):119‐127. [DOI] [PubMed] [Google Scholar]
- 11. Graff EC, Fang H, Wanders D, Judd RL. Anti‐inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism. 2016;65(2):102‐113. [DOI] [PubMed] [Google Scholar]
- 12. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by beta‐hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity‐induced inflammation and insulin resistance. Nat Med. 2011;17(2):179‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wree A, Eguchi A, McGeough MD, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta‐hydroxybutyrate blocks NLRP3 inflammasome‐mediated inflammatory disease. Nat Med. 2015;21(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247‐270. [DOI] [PubMed] [Google Scholar]
- 17. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507‐521. [DOI] [PubMed] [Google Scholar]
- 18. Hochberg Z. An evolutionary perspective on the obesity epidemic. Trends Endocrinol Metab. 2018;29(12):819‐826. [DOI] [PubMed] [Google Scholar]
- 19. Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low‐carbohydrate, ketogenic diet versus a low‐fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769‐777. [DOI] [PubMed] [Google Scholar]
- 20. Gibson AA, Seimon RV, Lee CM, et al. Do ketogenic diets really suppress appetite? A systematic review and meta‐analysis. Obes Rev. 2015;16(1):64‐76. [DOI] [PubMed] [Google Scholar]
- 21. Terzikhan N, Doets EL, Vonk Noordegraaf‐Schouten M. Extensive literature search and review as preparatory work for the evaluation of the essential composition of total diet replacement products for weight control. EFSA Supporting Publications. 2015;12:590E. [Google Scholar]
- 22. Accurso A, Bernstein RK, Dahlqvist A, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab (Lond). 2008;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hite AH, Berkowitz VG, Berkowitz K. Low‐carbohydrate diet review: shifting the paradigm. Nutr Clin Pract. 2011;26:300‐308. [DOI] [PubMed] [Google Scholar]
- 24. Banting W. Letter on corpulence, addressed to the public. 1869. Obes Res. 1993;1(2):153‐163. [DOI] [PubMed] [Google Scholar]
- 25. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12:e593‐e601. [DOI] [PubMed] [Google Scholar]
- 27. Arguin H, Dionne IJ, Senechal M, et al. Short‐ and long‐term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause. 2012;19(8):870‐876. [DOI] [PubMed] [Google Scholar]
- 28. Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164(4):302‐311. [DOI] [PubMed] [Google Scholar]
- 29. Harvie M, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anton SD, Moehl K, Donahoo WT, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity (Silver Spring). 2018;26(2):254‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parry SA, Hodson L. Influence of dietary macronutrients on liver fat accumulation and metabolism. J Invest Med. 2017;65(8):1102‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe M, Singhal G, Fisher FM, et al. Liver‐derived FGF21 is essential for full adaptation to ketogenic diet but does not regulate glucose homeostasis. Endocrine. 2019;1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desai BN, Singhal G, Watanabe M, et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab. 2017;6(11):1395‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garbow JR, Doherty JM, Schugar RC, et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low‐carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G956‐G967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang X, Qin J, Zhao Y, et al. Long‐term ketogenic diet contributes to glycemic control but promotes lipid accumulation and hepatic steatosis in type 2 diabetic mice. Nutr Res. 2016;36(4):349‐358. [DOI] [PubMed] [Google Scholar]
- 37. Schugar RC, Huang X, Moll AR, Brunt EM, Crawford PA. Role of choline deficiency in the fatty liver phenotype of mice fed a low protein, very low carbohydrate ketogenic diet. PLoS ONE. 2013;8:e74806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ballaban‐gil K. Complications of the ketogenic diet 1998. [DOI] [PubMed]
- 39. Kang HC, Chung DE, Kim DW, Kim HD. Early‐ and late‐onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45(9):1116‐1123. [DOI] [PubMed] [Google Scholar]
- 40. Raju KN, Gulati S, Kabra M, et al. Efficacy of 4:1 (classic) versus 2.5:1 ketogenic ratio diets in refractory epilepsy in young children: a randomized open labeled study. Epilepsy Res. 2011;96(1‐2):96‐100. [DOI] [PubMed] [Google Scholar]
- 41. Sharma S, Gulati S, Kalra V, Agarwala A, Kabra M. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy—Indian experience. Seizure. 2009;18(6):446‐449. [DOI] [PubMed] [Google Scholar]
- 42. Groesbeck DK, Bluml RM, Kossoff EH. Long‐term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006;48(12):978‐981. [DOI] [PubMed] [Google Scholar]
- 43. Stevens CE, Turner Z, Kossoff EH. Hepatic dysfunction as a complication of combined valproate and ketogenic diet. Pediatr Neurol. 2016;54:82‐84. [DOI] [PubMed] [Google Scholar]
- 44. Paoli A, Bianco A, Grimaldi KA, Lodi A, Bosco G. Long term successful weight loss with a combination biphasic ketogenic Mediterranean diet and Mediterranean diet maintenance protocol. Nutrients. 2013;5:5205‐5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mardinoglu A, Wu H, Bjornson E, et al. An integrated understanding of the rapid metabolic benefits of a carbohydrate‐restricted diet on hepatic steatosis in humans. Cell Metab. 2018;27:559‐571. e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taggart AK, Kero J, Gan X, et al. (D)‐β‐hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA‐G. J Biol Chem. 2005;280(29):26649‐26652. [DOI] [PubMed] [Google Scholar]
- 47. Tendler D, Lin S, Yancy WS Jr, et al. The effect of a low‐carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52(2):589‐593. [DOI] [PubMed] [Google Scholar]
- 48. Perez‐Guisado J, Munoz‐Serrano A. The effect of the Spanish Ketogenic Mediterranean Diet on nonalcoholic fatty liver disease: a pilot study. J Med Food. 2011;14(7‐8):677‐680. [DOI] [PubMed] [Google Scholar]
- 49. Schiavo L, Pilone V, Rossetti G, Barbarisi A, Cesaretti M, Iannelli A. A 4‐week preoperative ketogenic micronutrient‐enriched diet is effective in reducing body weight, left hepatic lobe volume, and micronutrient deficiencies in patients undergoing bariatric surgery: a prospective pilot study. Obes Surg. 2018;28(8):2215‐2224. [DOI] [PubMed] [Google Scholar]
- 50. Paoli A, Cenci L, Grimaldi KA. Effect of ketogenic Mediterranean diet with phytoextracts and low carbohydrates/high‐protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr J. 2011;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Basciani S, Costantini D, Contini S, et al. Safety and efficacy of a multiphase dietetic protocol with meal replacements including a step with very low calorie diet. Endocrine. 2015;48(3):863‐870. [DOI] [PubMed] [Google Scholar]
- 52. Leonetti F, Campanile FC, Coccia F, et al. Very low‐carbohydrate ketogenic diet before bariatric surgery: prospective evaluation of a sequential diet. Obes Surg. 2015;25(1):64‐71. [DOI] [PubMed] [Google Scholar]
- 53. Colles SL, Dixon JB, Marks P, Strauss BJ, O'Brien PE. Preoperative weight loss with a very‐low‐energy diet: quantitation of changes in liver and abdominal fat by serial imaging. Am J Clin Nutr. 2006;84(2):304‐311. [DOI] [PubMed] [Google Scholar]
- 54. Bruci A, Tuccinardi D, Tozzi R, et al. Very low‐calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients. 2020;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benjaminov O, Beglaibter N, Gindy L, et al. The effect of a low‐carbohydrate diet on the nonalcoholic fatty liver in morbidly obese patients before bariatric surgery. Surg Endosc. 2007;21(8):1423‐1427. [DOI] [PubMed] [Google Scholar]
- 56. Lewis MC, Phillips ML, Slavotinek JP, Kow L, Thompson C, Toouli J. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16(6):697‐701. [DOI] [PubMed] [Google Scholar]
- 57. Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short‐term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011;93(5):1048‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well‐being during a 4 to 21‐day fasting period in an observational study including 1422 subjects. PLoS ONE. 2019;14(1):e0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136(5):1552‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ministrini S, Calzini L, Nulli Migliola E, et al. Lysosomal acid lipase as a molecular target of the very low carbohydrate ketogenic diet in morbidly obese patients: the potential effects on liver steatosis and cardiovascular risk factors. J Clin Med. 2019;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haufe S, Engeli S, Kast P, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53(5):1504‐1514. [DOI] [PubMed] [Google Scholar]
- 62. Westerbacka J, Lammi K, Hakkinen AM, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90(5):2804‐2809. [DOI] [PubMed] [Google Scholar]
- 63. Lin WY, Wu CH, Chu NF, Chang CJ. Efficacy and safety of very‐low‐calorie diet in Taiwanese: a multicenter randomized, controlled trial. Nutrition. 2009;25(11‐12):1129‐1136. [DOI] [PubMed] [Google Scholar]
- 64. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541‐2551. [DOI] [PubMed] [Google Scholar]
- 65. Stekovic S, Hofer SJ, Tripolt N, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non‐obese humans. Cell Metab. 2019;30(3):462‐476 e5. [DOI] [PubMed] [Google Scholar]
- 66. Lazo M, Solga SF, Horska A, et al. Effect of a 12‐month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33(10):2156‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non‐alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56(1):255‐266. [DOI] [PubMed] [Google Scholar]
- 68. Alferink LJ, Kiefte‐de Jong JC, Erler NS, et al. Association of dietary macronutrient composition and non‐alcoholic fatty liver disease in an ageing population: the Rotterdam study. Gut. 2019;68(6):1088‐1098. [DOI] [PubMed] [Google Scholar]
- 69. Lundsgaard AM, Holm JB, Sjoberg KA, et al. Mechanisms preserving insulin action during high dietary fat intake. Cell Metab. 2019;29(1):50‐63 e4. [DOI] [PubMed] [Google Scholar]
- 70. Fabbrini E, Magkos F. Hepatic Steatosis as a marker of metabolic dysfunction. Nutrients. 2015;7(6):4995‐5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256‐268. [DOI] [PubMed] [Google Scholar]


