Abstract
Objective
The aim of this study was to examine the long‐term efficacy of combination of tamsulosin 0.2 mg + dutasteride 0.5 mg + imidafenacin 0.2 mg (TDI) therapy compared with tamsulosin + dutasteride (TD) therapy for 52 weeks in benign prostatic hyperplasia (BPH) patients with a prostate volume (PV) ≥30 mL and remaining overactive bladder (OAB) symptoms after having received tamsulosin for ≥8 weeks. Previously, we reported that the improvement in OAB symptoms at 24 weeks was significantly greater in the TDI than TD group.
Methods
BPH patients with OAB symptoms after ≥8 weeks tamsulosin were randomly assigned to the TDI or TD group in a ratio of 1:1 ratio, and followed‐up for 52 weeks. Changes in the OAB Symptom Score (OABSS), International Prostate Symptom Score (IPSS), and post‐void residual (PVR) were evaluated.
Results
In all, 163 patients were randomized, and 125 patients (76.7%) completed 52 weeks of treatment. At Week 52, there were significant decreases in the OABSS and IPSS storage subscore compared with baseline in the TDI versus TD group, but the change in the total IPSS did not differ significantly between the two groups. There was no change in PVR from Week 24 to Week 52 in either group.
Conclusions
For BPH patients with PVR ≥30 mL and remaining storage symptoms despite tamsulosin monotherapy, TDI treatment showed better results in terms of improved OAB symptoms than TD treatment up to 52 weeks.
Keywords: 5α‐reductase inhibitor, benign prostatic hyperplasia, cholinergic antagonist, combination therapy, long‐term efficacy, overactive bladder
1. INTRODUCTION
In benign prostatic hyperplasia (BPH) patients, α1‐adreceptor antagonists (hereafter “alpha‐blockers”) are used as first‐line pharmacotherapy. If overactive bladder (OAB) persists despite this therapy, the addition of an anticholinergic is recommended.1 A post hoc analysis of the Combination of Avodart and Tamsulosin (the CombAT) study, which compared combination tamsulosin and dutasteride therapy with tamsulosin or dutasteride monotherapy for 4 years, showed that the combination therapy was more effective against storage symptoms than monotherapy with either drug alone.2 Thus, for a BPH patient with a prostate volume (PV) ≥30 mL, the combination of an alpha‐blocker and a 5α‐reductase inhibitor may be effective even for storage symptoms. In a previous study, combination therapy with tamsulosin 0.2 mg and imidafenacin 0.2 mg (an anticholinergic drug) was reported to be more effective against OAB symptoms, evaluated in terms of OAB Symptom Score (OABSS), than tamsulosin monotherapy in BPH patients.3 However, that study was performed in patients with a small prostate and was only a short‐term evaluation.
It is unknown whether a combination of the three drug types (i.e. an alpha‐blocker, 5α‐reductase inhibitor, and an anticholinergic) would be more effective than a combination of two drug types (alpha‐blocker and 5α‐reductase inhibitor) against male OAB associated with a large prostate, and few, if any, studies have examined the long‐term usefulness of a combination of these drugs.
The DIrecT (Dutasteride and Imidafenacin in BPH/OAB patients not respond to eight weeks' continuous treatment of Tamsulosin) study was performed over a period of 52 weeks. Outcomes of the DIrecT study up to 24 weeks have been reported previously; the addition of dutasteride and imidafenacin (TDI group) to tamsulosin had better results than the addition of dutasteride alone (TD group) in terms of improving OAB symptoms after 24 weeks in BPH patients who had a PV ≥30 mL and OAB symptoms that had persisted despite tamsulosin monotherapy for ≥8 weeks.4 The aim of the present study was to examine the long‐term outcomes of the DIrecT study up to 52 weeks.
2. METHODS
The DIrecT study was a multicenter randomized open‐label controlled trial conducted over a period of 52 weeks.
To be eligible for inclusion, patients had to have an OABSS urinary urgency score of ≥2 (one or more urinary urgency episodes/week) and an OABSS total score of ≥3 despite ≥8 weeks treatment with tamsulosin 0.2 mg/d (the recommended dose in Japan and other Asian countries), be aged between 50 and 89 years, and have a PV ≥30 mL. Exclusion criteria were a maximal urinary flow rate (Qmax) <5 mL/s, post‐void residual (PVR) volume > 100 mL, prostate‐specific antigen (PSA) >10 ng/mL (although patients remained eligible for the study if cancer comorbidity could be ruled out by biopsy etc.), suspected polyuria, or being treated with medication for frequent urination and/or urinary incontinence within the 2 weeks prior to study enrollment.
Patients who met the eligibility criteria were randomly assigned (in a ratio of 1:1) to receive dutasteride 0.5 mg/d (TD group) or dutasteride 0.5 mg/d + imidafenacin 0.2 mg/d (TDI group) in addition to continuing tamsulosin. Tamsulosin and dutasteride were taken orally once daily after breakfast, and imidafenacin was taken orally twice daily after breakfast and dinner for 52 weeks. Subjects visited the participating medical institutions at Weeks 0, 4, 12, 24, 36, and 52 after starting drug treatment. To assess lower urinary tract symptoms (LUTS) and quality of life (QOL), self‐administered questionnaires (OABSS, International Prostate Symptom Score [IPSS], IPSS‐QOL, and the benign prostatic hyperplasia impact index [BII]) were completed at Weeks 0, 4, 12, 24, 36, and 52. In addition, frequency volume chart (FVC) assessment and hematology test (PSA and testosterone) were conducted at Weeks 0, 12, 24, and 52, and PV, Qmax, and PVR were determined at Weeks 0, 24, and 52. Subjects were asked about adverse events at each visit.
The subjects completed the questionnaires at outpatient visits. The FVC was completed by the patients, in principle, 3 days before each visit and brought to the study site at each visit. PV was determined by transabdominal or transrectal ultrasonography, PVR was determined by transabdominal ultrasonography, and Qmax was determined by uroflowmetry at each institution.
The primary endpoint was change in total OABSS. Secondary endpoints were changes in response to each question of the OABSS, IPSS (total score and storage and voiding symptoms subscores), QOL index, BII, FVC variables (number of voids/24 h, number of urinary urgency episodes/24 h, number of urgency incontinence episodes/24 h, urine volume/24 h, and voided volume/micturition), PV, PVR, Qmax, PSA concentration, and testosterone concentration.
A web‐based system (eClinical Base; Translational Research Informatics Center, Kobe, Japan) was used for to assign patients to the two treatment groups after necessary data for patient registration were input by investigators and the patients were confirmed to meet the eligibility criteria. A minimization method incorporating an element of randomization with institutions as a stratifying factor was used to allocate patients to the two treatment groups. The detailed method of allocation was not disclosed until completion of the study.
Based on a previous report,2 the treatment difference in sample size‐weighted OABSS total score was estimated to be 1.4. The number of patients to be enrolled was estimated on the basis of the sample size needed to detect a between‐group mean score difference of 1.4 with a significance level of two‐sided α = 0.05 on t test. Assuming an SD of 2.4 based on the previous study, 63 patients per group were required to achieve statistical power of 90%. Assuming a drop‐out rate of approximately 25%, we planned to enroll 170 patients in the two groups.4
The full analysis set (FAS) was determined to be all patients excluding those who received no study drugs after randomization, those for whom there were no data after initial evaluation, and those who were later found to be ineligible for the study. Point estimates of means and proportions for the endpoints and their 95% confidence intervals (CIs) were calculated and compared between groups and within each group for changes from baseline. On the assumption that the variables could approximate continuous variables, a linear mixed‐effects model was used for statistical tests and, if needed, other models were used to validate the approximation. Two‐sided P < 0.05 was considered significant. All statistical analyses were performed using Stata version 13 (Stata, College Station, Texas).
The study protocol (UMIN000011980) was reviewed and approved by the institutional review board at each clinical site. The study was performed in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies (revised in July 2008).5 Each patient provided written informed consent before enrollment.
3. RESULTS
In all, 163 patients were randomized, and 125 patients (76.7%) completed 52 weeks of treatment (Figure 1). Reasons for discontinuation in each group are shown in Figure 1. Patient characteristics are given in Table 1.
Figure 1.
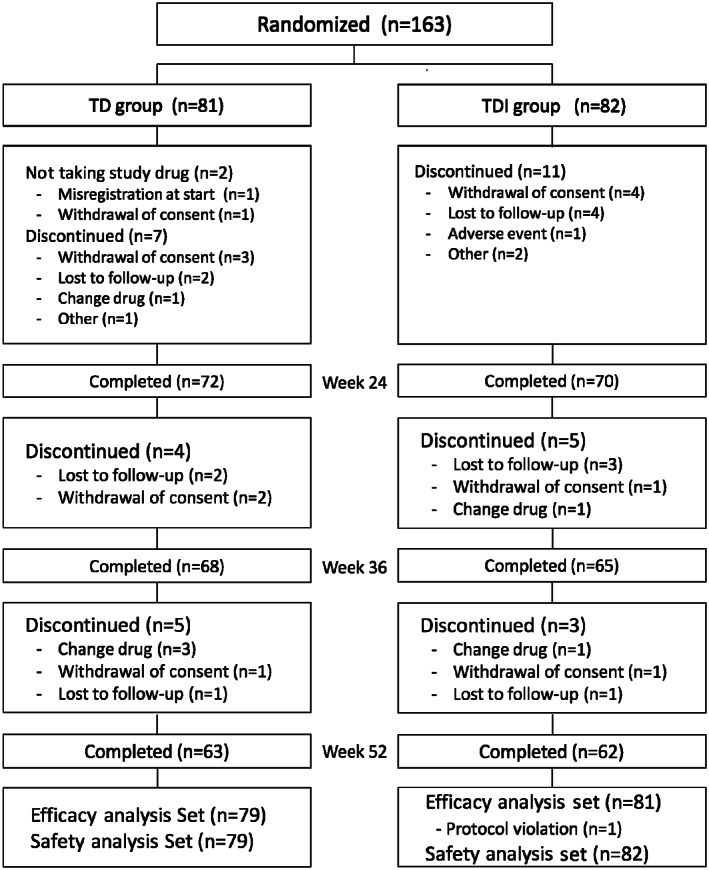
Subject disposition. TD, tamsulosin + dutasteride; TDI, tamsulosin + dutasteride + imidafenacin
Table 1.
Patient characteristics
| TD group (n = 79) | TDI group (n = 82) | |
|---|---|---|
| Age (y) | 73.6 ± 7.0 | 72.9 ± 7.6 |
| Duration of prior tamsulosin treatment (wk) | ||
| <26 | 48 (60.8) | 57 (69.5) |
| 26 to 52 | 6 (7.6) | 7 (8.5) |
| >52 | 25 (31.6) | 18 (22.0) |
| OABSS total | 7.7 (2.7) | 7.9 (2.3) |
| IPSS | ||
| Total score | 14.1 ± 5.9 | 13.7 ± 5.8 |
| Storage score | 7.2 ± 2.7 | 7.3 ± 2.8 |
| Voiding score | 6.8 ± 4.7 | 6.4 ± 4.3 |
| IPSS‐QOL score | 4.5 ± 1.1 | 4.4 ± 1.1 |
| BPH impact index | 5.0 ± 3.7 | 5.2 ± 3.8 |
| No. voids/24 ha | 11.4 ± 3.5 (n = 64) | 10.7 ± 2.5 (n = 66) |
| Urgency episodes/24 ha | 3.1 ± 3.3 (n = 48) | 3.0 ± 3.0 (n = 58) |
| Urgency incontinence episodes/24 ha | 1.9 ± 3.3 (n = 13) | 1.1 ± 1.1 (n = 18) |
| Urine volume/24 h (mL)a | 1677 ± 620 (n = 64) | 1637 ± 564 (n = 68) |
| Mean volume voided/micturition (mL)a | 160 ± 59 (n = 63) | 166 ± 48 (n = 64) |
| Prostate volume (mL) | 43.7 ± 15.1 | 44.6 ± 18.8 |
| Qmax (mL/s) | 11.3 ± 4.8 | 11.7 ± 6.1 |
| PVR (mL) | 24.2 ± 25.8 | 22.3 ± 25.6 |
| PSA (ng/mL) | 4.1 ± 4.1 | 3.3 ± 2.6 |
Abbreviations: BPH, benign prostatic hyperplasia; IPSS, International Prostate Symptom Score; OABSS, Overactive Bladder Symptom Score; PSA, prostate‐specific antigen; PVR, post‐void residual; Qmax, maximum flow rate; TD, tamsulosin + dutasteride; TDI, tamsulosin + dutasteride + imidafenacin
Data are given as the mean ± SD or as n (%).
Evaluated using a frequency volume chart.
Changes in the total OABSS from Week 0 to Week 52 are shown in Figure 2; changes in total score and the storage symptoms subscore on the IPSS, as well as the IPSS‐QOL index, are shown in Figure 3. We had reported the results of the OABSS and IPSS up to 24 weeks previously.4 The changes in total OABSS, each OABSS subscore, IPSS, and IPSS‐QOL index from Week 0 to Weeks 36 and 52, as well as differences in the changes in these parameters between groups are given in Table 2.
Figure 2.
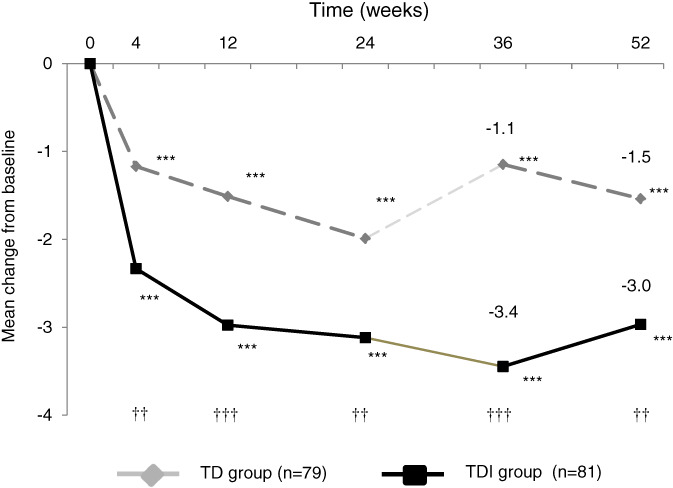
Changes in the total Overactive Bladder Questionnaire Symptom Score from the beginning of the study to 52 weeks in the tamsulosin + dutasteride (TD) and tamsulosin + dutasteride + imidafenacin (TDI) groups. Results for weeks 4, 12 and 24 relative to week 0 have been reported previously.4 Results of for weeks 36 and 52 relative to week 0 were analyzed in the present study using a mixed‐effects model. ***P < 0.001 compared with baseline; † P < 0.05, †† P < 0.01, ††† P < 0.001 between groups
Figure 3.
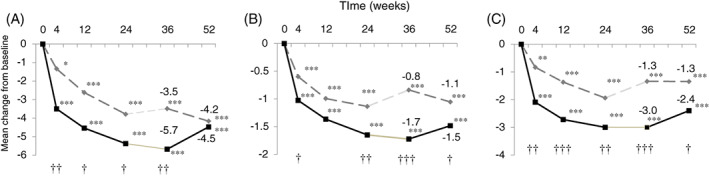
Changes in the A, total International Prostate Symptom Score (IPSS) score and B, quality of life and C, storage symptom subscores on the IPSS from the start of the study to 52 weeks. Results for weeks 4, 12 and 24 relative to week 0 have been reported previously.4 Results of for weeks 36 and 52 relative to week 0 were analyzed in the present study using a mixed‐effects model. *P < 0.05, **P < 0.01, ***P < 0.001 compared with baseline; † P < 0.05, †† P < 0.01, ††† P < 0.001 between groups
Table 2.
Changes from baseline (Week 0) at Weeks 36 and 52
| Baseline | Mean change from baseline (P‐value) | P‐value between groups | |||
|---|---|---|---|---|---|
| Week 36 | Week 52 | Week 36 | Week 52 | ||
| OABSS | |||||
| Total score | |||||
| TD group | 7.7 ± 2.7 | −1.15 (<0.001) | −1.54 (<0.001) | <0.001 | 0.002 |
| TDI group | 7.9 ± 2.3 | −3.45 (<0.001) | −2.97 (<0.001) | ||
| Daytime frequency score | |||||
| TD group | 0.9 ± 0.5 | 0.04 (0.52) | −0.13 (0.06) | <0.001 | 0.03 |
| TDI group | 0.9 ± 0.5 | −0.31 (<0.001) | −0.34 (<0.001) | ||
| Nocturia score | |||||
| TD group | 2.3 ± 0.9 | −0.15 (0.13) | −0.25 (0.01) | 0.001 | 0.02 |
| TDI group | 2.2 ± 0.9 | −0.57 (<0.001) | −0.56 (<0.001) | ||
| Urgency score | |||||
| TD group | 3.2 ± 1.0 | −0.93 (<0.001) | −0.99 (<0.001) | <0.001 | 0.02 |
| TDI group | 3.3 ± 1.0 | −1.82 (<0.001) | −1.54 (<0.001) | ||
| Urgency incontinence score | |||||
| TD group | 1.4 ± 1.5 | −0.12 (0.44) | −0.17 (0.29) | 0.003 | 0.10 |
| TDI group | 1.4 ± 1.3 | −0.75 (<0.001) | −0.53 (<0.001) | ||
| IPSS | |||||
| Total score | |||||
| TD group | 14.1 ± 5.9 | −3.49 (<0.001) | −4.16 (<0.001) | 0.01 | 0.66 |
| TDI group | 13.8 ± 5.9 | −5.68 (<0.001) | −4.47 (<0.001) | ||
| Voiding score | |||||
| TD group | 6.8 ± 4.7 | −2.10 (<0.001) | −2.81 (<0.001) | 0.36 | 0.24 |
| TDI group | 6.5 ± 4.3 | −2.68 (<0.001) | −2.08 (<0.001) | ||
| Storage score | |||||
| TD group | 7.2 ± 2.7 | −1.34 (<0.001) | −1.35 (<0.001) | <0.001 | 0.02 |
| TDI group | 7.3 ± 2.8 | −3.00 (<0.001) | −2.40 (<0.001) | ||
| IPSS‐QOL score | |||||
| TD group | 4.5 ± 1.0 | −0.84 (<0.001) | −1.05 (<0.001) | <0.001 | 0.048 |
| TDI group | 4.4 ± 1.1 | −1.72 (<0.001) | −1.48 (<0.001) | ||
| BPH impact index | |||||
| TD group | 5.0 ± 3.7 | −1.32 (<0.001) | −1.36 (<0.001) | 0.01 | 0.04 |
| TDI group | 5.2 ± 3.8 | −2.54 (<0.001) | −2.35 (<0.001) | ||
| No. voids/24 ha | |||||
| TD group | 11.4 ± 3.5 | – | −0.39 (0.30) | – | 0.23 |
| TDI group | 10.7 ± 2.5 | – | −0.82 (0.01) | ||
| No. episodes urgency/24 ha | |||||
| TD group | 3.1 ± 3.3 | – | −1.12 (0.09) | – | 0.84 |
| TDI group | 3.0 ± 3.0 | – | −0.99 (0.01) | ||
| No. episodes urgency incontinence/24 ha | |||||
| TD group | 1.9 ± 3.3 | – | −1.50 (0.38) | – | 0.19 |
| TDI group | 1.1 ± 1.1 | – | −0.31 (0.21) | ||
| Urine volume/24 h (mL)a | |||||
| TD group | 1677 ± 620 | – | 21.4 (0.77) | – | 0.33 |
| TDI group | 1637 ± 564 | – | 91.4 (0.09) | ||
| Mean voided volume/micturition (mL)a | |||||
| TD group | 160 ± 59 | – | 3.2 (0.65) | – | 0.004 |
| TDI group | 195 ± 92 | – | 24.2 (<0.001) | ||
| Prostate volume (% change) | |||||
| TD group | 43.7 ± 15.1 | – | −25.8 (<0.001) | – | 0.69 |
| TDI group | 44.6 ± 18.8 | – | −27.2 (<0.001) | ||
| Qmax (mL/s) | |||||
| TD group | 11.3 ± 4.8 | – | 0.89 (0.21) | – | 0.16 |
| TDI group | 11.7 ± 6.1 | – | 2.88 (0.13) | ||
| PVR (mL) | |||||
| TD group | 24.2 ± 25.8 | – | 0.33 (0.95) | – | 0.01 |
| TDI group | 22.3 ± 25.6 | – | 17.2 (0.01) | ||
| PSA (% change) | |||||
| TD group | 4.1 ± 4.1 | – | −49.9 (<0.001) | – | 0.98 |
| TDI group | 3.3 ± 2.6 | – | −50.0 (<0.001) | ||
| Testosterone (% change) | |||||
| TD group | 7.6 ± 12.7 | – | 13.3 (0.006) | – | 0.84 |
| TDI group | 6.0 ± 10.5 | – | 12.0, 0.04 | ||
Abbreviations: BPH, benign prostatic hyperplasia; IPSS, International Prostate Symptom Score; OABSS, Overactive Bladder Symptom Score; PSA, prostate‐specific antigen; PVR, post‐void residual; Qmax, maximum flow rate; QOL, quality of life; TD, tamsulosin + dutasteride; TDI, tamsulosin + dutasteride + imidafenacin.
Baseline data are given as the mean ± SD.
Evaluated using a frequency volume chart.
The total OABSS at Weeks 36 and 52 was significantly lower compared with Week 0 in both groups. Significant decreases in total OABSS and each OABSS subscore (except for urgency incontinence) were noted in the TDI versus TD group up to Weeks 36 and 52 (Table 2).
IPSS total scores were significantly lower at Weeks 36 and 52 compared with Week 0 in both groups. The IPSS total score was significantly lower in the TDI than TD group at Week 36, but no significant difference was detected between the groups at Week 52. The IPSS storage symptom subscore and QOL index were significantly improved at Weeks 36 and 52 compared with Week 0 in both groups, and significant intergroup differences were detected at Weeks 36 and 52. IPSS voiding symptom subscores were also significantly improved at Weeks 36 and 52 compared with Week 0 in both groups, and no significant differences were detected between the two groups. The BII improved significantly in the TDI group compared with the TD group.
Changes from Week 0 in FVC variables and laboratory data at Week 52 are given in Table 2. Mean voided volume/micturition improved significantly in the TDI compared with TD group. PVR at Week 52 was increased by 0.3 mL (95% CI −9.4, 10.1) in the TD group and by 17.2 mL (95% CI 4.2, 30.3) in the TD group compared with Week 0, demonstrating a significant difference between groups (intergroup difference in change: 17.1 mL; 95% CI 3.8, 30.3; P = 0.01). However, the change in PVR from Week 24 to Week 52 was not significant in either the TD or TDI group (−3.4 mL [95% CI −13.6, 6.8] and −3.0 mL [95% CI −17.9, 11.8], respectively) or between groups (intergroup difference in change: 0.8 mL; 95% CI −14.1, 15.7; P = 0.92).
There were two adverse events related to the study drugs in the TDI group, and none in the TD group. The two events were dry mouth and erectile dysfunction, neither of which was serious. The subject with dry mouth was the only patient who prematurely discontinued the study drugs for safety reasons.
4. DISCUSSION
In the present study, the addition of dutasteride and imidafenacin for 52 weeks to tamsulosin was evaluated in BPH patients with PV ≥30 mL and persistent OAB symptoms despite ≥8 weeks treatment with tamsulosin. Following from our previous study,4 we evaluated the efficacy and safety of TDI versus TD therapy until Week 52. In the TDI group, OABSS was significantly improved compared with the TD group up to 52 weeks; there were also significant improvements in the IPSS storage symptom subscore, IPSS‐QOL, and BII in the TDI versus TD group. Moreover, in terms of safety, there were no serious or unknown events for which a causal relationship to the study drugs could not be ruled out. These results seem to demonstrate the superiority of the TDI triple combination over the TD dual combination in the treatment of OAB associated with larger BPH up to 52 weeks.
The rates of reduction in PV at Week 52 were similar in the TD and TDI groups, and were similar to reductions with dual treatment (tamsulosin and dutasteride) reported in previous studies.6, 7 In the CombAT study,2 the IPSS total score and storage subscore at baseline were higher than in the TD group in the present study. In addition, although tamsulosin and dutasteride administration started at the same time in the combination group of the CombAT study, the addition of dutasteride after tamsulosin was found to be insufficiently effective in the present study. Despite these differences between the two studies, the changes in the IPSS total score and storage subscore in the TD group were similar to those in the tamsulosin and dutasteride combination group in the CombAT study, suggesting that the tamsulosin and dutasteride treatment in the present study can be considered reasonable.
Because the scores related to storage symptoms and QOL improved significantly in the TDI compared with TD group, the addition of imidafenacin was expected to improve storage symptoms and QOL for 52 weeks. In terms of the long‐term combination therapy with an alpha‐blocker and an anticholinergic, there is one report regarding the administration of a combination of tamsulosin and solifenacin for 52 weeks8 in which improvement of both IPSS total score and storage symptom subscore was maintained for 52 weeks.
There were no adverse events related to the drugs after Week 24 in the TD group, but two adverse events were noted in the TDI group. Although one of the two events led to study discontinuation, it was not a notable event compared with other reports. PVR was significantly higher at Week 52 compared with Week 0 in the TDI group. However, this increase occurred only between Weeks 0 and 24. There was neither an increase in PVR nor the occurrence of urinary retention between Weeks 25 and 52.
When using anticholinergics, it is necessary to consider their effect on cognitive function. According to post‐marketing surveillance results of imidafenacin administration in patients with mild cognitive impairment (MCI),9 the conversion rate of MCI to dementia was 3.6%, which was not considered to exceed that reported in past epidemiological studies (6.8%‐16.1% per year). In the present study, no patients exhibited cognitive dysfunction, including memory disorder.
Although the present study had a randomized parallel‐group comparison design, it was not a double‐blind placebo‐controlled study. In addition, the efficacy and safety of administration for longer than 52 weeks is not known. Considering that the cost burden of administering three drugs is high, it is necessary to investigate when and which drug to discontinue. In the future, studies investigating which patient background characteristics will result in better outcomes after TDI triple administration are needed.
In conclusion, TDI treatment showed better results in terms of improvements in OAB symptoms than TD treatment up to 52 weeks for BPH patients with PV ≥30 mL and remaining storage symptoms despite treatment with tamsulosin monotherapy.
ACKNOWLEDGEMENTS
This study was funded by Kyorin Pharmaceutical. The funding body had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors thank H. Kakizaki and N. Sekido (Safety Review Committee) for the safety review. The authors acknowledge the Clinical Research Support Center Kyushu for supporting individual study centers, managing study progress, and data management. The authors thank WILL Medical Communications (Osaka Japan) for help writing the manuscript.
Disclosure
The authors declare no conflicts of interest.
Yamanishi T, Asakura H, Seki N, Tokunaga S. A 52‐week multicenter randomized controlled study of the efficacy and safety of add‐on dutasteride and imidafenacin to tamsulosin in patients with benign prostatic hyperplasia with remaining overactive bladder symptoms (DIrecT study). Lower Urinary Tract Symptoms. 2019;11:115–121. 10.1111/luts.12243
Funding information Kyorin Pharmaceutical Co., Ltd.; Kyorin Pharmaceutical
REFERENCES
- 1. Homma Y, Gotoh M, Kawauchi A, et al. Clinical guidelines for male lower urinary tract symptoms and benign prostatic hyperplasia. Int J Urol. 2017;24:716‐729. [DOI] [PubMed] [Google Scholar]
- 2. Montorsi F, Roehrborn C, Garcia‐Penit J, et al. The effects of dutasteride or tamsulosin alone and in combination on storage and voiding symptoms in men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH): 4‐year data from the Combination of Avodart and Tamsulosin (CombAT) study. BJU Int. 2011;107:1426‐1431. [DOI] [PubMed] [Google Scholar]
- 3. Takeda M, Nishizawa O, Gotoh M, Yoshida M, Takahashi S, Masumori N. Clinical efficacy and safety of imidafenacin as add‐on treatment for persistent overactive bladder symptoms despite alpha‐blocker treatment in patients with BPH: the ADDITION STUDY. Urology. 2013;82:887‐893. [DOI] [PubMed] [Google Scholar]
- 4. Yamanishi T, Asakura H, Seki N, Tokunaga S. Efficacy and safety of combination therapy with tamsulosin, dutasteride and imidafenacin for the management of overactive bladder symptoms associated with benign prostatic hyperplasia: a multicenter, randomized, open‐label, controlled trial (DIrecT Study). Int J Urol. 2017;24:525‐531. [DOI] [PubMed] [Google Scholar]
- 5. Iijima Y. Ethical guidelines for clinical studies: overview. Rinsho Shinkeigaku. 2011;51:830‐833. www.ncbi.nlm.nih.gov/pubmed/22277384 [DOI] [PubMed] [Google Scholar]
- 6. Joo KJ, Sung WS, Park SH, Yang WJ, Kim TH. Comparison of alpha‐blocker monotherapy and alpha‐blocker plus 5alpha‐reductase inhibitor combination therapy based on prostate volume for treatment of benign prostatic hyperplasia. J Int Med Res. 2012;40:899‐908. [DOI] [PubMed] [Google Scholar]
- 7. Chung BH, Roehrborn CG, Siami P, et al. Efficacy and safety of dutasteride, tamsulosin and their combination in a subpopulation of the CombAT study: 2‐year results in Asian men with moderate‐to‐severe BPH. Prostate Cancer Prostatic Dis. 2009;12:152‐159. [DOI] [PubMed] [Google Scholar]
- 8. Drake MJ, Chapple C, Sokol R, et al. Long‐term safety and efficacy of single‐tablet combinations of solifenacin and tamsulosin oral controlled absorption system in men with storage and voiding lower urinary tract symptoms: results from NEPTUNE Study and NEPTUNE II open‐label extension. Eur Urol. 2015;67:262‐270. [DOI] [PubMed] [Google Scholar]
- 9. Sakakibara R, Hamano H, Yagi H. Cognitive safety and overall tolerability of imidafenacin in clinical use: a long‐term, open‐label, post‐marketing surveillance study. LUTS. 2014;6:138‐144. [DOI] [PubMed] [Google Scholar]


