Abstract
Sleep deprivation commonly impairs affective regulation and causes worse mood. However, the majority of previous research concerns young adults. Because susceptibility to sleep deprivation and emotion regulation change distinctively across adult age, we tested here the hypothesis that the effect of sleep deprivation on mood is stronger in young than in older adults. In an experimental design, young (18–30 years) and older adults (60–72 years) participated in either a sleep control (young, n = 63; older, n = 47) or a total sleep deprivation condition (young, n = 61; older, n = 47). Sleepiness, mood and common symptoms of sleep deprivation were measured using established questionnaires and ratings. Sleep‐deprived participants felt more sleepy, stressed and cold, and reported lower vigour and positive affect, regardless of age. All the other outcome measures (negative affect, depression, confusion, tension, anger, fatigue, total mood disturbance, hunger, cognitive attenuation, irritability) showed a weaker response to sleep deprivation in the older group, as indicated by age*sleep deprivation interactions (ps < 0.05). The results show that older adults are emotionally less affected by sleep deprivation than young adults. This tolerance was mainly related to an attenuated increase in negative mood. This could possibly be related to the well‐known positivity effect, which suggests that older adults prioritize regulating their emotions to optimize well‐being. The results also highlight that caution is warranted when generalizing results from sleep deprivation studies across the adult lifespan.
Keywords: affect, age differences, emotion, KSS, sleep deprivation
1. INTRODUCTION
Good sleep is not only essential for physical health and cognitive performance, but also plays a critical role in emotional functioning, as highlighted by several studies during the past years (Goldstein & Walker, 2014). So far though, hardly any research has investigated whether the affective consequences of sleep deprivation observed in young adults can be generalized to the later part of the lifespan. In general, well‐being and positive affect increase and negative emotions decline with older age (Carstensen, Isaacowitz, & Charles, 1999; Reed & Carstensen, 2012), which seems somewhat paradoxical considering the challenges inherent to older age, such as deteriorating health. A main reason appears to be that older compared with young adults prioritize positive over negative information (Reed, Chan, & Mikels, 2014). However, it is so far unknown whether this positivity effect is also reflected by less mood deterioration when undergoing sleep loss.
Sleep deprivation increases negative mood (Kahn, Sheppes, & Sadeh, 2013) and impairs emotion regulation (Goldstein & Walker, 2014) and socio‐emotional functioning (Beattie, Kyle, Espie, & Biello, 2015), which is likely to have adverse consequences for everyday life. An early meta‐analysis by Pilcher and Huffcut suggests that the effect of sleep deprivation on mood is even larger than the effect on cognitive performance (1996). However, the vast majority of research has been conducted in young adults. Prior studies suggest that older adults are less affected by sleep deprivation in terms of perceived sleepiness (Duffy, Willson, Wang, & Czeisler, 2009) and cognitive performance decrements (Duffy et al., 2009; Philip et al., 2004), yet knowledge on the affective consequences of sleep deprivation in older adults is very scarce. To our knowledge, only one prior study (Birchler‐Pedross et al., 2009) included a more general mood measure. The results showed that older adults’ well‐being was more impaired than young adults’ by sleep deprivation. However, this study was limited by a small sample size and the lack of established outcome measures that differentiate between negative and positive dimensions of affect. Using an empathy for pain paradigm, our recent findings indicate that the effect of partial sleep deprivation in older adults is opposite to that in younger adults (Tamm et al., 2017). This study focused specifically on empathy, and not on more general aspects of emotion, but nevertheless further underscores the need to study sleep manipulations in different age groups. Thus, it is an open challenge to investigate how total sleep deprivation affects positive and negative dimensions of affect in older compared with young adults. Subjective measures are increasingly considered important outcomes, as, for instance, hedonic well‐being is not only bidirectionally linked with health but may even prospectively predict health outcomes in older age (Steptoe, Deaton, & Stone, 2015).
Older adults may be emotionally protected from sleep loss because they better tolerate sleep deprivation as shown with cognitive functioning (Duffy et al., 2009; Philip et al., 2004), and because of a shift in emotion regulation strategies in later life. The majority of studies indicate that sleep deprivation has a less adverse impact on cognition in older adults, but the mechanisms driving the age differences are still poorly understand (Scullin & Bliwise, 2015). Potential reasons that have been discussed are that older adults may already be impaired at baseline as a result of generally sleeping more poorly than young adults (i.e. flooring effect), or alternatively may have a lower need for sleep (Scullin & Bliwise, 2015). Moreover, changes in circadian regulation such as an attenuated amplitude could contribute to the observed age differences (Schmidt, Peigneux, & Cajochen, 2012). With respect to age‐dependent effects of sleep deprivation on mood, general changes in emotion regulation across the adult lifespan may also need to be considered. According to the socioemotional selectivity theory (Carstensen et al., 1999), one of the most prominent lifespan theories, young adults perceive their time horizon as more open ended and prioritize future‐oriented goals such as knowledge gain. On the other hand, because of perceiving their remaining lifetime as more limited, older adults focus on maximizing positive affective states and minimizing negative ones in order to achieve emotional gratification (Reed & Carstensen, 2012; Reed et al., 2014). This positivity effect in older age has been confirmed in several experiments, and a recent meta‐analysis concluded that the support for the positivity effect is indeed stable (Reed et al., 2014). Moreover, brain imaging findings confirm that young and older adults process emotional stimuli differentially (Mather, 2012). There is also support for the persistence of the positivity effect in everyday life: older adults are more likely to interpret ambiguous situations positively than young adults are (Juang & Knight, 2016), and recall more daily positive experiences (English & Carstensen, 2014). Yet, it is unclear whether the positivity effect in older age withstands more severe challenges. Only recently, Everaerd, Klumpers, Oude Voshaar, Fernández, and Tendolkar (2017) reported that the typically attenuated response to negative stimuli in older adults is less pronounced when individuals are also challenged by a stress task, compared with when they are in a more relaxed state. Of note, putative psychological and biological explanations of age differences in mood and affective regulation in response to sleep deprivation may in fact be very closely connected with each other, as recent advances in affective neuroscience suggest that emotions are strongly related to and likely rooted in bodily changes such as altered metabolism and energy regulation (Barrett, Quigley, & Hamilton, 2016).
Against the backdrop of the lower susceptibility to sleep deprivation shown in prior studies and the changes in emotion regulation strategies in older adults, the aim of this study was to test the hypothesis that sleep deprivation has a less strong effect on mood in older adults compared with young adults.
2. MATERIALS AND METHODS
2.1. Sample
The final sample consisted of 218 participants. Background information for the sample is displayed in Table 1. Participants were recruited via newspapers, online platforms and posters. They filled in online forms assessing demographic variables, health and sleep habits. Inclusion criteria were being 18–30 or 60–72 years old, being fluent in Swedish and living in the greater Stockholm area. Individuals who reported current severe somatic disease, psychiatric or neurological disorders or intake of relevant medication, daily use of nicotine, drinking more than four cups of caffeinated drinks/day or inability to abstain from caffeine were excluded. Participants were screened for sleep disorders and current stress (see for more details Schwarz et al. (2018)). Participants who fulfilled the inclusion criteria were invited to an initial meeting approximately 1 week before the test session. Participants were economically compensated and gave written informed consent. The study was approved by the Regional Ethical Review Board in Stockholm.
Table 1.
Sample characteristics (mean [standard deviation])
| Young adults (18–30 years) | Older adults (60–72 years) | |||
|---|---|---|---|---|
| Normal night's sleep | Sleep deprivation | Normal night's sleep | Sleep deprivation | |
| Total, n (male) | 63 (36) | 61 (37) | 47 (16) | 47 (16) |
| Age (years) | 23.08 (3.37) | 23.52 (3.1) | 66.30 (3.30) | 67.26 (3.24) |
| BMI (kg/m2) | 22.33 (2.46) | 22.93 (2.41) | 23.54 (2.26) | 23.28 (2.32) |
| Insomnia severity index | 3.56 (2.35) | 3.08 (2.44) | 3.79 (2.84) | 4.19 (2.42) |
| Epworth Sleepiness Scale | 5.40 (2.84) | 5.13 (2.94) | 5.91 (3.37) | 5.77 (3.20) |
| Duration of sleep period 1 night before (hh:mm, A)a | 07:35 (00:56) | . (.) | 08:06 (01:04) | . (.) |
| Sleep start 1 night before (hh:mm, A)a | 23:58 (00:51) | . (.) | 23:08 (01:00) | . (.) |
| Sleep end 1 night before (hh:mm, A)a | 07:34 (00:50) | . (.) | 07:15 (00:39) | . (.) |
| Duration of sleep period 2–3 nights before (hh:mm, A)b | 07:44 (01:01) | 07:49 (00:53) | 07:43 (00:51) | 07:54 (00:45) |
| Sleep start 2–3 nights before (hh:mm, A)b | 00:23 (01:12) | 00:12 (00:53) | 23:41 (00:55) | 23:13 (00:47) |
| Sleep end 2–3 nights before (hh:mm, A)b | 08:07 (01:08) | 08:02 (00:56) | 07:25 (00:40) | 07:07 (00:55) |
| Start time 13.00/16.00 | 32/31 | 30/31 | 25/22 | 27/20 |
n = 6 missing;
n = 15 missing.
A, Actigraphy; BMI, body mass index.
2.2. Design
This study is part of a larger project (Schwarz et al., 2018). Stratified by age and gender, participants were randomized to either one night of normal sleep or sleep deprivation for one night, as well as session start time. There was no significant difference between conditions regarding the allocation to session start at 13.00 or 16.00 hours (χ2 3 = 0.81, p = 0.847). Moreover, half of the participants were allocated to a stress condition, during which they participated in a psychosocial challenge in the latter part of the test session. Participants were blinded for the stress condition, and all measures that were used in the current analysis were collected before the psychosocial challenge. Therefore, data were aggregated across stress and no‐stress conditions.
Participants were asked to keep their regular sleep–wake schedule during the week before the test session. Participants completed sleep diaries and wore actigraphs (Actiwatch 4, CamNtech) for 3 days prior to the test session. Moreover, they were instructed to refrain from caffeine, alcohol, napping and physical training on the day before and the day of the test session.
Participants assigned to the sleep deprivation condition arrived at the laboratory at 22:00 hours the day before and stayed until the test session. During the night, they rated stress and sleepiness hourly, and completed a 30‐min cognitive test battery every other hour. The majority of the time, they were free to engage in low‐demanding leisure activities. After the test session, sleep‐deprived participants were offered a taxi ride home for safety reasons.
Participants in the normal sleep condition slept at home in order to avoid the laboratory environment negatively affecting their sleep, and were asked to sleep for at least 7 hr and to wake up at 08:00 hours at the latest. They arrived in time for their test session at the laboratory.
Daytime test sessions started at either 13.00 or 16.00 hours and took place in a different building to the night‐time sleep deprivation. Upon arrival at the laboratory, participants gave a saliva sample, were set up with a heart rate monitor and performed a short gait analysis test. Following this, they filled in a range of questionnaires on their mood and well‐being (the outcome of interest in the current study), which are described below. Scales were administered at approximately 13.20 or 16.20 hours in the following order: CHICa, Profile of Mood States, Positive and Negative Affect Schedule, Karolinska Sleepiness Scale (KSS) and subjective stress rating.
2.3. Outcome measures
Subjective sleepiness was measured using the KSS (Åkerstedt & Gillberg, 1990). Participants were instructed to rate their sleepiness during the past 5 min.
Participants also rated their subjective stress levels using a single‐item question with ordinal anchored response alternatives ranging from 1 = very relaxed to 9 = extremely stressed. Participants were instructed to rate their stress levels during the past 5 min. The stress scale has not yet been formally validated, but our recent results suggest that it is a sensitive measure when participants are exposed to an acute stress paradigm (Schwarz et al., 2018).
The Positive and Negative Affect Schedule (PANAS) was used to assess positive and negative affect (Watson, Clark, & Tellegen, 1988). Participants were instructed to rate how they felt right now.
The Profile of Mood States (POMS) (McNair, Lorr, & Droppleman, 1992) is a well‐established rating scale containing 65 items that assesses transient mood states. It comprises six subscales: tension, depression, anger, fatigue, confusion and vigour. The overall score of ‘total mood disturbance’ is an aggregate measure calculated from the six subscales, with vigour subtracted from the sum of the other subscales. In the current study, the participants were instructed to rate how they felt right now.
The CHICa scale is a 24‐item rating scale (ratings from 0 to 3) that measures subjective consequences of sleep loss on four subscales covering thermoregulation (cold), disrupted appetite (hunger), affective problems (irritability) and cognitive functioning (cognitive attenuation) (Oginska, Mojsa‐Kaja, Fafrowicz, & Marek, 2014). We calculated the average score for each subscale. In the present context, the irritability scale is of primary interest, but for completeness all subscales are reported. Because the questionnaire was translated to Swedish for the purpose of this study, we calculated Cronbach's alpha, showing a good internal consistency for all subscales (cold α = 0.88, hunger α = 0.87, irritability α = 0.87, cognitive attenuation α = 0.90).
2.4. Statistical analysis
To investigate whether the effect of sleep deprivation on mood is age dependent, data were analyzed using a 2 × 2 ANOVA with age and sleep deprivation as between‐subject factors. The significance level was set at p < 0.05 (two sided).
Inferential analysis was complemented by omega squared as the effect size measure for interaction effects, which was calculated using the formula (Lakens, 2013):
Bias‐corrected and accelerated confidence intervals for effect sizes were calculated using bootstrapping with 1000 samples (stratified selection for condition). According to Cohen (1988, cited after Kim (2016)), small effect sizes are defined as 0.01 < ω2 < 0.06, medium effect sizes as 0.06 < ω2 < 0.15 and large effect sizes as ω2 > 0.15.
We only report effect sizes for interaction effects, as this is the main scope of the present paper. Moreover, in the presence of significant interactions, main effects cannot always be interpreted in a meaningful way.
2.5. Supplementary analysis
Older adults compared with younger adults are more prone to be morning types (Roenneberg et al., 2007), which in turn has been associated with more positive affect (Biss & Hasher, 2012). Thus, diurnal type could be a mediator of putative age differences. Therefore, we conducted a supplementary analysis using diurnal preference (measured using the Diurnal Type Scale (Torsvall & Akerstedt, 1980)) as covariate. Results, which by and large did not affect the conclusions of the main analysis, are presented as Supporting Information, Table S1.
3. RESULTS
We investigated the age‐dependent effects of sleep deprivation across a wide range of mood measures. Descriptive values are displayed in Figure 1; results of the 2 × 2 ANOVA are presented in Table 2. For some outcome measures, values of two participants were missing because of technical error, as denoted in Table 2. Effect sizes for the sleep by age interaction are presented in Figure 2.
Figure 1.
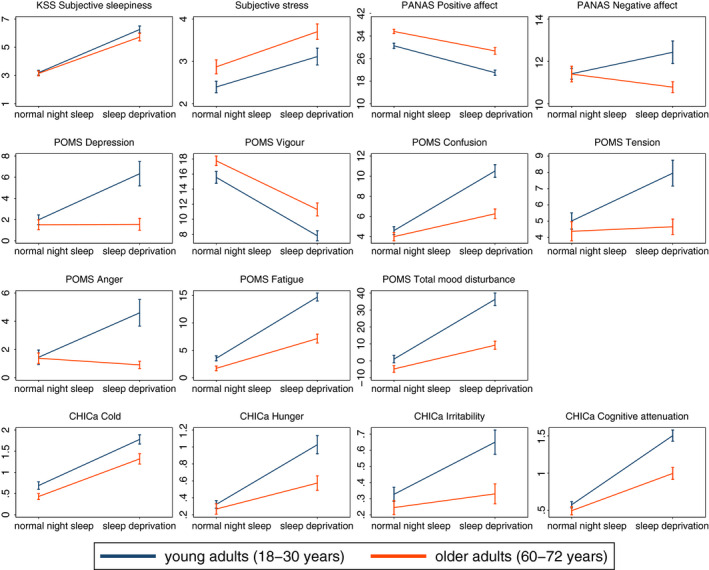
Descriptive data for the outcome measures (Mean ± Standard Error of the Mean) in the four conditions. KSS, Karolinska Sleepiness Scale; PANAS, positive and negative affect schedule; POMS, profile of mood states
Table 2.
Results from age by sleep deprivation ANOVA
| Age | Sleep deprivation | Age × Sleep deprivation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | F | df | p | |
| KSS | 1.84 | 1,214 | 0.176 | 164.42 | 1,214 | <0.001 | 1.2 | 1,214 | 0.274 |
| Subjective stress | 9.33 | 1,214 | 0.003 | 19.78 | 1,214 | <0.001 | 0.1 | 1,214 | 0.748 |
| Positive and negative affect schedulea | |||||||||
| Positive affect | 44.44 | 1,212 | <0.001 | 73.46 | 1,212 | <0.001 | 1.9 | 1,212 | 0.170 |
| Negative affect | 4.59 | 1,212 | 0.033 | 0.26 | 1,212 | 0.609 | 4.43 | 1,212 | 0.037 |
| Profile of mood statesa | |||||||||
| Depression | 11.72 | 1,212 | 0.001 | 8.19 | 1,212 | 0.005 | 7.88 | 1,212 | 0.005 |
| Vigour | 14.41 | 1,212 | <0.001 | 89.85 | 1,212 | <0.001 | 0.75 | 1,212 | 0.389 |
| Confusion | 23.51 | 1,212 | <0.001 | 67.06 | 1,212 | <0.001 | 13.31 | 1,212 | <0.001 |
| Tension | 9.87 | 1,212 | 0.002 | 6.61 | 1,212 | 0.011 | 4.53 | 1,212 | 0.035 |
| Anger | 8.68 | 1,212 | 0.004 | 4.43 | 1,212 | 0.037 | 8.06 | 1,212 | 0.005 |
| Fatigue | 55.52 | 1,212 | <0.001 | 173.28 | 1,212 | <0.001 | 20.75 | 1,212 | <0.001 |
| Total mood disturbance | 34.93 | 1,212 | <0.001 | 78.56 | 1,212 | <0.001 | 14.62 | 1,212 | <0.001 |
| CHICaa | |||||||||
| Cold | 12.69 | 1,212 | <0.001 | 96.12 | 1,212 | <0.001 | 1.00 | 1,212 | 0.319 |
| Hunger | 10.02 | 1,212 | 0.002 | 39.87 | 1,212 | <0.001 | 6.18 | 1,212 | 0.014 |
| Irritability | 11.52 | 1,212 | 0.001 | 11.89 | 1,212 | 0.001 | 4.05 | 1,212 | 0.046 |
| Cognitive attenuation | 20.71 | 1,212 | <0.001 | 122.79 | 1,212 | <0.001 | 11.03 | 1,212 | 0.001 |
n = 2 missing. KSS, Karolinska Sleepiness Scale.
Figure 2.
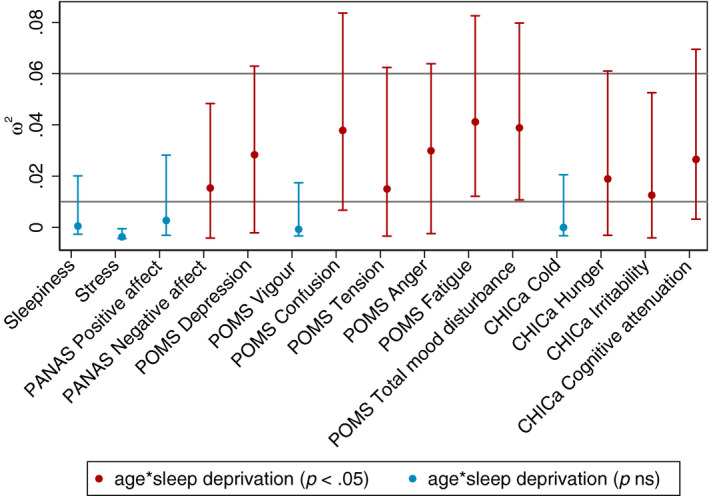
Effect size w2 for age by sleep deprivation interactions with bias‐corrected and accelerated 95% confidence intervals. Horizontal lines indicate cut‐offs for small (0.01) and medium effect sizes (0.06), respectively. PANAS, positive and negative affect schedule; POMS, profile of mood states; Total Mood D, total mood disturbance
3.1. Subjective sleepiness and stress
The age by sleep deprivation interactions were not significant for sleepiness and stress. Sleep deprivation resulted in increased sleepiness and more subjective stress see (Figure 1). Older adults were more stressed than younger adults, but showed no difference with respect to sleepiness.
3.2. Positive and Negative Affect Schedule (PANAS)
For PANAS positive affect, we observed significant main effects of age and sleep deprivation; positive affect was lower in young adults and after sleep deprivation.
For PANAS negative affect, the age by sleep deprivation interaction and the main effect for age were significant. According to follow‐up t tests, the effect of sleep deprivation was not significant in either age group. After a normal night's sleep no age differences were seen but after sleep deprivation young adults reported more negative affect than older adults (normal night's sleep, t 107 = 0.03, p = 0.973; sleep deprivation, t 105 = 2.56, p = 0.012).
3.3. Profile of Mood States (POMS)
Significant age by sleep deprivation interactions occurred for total mood disturbance and for all subscales but vigour, indicating that the effect of sleep deprivation was age dependent. Moreover, main effects for age and sleep were significant for all POMS subscales and total mood disturbance.
Follow‐up t tests for the interactions showed that in the young group, sleep deprivation was related to changes in all subscales and total mood disturbance (ps < 0.01). In the older participants, confusion, fatigue and total mood disturbance were significantly increased after sleep deprivation (ps < 0.001), but the changes were smaller compared with the young adults. Depression, tension and anger did not significantly differ between sleep‐deprived and rested older adults.
Vigour was higher in older compared with young participants, and lower in the sleep deprivation condition.
In the non‐sleep deprived participants, depression, confusion, tension, anger and total mood disturbance were not significantly different in young and older adults (all ps > 0.05), but vigour was higher in older compared with young participants (t 107 = −2.1, p = 0.038) and fatigue lower (t 107 = 2.85, p = 0.005).
3.4. CHICa
Age by sleep deprivation interactions were significant for the CHICa subscales hunger, irritability and cognitive attenuation. Sleep deprivation was associated with an increase in irritability in young (t 120 = −3.75, p < 0.001) but not older participants (t 92 = −1.13, p = 0.261). Hunger and cognitive problems were higher after sleep deprivation in both young and older adults, but the effect of sleep deprivation was smaller for the older adults (hunger (young), t 120 = −6.14, p < 0.001; hunger (older) t 92 = −2.91, p = 0.004; cognitive attenuation (young), t 120 = −10.92, p < 0.001; cognitive attenuation (older), t 92 = −5.16, p < 0.001).
Both young (t 120 = −7.77, p < 0.001) and older participants (t 92 = −6.33, p < 0.001) felt significantly colder in the sleep deprivation than in the normal night's sleep condition.
In the normal sleep condition, young and older adults did not differ regarding irritability (t 107 = 1.31, p = 0.194), hunger (t 107 = 0.73, p = 0.464) and cognitive attenuation (t 107 = 1.17, p = 0.243), but young participants felt significantly more cold (t 107 = 2.17, p = 0.033).
4. DISCUSSION
The aim of the present study was to investigate whether the effect of sleep deprivation differs between young and older adults. The sleep‐deprived group showed more sleepiness, subjective stress and feeling cold, and less vigour and positive affect, regardless of age. All the other outcome measures (negative affect, depression, confusion, tension, anger, fatigue, total mood disturbance, hunger, cognitive attenuation and irritability) showed a weaker response in the older group. Thus, the present results suggest that older adults are not only less vulnerable to sleep deprivation in terms of cognitive performance, as highlighted by previous studies (Duffy et al., 2009; Philip et al., 2004), but also in terms of being more resilient to mood deterioration.
The increased sleepiness levels confirm that the sleep deprivation protocol was successful, as sleepiness is a sensitive indicator of sleep deprivation (Akerstedt, Anund, Axelsson, & Kecklund, 2014). No age differences occurred with respect to rated sleepiness. Previous results on age differences in sleepiness after prolonged wakefulness are variable (Duffy et al., 2009; Philip et al., 2004; Sagaspe et al., 2012). The increased subjective stress levels after sleep deprivation are in line with previous research (Franzen et al., 2011; Wright et al., 2015).
Sleep deprivation had pronounced effects on mood and other symptoms of sleep loss. Using the PANAS scale, we found that positive affect was lower both in young and older adults who participated in the sleep deprivation condition. Moreover, negative affect was lower after sleep deprivation in older adults compared with the young group. The results in the young group parallel previous research that showed an effect of sleep deprivation on positive but not on negative affect on the PANAS scale (Franzen, Siegle, & Buysse, 2008).
The changes in the other mood measures, foremost in the young group (i.e. the decrease in vigour, and the increases in depression, confusion, tension, anger, fatigue, total mood disturbance and irritability), are in accordance with several previous studies that reported higher fatigue (Drake et al., 2001; Scott, Mcnaughton, & Polman, 2006), anxiety (Pires, Bezerra, Tufik, & Andersen, 2016), depressive symptoms (Paterson et al., 2011; Scott et al., 2006) and confusion (Drake et al., 2001), as well as reduced vigour (Drake et al., 2001; Scott et al., 2006), happiness (Paterson et al., 2011) and activation (Paterson et al., 2011) after sleep deprivation. In the present study, participants also felt more hungry and cold after sleep deprivation, which could be interpreted in line with the increased appetite (Knutson, Spiegel, Penev, & Van Cauter, 2007) and altered thermoregulation (Romeijn et al., 2012) described previously.
Consistent with our hypothesis, older adults were less affected by sleep loss for the majority of outcome measures. They had a slightly lower negative affect in the sleep deprivation condition than young adults, and the increases in confusion, fatigue, total mood disturbance, hunger and cognitive attenuation in response to sleep deprivation were mitigated. Moreover, ratings of depression, tension, anger and irritability were not significantly changed by sleep deprivation. Thus, sleep deprivation had a distinctively different effect on mood in young and older adults, despite sleepiness being similar in both age groups.
Examining the overall pattern of the variables in more detail, it seems that the effect of sleep deprivation differed between young and older adults exclusively on the scales that are targeting the negative affect dimension (i.e. PANAS negative affect, POMS depression, confusion, anger, tension and fatigue, CHICa irritation and cognitive attenuation). On the other hand, the scales related to positive affect (i.e. PANAS positive affect, POMS vigour) and scales that target other common subjective consequences of sleep loss (sleepiness, stress and cold) show a similar pattern in young and older individuals. It may seem surprising that we found an interaction effect for fatigue, but not for KSS. Yet it is worth noting that the POMS fatigue scale contains negatively valenced items (i.e. worn out, listless, fatigued, exhausted, sluggish, weary, bushed). KSS, on the other hand, assesses sleepiness without directly implying a negative valence. This may be an important difference between the two measures.
The current results indicate that older adults are showing a similar decline in positive affect after sleep deprivation to young adults, but they seem superior at regulating negative affect. This is particularly interesting as negative items on mood scales cannot merely be considered antonyms of positive items (Steptoe et al., 2015). Both the negative and the positive dimension of affect carry unique information that is essential for assessing hedonic well‐being (Steptoe et al., 2015). One emotion regulation strategy commonly used by older adults is to shift attention away from and selectively ignore stimuli and situations that are likely to elicit a negative mood (see review by Mather, 2012). Thus, a key component of the positivity effect in older age is a bias towards attending less to negative information, rather than merely focusing on positive information. Assuming that in a sleep deprivation protocol participants are likely to be exposed to a situation that elicits negative rather than positive feelings, it seems logical that we found the interaction effect mainly on scales targeting negative affect.
The current results are at odds with one small previous study that found that older persons’ (n = 16) well‐being was more impaired by sleep deprivation than that of young adults (n = 16) (Birchler‐Pedross et al., 2009). Yet it is difficult to directly compare results, as in that study well‐being was calculated as an integrative measure that combined different aspects (visual analogue scales for mood, tension and physical comfort) and it is unclear whether, for instance, older adults may have suffered mostly from lower comfort in the constant routine situation. However, our results fit overall very well with studies on cognition that demonstrate that older adults outperform young adults in cognitive tasks after sleep deprivation (Duffy et al., 2009; Philip et al., 2004). Because cognition and emotion are closely intertwined and affect each other (for review see, for example, Okon‐Singer, Hendler, Pessoa, and Shackman, 2015), it would be interesting to investigate in future studies whether the individuals that show less impairment regarding their affect are also the ones who are less susceptible regarding cognitive performance, in particular with respect to an ageing perspective. Beyond the impact on cognition, positive affect and well‐being are considered protective factors for health and longevity (Chida & Steptoe, 2008; Steptoe & Wardle, 2011). Thus, a less strong affective response to challenges such as sleep loss could contribute to maintaining better health in older age.
4.1. Limitations
Here we primarily interpret the current findings in relation to a positivity effect as a function of adult ageing, as suggested by the socioemotional selectivity theory (Carstensen et al., 1999). However, the effect of sleep deprivation on mood may not only be attributable to emotion regulation strategies but could in part also originate from biologically driven processes. Sleep duration and structure change across adult ageing (Schwarz et al., 2017), the circadian amplitude is reduced (Duffy, Zitting, & Chinoy, 2015), and homeostatic sleep pressure after curtailed sleep is lower in older age, which may indicate a reduced need for sleep (Akerstedt et al., 2018). In the current sample, sleep deprivation had a similar effect on the activity of baseline stress systems (cortisol and alpha amylase) in the two age groups (Schwarz et al., 2018) but other relevant aspects related to sleep–wake regulation may still have been impacted differentially in the young and older participants. Therefore, it is important to include more biological measures that better address whether differences in sleep drive (e.g. the homeostatic slow wave sleep response to sleep deprivation) are linked to any age‐dependent effects. Moreover, given that physiological changes and energy regulation may be also at the core of an affective experience (Barrett et al., 2016), it would be interesting to directly link the physiological consequences of sleep deprivation to the affective experience.
We here use self‐ratings of affective status, and thus cannot fully exclude any effects of reporting bias. On the other hand, self‐reported well‐being (e.g. mood rating) is related to health in older age (Steptoe et al., 2015), which underscores the value of self‐report data. As with most studies on ageing, we cannot distinguish between cohort effects and true effects of ageing because of the between‐subject design. Moreover, participants slept at their home in the normal night's sleep condition and arrived at the laboratory in time for the test session, whereas sleep‐deprived individuals stayed in the laboratory from the evening before. However, the same procedure was used for young and older adults, and the results in young adults correspond very well with previous studies. It would also have been interesting to include further age groups, such as adolescence and midlife, to elucidate whether the effect of sleep deprivation on mood is linear or curvilinear across age.
This is a selected sample of healthy participants, so it is unclear how the results generalize to the general population. Because it has been proposed that older adults focus more on emotional control than younger adults (Lawton, Kleban, Rajagopal, & Dean, 1992), it is, for instance, possible that older individuals vulnerable to sleep loss would not sign up for a sleep deprivation study. We here address only the response to sleep deprivation for one night. However, in daily life many individuals experience chronic sleep restriction rather than short‐term total sleep deprivation. Therefore, it remains an important challenge to address whether similar age‐dependent effects on mood occur when participants experience longer‐term sleep curtailment, both inside and outside the laboratory. Lastly, because circadian aspects, in combination with homeostatic aspects, may impact on mood (Boivin et al., 1997), it is important to investigate in future studies whether the age‐dependent effect of sleep deprivation varies across the day/circadian phase. A strength of the current study is the relatively large dataset, as well as the use of established questionnaires that address both the positive and negative dimension of affect.
4.2. Overall conclusion
Overall, the results of the current study show that young participants are emotionally more affected by sleep deprivation than older adults, especially regarding negative aspects of mood. This could possibly be related to the well‐known positivity effect in older age (Reed & Carstensen, 2012), which suggests that older adults prioritize regulating their emotions to optimize well‐being and therefore strategically shift attention away from stimuli and situations that may elicit negative affect, and may also do this during sleep deprivation. The results also highlight that caution is warranted when generalizing results from sleep deprivation studies across the adult lifespan.
AUTHOR CONTRIBUTIONS
Johanna Schwarz: study design, data collection, data analysis, interpretation of results and preparation of the manuscript. John Axelsson: study design, interpretation of results and preparation of the manuscript. Andreas Gerhardsson: data collection, interpretation of results and preparation of the manuscript. Sandra Tamm: interpretation of results and preparation of the manuscript. Håkan Fischer: study design, interpretation of results and preparation of the manuscript. Göran Kecklund: study design, interpretation of results and preparation of the manuscript. Torbjörn Åkerstedt: study design, interpretation of results and preparation of the manuscript.
CONFLICT OF INTEREST
This study was supported by a grant from Riksbankens Jubileumsfond (P13‐0649:1). None of the authors has any relevant conflict of interest to declare.
Supporting information
Table S1
Schwarz J, Axelsson J, Gerhardsson A, et al. Mood impairment is stronger in young than in older adults after sleep deprivation. J Sleep Res. 2019;28:e12801 10.1111/jsr.12801
REFERENCES
- Akerstedt, T. , Anund, A. , Axelsson, J. , & Kecklund, G. (2014). Subjective sleepiness is a sensitive indicator of insufficient sleep and impaired waking function. Journal of Sleep Research, 23, 240–252. [DOI] [PubMed] [Google Scholar]
- Åkerstedt, T. , & Gillberg, M. (1990). Subjective and objective sleepiness in the active individual. International Journal of Neuroscience, 52, 29–37. 10.3109/00207459008994241 [DOI] [PubMed] [Google Scholar]
- Akerstedt, T. , Lekander, M. , Nilsonne, G. , Tamm, S. , D'onofrio, P. , Kecklund, G. , … Schwarz, J. (2018). Effects of late‐night short‐sleep on in‐home polysomnography: Relation to adult age and sex. Journal of Sleep Research, 27, e12626 10.1111/jsr.12626 [DOI] [PubMed] [Google Scholar]
- Barrett, L. F. , Quigley, K. S. , & Hamilton, P. (2016). An active inference theory of allostasis and interoception in depression. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 371, 20160011 10.1098/rstb.2016.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie, L. , Kyle, S. D. , Espie, C. A. , & Biello, S. M. (2015). Social interactions, emotion and sleep: A systematic review and research agenda. Sleep Medicine Reviews, 24, 83–100. 10.1016/j.smrv.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Birchler‐Pedross, A. , Schroder, C. M. , Munch, M. , Knoblauch, V. , Blatter, K. , Schnitzler‐Sack, C. , … Cajochen, C. (2009). Subjective well‐being is modulated by circadian phase, sleep pressure, age, and gender. Journal of Biological Rhythms, 24, 232–242. 10.1177/0748730409335546 [DOI] [PubMed] [Google Scholar]
- Biss, R. K. , & Hasher, L. (2012). Happy as a lark: Morning‐type younger and older adults are higher in positive affect. Emotion, 12, 437–441. 10.1037/a0027071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin, D. B. , Czeisler, C. A. , Dijk, D. J. , Duffy, J. F. , Folkard, S. , Minors, D. S. , … Waterhouse, J. M. (1997). Complex interaction of the sleep‐wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry, 54, 145–152. 10.1001/archpsyc.1997.01830140055010 [DOI] [PubMed] [Google Scholar]
- Carstensen, L. L. , Isaacowitz, D. M. , & Charles, S. T. (1999). Taking time seriously. A theory of socioemotional selectivity. The American Psychologist, 54, 165–181. 10.1037/0003-066x.54.3.165 [DOI] [PubMed] [Google Scholar]
- Chida, Y. , & Steptoe, A. (2008). Positive psychological well‐being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine, 70, 741–756. 10.1097/psy.0b013e31818105ba [DOI] [PubMed] [Google Scholar]
- Drake, C. L. , Roehrs, T. A. , Burduvali, E. , Bonahoom, A. , Rosekind, M. , & Roth, T. (2001). Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology, 38, 979–987. 10.1111/1469-8986.3860979 [DOI] [PubMed] [Google Scholar]
- Duffy, J. F. , Willson, H. J. , Wang, W. , & Czeisler, C. A. (2009). Healthy older adults better tolerate sleep deprivation than young adults. Journal of the American Geriatrics Society, 57, 1245–1251. 10.1111/(issn)1532-5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, J. F. , Zitting, K.‐M. , & Chinoy, E. D. (2015). Aging and circadian rhythms. Sleep Medicine Clinics, 10, 423–434. 10.1016/j.jsmc.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English, T. , & Carstensen, L. L. (2014). Emotional experience in the mornings and the evenings: Consideration of age differences in specific emotions by time of day. Frontiers in Psychology, 5, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerd, D. , Klumpers, F. , Oude Voshaar, R. , Fernández, G. , & Tendolkar, I. (2017). Acute stress enhances emotional face processing in the aging brain. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2, 591–598. [DOI] [PubMed] [Google Scholar]
- Franzen, P. L. , Gianaros, P. J. , Marsland, A. L. , Hall, M. H. , Siegle, G. J. , Dahl, R. E. , & Buysse, D. J. (2011). Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosomatic Medicine, 73, 679–682. 10.1097/psy.0b013e31822ff440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen, P. L. , Siegle, G. J. , & Buysse, D. J. (2008). Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research, 17, 34–41. 10.1111/j.1365-2869.2008.00635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A. N. , & Walker, M. P. (2014). The role of sleep in emotional brain function. Annual Review of Clinical Psychology, 10, 679–708. 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang, C. , & Knight, B. G. (2016). Age differences in interpreting ambiguous situations: The effects of content themes and depressed mood. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 71, 1024–1033. 10.1093/geronb/gbv037 [DOI] [PubMed] [Google Scholar]
- Kahn, M. , Sheppes, G. , & Sadeh, A. (2013). Sleep and emotions: Bidirectional links and underlying mechanisms. International Journal of Psychophysiology, 89, 218–228. 10.1016/j.ijpsycho.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Kim, H. Y. (2016). Statistical notes for clinical researchers: Sample size calculation 3. Comparison of several means using one‐way ANOVA. Restorative Dentistry & Endodontics, 41, 231–234. 10.5395/rde.2016.41.3.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, K. L. , Spiegel, K. , Penev, P. , & Van Cauter, E. (2007). The metabolic consequences of sleep deprivation. Sleep Medicine Reviews, 11, 163–178. 10.1016/j.smrv.2007.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t‐tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton, M. P. , Kleban, M. H. , Rajagopal, D. , & Dean, J. (1992). Dimensions of affective experience in three age groups. Psychology and Aging, 7, 171–184. 10.1037/0882-7974.7.2.171 [DOI] [PubMed] [Google Scholar]
- Mather, M. (2012). The emotion paradox in the aging brain. Annals of the New York Academy of Sciences, 1251, 33–49. 10.1111/j.1749-6632.2012.06471.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair, D. M. , Lorr, M. , & Droppleman, L. F. (1992). Profile of Mood States (POMS) manual. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Oginska, H. , Mojsa‐Kaja, J. , Fafrowicz, M. , & Marek, T. (2014). Measuring individual vulnerability to sleep loss–the CHICa scale. Journal of Sleep Research, 23, 339–346. [DOI] [PubMed] [Google Scholar]
- Okon‐Singer, H. , Hendler, T. , Pessoa, L. , & Shackman, A. J. (2015). The neurobiology of emotion‐cognition interactions: Fundamental questions and strategies for future research. Frontiers in Human Neuroscience, 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, J. L. , Dorrian, J. , Ferguson, S. A. , Jay, S. M. , Lamond, N. , Murphy, P. J. , … Dawson, D. (2011). Changes in structural aspects of mood during 39‐66 h of sleep loss using matched controls. Applied Ergonomics, 42, 196–201. 10.1016/j.apergo.2010.06.014 [DOI] [PubMed] [Google Scholar]
- Philip, P. , Taillard, J. , Sagaspe, P. , Valtat, C. , Sanchez‐Ortuno, M. , Moore, N. , … Bioulac, B. (2004). Age, performance and sleep deprivation. Journal of Sleep Research, 13, 105–110. 10.1111/j.1365-2869.2004.00399.x [DOI] [PubMed] [Google Scholar]
- Pilcher, J. J. , & Huffcutt, A. I. (1996). Effects of sleep deprivation on performance: A meta‐analysis. Sleep, 19, 318–326. 10.1093/sleep/19.4.318 [DOI] [PubMed] [Google Scholar]
- Pires, G. N. , Bezerra, A. G. , Tufik, S. , & Andersen, M. L. (2016). Effects of acute sleep deprivation on state anxiety levels: A systematic review and meta‐analysis. Sleep Medicine, 24, 109–118. 10.1016/j.sleep.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Reed, A. E. , & Carstensen, L. L. (2012). The theory behind the age‐related positivity effect. Frontiers in Psychology, 3, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, A. E. , Chan, L. , & Mikels, J. A. (2014). Meta‐analysis of the age‐related positivity effect: Age differences in preferences for positive over negative information. Psychology and Aging, 29, 1–15. 10.1037/a0035194 [DOI] [PubMed] [Google Scholar]
- Roenneberg, T. , Kuehnle, T. , Juda, M. , Kantermann, T. , Allebrandt, K. , Gordijn, M. , & Merrow, M. (2007). Epidemiology of the human circadian clock. Sleep Medicine Reviews, 11, 429–438. 10.1016/j.smrv.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Romeijn, N. , Verweij, I. M. , Koeleman, A. , Mooij, A. , Steimke, R. , Virkkala, J. , … Van Someren, E. J. (2012). Cold hands, warm feet: Sleep deprivation disrupts thermoregulation and its association with vigilance. Sleep, 35, 1673–1683. 10.5665/sleep.2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagaspe, P. , Taillard, J. , Amieva, H. , Beck, A. , Rascol, O. , Dartigues, J. F. , … Philip, P. (2012). Influence of age, circadian and homeostatic processes on inhibitory motor control: A Go/Nogo task study. PLoS ONE, 7, e39410 10.1371/journal.pone.0039410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C. , Peigneux, P. , & Cajochen, C. (2012). Age‐related changes in sleep and circadian rhythms: Impact on cognitive performance and underlying neuroanatomical networks. Frontiers in Neurology, 3, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, J. F. A. , Akerstedt, T. , Lindberg, E. , Gruber, G. , Fischer, H. , & Theorell‐Haglow, J. (2017). Age affects sleep microstructure more than sleep macrostructure. Journal of Sleep Research, 26, 277–287. 10.1111/jsr.12478 [DOI] [PubMed] [Google Scholar]
- Schwarz, J. , Gerhardsson, A. , Van Leeuwen, W. , Lekander, M. , Ericson, M. , Fischer, H. , … Åkerstedt, T. (2018). Does sleep deprivation increase the vulnerability to acute psychosocial stress in young and older adults? Psychoneuroendocrinology, 96, 155–165. 10.1016/j.psyneuen.2018.06.003 [DOI] [PubMed] [Google Scholar]
- Scott, J. P. , Mcnaughton, L. R. , & Polman, R. C. (2006). Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiology and Behavior, 87, 396–408. 10.1016/j.physbeh.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Scullin, M. K. , & Bliwise, D. L. (2015). Sleep, cognition, and normal aging: Integrating a half‐century of multidisciplinary research. Perspectives on Psychological Science, 10, 97–137. 10.1177/1745691614556680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe, A. , Deaton, A. , & Stone, A. A. (2015). Subjective wellbeing, health, and ageing. The Lancet, 385, 640–648. 10.1016/s0140-6736(13)61489-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe, A. , & Wardle, J. (2011). Positive affect measured using ecological momentary assessment and survival in older men and women. Proceedings of the National Academy of Sciences of United States of America, 108, 18244–18248. 10.1073/pnas.1110892108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm, S. , Nilsonne, G. , Schwarz, J. , Lamm, C. , Kecklund, G. , Petrovic, P. , … Lekander, M. (2017). The effect of sleep restriction on empathy for pain: An fMRI study in younger and older adults. Scientific Reports, 7, 12236 10.1038/s41598-017-12098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsvall, L. , & Akerstedt, T. (1980). A diurnal type scale Construction, consistency and validation in shift work. Scandinavian Journal of Work, Environment & Health, 6, 283–290. 10.5271/sjweh.2608 [DOI] [PubMed] [Google Scholar]
- Watson, D. , Clark, L. A. , & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Wright, K. P. Jr , Drake, A. L. , Frey, D. J. , Fleshner, M. , Desouza, C. A. , Gronfier, C. , & Czeisler, C. A. (2015). Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain, Behavior, and Immunity, 47, 24–34. 10.1016/j.bbi.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


