Abstract
The emotional dysregulation and impaired working memory found after sleep loss can have severe implications for our daily functioning. Considering the intertwined relationship between emotion and cognition in stimuli processing, there could be further implications of sleep deprivation in high‐complex emotional situations. Although studied separately, this interaction between emotion and cognitive processes has been neglected in sleep research. The aim of the present study was to investigate the effect of 1 night of sleep deprivation on emotional working memory. Sixty‐one healthy participants (mean age: 23.4 years) were either sleep deprived for 1 night (n = 30) or had a normal night’s sleep (n = 31). They performed an N‐back task with two levels of working memory load (1‐back and 3‐back) using positive, neutral and negative picture scenes. Sleep deprivation, compared with full night sleep, impaired emotional working memory accuracy, but not reaction times. The sleep‐deprived participants, but not the controls, responded faster to positive than to negative and neutral pictures. The effect of sleep deprivation was similar for both high and low working memory loads. The results showed that although detrimental in terms of accuracy, sleep deprivation did not impair working memory speed. In fact, our findings indicate that positive stimuli may facilitate working memory processing speed after sleep deprivation.
Keywords: affective significance, executive functions, positivity effect, sleep loss, sustained wakefulness
1. INTRODUCTION
May I say that I have not thoroughly enjoyed serving with humans? I find their illogic and foolish emotions a constant irritant. (Spock, Star Trek, 1968)
According to the alien character Spock, having emotions that interfere with reasoning is a typical human trait. This trait may be even more prominent after a night without sleep as sleep deprivation causes cognitive and emotional dysfunction (Goldstein & Walker, 2014; Lim & Dinges, 2010) that could impair everyday activities and work performance. Although studied separately, the integrative effect of sleep deprivation on cognition and emotion has received little attention.
Sleep deprivation has detrimental effects on a wide array of cognitive functioning, with an especially high impact on sustained attention and working memory tasks as shown in a meta‐analysis (Lim & Dinges, 2010). Working memory comprises both executive and non‐executive components, and to fully understand the effects of sleep deprivation on working memory these components must be disentangled (Whitney & Hinson, 2010). By using two levels of working memory load, recent work shows that sleep deprivation mainly impairs the non‐executive processes of working memory such as vigilance and attention (Lo et al., 2012; Tucker, Whitney, Belenky, Hinson, & Van Dongen, 2010). A reason for this may be an activation of compensatory mechanisms associated with increased task demands (Drummond, Brown, Salamat, & Gillin, 2004).
Sleep deprivation is also associated with emotional dysregulation (Goldstein & Walker, 2014). Neural evidence of this dysregulation indicates both an increased emotional reactivity and decreased cognitive control of the emotional reaction towards positive and negative affective stimuli (Gujar, Yoo, Hu, & Walker, 2011; Yoo, Gujar, Hu, Jolesz, & Walker, 2007). This emotional dysregulation has been further supported by behavioural findings of impulsivity (Anderson & Platten, 2011), increased attention towards negative stimuli (Cote, Jancsar, & Hunt, 2015), increased negativity towards neutral stimuli (Tempesta et al., 2010), less decrease in emotional response (Van Der Helm et al., 2011) and higher distractibility by negative images during working memory performance (Chuah et al., 2010) following sleep deprivation. Sleep deprivation has also been found to increase neural activity in reward networks when presented with positive pictures (Gujar et al., 2011). On the other hand, some evidence points to a dampened emotional response after sleep deprivation (Pilcher, Callan, & Posey, 2015). Taken together, previous research shows emotional dysregulation after sleep deprivation. However, it is less known how that impacts higher cognitive functions after sleep loss.
Non‐sleep‐related affective psychology research shows that emotional stimuli are prioritized in attentional processing, which in turn can impair or facilitate cognitive functions (Pessoa, 2009). It has been reported that both positive and negative affective picture stimuli improve working memory performance (Levens & Phelps, 2008; Lindström & Bohlin, 2011). Other studies have found that while positive stimuli are beneficial for working memory performance, negative stimuli impair it (Osaka, Yaoi, Minamoto, & Osaka, 2013; Perlstein, Elbert, & Stenger, 2002). A reason for the diverging results might be that when stimuli are perceived as threatening, the attentional resources are directed towards the threat, disrupting working memory performance (Pessoa, 2009). On the other hand, a high level of working memory load may absorb more attentional resources and reduce the impact of a distracting emotional component (Hur, Iordan, Dolcos, & Berenbaum, 2016). Given an emotional dysregulation after sleep deprivation (Gujar et al., 2011; Yoo et al., 2007), working memory performance could be further modulated by emotional content.
To the best of our knowledge, the effects of sleep deprivation on emotional working memory have only been investigated once and the study reported no significant effects of valence after a full night of sleep deprivation (Tempesta, De Gennaro, Presaghi, & Ferrara, 2014). The authors used a task with a single working memory load (2‐back), thus the dissociation between executive and non‐executive components (Tucker et al., 2010) of emotional working memory following sleep deprivation has not previously been addressed. The aim of the present study was to examine the emotion–working memory interaction after 1 night of sleep deprivation by using positive, negative and neutral pictures in a task with two levels of working memory load.
2. Materials and methods
2.1. Participants
Data were collected from 61 healthy participants (age: M = 23.4 years, SD = 3.4 years, range = 18–30 years, 40% women). The participants were randomized to a control or a total sleep deprivation (TSD) condition as part of a larger project (Schwarz et al., 2018). Here, we focused on the young sample (see Table 1 for demographics and sleep characteristics). Before inclusion, all participants filled in online screening questionnaires assessing demography, health, sleep habits and sleep disorders. The included individuals had no current or previous psychiatric disorders, where besides self‐assessment we used the Hospital Anxiety and Depression Scale (Zigmond & Snaith, 1983) with exclusion criteria set to 11 or above. They reported no severe illness and no sleep disorders. Recruitment was done via an online platform, paper notes at public places and newspaper advertisement. Participants who fulfilled the inclusion criteria were invited to the lab approximately 1 week before testing for a screening interview and to get familiarized with the task. Participants were instructed to keep a regular sleep schedule (bedtime 21:30–00:30 hours and wake‐up time no later than 08:00 hours) the week before coming to the lab, and to refrain from caffeine, alcohol and training 24 hrs before the test session, which started 13:00 hours for half of the group and 16:00 hours for the other half (balanced across the sleep conditions) and lasted for 3 hrs in total. The emotional working memory task was performed 2 hrs into the test session (15:00 hours/18:00 hours). Sleeping habits were monitored by sleep diary and wrist actigraphy (Cambridge Neurotechnology®, Cambridge, UK) for 3 days prior to the experiment (Table 1). All participants signed a written informed consent and were economically compensated for participating. The study was approved by the Regional Ethical Review Board in Stockholm.
Table 1.
Descriptive table of age and sleep
| Control | Sleep deprivation | Test statistics | |||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||
| Age (years) | |||||
| Women | 14 | 25.14 (3.48) | 12 | 22.75 (3.49) | t = 1.74 |
| Men | 17 | 22.00 (3.18) | 18 | 23.72 (3.06) | t = −1.63 |
| Total | 31 | 23.42 (3.63) | 30 | 23.33 (3.22) | t = 0.10 |
| Education (%) | χ2 = 3.12 | ||||
| Secondary school | 0 | 0.00% | 1 | 1.64% | |
| High school | 13 | 21.31% | 13 | 21.31% | |
| University < 2.5 years | 8 | 13.11% | 11 | 18.03% | |
| University > 3 years | 10 | 16.39% | 5 | 8.20% | |
| Habitual sleep (hh:mm) | |||||
| Work sleep time | 31 | 23:12 (00:50) | 30 | 23:29 (00:49) | t = −1.30 |
| Work wake time | 31 | 07:29 (01:02) | 30 | 07:40 (01:13) | t = −0.59 |
| Work sleep duration | 31 | 08:17 (00:59) | 30 | 08:11 (01:06) | t = 0.38 |
| Free sleep time | 31 | 00:02 (00:57) | 30 | 00:35 (01:01) | t = −2.16* |
| Free wake time | 31 | 08:44 (01:05) | 30 | 09:19 (00:56) | t = −2.24* |
| Free sleep duration | 31 | 08:41 (01:07) | 30 | 08:43 (00:59) | t = −0.14 |
| Actiwatch (assumed sleep) | |||||
| Nights 2 & 3 before | 29 | 07:50 (00:55) | 28 | 07:50 (00:56) | t = −0.03 |
| Night before | 29 | 07:53 (01:02) | – | – | – |
| Sleep measures | |||||
| ISI | 31 | 3.77 (2.59) | 30 | 3.30 (2.41) | t = 0.74 |
| KSQ sleepiness | 31 | 5.39 (0.47) | 30 | 5.38 (0.38) | t = 0.06 |
| ESS | 31 | 4.87 (2.51) | 30 | 5.53 (2.85) | t = −0.96 |
ESS, Epworth Sleepiness Scale; ISI: Insomnia Severity Index; KSQ: Karolinska Sleep Questionnaire.
Four participants were omitted from actigraphy analysis due to technical issues. Actiwatch assumed sleep: nights 2 & 3 before: mean of 2 nights prior to the night before the experiment.
p < 0.05.
2.2. Design and procedure
2.2.1. Total sleep deprivation
Participants in the TSD condition arrived at the laboratory at 22:00 hours after being awake during the day, and stayed until the end of the test session the following day. During the night, participants performed cognitive tests every other hour, and rated their sleepiness using the Karolinska Sleepiness Scale (KSS; Åkerstedt & Gillberg, 1990) The majority of the time was spent doing low‐demanding leisure activities. From 07:00 hours until the testing session started, no cognitive tests were administered and participants were allowed to take a shower in the morning and a short walk outdoors with a research assistant. Window blinds were used during the night to keep light conditions constant. In addition to the actigraphs, participants were monitored by the experimenters. Standardized meals and snacks were provided throughout their time in the laboratory. No food was allowed from 1 hr before the start of the test session until the end of the session.
2.2.2. Sleep control
Participants in the control condition slept at home the night before the test session, monitored by wrist actigraphy (Table 1). They were instructed not to engage in any stressful activities 24 hrs before the test session and had been given a standardized lunch to be eaten at home, but were asked to refrain from eating from 1 hr before the start of the test session.
2.3. Apparatus
The task was performed on a 17.3‐inch display laptop with a NVIDA GeForce GTX 850M graphics card (resolution 1,920 × 1,080). Response keys (A and L on the keyboard) were marked with a red and a green sticker, respectively.
2.4. Emotional N‐back task
The N‐back task (Figure 1) is a common and valid measure of working memory where participants are to respond to whether a current stimulus matches the one presented N steps back in the sequence. The task was designed with two levels of working memory load (1‐back and 3‐back). Stimuli consisted of positive, neutral and negative scenes from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2008). Emotional pictures were matched on arousal (Negative: 4.93 ± 0.57; Positive: 4.63 ± 0.64, t 46 = 1.65, p > 0.05) and differed significantly compared with the neutral pictures (3.84 ± 0.82, p < 0.001; Table S1). In order to try to isolate the emotional component of the pictures, food items and close‐up faces were not used. Each trial consisted of a stimulus that was presented for 900 ms followed by an interstimulus interval of 1,000 ms. Participants were instructed to respond (as fast and correct as possible) within the full trial duration (1,900 ms) if the current stimulus was the same as the one N steps back in the sequence, by pressing the L‐key for YES and the A‐key for NO on the keyboard. Each participant performed a 1‐back block and a 3‐back block twice, that was either neutral‐negative or neutral‐positive (balanced). This was done because working memory updating has been found to be affected by switching between valences (Levens & Gotlib, 2010). Each block consisted of 72 trials, which means that they performed 288 trials in total. Order and block valence was balanced between participants. Within each block, stimulus order was pseudo‐randomized so that each stimulus (n = 24) functioned as a probe, target and a non‐target (no inference trials were included) an equal number of times within each block. The picture sets were unique for each block. Before starting the experiment task, participants were shown a demonstration and performed two shorter training blocks. The entire test took about 12 min to complete. Reaction time (RT) and response (yes, no or missed) were registered; the task was programmed using PsychoPy (Peirce, 2007).
Figure 1.
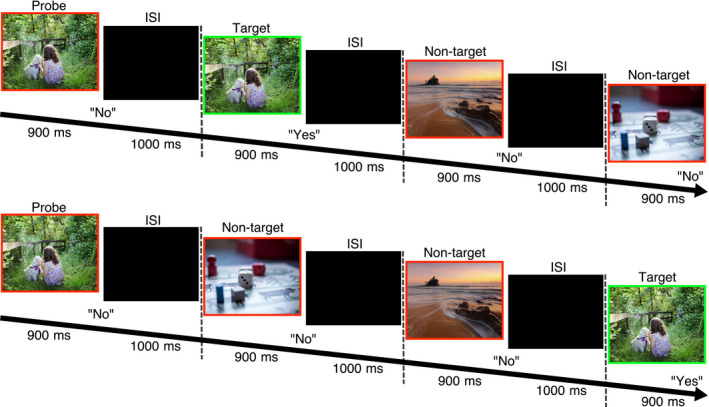
Emotional pictures and correct responses. NO responses were made on A‐key and YES on the L‐key on the keyboard. ISI = inter‐stimulus interval. Response was recorded during the entire trial duration (picture + ISI, 1900ms). Pictures are examples from www.pixabay.com, not used in experiment
2.5. Subjective measures
Emotional state was assessed before the task using the Positive and Negative Affect Schedule (PANAS), a two‐dimensional affective state scale with scores ranging from 10 to 50 (Watson, Clark, & Tellegen, 1988). Subjective sleepiness before the task was measured with the KSS (Åkerstedt & Gillberg, 1990).
2.6. Statistical analysis
As a measure of accuracy, we performed a 2 × 2 × 3 mixed analysis of variance (ANOVA) for d′ and omissions, with sleep as a between‐subjects variable (TSD, Control), and load (1‐back, 3‐back) and valence (positive, negative, neutral) as within‐subject variables. We excluded three participants that had all missing answers in at least one condition to avoid causing unbalance in the mixed ANOVA. Hence, data from 58 participants were used in the analysis (TSD: 27; Control: 31). When calculating the d′ we adjusted for extreme proportions by adding 0.5 to the sum of each cell and 1.0 to the sum of each row (Hautus, 1995). Moreover, we did not include omissions in the d′‐calculation, but treated omission percent as a separate outcome. d′ accounts for any response bias tendency, from the difference between the standardized hit rate and false alarm rate (Macmillan & Kaplan, 1985). RTs were analysed using mixed‐effects modelling (Bates, Maechler, Bolker, & Walker, 2015), with sleep, load and valence as fixed factors. Before model fitting, responses faster than 200 ms (n = 6; 0.14%) were omitted. RTs were log‐transformed to meet normality assumption, and only correct responses to target stimuli were used for the analysis. We applied a forward model selection procedure for the random effects structure, adding random parameters to a model that contained all fixed factors until the model was no longer significantly improved (Table S2). The best fitting model included a random intercept for participant, a random intercept for picture and a random slope for load within participant, as well as the covariance between load and the participant intercept. Including picture as a random variable was done to improve the generalizability of the picture sample to a population of pictures (Bates et al., 2015). The model was trimmed by removing data points exceeding 2.5 standard deviations (n = 78; 1.87%) from the residual mean and refitted with the trimmed data set (n = 4,094) using a restricted maximal likelihood estimation (DeVaan, Schreuder, & Baayen, 2007). We performed a full contrast analysis and post hoc t‐tests with a Kenward–Rogers degrees of freedom approximation for estimation of p‐values (Halekoh & Højsgaard, 2014). Welch t‐test were used for KSS and PANAS. Analyses were performed in R (R Core Team, 2016). Data are presented as M ± SD unless stated otherwise.
3. RESULTS
3.1. Subjective measures
Karolinska Sleepiness Scale ratings showed that the TSD group was significantly sleepier (8.1 ± 1.2) than the control group (4.7 ± 1.9, t 51.1 = 8.2, p < 0.001). PANAS scores rated before the test indicated that the TSD group was less positive (22.9 ± 8.4) than the control group (28.8 ± 6.6, t 47.5 = 2.9, p = 0.006). There was no significant difference in negative affect between the two groups (Control: 10.9 ± 1.6; TSD: 11.2 ± 2.3, p = 0.53).
3.2. Emotional working memory
Table 2 shows the results from the full mixed ANOVAs and the contrast analysis of the mixed‐effects model. Means for all outcomes are visualized in Figure 2, for main and simple effects see Figure S1. The TSD group was less accurate (d′) (2.21 ± 0.93) than the control group (2.64 ± 0.80, F 1,56 = 7.85, p = 0.007), and the TSD group had a higher omissions percentage (8.57 ± 16.10%) than the control group (0.78 ± 2.65%, F 1,56 = 8.00, p = 0.006). The accuracy (d′) for 1‐back was higher (2.87 ± 0.76) than for the 3‐back (2.00 ± 0.79, F 1,56 = 116.02, p < 0.001). No other statistically significant main or interaction effects on accuracy (d′) or omission percent were evident.
Table 2.
ANOVA style contrast table of outcome variables with degrees of freedom estimated from Kenward–Roger approximations
| d′ | Omissions (%) | RT (log) | ||||
|---|---|---|---|---|---|---|
| F (df) | p | F (df) | p | F (df) | p | |
| Sleep | 7.85 (1, 56) | 0.007 | 8.00 (1, 56) | 0.006 | 0.28 (1, 56.6) | 0.601 |
| Load | 116.02 (1, 56) | <0.001 | 0.59 (1, 56) | 0.445 | 278.02 (1, 58.5) | <0.001 |
| Valence | 2.24 (2, 112) | 0.112 | 0.08 (2, 112) | 0.924 | 6.35 (2, 96) | 0.003 |
| Sleep × load | 0.83 (1, 56) | 0.367 | 0.12 (1, 56) | 0.732 | 2.54 (1, 58.4) | 0.116 |
| Sleep × valence | 1.6 (2, 112) | 0.206 | 0.39 (2, 112) | 0.681 | 5.55 (2, 3,916.3) | 0.004 |
| Load × valence | 1.37 (2, 112) | 0.259 | 0.7 (2, 112) | 0.498 | 3.53 (2, 3,954) | 0.029 |
| Sleep × load × valence | 0.06 (2, 112) | 0.946 | 0.34 (2, 112) | 0.713 | 0.25 (2, 3,928.3) | 0.781 |
RT: reaction time.
The number of observations is larger for RT as it was calculated using a mixed model design without aggregated means. Significant effects are marked in bold font.
Figure 2.
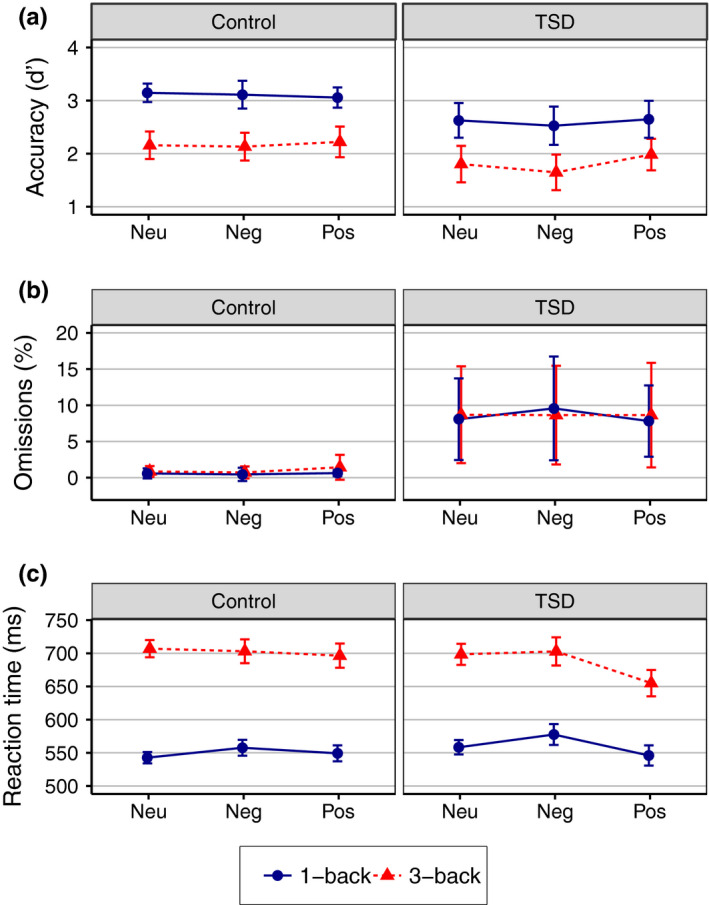
Performance on emotional N‐back task. Panels display (a) accuracy, (b) omission percent and (c) reaction time for Control and sleep deprivation (TSD) condition on negative (Neg), neutral (Neu) and positive (Pos) pictures, by 1‐back (blue, solid) and 3‐back (red, dashed) load. Error bars represent 95% CI
There was no overall significant difference in mean RT between the TSD (610.86 ± 150.72 ms) and Control (618.00 ± 151.40 ms) conditions. RTs were slower for 3‐back (696.80 ± 192.28 ms) than for 1‐back (553.17 ± 151.50 ms, F 2,58.5 = 278.02, p < .001). There was a main effect of valence (F 2,96 = 6.35, p = 0.003). However, following up a significant sleep × valence interaction (F 2,3916.3 = 5.55, p = 0.004) revealed that the effect of valence was only present in the sleep deprivation group (F 2,90.3 = 10.91, p < 0.001; Control: F 2,91.0 = 0.68, p = 0.51), with responses to positive pictures (590.46 ± 160.93 ms) being faster than to neutral (612.44 ± 175.53 ms, t 88.6 = 3.27, p = .002) and negative pictures (628.76 ± 169.31 ms, t 90.1 = 4.61, p < 0.001), and a significant difference between neutral and negative pictures (t 92.3 = 2.05, p = 0.043). Additionally, there was a significant load × valence interaction (F 2,3,954 = 3.53, p = 0.029), indicating that in the 3‐back load responses were faster for positive compared with neutral and with negative pictures (p < .05), with no significant difference between neutral and negative (p = 0.99). In the 1‐back load the positive and neutral were faster than the negative (p < .01), with no significant difference between neutral and positive pictures (p = 0.79).
An exploratory analysis showed no support for a speed–accuracy trade‐off in the present data as RT was not significantly correlated with d′ within each cell in the 2 × 2 × 3 model (p > 0.05).
4. DISCUSSION
In the present study, we investigated if emotional content modulates the effect of TSD on two levels of working memory load. Our results showed that 1 night of sleep deprivation impaired working memory accuracy and omission rate, but not RTs, with similar effects for high and low working memory load. The finding that the sleep‐deprived group had faster RTs to positive pictures than to neutral, which in turn were faster than to negative pictures indicates that consequences of sleep loss may depend on emotional content.
Using two levels of working memory load, we found an impairment in working memory accuracy and omission rate following sleep deprivation which is in line with previous research (Lim & Dinges, 2010). The lack of interaction effect between sleep deprivation and load is also in agreement with previous research using non‐emotional working memory tasks (Lo et al., 2012; Tucker et al., 2010), suggesting that impairments after sleep deprivation are more likely to be related to non‐executive components common to both high and low working memory load, such as vigilance and attention.
The impaired accuracy and omission rate after sleep deprivation did not depend on valence. These findings are in line with previous research on emotional working memory after a full night of sleep deprivation (Tempesta et al., 2014). Sleep deprivation has previously been found to cause increased interference by negative pictures on working memory when the pictures were used as distractors (Chuah et al., 2010). However, integrating the emotional stimuli in the task we found no such interference effect on accuracy. Moreover, our results do not corroborate non‐sleep‐related findings of emotional stimuli facilitating working memory accuracy (Lindström & Bohlin, 2011). One reason could be that compared with this study we used negative and positive pictures that had an average lower normative arousal score.
We did not find a main effect of sleep deprivation on RT, but an interaction between sleep deprivation and valence. While sleep deprivation has been found to impair RT in many working memory tasks (Lim & Dinges, 2010), studies that use tasks that require more complex stimuli processing do not show a consistent effect of sleep deprivation on RT (Anderson & Platten, 2011; Tempesta et al., 2014). For example, Maccari et al. (2014) demonstrated that the difference in RTs between sleep‐deprived and controls were less for face stimuli compared with word stimuli, and that there was no difference between the groups for the positive faces. Further research is needed to investigate how stimulus type and emotional content modulate working memory processing speed after sleep deprivation. The contrasting effects of sleep deprivation on accuracy and RT did not appear to be biased by a speed accuracy trade‐off, which is in line with previous experimental findings (Tucker et al., 2010) and fits with the conclusions from a recent meta‐analysis (Lim & Dinges, 2010).
Emotional stimuli are considered to be more salient and therefore requiring more attentional resources, which, when triggering a reward state, could lead to enhanced executive processing or, when signalling threat, be disruptive (Pessoa, 2009). This pattern was indeed found for the RTs in the sleep‐deprived group, with faster responses to positive pictures compared with neutral pictures, which in turn were faster than with negative pictures. The increased amygdala activity and disability to regulate emotional response after sleep deprivation (Gujar et al., 2011; Yoo et al., 2007) could therefore have acted beneficial for positive stimuli and disruptive for negative stimuli. However, further imaging studies are needed to determine if these neural responses after sleep deprivation can be directly linked to emotional working memory processing speed. Unlike Tempesta et al. (2014), we used positive and negative pictures in separate blocks to avoid possible carry‐over effects between positive and negative stimuli (Levens & Gotlib, 2010). This block separation is likely to have made the distinction between the two valences more pronounced. Whereas RTs to positive and negative pictures were consistent, the RTs to neutral pictures varied between the loads. Responses were faster for positive compared with neutral and negative pictures in the 3‐back condition, and responses to positive and neutral pictures were faster compared with negative pictures in the 1‐back condition. These results do not alter our conclusions that positive stimuli promoted faster responses and negative stimuli promoted slower responses after sleep deprivation. Worth noting is that as there were no significant differences between the valences in accuracy or omission rate, the effect on RT is not likely to have been at the expense of these other performance measures (Levens & Phelps, 2008). Instead, we believe that the change in RT represents a change in working memory processing speed of positive and negative pictures after sleep deprivation. Tempesta et al. (2014) found improved performance to negative pictures during the night, but no effect of valence after a full night of sleep deprivation. As we found changes in RTs related to picture valence after a full night of sleep deprivation, it seems important to replicate the findings in future studies.
Both groups were less accurate and slower on the 3‐back compared with the 1‐back task, but with similar levels of omission. In addition to the impaired performance in terms of working memory accuracy, the participants in the sleep group were sleepier and less positive but not more negative, which is in line with previous research (Franzen, Buysse, Dahl, Thompson, & Siegle, 2009). A somewhat speculative interpretation of our findings is that the decreased positivity affect after sleep deprivation lowered the expectation of a rewarding stimulus (positive picture), which generated a positive prediction error associated with increased dopamine release (Schultz, 2016).
One possible limitation of the current study is that participants in the sleep control condition as compared with those that were sleep deprived did not spend the night or the daytime before the test session under full supervision, but were asked to sleep as usual at home and were monitored by actigraphy. On the other hand, it is likely that they had better sleep than if they would have slept in the lab (Iber et al., 2004). Moreover, they were instructed not to engage in any stressful activity 24 hrs before the test, and the 2 hrs before the start of the emotional working memory task was standardized for all participants. Although full control would have been preferable, our actigraphy data of the night before the test session indicated that subjects were compliant. Another limitation, common to much research, is that we used data from a young healthy sample (18–30 years), thus generalizing our findings to a broader age span or to an unhealthy population might be difficult. Moreover, it should be noted that we compensated the use of a between‐subjects design with a large sample size to reduce the risk of insufficient power. Because sleeping a few hours or at odd (not night) hours is also common in the general population, more research is needed to see how emotion and working memory interacts after other types of insufficient sleep and sleep disturbances compared with a full night of sleep deprivation.
To conclude, our results support the notion that sleep deprivation has detrimental effects on working memory accuracy and omission rate, and that this effect is independent of working memory load and emotional valence. Moreover, our study showed that sleep deprivation can have effects on emotional working memory processing speed that are beneficial for positive, but harmful for negative picture scenes. The findings contribute to a better understanding of how emotional valence influences working memory in sleep‐deprived individuals.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
AUTHOR CONTRIBUTIONS
Study design: AG, TÅ, JA, HF, ML, JS; data collection: AG, JS; data analysis: AG, JS; interpretation of results: AG, TÅ, JA, HF, ML, JS; preparation of manuscript: AG, TÅ, JA, HF, ML, JS.
Supporting information
ACKNOWLEDGEMENTS
This study was funded through a grant from the Riksbankens Jubileumsfond (Swedish Foundation for Humanities and Social Sciences) (P13‐0649:1). The authors also would like to thank their research assistants and colleagues for their invaluable work in conducting the study, day and night.
Gerhardsson A, Åkerstedt T, Axelsson J, et al. Effect of sleep deprivation on emotional working memory. J Sleep Res. 2019;28:e12744 10.1111/jsr.12744
REFERENCES
- Åkerstedt, T. & Gillberg, M. (1990). Subjective and objective sleepiness in the active individual. International Journal of Neuroscience, 1, 29–37. 10.3109/00207459008994241 [DOI] [PubMed] [Google Scholar]
- Anderson, C. & Platten, C. R. (2011). Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behavioral Brain Research, 217, 463–466. 10.1016/j.bbr.2010.09.020 [DOI] [PubMed] [Google Scholar]
- Bates, D. M. , Maechler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Chuah, L. Y. M. , Dolcos, F. , Chen, A. K. , Zheng, H. , Parimal, S. , & Chee, M. W. L. (2010). Sleep deprivation and interference by emotional distracters. Sleep, 33, 1,305–1,313. 10.1093/sleep/33.10.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, K. , Jancsar, C. , & Hunt, B. (2015). Event‐related neural response to emotional picture stimuli following sleep deprivation. Psychology & Neuroscience, 8, 102–113. 10.1037/h0100354 [DOI] [Google Scholar]
- DeVaan, L. , Schreuder, R. , & Baayen, R. H. (2007). Regular morphologically complex neologisms leave detectable traces in the mental lexicon. The Mental Lexicon, 2, 1–23. 10.1075/ml.2.1.02vaa [DOI] [Google Scholar]
- Drummond, S. P. A. , Brown, G. G. , Salamat, J. S. , & Gillin, J. C. (2004). Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep, 27, 445–451. [PubMed] [Google Scholar]
- Franzen, P. L. , Buysse, D. J. , Dahl, R. E. , Thompson, W. , & Siegle, G. J. (2009). Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology, 80, 300–305. 10.1016/j.biopsycho.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A. N. & Walker, M. P. (2014). The role of sleep in emotional brain function. Annual Review of Clinical Psychology, 10, 679–708. 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar, N. , Yoo, S.‐S. , Hu, P. , & Walker, M. (2011). Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. Journal of Neuroscience, 31, 4,466–4,474. 10.1523/JNEUROSCI.3220-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halekoh, U. & Højsgaard, S. (2014). A Kenward‐Roger approximation and parametric bootstrap methods for tests in linear mixed models ‐ The R Package pbkrtest. Journal of Statistical Software, 59, 1–32. 10.18637/jss.v059.i09 26917999 [DOI] [Google Scholar]
- Hautus, M. J. (1995). Corrections for extreme proportions and their biasing effects on estimated values of d′. Behavior Research Methods, Instruments, & Computers, 27, 46–51. 10.3758/BF03203619 [DOI] [Google Scholar]
- Hur, J. , Iordan, A. D. , Dolcos, F. , & Berenbaum, H. (2016). Emotional influences on perception and working memory. Cognition and Emotion, 9,931, 1–9. 10.1080/02699931.2016.1213703 [DOI] [PubMed] [Google Scholar]
- Iber, C. , Redline, S. , Kaplan Gilpin, A. M. , Quan, S. F. , Zhang, L. , Gottlieb, D. J. , … Smith, P. (2004). Polysomnography performed in the unattended home versus the attended laboratory setting–Sleep Heart Health Study methodology. Sleep, 27, 536–540. 10.1093/sleep/27.3.536 [DOI] [PubMed] [Google Scholar]
- Lang, P. J. , Bradley, M. M. , & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A‐8 University of Florida, Gainesville, FL. [Google Scholar]
- Levens, S. M. & Gotlib, I. H. (2010). Updating positive and negative stimuli in working memory in depression. Journal of Experimental Psychology: General, 139, 654–664. 10.1037/a0020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens, S. M. & Phelps, E. A. (2008). Emotion processing effects on interference resolution in working memory. Emotion, 8, 267–280. 10.1037/1528-3542.8.2.267 [DOI] [PubMed] [Google Scholar]
- Lim, J. & Dinges, D. F. (2010). A meta‐analysis of the impact of short‐term sleep deprivation on cognitive variables. Psychological Bulletin, 136, 375–389. 10.1037/a0018883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström, B. R. & Bohlin, G. (2011). Emotion processing facilitates working memory performance. Cognition and Emotion, 25, 1,196–1,204. 10.1080/02699931.2010.527703 [DOI] [PubMed] [Google Scholar]
- Lo, J. C. , Groeger, J. A. , Santhi, N. , Arbon, E. L. , Lazar, A. S. , Hasan, S. , … Dijk, D.‐J. (2012). Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One, 7, e45987 10.1371/journal.pone.0045987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari, L. , Martella, D. , Marotta, A. , Sebastiani, M. , Banaj, N. , Fuentes, L. J. , & Casagrande, M. (2014). Effects of sleep loss on emotion recognition: A dissociation between face and word stimuli. Experimental Brain Research, 232, 3,147–3,157. 10.1007/s00221-014-3995-9 [DOI] [PubMed] [Google Scholar]
- Macmillan, N. A. & Kaplan, H. L. (1985). Detection theory analysis of group data: Estimating sensitivity from average hit and false‐alarm rates. Psychological Bulletin, 98, 185–199. 10.1037/0033-2909.98.1.185 [DOI] [PubMed] [Google Scholar]
- Osaka, M. , Yaoi, K. , Minamoto, T. , & Osaka, N. (2013). When do negative and positive emotions modulate working memory performance? Scientific Reports, 3, 1,375 10.1038/srep01375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce, J. W. (2007). PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods, 162, 8–13. 10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein, W. M. , Elbert, T. , & Stenger, V. A. (2002). Dissociation in human prefrontal cortex of affective influences on working memory‐related activity. Proceedings of the National Academy of Sciences, 99, 1,736–1,741. 10.1073/pnas.241650598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa, L. (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13, 160–166. 10.1016/j.tics.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher, J. J. , Callan, C. , & Posey, J. L. (2015). Sleep deprivation affects reactivity to positive but not negative stimuli. Journal of Psychosomatic Research, 79, 657–662. 10.1016/j.jpsychores.2015.05.003 [DOI] [PubMed] [Google Scholar]
- R Core Team (2016). R: A Language and Environment for Statistical Computing. Retrieved from https://www.r-project.org/.
- Schultz, W. (2016). Dopamine reward prediction error coding. Dialogues in Clinical Neuroscience, 18, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, J. , Gerhardsson, A. , van Leeuwen, W. , et al. (2018). Does sleep deprivation increase the vulnerability to acute psychosocial stress in young and older adults? Psychoneuroendocrinology, 96, 155–165. [DOI] [PubMed] [Google Scholar]
- Tempesta, D. , Couyoumdjian, A. , Curcio, G. , Moroni, F. , Marzano, C. , DeGennaro, L. , & Ferrara, M. (2010). Lack of sleep affects the evaluation of emotional stimuli. Brain Research Bulletin, 82, 104–108. 10.1016/j.brainresbull.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Tempesta, D. , De Gennaro, L. , Presaghi, F. , & Ferrara, M. (2014). Emotional working memory during sustained wakefulness. Journal of Sleep Research, 23, 646–656. 10.1111/jsr.12170 [DOI] [PubMed] [Google Scholar]
- Tucker, A. M. , Whitney, P. , Belenky, G. , Hinson, J. M. , & Van Dongen, H. P. A. (2010). Effects of sleep deprivation on dissociated components of executive functioning. Sleep, 33, 47–57. 10.1093/sleep/33.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Helm, E. , Yao, J. , Dutt, S. , Rao, V. , Saletin, J. M. , & Walker, M. P. (2011). REM sleep depotentiates amygdala activity to previous emotional experiences. Current Biology, 21, 2,029–2,032. 10.1016/j.cub.2011.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, D. , Clark, L. A. , & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1,063–1,070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Whitney, P. & Hinson, J. M. (2010). Measurement of cognition in studies of sleep deprivation. Progress in Brain Research, 185, 37–48. [DOI] [PubMed] [Google Scholar]
- Yoo, S.‐S. , Gujar, N. , Hu, P. , Jolesz, F. A. , & Walker, M. P. (2007). The human emotional brain without sleep – a prefrontal amygdala disconnect. Current Biology, 17, R877–R878. 10.1016/j.cub.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Zigmond, A. S. & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67, 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


