Abstract
Background: Enteral nutrition (EN) increases hyperglycemia due to high carbohydrate concentrations while providing insufficient protein. The study tested whether an EN formula with very high‐protein‐ and low‐carbohydrate‐facilitated glucose control delivered higher protein concentrations within a hypocaloric protocol. Methods: This was a multicenter, randomized, open‐label clinical trial with parallel design in overweight/obese mechanically ventilated critically ill patients prescribed 1.5 g protein/kg ideal body weight/day. Patients received either an experimental very high‐protein (37%) and low‐carbohydrate (29%) or control high‐protein (25%) and conventional‐carbohydrate (45%) EN formula. Results: A prespecified interim analysis was performed after enrollment of 105 patients (52 experimental, 53 control). Protein and energy delivery for controls and experimental groups on days 1–5 were 1.2 ± 0.4 and 1.1 ± 0.3 g/kg ideal body weight/day (P = .83), and 18.2 ± 6.0 and 12.5 ± 3.7 kcals/kg ideal body weight/day (P < .0001), respectively. The combined rate of glucose events outside the range of >110 and ≤150 mg/dL were not different (P = .54, primary endpoint); thereby the trial was terminated. The mean blood glucose for the control and the experimental groups were 138 (−SD 108, +SD 177) and 126 (−SD 99, +SD 160) mg/dL (P = .004), respectively. Mean rate of glucose events >150 mg/dL decreased (Δ = −13%, P = .015), whereas that of 80–110 mg/dL increased (Δ = 14%, P = .0007). Insulin administration decreased 10.9% (95% CI, −22% to 0.1%; P = .048) in the experimental group relative to the controls. Glycemic events ≤80 mg/dL and rescue dextrose use were not different (P = .23 and P = .53). Conclusions: A very high‐protein and low‐carbohydrate EN formula in a hypocaloric protocol reduces hyperglycemic events and insulin requirements while increasing glycemic events between 80–110 mg/dL.
Keywords: nutrition, enteral nutrition, enteral formula, nutrition support practice, critical care, research and diseases
Clinical Relevancy Statement
Stress hyperglycemia frequently occurs in critically ill patients independently worsening clinical outcomes. Insulin is currently the main treatment for stress hyperglycemia, but it is associated with side effects, limiting its utility. This article pragmatically explores whether an enteral nutrition formula containing higher protein and lower carbohydrate concentrations may facilitate glucose control, decreasing hyperglycemic events while reducing insulin utilization when used in a hypocaloric nutrition protocol.
Introduction
Nutrition for critically ill patients traditionally involves the provision of moderate amounts of protein (0.6–0.8 g/kg ideal body weight/day [IBW/d]) while delivering energy aimed at meeting energy expenditures (22–25 kcals/kg IBW/d), which are similar to the basal metabolic needs of normal adult individuals.1 Carbohydrates are considered the main source of energy based on the concept that they inhibit protein catabolism and could curtail muscle loss in healthy individuals. Thus, standard clinical practice is aimed at meeting caloric goals (so called normocaloric nutrition),2 providing up to 50% of the energy in the form of carbohydrates. The results of clinical trials, however, have failed to show that a significant benefit is derived from meeting caloric goals in an intensive care unit (ICU), suggesting that individualized nutrition interventions need to be considered. In fact, attempting to meet caloric goals using traditional carbohydrate loads may be associated with significant side effects.3, 4, 5
A particularly important side effect of normocaloric nutrition is the development and/or worsening of existing hyperglycemia.6 Hyperglycemia is frequently observed in critically ill patients. Hyperglycemia of critical illness (stress hyperglycemia) is thought to be secondary to metabolic, hormonal, and inflammatory factors and is independent of the patient's preexisting metabolic status, including the presence of diabetes or obesity.7, 8, 9 ICU hyperglycemia is considered a causative factor of poor clinical outcomes10 and is treated mainly with insulin.11 Following a landmark clinical trial in 2001 and a pragmatic trial in 2009, target glucose levels above 110 mg/dL and <180 mg/dL were considered acceptable even though optimum glucose targets are still debated.12, 13 A recent assessment of the quality of glycemic control, however, reported at least 1 episode of hyperglycemia above 10 mmol/L (180 mg/dL) in 63% of patients, whereas episodes of moderate and severe hypoglycemia were documented in 9% and 1%, respectively.14 These data suggest that, in the real world, there is ample room for improvement, and new methods to achieve adequate glucose control are needed.
The changes in understanding energy requirements has also prompted a reassessment of the role of protein. Traditional goals for protein delivery are 0.8 g/kg/d, even though in clinical practice patients tend to receive lower amounts than those suggested.15 A positive nitrogen balance, reflecting an improvement in protein anabolism, is observed by increasing protein loads above 0.8 g/kg/d.16, 17, 18 Furthermore, observational trials suggest that the provision of at least 1.2 g/kg/d is safe and may be associated with improved clinical outcomes.19 Although the ideal concentration is yet to be determined, new guidelines suggest that an increase in protein requirements could be beneficial.2
Most currently available enteral and premade parenteral nutrition formulas were created based on traditional nutrition goals, may be inadequate at meeting new guidelines, and are associated with side effects, including worsening hyperglycemia. We thus hypothesized that a formula containing lower carbohydrate concentrations (providing 29% of total calories) and increased amounts of protein (providing 37% of total calories) would facilitate improved glucose control and avoid worsening of hyperglycemia (>150 mg/dL) while delivering protein requirements suggested by new guidelines. Herein we report testing this hypothesis in an open‐label multicenter trial focusing on overweight and obese critically ill patients in medical ICUs in multiple centers in the United States and Canada.
Methods
Study Participants
We conducted a multicenter, randomized, open‐label clinical trial with parallel design in the United States (6 sites) and Canada (1 site; clinicaltrials.gov identifier: NCT02337556). The design of the study was approved by the ethics board of each hospital. Informed consent was obtained from all patients prior to any study procedure.
Patients were eligible if they were mechanically ventilated, critically ill, overweight, and obese (body mass index [BMI] 26–45) and required enteral nutrition (EN) for 5 days or longer. Exclusion criteria included a history of surgery, trauma, type I diabetes mellitus or diabetic ketoacidosis, pregnancy, and requirement for parenteral nutrition. Patients with type II diabetes who were on insulin prior to their admission were initially excluded; however, due to slow recruitment, the protocol was amended to include these patients.
Randomization
Patients were randomized to experimental or control EN formulas. Randomization was dynamically stratified by center using Medidata‐Balance with equal allocation ratio.
Interventions
The experimental formula contained high‐whey peptides (37%) and low carbohydrates (29%) (Peptamen Intense VHP, Nestlé Health Science, Bridgewater, NJ). The control formula contained a standard polymeric protein (25%) and high carbohydrates (45%; Replete, Nestlé Health Science, Bridgewater, NJ; Table 1). Both EN formulae had the same caloric density (1 kcal/mL). EN was initiated within 48 hours of admission into the study and advanced until protein goal (1.5 g/kg IBW/d) was achieved. Because of the difference in concentrations in nonprotein calories between the 2 formulas, a protocol aimed at achieving the same amount of protein would result in lower delivery of calories in the experimental group conforming to a hypocaloric regimen. Similarly, as the concentrations of protein in the 2 formulas were different, the volume of delivery of the control formula was higher when compared with the experimental formula to meet protein goals (Table 2). Analysis of nutrition intake excluded the last ICU day because patients were likely to be taken off EN around this time.
Table 1.
Composition Control and Experimental Enteral Nutrition Formulas
| Control | Experimental | |
|---|---|---|
| Caloric density (kcal/mL) | 1 | 1 |
| Protein (%) | 25 | 37 |
| Protein Source | Casein | Enzymatically hydrolyzed whey |
| Carbohydrate (%) | 45 | 29 |
| Carbohydrate source | Corn syrup, maltodextrin, dextrose | Maltodextrin, corn starch |
| Dietary fiber (g/L) | 0 | 4 |
| Dietary fiber source | Fructooligosaccharide, inulin | |
| Fat (%) | 30 | 34 |
| Fat source | Canola oil, MCT | MCT, fish oil, high linoleic safflower oil, soy bean oil |
| MCT:LCT | 20:80 | 50:50 |
| n6:n3 ratio | 2.4:1 | 1.8:1 |
| EPA and DHA (g/L) | 0 | 2 |
| Free water (mL/L) | 840 | 840 |
| Osmolality (mOsm/kg water) | 300 | 345 |
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LCT, long‐chain triglycerides; MCT, medium‐chain triglycerides; N6:n3 ratio, ω‐6 to ω‐3 fatty acid ratio; mOsm/kg, milliosmoles/kilogram.
Table 2.
Calculated Nutrition Intake Per Treatment Group Based on IBW
| Group | Protein Goal (g/kg) | CHO (g/kg) | Lipid (g/kg) | Calories (kcal/kg) | Volume (mL/hr) |
|---|---|---|---|---|---|
| Control | 1.5 | 2.6 | 0.8 | 23.4 | 75.0 |
| Experimental | 1.5 | 1.2 | 0.6 | 16.3 | 51.6 |
| Percent difference experimental vs control | 0 | −53 | −22 | −30 | −31 |
A different volume of the control and experimental formulas was necessary to achieve adequate protein goals (1.5 g/kg/d IBW) as is demonstrated for a theoretical 75‐kg individual. Differences in the delivery of total calories, carbohydrates, and lipids are also described. IBW, ideal body weight.
Outcomes and Data Sources
The primary endpoint was the difference in the mean rate of glycemic events outside the range of >110 and ≤150 mg/dL in the first 7 days in the ICU between the control and the experimental groups. This was created by a composite score of the following 2 secondary endpoints: (1) the mean rate of glycemic events >150 mg/dL (hyperglycemia) added to (2) the mean rate of glycemic events ≤110 mg/dL.
Secondary endpoints evaluated the distribution of glucose events >80 and ≤110 mg/dL (normoglycemia), >110 and ≤150 mg/dL, and >150 mg/dL. The presence of hypoglycemic events (≤80 mg/dL) was also collected. Other secondary endpoints included average glucose levels, exogenous insulin, and dextrose administration. Analysis of biochemical differences between the 2 groups included complete blood count, renal function tests, liver function tests, serum electrolytes, C‐reactive protein, ketones, serum albumin level, and pre–serum albumin level. Clinical effects including tolerance and adverse events were also followed and coded according to the MedDRA dictionary.
Blood glucose levels were measured using point‐of‐care bedside monitors or laboratory equipment available, allowing each hospital to use their own quality‐of‐care standards. Discretion was given to clinicians to prescribe insulin or dextrose as clinically indicated through hospital protocols.
Data were captured online using electronic case report forms. Computerized edit checks were performed to detect discrepancies. The Clinical Data Management System (Medidata) complied with Good Clinical Practice laws and regulations for clinical study.
Sample Size
A sample size of 208 participants was required to detect a difference of 10% between the 2 groups, with an assumed standard deviation (SD) of 24% in glycemic event rate. The sample size calculation considered an experiment wise false positive rate of 5%, a power of 80%, and 2 interim analyses using O'Brien and Fleming stopping boundaries. The design included 2 planned interim analyses by an independent data‐monitoring committee for futility and safety. The trial was terminated for futility after approximately half of the patients were enrolled.20
Statistical Analysis
Glycemic events were calculated for each patient as a rate of events outside the range of >110 and ≤150 mg/dL divided by the number of glucose measurements during the ICU stay. The difference of the rate of glycemic events between the groups was analyzed by analysis of covariance correcting for baseline glucose level and center. The analysis was weighted by the number of measurements performed for a participant.21
Frequency of corrective actions with the use of insulin and the need for dextrose for hypoglycemia were analyzed in a similar way as the rate of glycemic events. Serum glucose, serum albumin level, pre–serum albumin level, C‐reactive protein, alkaline phosphatase, carbon dioxide, bicarbonate, creatinine, hemoglobin A1C, and white blood cell count were approximately log‐normally distributed. For log‐normally distributed biochemistry variables, the variation around the geometric mean, the mean ± SD on the log scale was back‐transformed to the original scale. The following convention for normally distributed data was used: mean ± SD, and for log‐normal data: , , . Treatment differences on the log‐scale were interpreted as percent changes: . Biochemistry values were analyzed by analysis of covariance correcting for baseline and center. Serum ketones were measured semiquantitatively (negative, trace, positive), assigned categories of 0, 0.5, and 1, respectively, and analyzed by a negative binomial model.
A post hoc analysis of the dispersion of blood glucose levels was also performed. Dispersion was defined as the differences in SD from the mean and compared between groups.22 Because blood glucose is approximately log‐normally distributed, the mean and SD at the log‐scale were back transformed to the original scale, and a statistical test on the ratio of the back‐transformed SD of the 2 groups was constructed using the Δ method.
Results
Enrollment concluded at the first planned interim analysis after approximately half the patients were enrolled (June 2013–November 2016). Of the 106 patients that signed informed consent, 105 were randomized, 102 patients provided glucose measurements, and 98 patients received EN. Intention‐to‐treat analysis comprised 102 participants (52 control, 50 experimental; Figure 1). Mean hospital length of stay was 4.17 ± 2.37 vs 4.12 ± 2.32 days in the control vs experimental groups, respectively (P = .87). There was a progressive daily attrition of patients mostly as a result of clinical improvement including removal of the feeding tube and discharge from the ICU (Figure S1, Table S1).
Figure 1.
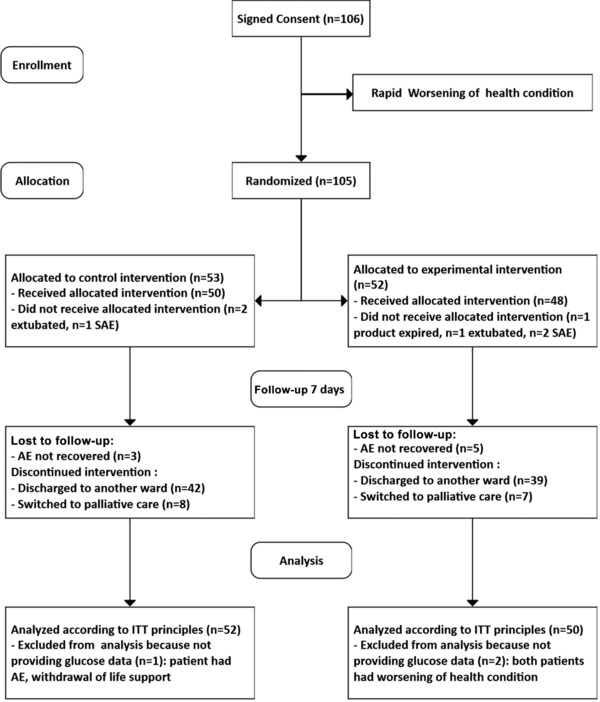
Consort diagram of patients enrolled and followed during the study. AE, adverse event; ITT, intention to treat; SAE, serious adverse event.
Demographics
Both groups were similar with regard to distribution of diagnoses, age, BMI, and APACHE II scores. There were more men and African Americans in the experimental group. Most frequent diagnoses included acute respiratory failure, pneumonia, and sepsis (Table 3). The average BMI was 33.0 ± 5.8 kg/m2 and 33.4 ± 4.6 kg/m2 for the control and experimental groups, respectively (Table 3). A total of 37 patients (16 in the control and 21 in the experimental groups) were reported to have type II diabetes mellitus. Average hemoglobin A1C at the time of randomization was similar between groups: 6.1 (5.3, 7.1) g/dl and 6.1 (5.0, 7.4) g/dl in the control and experimental groups, respectively.
Table 3.
Primary Admission Diagnostics and Baseline Patient Characteristics by Feeding Regimen Group
| Diagnosis | Control n = 53 | Experimental n = 52 | P‐Value | ||
|---|---|---|---|---|---|
| Acute respiratory failure, n (%) | 18 | (34.0) | 16 | (30.8) | .727 |
| Sepsis, n (%) | 14 | (26.4) | 11 | (21.2) | .527 |
| Pneumonia, n (%) | 9 | (17.0) | 9 | (17.3) | .965 |
| Neurological, n (%) | 6 | (11.3) | 9 | (17.3) | .384 |
| Cardiac, n (%) | 4 | (7.5) | 5 | (9.6) | .706 |
| Pulmonary embolism, n (%) | 1 | (1.9) | 1 | (1.9) | .989 |
| Kidney injury, n (%) | 1 | (1.9) | 0 | (0.0) | NA |
| Hemorrhagic shock, n (%) | 0 | (0.0) | 1 | (1.9) | 1.000 |
| Age (years), mean ± SD | 63.3 ± 11.9 | 61.0 ± 14.6 | .371 | ||
| Weight (kg), mean ± SD | 94.3 ± 18.7 | 97.8 ± 18.9 | .337 | ||
| Height (cm), mean ± SD | 169.0 ± 12.3 | 170.9 ± 11.3 | .414 | ||
| BMI (kg/m2), mean ± SD | 33.0 ± 5.8 | 33.4 ± 4.6 | .753 | ||
| APACHE II score,a mean ± SD | 25.9 ± 9.2 | 24.8 ± 8.8 | .535 | ||
| Race: black, % | 9.4 | 17.3 | .242 | ||
| Sex: female, % | 54.7 | 42.3 | .205 | ||
| HgbA1cb | 6.1 (5.3, 7.1) | 6.1 (5.0, 7.4) | .787 | ||
| DMII, n | 16 | 21 | .276 |
BMI, body mass index; DMII, Diabetes Mellitus type 2; HgbA1c, hemoglobin A1C; n, number of participants.
Experimental group based on 51 participants.
Geometric mean. Experimental group based on 49 participants.
Nutrition Intake
Protein intake was similar between the groups on days 1–5: 1.2 ± 0.4 g/kg IBW/d in the control group and 1.1 ± 0.3 g/kg/day in the experimental group (P = .83), achieving approximately 80% of expected both protein and caloric goals (Figure 2A). Total energy intake increased progressively in the first 24 hours reaching 17.3 ± 7.7 kcals/kg IBW/d in the control group and 13.7 ± 3.8 kcals/kg IBW/d in the experimental group. These levels were maintained throughout the ICU stay; the control group received 18.2 ± 6.0 kcals/kg IBW/d on average from days 1–5, whereas the experimental group received 12.5 ± 3.7 kcals/kg IBW/d (P < .0001; Figure 2B). The decrease in total energy intake in the experimental group relative to the control group reflected a decrease in carbohydrate load; the control patients received 126 ± 48 g/day on average on days 1–5, whereas the experimental patients received 61 ± 22 g/day (P < .0001).
Figure 2.
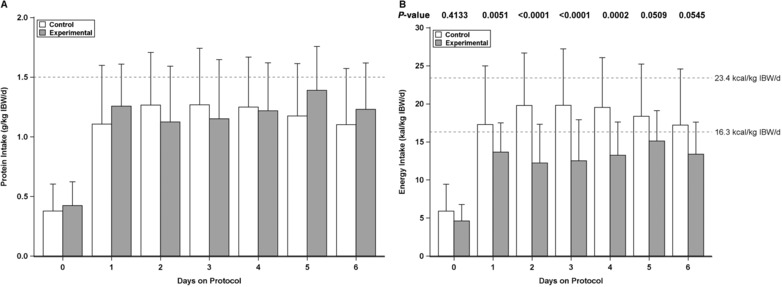
Protein and energy intake by day by intervention groups. (A) Protein intake by day (in g/kg IBW/d). (B) Total energy intake per day (in kcal/kg IBW/d). IBW/d, ideal body weight/day.
Effect of the EN Formula on Blood Glucose
A planned interim analysis revealed a lack of statistical difference in the primary endpoint, defined as the difference in the mean rate of glycemic events outside the range of >110 and ≤150 mg/dL between groups (2.7%; 95% confidence interval [CI], −6% to 11.5%; P = .54). As a result, the study was stopped early, and analyses of safety and secondary outcomes were completed.
There was a significant decrease in the mean rate of hyperglycemic events >150 mg/dL in the experimental group (−13%; 95% CI, −24% to −3%; P = .015) and a corresponding increase in the mean rate of glycemic events between >80 and ≤110 mg/dL (14%; 95% CI, 6% to 21%; P = .0007), relative to the control group. There was no difference in the mean rate of glycemic events in >110 and ≤150 mg/dL (Figure 3). Therefore, the decrease in the mean rate of glycemic events >150 mg/dL was similar in number to the increase in the mean rate of glycemic events in >80 and ≤110 mg/dL, thus preventing the demonstration of a statistical difference for the primary outcome.
Figure 3.
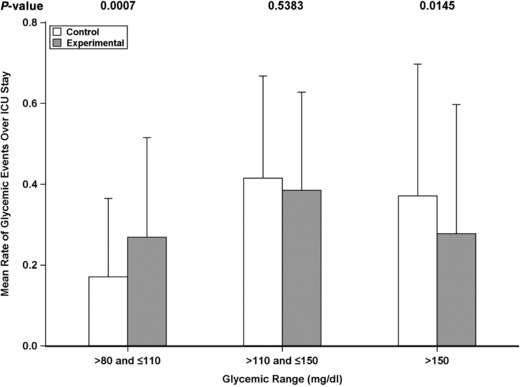
Mean rate of glycemic events during intensive care unit (ICU) stay by intervention groups. Mean rate of glycemic events (mg/dL) for the control (white) and experimental groups (gray) are shown. P‐values for the hypothesis test of no difference are shown on the top.
Additional Analyses on Blood Glucose
No significant difference in the number of glucose events ≤80 mg/dL (P = .23), events ≤60 mg/dL (P = .94), nor mean frequencies of dextrose utilization (P = .53) was observed.
Geometric mean glucose levels in the control group during the first 7 days was 138 mg/dL (−SD 108, +SD 177) compared with 126 mg/dL (−SD 99, +SD 160), reflecting a 10.8% decrease in average blood glucose (P = .004) in the experimental group (Figure 4). In addition, in a post hoc analysis, a significant reduction in dispersion from average glucose levels (measured as the SD) was observed (−11%) in the experimental group when compared with the controls (P = .0015).
Figure 4.
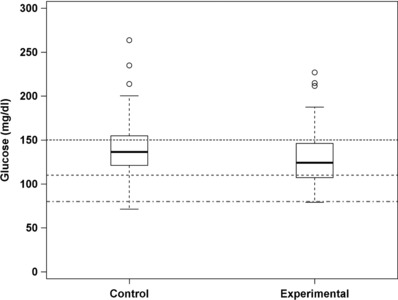
Boxplot of average blood glucose levels. The boxes include first and third quartiles. Medians are shown in thick black lines. Whiskers extend within 1.5*(interquartile range). Thresholds 150, 110, and 80 mg/dL are depicted by pointed, slashed, dot‐slashed horizontal lines, respectively.
Insulin Utilization
There was a significant decrease in the number of times insulin was administered in patients receiving the experimental formula (−10.9%; 95% CI, −21.9% to 0.1%; P = .048) when compared with the controls. The average daily doses of insulin for the control and experimental groups were 52.9 ± 93.2 and 43.8 ± 95.8 units/day, respectively (P = .25).
Safety and Tolerability
There were no significant differences in adverse neurologic, cardiovascular, or respiratory effects between the groups. There was an increase in the number of patients with abdominal distention in the experimental group without a difference in the number of patients requiring EN discontinuation (P = .02).
During the feeding protocol, 2 and 6 patients died in the experimental and control groups, respectively (P = .27). Further follow‐up during the hospital stay demonstrated that overall, 7 and 8 patients in the experimental and control groups, respectively, either died in wards or transferred to palliative care (P = 1).
Laboratory Values
Laboratory values measured are displayed in Table S2. For serum albumin level, pre–serum albumin level, C‐reactive protein, and creatinine, no statistically significant differences were observed across time. However, there was a trend toward lower total circulating protein and a lower white blood cell count in the experimental group on days 6 and 7. There was an increase in serum alkaline phosphatase across time in the control group (P < .05 on days 4–7). Serum carbon dioxide concentrations were higher in the control group (P < .05 on days 2 and 4–7), and serum bicarbonate concentrations were higher in the control group on days 3–7 (P < .05 on days 5 and 7). There was no statistical difference in ketone bodies detected in blood (mean rates 0.15 ± 0.46 and 0.26 ± 0.53 in the control and the experimental groups, respectively; P = .36). However, a small statistically significant increase in urinary ketones was observed in the experimental group when compared with the controls (mean rates 0.41 ± 0.74 and 0.08 ± 0.27, respectively; P = .012).
Discussion
Hyperglycemia is almost universally observed in critically ill patients contributing to poor clinical outcomes. EN formulas worsen hyperglycemia and may hinder efforts at controlling glucose.7 Increasing awareness of the problems associated with standard nutrition has prompted investigators to explore alternative options, including the substitution of high‐glycemic to low‐glycemic carbohydrates, addition of lipids as an energy source,6, 23 or allowing an energy deficit through hypocaloric nutrition or permissive underfeeding.24, 25, 26
This study explored a strategy of increasing the proportion of protein while reducing carbohydrate loads within a hypocaloric protocol in overweight and obese patients. It demonstrates that, although a reduction in the mean rate of glucose events outside >110 and ≤150 mg/dL was not achieved (primary endpoint), the use of an EN formula containing a high proportion of whey peptides and lower carbohydrate concentrations decreased hyperglycemic events (>150 mg/dL), increased normoglycemic events (>80 and ≤110 mg/dL), lowered average serum glucose, and decreased insulin requirements.
In standard polymeric formulas, carbohydrates make up 45%–60% of total energy along with lower protein concentrations. This composition reflects a long‐standing hypothesis that carbohydrates could avoid a caloric deficit and curtail protein catabolism in critical illness.27 Despite this theoretical benefit, several well‐designed prospective randomized clinical trials demonstrate that meeting short‐term caloric goals is of little or no significant clinical benefit.4, 5, 24, 25, 28, 29
The capacity to change the composition of EN formulae to lower carbohydrate loads while increasing protein concentrations may exert significant metabolic and potential clinical benefits26 because increased protein intake is associated with improved anabolism and nitrogen retention as has been described in trauma, sepsis, obese, and other critically ill patients.16, 30, 31, 32 In addition, observational studies suggest that the provision of protein ≥1.2 g/kg IBW/d is associated with improved outcomes, including lower mortality.19 The debate as to the ideal amount of protein is by no means complete, and caution should be used until adequately powered multicenter prospective randomized controlled trials are completed. Although it is possible to deliver higher amounts of protein with standard EN formulas, achieving this goal is associated with increased delivery of nonprotein calories, particularly carbohydrates, and worse blood sugar control.
This study failed to achieve the primary endpoint, which was a composite score that summed the mean rate of hyperglycemic events above 150 mg/dL and events in ≤110 mg/dL. As expected, there was a decrease in hyperglycemic events. However, there was an unexpected increase in normoglycemic events, >80 and ≤110 mg/dL, in the experimental group. The decrease in hyperglycemic events matched the increase in normoglycemic events, thus explaining why the primary endpoint was not achieved. In addition, there was also a significant decrease in average blood glucose levels and lower glucose dispersion in the experimental group.
The main mechanism of improvement in glycemic control appears to be the result of the lower carbohydrate load. However, hypocaloric nutrition alone has not been shown to be associated with normalization of blood glucose levels, and thus it is possible that other macronutrients could have also exerted an effect in increasing the number of glycemic events within 80–100 mg/dL.5 In noncritically ill individuals, for example, provision of protein of high biological value in the form of whey protein or its peptides is associated with improved glucose control, which has been described as an insulinotropic effect.33 Thus, it may be possible that the increased amounts of whey protein in the experimental formula may have contributed to an increase in normoglycemic events, an enticing hypothesis that remains to be tested.
Safety is central to the assessment of a nutrition regimen. Gastrointestinal side effects were modest and did not require stopping of the delivery of the experimental formula. Furthermore, there appeared to be no difference in side effects in other organs/systems. Attention was placed to measuring hypoglycemic episodes and rescue dextrose use, which were not increased in the experimental group. Decrease in glycemic dispersion was observed in the experimental group. Thus, this study supports the hypothesis that a hypocaloric regimen using a formula containing higher protein and lower carbohydrates is safe.
This study also explored other potential metabolic effects. A decrease in carbon dioxide accumulation would be expected because of the lower respiratory quotient associated with decreased carbohydrate intake. Lower serum bicarbonate also suggests a decreased need for renal compensation. Interestingly, the rise in ketones was found to be only modestly elevated in the experimental group through urinary ketone analysis and only observed in a small proportion of patients. This may suggest that the amount of carbohydrate delivered in the experimental group may have been sufficient to inhibit ketogenesis for most patients.
Early termination of the study significantly decreased the sample size, power, and strengths of the conclusions. This is particularly important in the interpretation of clinical side effects and the exploratory laboratory values. Thus, the authors urge the reader to carefully consider these limitations as they interpret the data presented.
This trial focused on overweight (BMI>26 kg/m2) and obese (BMI>30 kg/m2) individuals, extending the original indications for hypocaloric nutrition beyond that of morbidly obese patients.34 Further research in other patient populations is necessary to extend the observations of this study to patients with a BMI <26 kg/m2. Although we could deliver only approximately 80% of protein and caloric goals, the 2 groups demonstrated a significant difference in the number of calories received. In addition, both groups received protein approximately 1.1–1.2 g/kg IBW/d, which approached the new recommendations for protein delivery.2, 35
Conclusions
Whereas the study presented herein failed to meet the primary endpoint, the results showed that a very high‐protein, low‐carbohydrate EN formula facilitated glucose control in critically ill overweight/obese patients in a medical ICU. A lower incidence of hyperglycemic episodes and an increase in blood glucose levels within 80–110 mg/dL was observed along with a simultaneous decrease insulin utilization. This trial demonstrated that an EN formula with very high protein and low carbohydrate may be useful in maintaining protein delivery in critically ill overweight/obese patients when used within a hypocaloric protocol. The long‐term biological and clinical effects of the use of an EN formula that delivers higher protein while avoiding excessive calories, including lower carbohydrates, need confirmation through further trials.
Supporting information
Table S1: Reasons for early departure from the clinical trial
Table S2: Differences in laboratory values across time (experimental minus control)
Figure S1: Patient enrollment by ICU day.
Acknowledgments
We acknowledge Ileana Alexander for managing the trial, Emilie Ba for data management tasks, Sarah Cohen for statistical review, and Shinobu Yamamoto for quality control of the manuscript.
Statement of Authorship
T. W. Rice, M. B. Huhmann, and J. B. Ochoa equally contributed to the conception and design of the research. M. B. Huhmann, T. W. Rice, D. C. Files, P. E. Morris, A. C. Bernard, T. R. Ziegler, J. W. Drover, and J. B. Ochoa contributed to the design of the research. T. W. Rice, D. C. Files, P. E. Morris, A. C. Bernard, T. R. Ziegler, J. W. Drover, J. P. Kress, and K. R. Ham contributed to the acquisition of the data. D. J. Grathwohl, M. B. Huhmann, and J. B. Ochoa contributed to the analysis of the data. All authors contributed to the interpretation of the data. J. B. Ochoa drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Financial disclosure: None declared.
Conflicts of interest: This study was funded by Nestlé Health Science. MBH and JBO received salary from Nestlé Health Science. DJG received salary from Nestlé Research Center.
[Correction added on May 10, 2019 after initial online publication: The copyright line was changed to company owned work and a legal statement was added to it.]
References
- 1. Food and Nutrition Board Insitute of Medicine of the National Academies . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (2002/2005). www.nap.edu. Accessed April 26, 2018. [DOI] [PubMed]
- 2. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159‐211. [DOI] [PubMed] [Google Scholar]
- 3. Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365(6):506‐517. [DOI] [PubMed] [Google Scholar]
- 4. Ochoa Gautier JB, Machado FR. Early nutrition in critically ill patients: feed carefully and in moderation. JAMA. 2013;309(20):2165‐2166. [DOI] [PubMed] [Google Scholar]
- 5. Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mesejo A, Montejo‐Gonzalez JC, Vaquerizo‐Alonso C, et al. Diabetes‐specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: a prospective, open‐label, blind‐randomized, multicenter study. Crit Care. 2015;19:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davidson P, Kwiatkowski CA, Wien M. Management of hyperglycemia and enteral nutrition in the hospitalized patient. Nutr Clin Pract. 2015;30(5):652‐659. [DOI] [PubMed] [Google Scholar]
- 8. Maynard GA, Holdych J, Kendall H, Harrison K, Montgomery PA, Kulasa K. Improving glycemic control safely in critical care patients: a collaborative systems approach in nine hospitals. Endocr Pract. 2017;23(5):583‐593. [DOI] [PubMed] [Google Scholar]
- 9. Van den Berghe G. Intensive insulin therapy in the ICU—reconciling the evidence. Nat Rev Endocrinol. 2012;8(6):374‐378. [DOI] [PubMed] [Google Scholar]
- 10. Falciglia M, Freyberg RW, Almenoff PL, D'Alessio DA, Render ML. Hyperglycemia‐related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37(12):3001‐3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mesotten D, Preiser JC, Kosiborod M. Glucose management in critically ill adults and children. Lancet Diabetes Endocrinol. 2015;3(9):723‐733. [DOI] [PubMed] [Google Scholar]
- 12. Finfer S, Chittock D, Li Y, et al. Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: long‐term follow‐up of a subgroup of patients from the NICE‐SUGAR study. Intensive Care Med. 2015;41(6):1037‐1047. [DOI] [PubMed] [Google Scholar]
- 13. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359‐1367. [DOI] [PubMed] [Google Scholar]
- 14. Gauthier L, Ferguson J, Dube AI, Nguyen PV, Beauchesne MF, Boutin JM. Assessment of quality of glycemic control in intensive care patients treated with an insulin infusion at a teaching hospital. Can J Diabetes. 2014;38(2):139‐143. [DOI] [PubMed] [Google Scholar]
- 15. Heyland DK, Lemieux M, Shu L, Quisenberry K, Day AG. What is “best achievable” practice in implementing the enhanced protein‐energy provision via the enteral route feeding protocol in intensive care units in the United States? Results of a multicenter, quality improvement collaborative [published online ahead of print October 1, 2016]. JPEN J Parenter Enteral Nutr. 10.1177/0148607116673301 [DOI] [PubMed] [Google Scholar]
- 16. Dickerson RN, Pitts SL, Maish GO 3rd, et al. A reappraisal of nitrogen requirements for patients with critical illness and trauma. J Trauma Acute Care Surg. 2012;73(3):549‐557. [DOI] [PubMed] [Google Scholar]
- 17. Liebau F, Sundstrom M, van Loon LJ, Wernerman J, Rooyackers O. Short‐term amino acid infusion improves protein balance in critically ill patients. Crit Care. 2015;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN J Parenter Enteral Nutr. 1979;3(6):452‐456. [DOI] [PubMed] [Google Scholar]
- 19. Weijs PJ, Looijaard WG, Beishuizen A, Girbes AR, Oudemans‐van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non‐septic mechanically ventilated critically ill patients. Crit Care. 2014;18(6):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443‐453. [DOI] [PubMed] [Google Scholar]
- 21. Fisher RAM, Mackenzie WA. Studies in crop variation. II. The manurial response of different potato varieties. J Agri Sci. 1923;13(3):311‐320. [Google Scholar]
- 22. Finfer S, Wernerman J, Preiser JC, et al. Clinical review: consensus recommendations on measurement of blood glucose and reporting glycemic control in critically ill adults. Crit Care. 2013;17(3):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barazzoni R, Deutz NEP, Biolo G, et al. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clin Nutr. 2017;36(2):355‐363. [DOI] [PubMed] [Google Scholar]
- 24. Bryk J, Zenati M, Forsythe R, Peitzman A, Ochoa JB. Effect of calorically dense enteral nutrition formulas on outcome in critically ill trauma and surgical patients. JPEN J Parenter Enteral Nutr. 2008;32(1):6‐11. [DOI] [PubMed] [Google Scholar]
- 25. Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest. 2003;124(1):297‐305. [DOI] [PubMed] [Google Scholar]
- 26. Rugeles SJ, Ochoa Gautier JB, Dickerson RN, Coss‐Bu JA, Wernerman J, Paddon‐Jones D. How many nonprotein calories does a critically ill patient require? A case for hypocaloric nutrition in the critically ill patient. Nutr Clin Pract. 2017;32(1 suppl):72S‐76S. [DOI] [PubMed] [Google Scholar]
- 27. Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502‐509. [DOI] [PubMed] [Google Scholar]
- 28. Arabi YM, Aldawood AS, Haddad SH, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372(25):2398‐2408. [DOI] [PubMed] [Google Scholar]
- 29. Petros S, Horbach M, Seidel F, Weidhase L. Hypocaloric vs normocaloric nutrition in critically ill patients: a prospective randomized pilot trial. JPEN J Parenter Enteral Nutr. 2016;40(2):242‐249. [DOI] [PubMed] [Google Scholar]
- 30. Dickerson RN, Lynch AM, Maish GO 3rd, Croce MA, Minard G, Brown RO. Improved safety with intravenous insulin therapy for critically ill patients with renal failure. Nutrition. 2014;30(5):557‐562. [DOI] [PubMed] [Google Scholar]
- 31. Dickerson RN, Patel JJ, McClain CJ. Protein and calorie requirements associated with the presence of obesity. Nutr Clin Pract. 2017;32(1 suppl):86S‐93S. [DOI] [PubMed] [Google Scholar]
- 32. Long CL, Crosby F, Geiger JW, Kinney JM. Parenteral nutrition in the septic patient: nitrogen balance, limiting plasma amino acids, and calorie to nitrogen ratios. Am J Clin Nutr. 1976;29(4):380‐391. [DOI] [PubMed] [Google Scholar]
- 33. Jakubowicz D, Froy O, Ahren B, et al. Incretin, insulinotropic and glucose‐lowering effects of whey protein pre‐load in type 2 diabetes: a randomised clinical trial. Diabetologia. 2014;57(9):1807‐1811. [DOI] [PubMed] [Google Scholar]
- 34. McClave SA, Kushner R, Van Way CW 3rd, et al. Nutrition therapy of the severely obese, critically ill patient: summation of conclusions and recommendations. JPEN J Parenter Enteral Nutr. 2011;35(5 suppl):88S‐96S. [DOI] [PubMed] [Google Scholar]
- 35. Hurt RT, McClave SA, Martindale RG, et al. Summary points and consensus recommendations from the International Protein Summit. Nutr Clin Pract. 2017;32(1 suppl):142S‐151S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Reasons for early departure from the clinical trial
Table S2: Differences in laboratory values across time (experimental minus control)
Figure S1: Patient enrollment by ICU day.


