Summary
Acute sleep deprivation can lead to judgement errors and thereby increases the risk of accidents, possibly due to an impaired working memory. However, whether the adverse effects of acute sleep loss on working memory are modulated by auditory distraction in women and men are not known. Additionally, it is unknown whether sleep loss alters the way in which men and women perceive their working memory performance. Thus, 24 young adults (12 women using oral contraceptives at the time of investigation) participated in two experimental conditions: nocturnal sleep (scheduled between 22:30 and 06:30 hours) versus one night of total sleep loss. Participants were administered a digital working memory test in which eight‐digit sequences were learned and retrieved in the morning after each condition. Learning of digital sequences was accompanied by either silence or auditory distraction (equal distribution among trials). After sequence retrieval, each trial ended with a question regarding how certain participants were of the correctness of their response, as a self‐estimate of working memory performance. We found that sleep loss impaired objective but not self‐estimated working memory performance in women. In contrast, both measures remained unaffected by sleep loss in men. Auditory distraction impaired working memory performance, without modulation by sleep loss or sex. Being unaware of cognitive limitations when sleep‐deprived, as seen in our study, could lead to undesirable consequences in, for example, an occupational context. Our findings suggest that sleep‐deprived young women are at particular risk for overestimating their working memory performance.
Keywords: sound distraction, women and men, nocturnal wakefulness, subjective performance, cognition
Introduction
Our working memory holds and manipulates information for brief periods of time, facilitating processes related to reasoning, planning, learning and goal‐directed behaviour (Baddeley, 2003, 2010). Research has shown that components of working memory, such as vigilance and decision‐making, can be affected negatively by acute sleep loss (Goel et al., 2009; Lim and Dinges, 2010; Reichert et al., 2016). A recent study found that objective working memory performance (measured by n‐back tasks without auditory interference) deteriorates more in women than in men under extended wakefulness (Santhi et al., 2016), suggesting that sleep loss may impact working memory adversely in a sex‐dependent manner.
In addition to sleep loss, distracting task‐irrelevant auditory information may also interfere with working memory processes (Röer et al., 2014). This may be relevant for real‐world settings, such as when driving through dense inner‐city traffic while a fellow passenger is talking to you (for instance, a child in the back seat), a situation in which the traffic environment must be navigated safely while being distracted by the ongoing conversation. Surprisingly, to the best of our knowledge, no study to date has tested whether sleep loss modulates the negative effects of task‐irrelevant auditory information on working memory performance. Short sleep (typically defined as <6 h sleep per day) has been associated with a deficit in auditory novelty processing (Gumenyuk et al., 2011), suggesting that irrelevant auditory information may be less distracting when, for instance, performing a working memory task under conditions of sleep loss.
In addition to objective working memory performance, a positive relationship between misjudgement of working memory performance and sleep loss could potentially increase the risk for serious health and economic consequences in a variety of professional and social settings. These consequences include accidents, grave errors in decision‐making and production loss, exemplified by traffic accidents and medical treatment errors. In spite of these potentially harmful consequences, how sleep loss affects estimation of working memory capability in relation to objectively measured working memory performance is currently unknown.
Against this background, the present study aimed to examine the effects of acute total sleep loss versus a full night of sleep on objective and self‐estimated working memory performance under conditions of silence compared with auditory verbal distraction. Participants’ working memory performance was measured using a digit‐encoding task, in which the participants encoded sequences of numbers while being exposed to auditory verbal distraction or no distraction (silent condition). We included an equal number of young men and women in the present study, matched for age, body mass index (BMI), educational status, and chronotype—factors which can all influence working memory (Gonzales et al., 2010; Murphy et al., 2016; Nowack and Van Der Meer, 2014; Padgaonkar et al., 2017).
We hypothesized that a single night of sleep loss (compared with regular sleep) and auditory verbal distraction (versus silence) would independently impair objective working memory performance. We also predicted that participants’ nocturnal vigilance state (i.e. being sleep‐deprived versus being well‐rested) would modulate the putative negative effects of auditory verbal distraction on working memory. Finally, we hypothesized that working memory could be affected differentially by sleep loss in women and in men.
Methods
Participants
Twenty‐four healthy normal‐weight non‐smoking adult university students (12 participants stated their gender to be female and 12 stated their gender to be male) were included into the study. Given that menstrual cycle fluctuations have been linked to variations in memory performance (Hampson and Morley, 2013; Sundström Poromaa and Gingnell, 2014), only women using oral monophasic contraceptives (containing progesterone and oestrogen) at the time of the study were selected for participation in the study. All participants were otherwise free of medication. Participants reporting psychiatric, neurological, hormonal, metabolic and sleep‐related diseases in a screening interview were not considered eligible for inclusion into the present study. Participants’ chronotype and daytime sleepiness were assessed by standardized questionnaires (the morningness–eveningness questionnaire and the Epworth Sleepiness Scale) (Horne and Ostberg, 1976; Johns, 1991).
The study was conducted according to the Declaration of Helsinki and all participants provided written informed consent. The experimental procedure was approved by the Ethical Committee of Uppsala (dnr 2015/347).
Experimental procedure
For a schematic overview of the experimental procedure, see Fig. 1.
Figure 1.
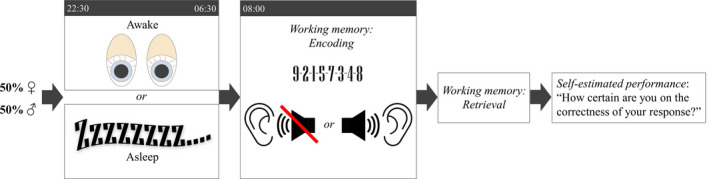
Schematic overview of the experimental procedure. Twenty‐four young adults (12 women and 12 men) participated in two experimental conditions separated by approximately 1 week: regular nocturnal sleep (scheduled between 22:30 and 06:30 hours) versus total sleep loss, in counterbalanced order. In the morning after sleep or nocturnal wakefulness, participants were administered a digital working memory test with 16 trials, in which eight‐digit sequences were learned and retrieved. Learning of digit sequences (exemplified in Fig. 1) was accompanied by either silence (eight trials) or auditory verbal distraction (eight trials) in a random order. After retrieval of the sequence, each trial ended with the question ‘How certain are you on the correctness of your response?’ (0 = not confident at all, 10 = very confident). The latter was regarded as a self‐estimate of working memory performance.
Each subject participated in two experimental conditions (regular nocturnal sleep versus nocturnal sleep loss), separated by approximately 1 week. The order of experimental conditions was counterbalanced across subjects. Neither of the experimental conditions of the female subjects were scheduled during their menstruation phase. Note that the amount of oestrogen and progestin (synthetic progestogens) are the same in each active monophasic contraceptive pill. This reduced the likelihood that fluctuating sex hormones would have differed between experimental sessions in participating women, as sessions were scheduled only on days when active pills were ingested.
In each experimental session, subjects arrived to the laboratory at ~18:00 hours. In the sleep condition, lights were turned off at ~22:30 hours and subjects were woken up at ~06:30 hours, to yield a sleep opportunity of 8 h. Conversely, in the sleep loss condition, participants were kept awake by the experimenters throughout the entire night. During all experimental wake periods, experimental room ceiling lights were switched on (~410 lux). However, experimental rooms were not exposed to any natural light. During the wake periods in the laboratory, participants were under supervision (mixed sex of experimenter; Chapman et al., 2018) and were allowed to spend their time with sedentary activities, e.g. reading a book, when no study‐related activities were scheduled.
The working memory task described below was administered to the participants in the morning at ~08:10 hours following regular sleep or nocturnal wakefulness.
Digital working memory task
This computerized working memory task (administered using the software PsychoPy; Peirce, 2007) included 16 trials (eight silent trials and eight trials with auditory verbal distraction; trials administered in a randomized order) in which sequences of numbers (digits 1–9) had to be encoded and retrieved in the order in which they were initially presented. Two test versions of the working memory task were utilized in a counterbalanced order across experimental conditions (i.e. half the participants were administered version A in the first experimental session and version B in the second experimental session, and vice versa). During the entire working memory task, participants wore bilateral headphones with high insulation.
Each of the 16 working memory trials started with a 24‐s‐long priming phase. During the priming phase, participants listened to an 8‐s‐long Russian phrase which was repeated consecutively three times. Eight phrases were used (i.e. each phrase was used twice in each of the working memory versions), all of which were standardized with respect to amplitude variation and peak volume (restricted to below 65 dB). All phrases were prerecorded and spoken by the same female native Russian speaker. Russian phrases were taken from the literature, popular science papers and news articles, and were of similar length (~10–12 words). According to the screening interview, none of the participants understood Russian, which minimized the risk that the phrases would distract working memory differentially between individuals based on language comprehension.
Following the priming phase, participants were presented with eight numerical digits (1–9) in random order, one after the other (one at a time) in black print centred on a white background at a rate of 1 digit per second (800 ms followed by a blank white screen for 200 ms). This was the digit encoding phase. Digits were never used twice within a sequence (i.e. sequences such as 1‐1‐… or 1‐2‐1… were not used). The 8‐s‐long digit encoding phase was accompanied by either auditory verbal distraction (using the same phrase as presented during the priming phase) or silence (50/50%).
Following the digit encoding phase, participants were prompted to input the eight‐digit sequence in the same forward order as it was presented during encoding. This was called the retrieval phase. The participants were instructed that if they could not recall one of the digits, they should guess which digit may have been at that position of the sequence. The retrieval was not time‐limited, and the sequence could be corrected during input. Of note, no auditory distraction was administered during retrieval. Once participants had completed the retrieval, they were subsequently requested by the program to self‐estimate their working memory performance for the completed trial. More specifically, they were asked to indicate, on a continuous scale ranging from 0 to 10, how certain they were about the correctness of their digit input (0 = not confident at all; 10 = very confident).
For the analysis, objective working memory performance was defined as the averaged scores from the three best trials during auditory distraction and silence, respectively. Self‐estimated working memory performance was extracted for the corresponding trials for the three trials with the best scores (objectively measured), and the self‐estimated scores from these three trials were averaged. We focused upon the mean of the best three trials as it reflects participants’ maximum obtainable working memory performance. We also chose to estimate working memory performance in this way, as it is robust towards bottom performances that may have been due to, for example, a lack of motivation.
Sleep assessment
In the experimental sleep condition, electroencephalography (EEG) was measured by Embla A10 recorders (Flaga hf, Reykjavik, Iceland) with a sampling frequency of 200 Hz. Electroencephalographic (EEG) signals were derived from F3, F4, C4, O1 and O2 electrodes and referenced to the contralateral mastoids. In addition, bilateral electro‐oculogram (EOG) and chin electromyogram (EMG) were recorded. Sleep was scored by an experienced scorer (FR), according to standard criteria (Silber et al., 2007). Sleep parameters of interest were total sleep time (TST), sleep onset latency (SOL; time from lights out at 22:30 hours to the first 30‐s epoch out of the first three consecutive epochs of sleep ≥ sleep stage 1; N1), minutes spent awake after sleep onset (WASO), slow‐wave sleep (SWS) latency and rapid eye movement (REM) sleep latency. Time spent in sleep stages N1, sleep stage 2 (N2), SWS and REM sleep were expressed as a percentage of TST.
A sleep diary completed by participants ensured that they had 7–9 h of sleep 3 nights prior to the onset of both experimental sessions (data not shown). Moreover, during the week before the first experimental session, participants had an in‐laboratory adaptation night. This was to reduce possible bias from the first‐night effect on sleep maintenance and quality in the experimental sleep night (Tamaki et al., 2016).
Statistical analysis
For statistical evaluation, SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) was used. Data are presented as mean ± standard deviation (SD), unless stated otherwise. Normal distribution of variables was assessed by visual inspection in combination with the Shapiro–Wilk test for normality. The effects of sleep loss and auditory verbal distraction on objectively measured and self‐estimated working memory were analysed by full‐factorial linear mixed models. Within‐subject factors experimental condition (i.e. being sleep‐deprived versus being well‐rested) and auditory distraction (auditory distraction versus silence during digit encoding) were entered as repeated fixed factors into the analyses. Sex was considered as a fixed between‐subjects factor. The fixed covariance matrix for each model was chosen based on tests for best fit using Akaike's information criterion (compound symmetry for the model for the objectively measured working memory scores and first‐order autoregressive for the self‐estimated working memory model). The restricted maximum likelihood method was used.
Sleep parameters were contrasted by sex based on two‐tailed independent Student's t‐tests and Mann–Whitney U‐tests for non‐normally distributed variables. Overall, a two‐sided P ≤ 0.05 was considered significant.
Results
Cohort characteristics
As revealed by the screening interview, the participating women and men were comparable with respect to age (women versus men, 22.3 ± 2.3 versus 22.7 ± 3.4 years; P = 0.78), BMI (22.4 ± 1.9 versus 22.1 ± 1.7 kg m−2; P = 0.67), chronotype score (52.4 ± 6.8 versus 51.8 ± 5.7 points; P = 0.82) and daytime sleepiness (9.2 ± 3.5 versus 7.6 ± 4.2 points; P = 0.33). Most of the participants were classified as having intermediate chronotypes (score of 42–58; 19 participants), and a few as being moderately morning types (score of 59–69; five participants).
Based on sleep diaries (mean ± SD), in the wake condition, participants woke up at 07:43 hours ± 57 min and spent 07:59 hours ± 49 min in bed during the night before the experimental day. In the sleep condition, participants reported that they woke up at 07:51 hours ± 55 min and spent 08:18 hours ± 55 min in bed during the night before the experimental session.
Sleep in the sleep condition was typical for laboratory conditions (Table 1). Whereas women spent slightly less time in the transitional sleep stage N1 than men (P ≤ 0.001; Table 1), no other sex differences in sleep were found (P ≥ 0.38; Table 1).
Table 1.
Sleep characteristics in the sleep condition
| Sleep variable | All | Women | Men | P‐value (women/men) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| SOL (min) | 15.5 | 9.8 | 17.0 | 11.5 | 14.1 | 8.1 | 0.49 |
| TST (min) | 441.1 | 13.7 | 441.0 | 16.2 | 441.1 | 11.4 | 0.99 |
| WASO (min) | 23.8 | 7.7 | 23.7 | 9.1 | 23.9 | 6.5 | 0.95 |
| N1 (% of TST) | 4.2 | 1.8 | 3.1 | 1.2 | 5.3 | 1.6 | <0.001 |
| N2 (% of TST) | 43.8 | 5.9 | 44.0 | 7.2 | 43.7 | 4.5 | 0.92 |
| SWS (% of TST) | 31.1 | 7.4 | 32.4 | 9.0 | 29.7 | 5.4 | 0.38 |
| REM (% of TST) | 20.9 | 4.4 | 20.5 | 4.3 | 21.2 | 4.7 | 0.71 |
| SWS latency (min) | 11.0 | 6.1 | 9.8 | 5.4 | 12.1 | 6.8 | 0.56M |
| REM latency (min) | 82.4 | 30.4 | 84.4 | 38.7 | 80.3 | 20.6 | 0.47M |
Characteristics of sleep in the sleep condition (i.e. sleep opportunity between approximately 02:30 and 06:30 hours). Statistical comparisons between women and men were performed with two‐tailed Student's t‐tests or Mann–Whitney U‐tests (M) for non‐normally distributed variables. SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset; N1, Stage 1 sleep; N2, Stage 2 sleep; SWS, slow‐wave sleep; REM, rapid eye movement sleep; SD, standard deviation.
Effects of sleep loss, language distraction and sex on objectively measured working memory performance
The effects of sleep, auditory verbal distraction and sex on objectively measured working memory performance are summarized in Fig. 2 and Table 2.
Figure 2.

Objective and self‐estimated working memory performance after sleep and sleep loss. The leftmost graphs display the mean across the two sound categories silent and auditory distraction. Upper panels: performance on the working memory task (number of correctly recalled digits, 8 is the maximum score) was estimated by the mean of the best three trials during which digit encoding was accompanied by either auditory distraction or silence. Lower panels: the three best working memory trials in each auditory distraction category (i.e. silence versus auditory distraction) were used to calculate averaged scores for self‐estimated working memory performance (0 = not at all confident in response; 10 = very confident in response). Note that participants were not asked to indicate how many of the digits that they thought they could recall correctly. *P < 0.05; **P < 0.01 for sleep loss versus sleep (paired two‐tailed Student's t‐tests). Data are mean ± standard deviation.
Table 2.
Objective and self‐estimated working memory performance following sleep and sleep loss
| Women | P (sleep/wake) | Men | P (sleep/wake) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sleep | Sleep loss | Sleep | Sleep loss | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Mean (silent and auditory distraction) | ||||||||||
| Objective working memory score | 6.9 | 0.8 | 6.1 | 1.2 | 0.024 | 6.6 | 1.2 | 6.5 | 1.0 | 0.714 |
| Self‐estimated working memory performance | 4.3 | 2.3 | 3.9 | 2.2 | 0.511 | 4.3 | 2.3 | 4.5 | 2.2 | 0.824 |
| Silent | ||||||||||
| Objective working memory score | 7.1 | 0.9 | 6.3 | 1.1 | 0.003 | 6.8 | 1.0 | 6.8 | 1.0 | 0.913 |
| Self‐estimated working memory performance | 4.7 | 2.6 | 4.1 | 2.4 | 0.308W | 4.4 | 2.3 | 4.7 | 2.2 | 0.717 |
| Auditory distraction | ||||||||||
| Objective working memory score | 6.7 | 0.9 | 5.9 | 1.4 | 0.125 | 6.5 | 1.5 | 6.3 | 1.2 | 0.611 |
| Self‐estimated working memory performance | 4.0 | 2.2 | 3.7 | 2.2 | 0.751 | 4.3 | 2.5 | 4.4 | 2.4 | 0.934 |
Objective working memory performance was estimated by the average of the best three trials, during which digit encoding was accompanied by either auditory verbal distraction or silence (minimum score = 0; maximum score = 8). The three best working memory trials in each auditory distraction category (i.e. silence versus auditory distraction) were furthermore used to calculate averaged scores for self‐estimated working memory performance for those trials (ranging from 0 to 10; 10 = very confident about the correctness of my response). The top row displays the mean across the two sound categories silent and auditory distraction. Note that participants were not asked to indicate how many of the digits that were correctly recalled. P < 0.05 (paired Student's t‐test or Wilcoxon's signed‐ranks tests, W) are indicated in bold type. SD, standard deviation.
Following sleep loss, participants’ performance on the digital working memory task was impaired, compared with that seen after a full night of sleep [sleep loss versus sleep, estimated mean ± standard error of the mean (SEM) from the linear mixed model: 6.3 ± 0.2 versus 6.8 ± 0.2 correct digits; F (1,66.0) = 8.46, P = 0.005]. It is noteworthy that this negative effect of sleep loss on working memory performance was seen only in women but not in men (experimental condition × sex, F (1,66.0) = 5.21, P = 0.026). Additionally, auditory verbal distraction administered during learning of the digit sequences impaired subsequent digit retrieval (auditory distraction versus silence, estimated mean ± SEM from the linear mixed model): 6.4 ± 0.2 versus 6.7 ± 0.2 correct digits; F (1,66.0) = 5.22, P = 0.025). Notwithstanding this main effect of auditory distraction on working memory performance, there was no interaction between auditory distraction and experimental condition (F (1,66.0) = 0.05, P = 0.825). No other main or interaction effects on working memory performance were found, including main effects of participants’ sex (P ≥ 0.757 for all main and interaction terms).
In order to investigate the robustness of our sex‐dependent effects of sleep loss on working memory, Bayesian statistics for related samples were applied as an alternative method to P‐value‐based statistics (results are shown in Supporting information, Table S1). Using the Bayes factor, we found support that the objective but not self‐estimated working memory performance was impaired in women after sleep loss versus sleep. For men, no differences in objective and self‐estimated working memory were observed between the sleep loss and sleep conditions.
Effects of sleep loss, language distraction and sex on self‐estimated working memory performance
Neither experimental condition, auditory distraction nor sex had an impact on self‐estimated working memory performance (P ≥ 0.134 for all main and interaction terms; Table 2 and Fig. 2).
Discussion
The current within‐subject study examined whether a night of sleep loss, in contrast to a night with regular sleep, would alter objectively measured as well as self‐estimated working memory performance in young adult men and women. In order to investigate whether task‐irrelevant auditory interference on working memory performance is modulated critically by acute sleep loss, we utilized a task paradigm in which sequences of eight digits had to be learned under either silence or auditory verbal distraction. Given that sleep–wake regulation and its impact on cognitive performance differs between men and women (Santhi et al., 2016), we also tested whether there would be sex differences in working memory performance following sleep loss versus sleep.
An important novel finding of our study is that sleep loss impaired objective but not self‐estimated working memory performance in women. In contrast, both working memory measures remained unaffected by sleep loss in men. This pattern of results indicates that women may have been less aware of the actual decline in their working memory performance following sleep loss. Such a discrepancy between objectively measured and self‐estimated working memory performance might be concerning, as it can lead to serious health and economic consequences in a variety of real‐world settings characterized by high working memory load and sleep loss (e.g. when driving a car in a highly trafficked area following night‐shift work). Women report longer ideal sleep duration than men (Tonetti et al., 2008) and complain more often about sleep problems (Sandlund et al., 2016; Tang et al., 2017; Zhang and Wing, 2006). Thus, aiding sleep may be of particular benefit to women regularly facing both high working memory load and disrupted sleep.
Our finding that working memory is impaired in women but not men following sleep loss adds to previous findings of sex‐specific differences on cognitive performance following sleep–wake disruption. A recent study demonstrated that 28‐h forced circadian desynchrony impairs working memory to a greater degree in women than in men, particularly in the early morning hours (Santhi et al., 2016). In this study, working memory performance declined more rapidly in women than in men after a similar time awake. The question, however, is why acute sleep loss seems to impair working memory performance only in women. A neuroimaging study has shown that compared with men, and despite similar performance, women exhibit greater signal intensity changes—a measure of neuronal activity and engagement—in brain circuits such as the prefrontal cortex when performing a working memory task under well‐rested conditions (Goldstein et al., 2005). This, in conjunction with the observation that increased neurocognitive activity is required to maintain working memory performance under conditions of sleep loss (Chee and Choo, 2004; Drummond et al., 2004; Goel et al., 2009; Reichert et al., 2016), could explain why working memory performance in women is particularly vulnerable to sleep loss. However, it is not appropriate to infer from our results that other cognitive functions (such as emotional processing, long‐term memory formation) might be affected in the same sex‐dependent manner by sleep loss as working memory in the present study. It must also be borne in mind that there might be a dose–response association between time spent awake and performance on a working memory task. Moreover, the observed association between sleep loss and working memory may be different at other circadian time‐points. Supporting this view, a recent study found that response times to the well‐established psychomotor vigilance task were prolonged (indicating worse performance) during the night when sleep‐deprived, but shorter during the day despite increased duration of sleep loss (Muto et al., 2016). Finally, the complexity of working memory tasks has been shown to modulate the impact of sleep loss on working memory performance (Lim and Dinges, 2010). For instance, whereas sleep loss appears to exert relatively strong effects on simple working memory tests, only small effects on more complicated working memory operations have been observed (Lim and Dinges, 2010). With this in mind, it cannot be ruled out that the sex differences in working memory performance following sleep loss observed in the present study are specific for the chosen working memory task investigating digit recall under verbal auditory distraction.
Extending previous findings demonstrating that task‐irrelevant auditory distraction can impair working memory in humans (Röer et al., 2014), in the present study, auditory verbal distraction (spoken Russian phrases) during digit encoding also influenced working memory performance negatively. This impairment of auditory verbal distraction on working memory was neither specific for sex nor was it modulated by experimental condition (i.e. being sleep‐deprived versus being well‐rested). The latter appears surprising as sleep loss, in contrast to being well‐rested, may be hypothesized to increase ‘passive’ gating of task‐irrelevant sensory information due to a compromised capacity to switch attention to novelty (Gumenyuk et al., 2011). Given that we utilized verbal distraction in the present study as task‐irrelevant auditory information, more studies are warranted to investigate how other types of auditory distractors (e.g. music) may interact with sleep and sleep loss with respect to working memory performance. In this context, it may also be worth investigating whether personality measures, such as openness and flexibility, alter the extent by which auditory distractors comprise working memory under sleep loss conditions. Finally, we cannot rule out that using familiar language (e.g. mother tongue) as auditory distractor instead of unfamiliar language, as employed in the present study, may have yielded different effects on working memory performance.
Our cross‐over study has several strengths and limitations. Although the number of participants was limited to 12 young men and 12 young women, sex groups were comparable with respect to age, BMI, chronotype, educational status and self‐reported daytime sleepiness. These factors have all been shown to affect working memory performance (Gonzales et al., 2010; Murphy et al., 2016; Nowack and Van Der Meer, 2014; Padgaonkar et al., 2017). Moreover, women in the present study all took monophasic contraceptive pills at the time of investigation to reduce potential confounding effects from fluctuations in the menstrual cycle on working memory performance (Hampson and Morley, 2013; Sundström Poromaa and Gingnell, 2014). Our results may therefore not extrapolate, for example, to women with freely cycling menstrual cycles or humans of other ages.
Conclusion
Working memory is central in cognitive functioning and key to perform efficiently and effectively in academic, professional and social settings. With this in mind, it is highly conceivable that a drop in working memory performance due to acute sleep loss represents a risk factor for harmful accidents and mistakes. For instance, sleep‐deprived humans have been shown to be more prone to cause both minor and severe accidents in traffic scenarios (Filtness et al., 2017). This drop in working memory performance following sleep loss might be especially worrisome in situations where those suffering from sleep deprivation may not notice their functional deficit. As suggested by our findings, in this context particular attention should be paid to young women facing challenges in which they have to cope with both a high working memory load and lack of sleep.
Conflict of Interest
The authors are unaware of any affiliation, funding or financial holdings that might be perceived as affecting the objectivity of this manuscript. The authors declare that there is no biomedical financial interest or potential conflict of interest.
Author Contributions
FHR and CB designed the study; FHR and CB wrote the protocol; FHR, SK, PA, LiL, MOB and LaL collected the data; FHR, SK and CB conducted the analyses. All authors interpreted the data; and all authors contributed to writing. All authors have approved the final manuscript.
Supporting information
Table S1. Bayesian statistics for related samples, split by sex
Acknowledgements
Work from the authors’ laboratory is supported by AFA Försäkring (Grant number 140006, CB), the Bissen Brainwalk Foundation, Erik, Karin and Gösta Selanders Foundation (JC), Fredrik och Ingrid Thurings Stiftelse (JC), the Lars Hiertas Minne Foundation (JC), Novo Nordisk Research Foundation (Grant number NNF14OC0009349, CB), the Tore Nilson Foundation (JC), the Swedish Research Society (JC), the Swedish Folksam Research Foundation (CB), the Swedish Society for Medicine (JC), the Swedish Brain Research Foundation (Grant number FO2016‐0092, CB), the Swedish Research Council (Grant number 2015‐03100, CB, JC) and the Åke Wiberg Foundation (JC). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Baddeley, A. Working memory: looking back and looking forward. Nat. Rev. Neurosci., 2003, 4: 829–839. [DOI] [PubMed] [Google Scholar]
- Baddeley, A. Working memory. Curr. Biol., 2010, 20: R136–R140. [DOI] [PubMed] [Google Scholar]
- Chapman, C. D. , Benedict, C. and Schiöth, H. B. Experimenter gender and replicability in science. Sci Adv., 2018, 4: e1701427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee, M. W. and Choo, W. C. Functional imaging of working memory after 24 hr of total sleep deprivation. J. Neurosci., 2004, 24: 4560–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, S. P. , Brown, G. G. , Salamat, J. S. and Gillin, J. C. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep, 2004, 27: 445–451. [PubMed] [Google Scholar]
- Filtness, A. J. , Armstrong, K. A. , Watson, A. and Smith, S. S. Sleep‐related vehicle crashes on low speed roads. Accid. Anal. Prev., 2017, 99: 279–286. [DOI] [PubMed] [Google Scholar]
- Goel, N. , Rao, H. , Durmer, J. S. and Dinges, D. F. Neurocognitive consequences of sleep deprivation. Semin. Neurol., 2009, 29: 320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, J. M. , Jerram, M. , Poldrack, R. et al Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology, 2005, 19: 509–519. [DOI] [PubMed] [Google Scholar]
- Gonzales, M. M. , Tarumi, T. , Miles, S. C. , Tanaka, H. , Shah, F. and Haley, A. P. Insulin sensitivity as a mediator of the relationship between BMI and working memory‐related brain activation. Obesity (Silver Spring), 2010, 18: 2131–2137. [DOI] [PubMed] [Google Scholar]
- Gumenyuk, V. , Roth, T. , Korzyukov, O. , Jefferson, C. , Bowyer, S. and Drake, C. L. Habitual short sleep impacts frontal switch mechanism in attention to novelty. Sleep, 2011, 34: 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, E. and Morley, E. E. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology, 2013, 38: 2897–2904. [DOI] [PubMed] [Google Scholar]
- Horne, J. A. and Ostberg, O. A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. Int. J. Chronobiol., 1976, 4: 97–110. [PubMed] [Google Scholar]
- Johns, M. W. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep, 1991, 14: 540–545. [DOI] [PubMed] [Google Scholar]
- Lim, J. and Dinges, D. F. A meta‐analysis of the impact of short‐term sleep deprivation on cognitive variables. Psychol. Bull., 2010, 136: 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C. F. , Rabelo, C. M. , Silagi, M. L. , Mansur, L. L. and Schochat, E. Impact of educational level on performance on auditory processing tests. Front. Neurosci., 2016, 10: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto, V. , Jaspar, M. , Meyer, C. et al Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science, 2016, 353: 687–690. [DOI] [PubMed] [Google Scholar]
- Nowack, K. and Van Der Meer, E. Impact of chronotype and time perspective on the processing of scripts. Int. J. Psychophysiol., 2014, 92: 49–58. [DOI] [PubMed] [Google Scholar]
- Padgaonkar, N. A. , Zanto, T. P. , Bollinger, J. and Gazzaley, A. Predictive cues and age‐related declines in working memory performance. Neurobiol. Aging, 2017, 49: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce, J. W. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods, 2007, 162: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert, C. F. , Maire, M. , Schmidt, C. and Cajochen, C. Sleep–wake regulation and its impact on working memory performance: the role of adenosine. Biology (Basel), 2016, 5: E11 10.3390/biology5010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röer, J. P. , Bell, R. and Buchner, A. What determines auditory distraction? On the roles of local auditory changes and expectation violations. PLoS ONE, 2014, 9: e84166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandlund, C. , Westman, J. and Hetta, J. Factors associated with self‐reported need for treatment of sleeping difficulties: a survey of the general Swedish population. Sleep Med., 2016, 22: 65–74. [DOI] [PubMed] [Google Scholar]
- Santhi, N. , Lazar, A. S. , Mccabe, P. J. , Lo, J. C. , Groeger, J. A. and Dijk, D. J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl Acad. Sci. USA, 2016, 113: E2730–E2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber, M. H. , Ancoli‐Israel, S. , Bonnet, M. H. et al The visual scoring of sleep in adults. J. Clin. Sleep Med., 2007, 3: 121–131. [PubMed] [Google Scholar]
- Sundström Poromaa, I. and Gingnell, M. Menstrual cycle influence on cognitive function and emotion processing‐from a reproductive perspective. Front. Neurosci., 2014, 8: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki, M. , Bang, J. W. , Watanabe, T. and Sasaki, Y. Night watch in one brain hemisphere during sleep associated with the first‐night effect in humans. Curr. Biol., 2016, 26: 1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J. , Liao, Y. , Kelly, B. C. et al Gender and Regional differences in sleep quality and insomnia: a general population‐based study in Hunan Province of China. Sci. Rep., 2017, 7: 43690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti, L. , Fabbri, M. and Natale, V. Sex difference in sleep‐time preference and sleep need: a cross‐sectional survey among Italian pre‐adolescents, adolescents, and adults. Chronobiol. Int., 2008, 25: 745–759. [DOI] [PubMed] [Google Scholar]
- Zhang, B. and Wing, Y. K. Sex differences in insomnia: a meta‐analysis. Sleep, 2006, 29: 85–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Bayesian statistics for related samples, split by sex


