Abstract
Uridine diphosphate (UDP)‐glycosyltransferases (UGTs) are widely distributed within living organisms and share roles in biotransformation of various lipophilic endo‐ and xenobiotics with activated UDP sugars. In this study, it was found that the activity of UGTs in abamectin‐resistant (AbR) strain was significantly higher (2.35‐fold) than that in susceptible strain (SS) of Tetranychus cinnabarinus. Further analysis showed that 5‐nitrouracil, the inhibitor of UGTs, could enhance the lethal effect of abamectin on mites. From the previous microarray results, we found an UGT gene (UGT201D3) overexpressed in AbR strain. Quantitative PCR analysis showed that UGT201D3 was highly expressed and more inducible with abamectin exposure in the AbR strain. After silencing the transcription of UGT201D3, the activity of UGTs was decreased and the susceptibility to abamectin was increased in AbR strain whereas it was not in SS. Furthermore, UGT201D3 gene was then successfully expressed in Escherichia coli. The recombinant UGT201D3 exhibited α‐naphthol activity (2.81 ± 0.43 nmol/mg protein/min), and the enzyme activity could be inhibited by abamectin (inhibitory concentration at 50%: 57.50 ± 3.54 μmol/L). High‐performance liquid chromatography analysis demonstrated that the recombinant UGT201D3 could effectively deplete abamectin (15.77% ± 3.72%) incubating with 150 μg protein for 6 h. These results provided direct evidence that UGT201D3 was involved in abamectin resistance in T. cinnabarinus.
Keywords: abamectin resistance, prokaryotic expression, RNA interference, Tetranychus cinnabarinus, UDP‐glycosyltransferases
Introduction
The carmine spider mite, Tetranychus cinnabarinus (Boisduval), is one of the major agricultural pests worldwide, infesting vegetables and many other crops including cotton, maize and tobacco (Guo et al., 1998; Cakmak & Baspinar, 2005). Many researchers believe that T. cinnabarinus and the two‐spotted mite, Tetranychus urticae, are two forms (red and green) of a single species (T. urticae) because of their similarity in morphological, biological and molecular characteristics (de Mendonca et al., 2011; Auger et al., 2013). So far, the control of phytophagous mites has mainly relied on chemical acaricides. However, the high reproductive potential and extremely short life cycle of these mites, result in rapid resistance development to many acaricides often after only a few applications, which is a major problem for the control of T. cinnabarinus (Devine et al., 2001; Stumpf & Nauen, 2001).
Abamectin is widely used as an insecticidal, acaricidal and nematicidal agent. As a macrocyclic lactones compound, it belongs to the family of avermectins (AVMs) which are derived from the soil organism Streptomyces avermitilis (Putter et al., 1981). Its target is most likely the γ‐aminobutyric acid chloride channels (GABACls), glutamate‐gated chloride channels (GluCls) and histamine‐gated chloride channels (HisCls) (Sigel & Baur, 1987; Zheng et al., 2002; McCavera et al., 2007). Even though abamectin is very efficient for the control of pests and mites, unfortunately many insects and mites have developed a high resistance to this pesticide (van Leeuwen et al., 2010). Up to now, most documents indicated that resistance against abamectin in insects and mites on one hand was dependent on the expression changes of a diverse set of proteins including metabolic enzymes and P‐glycoproteins (van Leeuwen et al., 2010; Riga et al., 2014; Xu et al., 2016), and on the other, was dependent on the mutations of the related chloride channels (Dermauw et al., 2012). Besides these factors, it is believed that some other factors may also contribute to abamectin resistance in arthropods.
Uridine diphosphate (UDP)‐glycosyltransferases (UGTs) are responsible for glycosylation by catalyzing the conjugation of a glycosyl group from an activated nucleotide sugars donor, UDP‐glycoside, with various small hydrophobic molecules, and it widely distributes in animals, plants, bacteria and viruses as a multifunctional protein superfamily (Bock, 2003; Mackenzie et al., 2005). The UGT protein structure is mainly divided into two parts: the N‐terminal aglycone substrate binding domain and the C‐terminal UDP‐glycoside binding domain (Magdalou et al., 2010). Unlike primary metabolic enzymes such as P450s or carboxylesterases (CarEs) which directly act on the toxin molecule to decrease its biological activity, UGTs work in the secondary metabolic process, adding bulky side groups onto toxic compounds to increase their hydrophilicity and facilitating their excretion from the organism (Ahn et al., 2012). The functions of UGT enzymes were well studied in mammals. They work as membrane‐bound protein in the lumen of the endoplasmic reticulum utilizing UDP‐glucuronic acid as a sugar donor to conjugate a large number of xenobiotics as well as endobiotics, such as bilirubin and drugs, pollutants, and odorants (Bock, 2003; Bon, 2010). Insect UGT enzymes were similar to plant UGTs, using UDP‐glucose rather than UDP‐glucuronic acid as a sugar donor (Bowles et al., 2005). It was reported that many endogenous compounds, like odorant binding proteins and ecdysteroid hormones are glycosylated by UGT enzymes in a range of insect species (Huang et al., 2008; Bozzolan et al., 2014). It was also reported that insect UGTs play an important role in detoxification of plant allelochemicals, like gossypol, which can partially be metabolized by UGTs via glycosylation in Helicoverpa armigera and Heliothis virescens (Krempl et al., 2016). Some insect UGT genes were up‐regulated in resistant strains and also were suspected to play a role in insecticide‐resistance development, including dichlorodiphenyltrichloroethane (DDT), pyrethroids, carbamates and neonicotinoids resistance (Pedra et al., 2004; Vontas et al., 2005; Silva et al., 2012; Yang et al., 2013). Moreover, the over‐expressions of UGT2 gene in Leptinotarsa decemlineata and UGT2B17 in Plutella xylostella, were proved to contribute to imidacloprid‐ and chlorantraniliprole‐resistance formation, respectively (Kaplanoglu et al., 2017; Li et al., 2017). All of the above studies suggested that the over‐expressions of UGT genes in insects might play important roles in the formation of insecticide resistance, however, the report about the direct interaction between UGT gene products and insecticides is still absent.
In this study, we detected the activity of UGTs in susceptible strain (SS) and abamectin‐resistant (AbR) strain of T. cinnabarinus. An UGT gene, UGT201D3, was fully sequenced and quantified for its messenger RNA (mRNA) expression profiles in different life stages and both strains, and the transcriptional response of this gene was investigated with exposure to abamectin. The RNA interference (RNAi) method was applied to investigate the role of UGT201D3 in abamectin resistance in T. cinnabarinus, then this gene was successfully expressed in Escherichia coli and the enzymatic properties were characterized. High‐perfomance liquid chromatography (HPLC) analysis was performed to confirm the interaction between the recombinant UGT201D3 protein and abamectin.
Materials and methods
Mites
Two strains of T. cinnabarinus were used in this study: SS, a susceptible strain reared for many years without exposure to insecticides in the laboratory, which was originally collected from a cropland of cowpea in Beibei, Chongqing, China in 1998; AbR, abamectin‐resistant strain, was generated from the SS strain with the selection of abamectin in the laboratory. Detailed information about the acaricide selection process is provided by He et al. (2009b). All the mites were cultivated on fresh potted cowpea leaves and kept in an artificial climate chamber at 26 ± 1 °C, 55%–70% relative humidity (RH) and a 14 : 10 h L : D photoperiod.
Reagents
The 95% abamectin was ordered from Bangnong Chemical Company (Guangzhou, China), 5‐nitrouracil was ordered from Adamas‐beta (Shanghai, China); iQ™ SYBR® Green Supermix was acquired from Bio‐Rad (Hercules, CA, USA); α‐naphthol was ordered from Shanghai Chemical Reagent Company of the Chinese Medical Group (Shanghai, China); pGEM‐T Easy Vector was ordered from Promega (Madison, WI, USA); pET‐28a expression vector and EcoR I, Nde I were obtained from Takara (Dalian, China). Trans5α and BL21 (DE3) competent cells were acquired from TransGen Biotech (Beijing, China); isopropyl b‐D‐1‐thiogalactopyranoside (IPTG), UDP‐glucose and kanamycin were obtained from Sigma Chemical Co. (St Louis, MO, USA). Other chemicals and reagents were high‐quality commercially available products supplied by local suppliers.
Determination of UGT activity
The total protein content of the enzyme solution was determined using the Enhanced BCA Protein Assay Kit (Beyotime Biotechnology, China) following the manufacturer's protocol. UGT activity was tested using α‐naphthol as substrate and UDP‐glucose as an activated sugar donor according to the modified method of Real et al. (1991) and Krempl et al. (2016). The 200 female adult mites (3–5 days old) from different strains were homogenized in 1.5 mL sodiumphosphate buffer (0.1 mol/L, pH 7.8) on ice, then centrifugation at 10 000 × g for 15 min at 4 °C, after which the extracted supernatant was tested. Specifically, UDP‐glucose (1 mmol/L), MgCl2 (10 mmol/L) and crude enzyme (100 μL) were added to the sodium phosphate buffer (0.1 mol/L, pH 7.8) resulting in a total volume of 500 μL and incubated in 1.5 mL centrifuge tubes for 20 min at 37 °C. Then α‐naphthol (10 μmol/L) was mixed to the incubation mixture conducted at 37 °C for 20 min. Finally, 250 μL of mixture was transferred to the 96‐well plates (Guangzhou JET Bio‐Filtration Products Co., Ltd., Guangzhou, China) with 50 μL of color developing agent (mixed as follows: mass fraction 5% SDS: mass fraction 1% fast blue B salt = 5:2 v/v). After incubation for 10 min at 37 °C, the optical density (OD) value was measured at 600 nm using a microplate reader (EON, BioTek Instruments Inc., Winooski, VT, USA). Control was detected in the absence of UDP‐glucose. The specific activity of UGTs was calculated based on an α‐naphthol standard curve and protein concentration of the enzyme source. All assays were performed in three biological replicates. Data were statistically analyzed by Student's t‐test with SPSS 19.0 (SPSS Inc., Chicago, IL, USA); a P‐value < 0.05 was considered statistically significant.
Bioassay
Bioassays of T. cinnabarinus were conducted using the modified residual coated vial (RCV) method as described by Feng et al. (2011). Abamectin was dissolved in acetone to various concentrations to keep the mortality at 20%–80%, then, thirty 3–5 days old healthy adult females were transferred into the abamectin‐coated centrifuge tube, each dose was repeated three times and the mites treated with acetone were used as control. The mites were checked under anatomical microscope after 24 h treatment. Mites showing immobility or with legs irregularly trembling were considered dead. The 5‐nitrouracil (the specific inhibitor of UGTs) was used to investigate the effect of UGTs on abamectin's toxicity and resistance formation in T. cinnabarinus according to relevant studies (Grancharov et al., 2001; Li et al., 2017). The only difference with the above was that each abamectin solution had 5‐nitrouracil added with a final concentration of 10 mg/L. There were three biological replicates. The median lethal concentrations (LC50) were calculated by PoloPlus (Probit and Logit Analysis, LeOra Software, Petaluma, USA).
RNA Extraction, reverse transcription and cloning of full‐length UGT201D3 cDNA
Total RNA was extracted from 200 female adults (3–5 days old) of T. cinnabarinus using the RNeasy® plus Micro Kit (Tiangen, Beijing, China). The RNA quantity and purity were checked by measuring the absorbance at 260 nm and the absorbance ratio of OD260/280 using a NanoVue UV‐Vis spectrophotometer (GE Healthcare Bio‐Science, Uppsala, Sweden), respectively. The RNA integrity was further verified by 1% agarose gel electrophoresis. The reverse transcription was carried out using a PrimeScript® 1st Strand cDNA Synthesis Kit (Takara Biotechnology Dalian Co., Ltd., Dalian, China) and stored at –20 °C for future use.
Based on the transcriptome data of T. cinnabarinus (NCBI: SRA052165) and the genome database of T. urticae (http://bioinformatics.psb.ugent.be/orcae/overview/Tetur), sequence information of UGT201D3 was acquired, and gene‐specific primers were designed and synthesized (Table S1) to directly amplify the complete open reading frame (ORF). Specific polymerase chain reaction (PCR) reaction was performed in a C1000™ Termal Cycler (Bio‐Rad, Hercules, CA, USA). The PCR conditions were set as follows: 3 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 55–65 °C (depending on gene‐specific primers) and 60 s at 72 °C, then 10 min at 72 °C. Cloning and sequence analyses of the complete ORF of UGT201D3 were repeated at least three times with different preparations of RNAs and further sequenced for confirmation (BGI, Beijing, China).
Bioinformatic analysis of UGT201D3
The DNA sequence of UGT201D3 was deduced by DNAMAN 5.2.2 (Lynnon Biosoft, San Ramon, CA, USA). The deduced sequence of amino acids was obtained through Primer Premier 5 (Version 5.00) and named by the UGT Nomenclature Committee (http://www.flinders.edu.au/medicine/sites/clinicalpharmacology/ugt-homepage.cfm) (Mackenzie et al., 2005). The multiple sequence alignment was performed online through ESPript 3.0 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi) (Robert et al., 2014). The signal peptide and transmembrane domain were predicted using SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP) (Bendtsen et al., 2004). The ExPASy proteomics tool (http://web.expasy.org/compute_pi/) was used to calculate the isoelectric point (pI) and molecular weight (MW) of UGT201D3 (Bairoch, 1993). A phylogenetic tree was constructed by the maximum likelihood method using MEGA 5.05 software (Tamura et al., 2011). A total of 1000 bootstrap replications were used to test the phylogeny.
Quantitative real‐time PCR (qPCR) of UGT201D3
In order to detect the expression level of UGT201D3 in different developmental stages of the AbR strain, approximately 2000 eggs (1–2 days old), 1000 larvae (1 day old), 800 nymphs (the first nymph [1–2 days old]) and 200 female adults (3–5 day old) of mites were prepared for RNA extraction. To quantify this UGT gene expression in different strains, we collected 200 female adult mites in SS and AbR strains, respectively, for each sample. To examine the effect of abamectin exposure on the expression of UGT201D3, we used abamectin to treat adult female mites from SS and AbR strains (LC10 [SS: 0.03 mg/L, AbR: 1.0 mg/L], dissolved in acetone) for 6, 12 and 24 h, respectively. Mites treated with acetone were used as a control. The survivors were collected for RNA extraction. For the induction experiment, we also adopted the modified RCV method described as above. Each treatment contained three biological replicates and three technique replicates.
Primers of UGT201D3 for qPCR study were designed by using primer 3.0 (http://frodo.wi.mit.edu/) (Rozen & Skaletsky, 2000) as shown in Table S1 along with a stable reference gene, ribosomal protein S18 (FJ608659) (Sun et al., 2010). Optimized PCR master mix (20 μL) contained the following components: 10 μL GoTaq® qPCR Master Mix (Promega, Madison, WI, USA), 1 μL diluted cDNA, 1 μL of each primer (10 μmol/L) and 7 μL ddH2O and the qPCR was performed on a Mx3000P thermal cycler (Agilent Technologies, Inc., Wilmington, NC, USA). The optimized thermal program was 95 °C for 2 min, then 40 cycles of denaturation at 95 °C for 15 s, 60 °C for 30 s. Finally, to ensure consistency and specificity of the amplified product, a melting curve analysis from 60 °C to 95 °C was applied. The qPCR experiments were performed according to minimum information for publication of qCR experiments (MIQE) guidelines (Garson et al., 2009). The relative expression of UGT201D3 was analyzed using the method (Livak & Schmittgen, 2001). The relative quantities of expression levels of UGT201D3 in the SS and AbR strains were determined by an independent sample t‐test with a significance level of P < 0.05 in SPSS 19.0 (SPSS Inc., Chicago, IL, USA). The differences in UGT201D3 expression levels among four developmental stages, UGT201D3 expression levels before and after induction were analyzed by one‐way analyses of variance (ANOVA), followed by Tukey's Honestly Significant Difference (HSD) tests, with a significance level of P < 0.05 in SPSS 19.0. There were three biological replicates and three technique replicates to minimize intra‐experiment variation.
RNAi of UGT201D3
RNAi was applied to further explore the toxicological function of UGT201D3 in T. cinnabarinus. The green fluorescent protein (GFP) (ACY56286) gene was used as a negative control and diethylpyrocarbonate (DEPC)‐treated water was used as a blank control. The UGT201D3 and the GFP genes were amplified by PCR using primers containing the T7 RNA polymerase promoter (Table S1). The double‐stranded RNAs (dsRNAs) were synthesized using TranscriptAid T7 High Yield Transcription Kit (Termo Scientifc, Lithuania, EU) on the basis of the manufacturer's instructions with the purified PCR products, and then purified the dsRNAs using the GeneJET RNA Purifcation Kit (Termo Scientifc, Lithuania, EU). The final dsRNAs were diluted with DEPC‐treated water to a concentration of 1000 ng/μL. The dsRNAs quantity and size were checked using a spectrophotometer and 1% agarose gel electrophoresis, respectively.
The RNAi was carried out according to a leaf‐disc feeding method described by Shi et al. (2015). After feeding for 48 h, the mites were collected to determine the reduction of transcription level of UGT201D3 using qPCR. Meanwhile, in order to analyze whether there existed an off‐target effect, we also detected the expression levels of another two UGT genes (UGT201B15 and UGT201E2) randomly from the same family with UGT201D3 (Fig. 2). The qPCR primers are also listed in Table S1.
Figure 2.
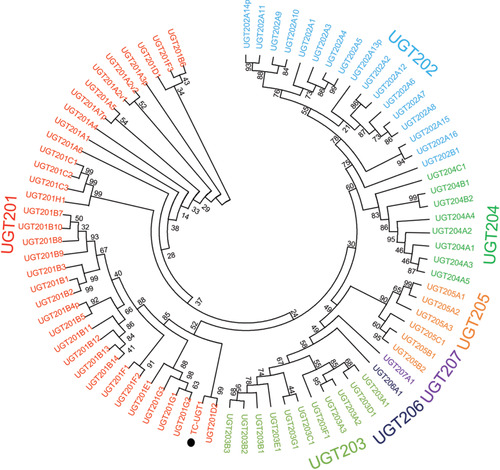
Phylogenetic relationship of UGT201D3 with uridine diphosphate‐glycosyltransferase (UGT) family of Tetranychus urticae. The phylogram was generated using the maximum likelihood method in MEGA 5.05 and the inferred phylogeny was tested by bootstrap analysis with 1000 replications. The sequences used for constructing the tree are listed in Table S2. Filled circles indicate the newly cloned UGT gene of T. cinnabarinus. Filled triangles mean the other two UGT genes of T. cinnabarinus were used to analyze whether there are off‐target effects in RNA interference (RNAi).
In this study, UGTs activity and bioassays with abamectin were tested after RNAi of UGT201D3. The 100–200 adult female mites at 48 h post‐feeding of UGT201D3 dsRNA was collected for determination of UGTs activity. Three doses of abamectin LC30, LC50 and LC70 (the LC30, LC50 and LC70 values of abamectin were 0.10 mg/L, 0.25 mg/L and 0.61 mg/L for SS, 2.0 mg/L, 3.3 mg/L and 5.9 mg/L for the AbR strain) were applied for the bioassays. We also used the same procedure as described above. The RNAi knockdown efficiencies, mortality rates and specific activities of UGTs were analyzed by one‐way analysis of variance (ANOVA), followed by Tukey's HSD tests (P‐value < 0.05).
Expression and purification of the UGT201D3
The specific primers of the original sequences of UGT201D3 gene were designed (Table S1). According to reports (Fink, 1998), in the folding process of protein expression, the formation of inclusion bodies is related to the intermolecular interaction of the hydrophobic surface of the protein, and hydrophobic groups and β‐sheets are more likely to aggregate to form inclusion bodies. Then, online analysis of the hydrophilicity/hydrophobicity of the target protein via ProtScale (https://web.expasy.org/protscale/) shows that there are multiple hydrophobic regions in the target protein (Score > 0 is a hydrophobic region) (Fig. S1). Hence, to ensure that no active sites and conserved regions of the protein were removed, we designed primers (UGT201D3q) that remove 15 amino acids from the 5' hydrophobic region. Moreover, Nde I and EcoR I restriction enzyme cutting sites were incorporated into forward and reverse primers (Table S1). Then the target gene sequences and the expression vector pET‐28a were ligated together using T4 DNA‐ligase (Takara Biotechnology Dalian Co., Ltd., Dalian, China) after being linearized with the same enzymes, respectively. The products of ligation were transformed into Trans5α competent cells to identify positive clones. After that, the prokaryotic expression vectors pET‐28a‐UGT201D3 and pET‐28a‐UGT201D3q were transformed into E. coli BL21 (DE3) strain and single colonies were cultured overnight with shaking at 37 °C. The grown bacterial cells with the proportion of 1% were inoculated into 500 mL Lysogeny broth‐kanamycin media shaken at 37 °C for 3–5 h until the OD600 reached 0.6–0.8. Then, IPTG was added to a final concentration of 0.1 mmol/L. The culture was subsequently incubated at 28 °C for 24 h at 180 r/min, and collected by centrifugation.
The cells were then harvested at 4 °C for 20 min by centrifugation at 4000 × g and re‐suspended in ice‐cold phosphate‐buffered saline (PBS) (0.04 mol/L, pH 7.4). Then the cells were disrupted by sonication (8 s, 150 W) on ice for 40 min and centrifuged at 9000 × g for 15 min at 4 °C. The supernatant was purified using a Ni2+‐NTA agarose gel column (Tiangen Biotech Co., Ltd., Beijing, China) with a linear imidazole gradient of 40–500 mmol/L in PBS (0.04 mol/L, pH 7.4), containing 0.5 mol/L NaCl. The quality of purified recombinant protein was determined by SDS‐PAGE (polyacrylamide gel electrophoresis) using a 5% (v/v) stacking gel and a 10% (v/v) resolving gel as well as western blotting with anti‐His tag monoclonal antibody.
Enzyme activity and inhibition with acaricide measurements
The purified UGT201D3 protein concentration and the specific activity were measured using a microplate reader as described above.
Recombinant UGT201D3 protein (purified) kinetic variables (K m [Michaelis constant] and V max [maximum velocity]) were evaluated in the enzymatic assay from a double‐reciprocal plot of data obtained under assay conditions with different α‐naphthol concentrations (3–30 μmol/L). To investigate the substrate competitions of the recombinant enzyme between α‐naphthol and abamectin, the median inhibitory concentration (IC50) was determined at different concentrations of abamectin (0–100 μmol/L) with α‐naphthol concentration equal to the K m of the recombinant UGT201D3 according to the enzymatic assay. For each combination of substrate and inhibitor at least three replicates were performed. In order to determine the type of inhibition, Dixon plot analysis were performed at three different concentrations of α‐naphthol (10, 20 and 40 μmol/L) and five different concentrations of abamectin (0, 30, 50, 80, 100 μmol/L). The experiments were repeated three times and results were analyzed with GraphPad prism 5 (GraphPad Software, Inc., San Diego, CA, USA).
HPLC analysis of abamectin metabolism
The ability of purified UGT201D3 to metabolize abamectin was determined by HPLC. Tested reactions were performed by incubation of abamectin (10 mg/L) with UGT201D3 protein (150 μg) and UDP‐glucose (1 mmol/L) in 5% acetonitrile water solution with MgCl2 (10 mmol/L); the total volume was 1 mL. Two controls were designed: one is the same volume of the elution buffer and the other is the same as the protein treatment only except that the expression vector pET‐28a was not ligated with the UGT201D3 gene. There were three biological replicates and three technique replicates. After incubating in a shaker at 28 °C, 180 r/min for 6 h, the reaction solution was transferred into a 15 mL centrifuge tube. The extraction of abamectin was conducted according to modified method of Zhang et al. (2012): 5 mL of ethyl acetate and 50 μL of 1% acetic acid aqueous solution were added to terminate the reactions and the samples were vortexed for 5 min. Then, after centrifuging for 5 min at 3500 r/min, the supernatant was totally transferred into a 100 mL flask. The extraction and centrifugation steps were repeated with fresh solvent. The extracts were combined and evaporated at 45 °C under vacuum (up to 100 mbar) with a rotary evaporator. Finally, the dry extract was redissolved to 1.0 mL with acetonitrile for HPLC analysis. Abamectin was separated on a SunFire C18 (5 μm, 150 mm × 4.6 mm) reverse phase analytical column (Waters Alliance 2695–2996). A mixture of acetonitrile and water (80 : 20, v/v) was used as mobile phase and the flow rate was 0.8 mL/min. The injection volume was 20 μL. Abamectin elution was monitored by changes in absorbance at 245 nm, and was quantified by peak integration. Data analyses were carried out using SPSS 19.0 (SPSS Inc., Chicago, IL, USA); the difference of the abamectin content between treatment and control was analyzed by an independent‐sample t‐test with a P‐value < 0.05.
Results
Activities of UGTs enzyme in SS and AbR strains of T. cinnabarinus
The specific activities of UGTs were 1.73 nmol/mg protein/min in AbR strain and 0.74 nmol/mg protein/min in SS, that is the activity of UGTs in AbR strain was 2.34‐fold of that in SS (Table 1), which suggested that the increase of UGT activity was probably correlated with T. cinnabarinus’s resistance against abamectin.
Table 1.
The specific activity of uridine diphosphate‐glycosyltransferases (UGTs) in different strains of Tetranychus cinnabarinus
| Strains | Specific activity (nmol/mg protein/min) |
|---|---|
| SS | 0.74 ± 0.04 |
| AbR | 1.73 ± 0.29* |
*Significant difference (P < 0.05).
SS, susceptible strain; AbR, abamectin‐resistant strain.
Effect of synergist
After inhibiting the activity of UGTs in mites, the lethal effect of abamectin on resistant mites was significantly enhanced. The LC50 of the SS was reduced from 0.25 mg/L to 0.20 mg/L, and that in AbR strain was reduced from 3.51 mg/L to 1.51 mg/L. The toxicity of abamectin to SS changed without significance in the synergist test (the 95% CI of LC50 overlapped between the treatments with and without synergist), however, that in AbR strain significantly increased by 56.98% in the synergist treatment. The synergistic effect of UGTs inhibiter was significant in AbR while it was not in SS, which indicated that UGTs played an important role in abamectin resistance in T. cinnabarinus (Table 2).
Table 2.
Toxicity of abamectin to SS and AbR strains with and without 5‐nitrouracil in Tetranychus cinnabarinus
| Strains | Treatments | LC50 (mg/L) 95% CI | Slope (±SE) | χ2 * | Increased toxicity |
|---|---|---|---|---|---|
| SS | Abamectin | 0.25 (0.20 – 0.34) | 1.37 ± 0.21 | 2.25 | – |
| Abamectin + 5‐nitrouracil | 0.20 (0.16 – 0.23) | 1.70 ± 0.23 | 2.49 | 20.00% | |
| AbR | Abamectin | 3.51 (2.68 – 5.21) | 2.27 ± 0.35 | 7.11 | – |
| Abamectin + 5‐nitrouracil | 1.51 (1.33 – 1.70) | 2.46 ± 0.30 | 1.88 | 56.98% |
*Chi‐square testing linearity, P < 0.05.
SS, susceptible strain; AbR, abamectin‐resistant strain; LC50, lethal concentration at 50% mortality.
Sequencing and annotation of UGT201D3 from T. cinnabarinus
From the previous microarray (GEO: GSE70824) results, we found an UGT gene over‐expressed in AbR strain. Then, this gene containing complete ORFs was obtained according to the transcriptome data of T. cinnabarinus and the genome of T. urticae. It was named as UGT201D3 by the UGT Nomenclature Committee. Sequence information has been submitted to GenBank and the accession number is KX905079. The ORF of UGT201D3 is 1383 bp, encoding 460 amino acid residues. According to the prediction results, UGT201D3 gene has no signal peptide and transmembrane domain. A characteristic signature sequence was identified in its C‐terminal, which contained the conserved amino acids: [FAV]‐[LIVMF]‐[TS]‐[HQ]‐[SGAC]‐G‐X(2)‐[STG]‐X(2)‐[DE]‐X(6)‐P‐[LIVMFA]‐[LIVMFF]‐X(2)‐P‐[LMVFIQ]‐X(2)‐[DE]‐Q (amino acids in square brackets can be arbitrary, and X represents any amino acid). The signature motif has been implicated in binding the UDP‐sugar (Mackenzie et al., 2005) (Fig. 1). The molecular weight of the predicted protein and estimated isoelectric point (pI) value were 53.49 kDa and 5.74, respectively. The phylogenetic analyses were performed by MEGA 5.05 with the maximum likelihood method on the basis of the deduced amino acid sequences of UGT201D3 and all UGT family genes of T. urticae (Ahn et al., 2014). The phylogenetic tree showed that the UGT201D3 shared 98% identity with UGT201D2 (tetur04g04350) from T. urticae (Fig. 2).
Figure 1.
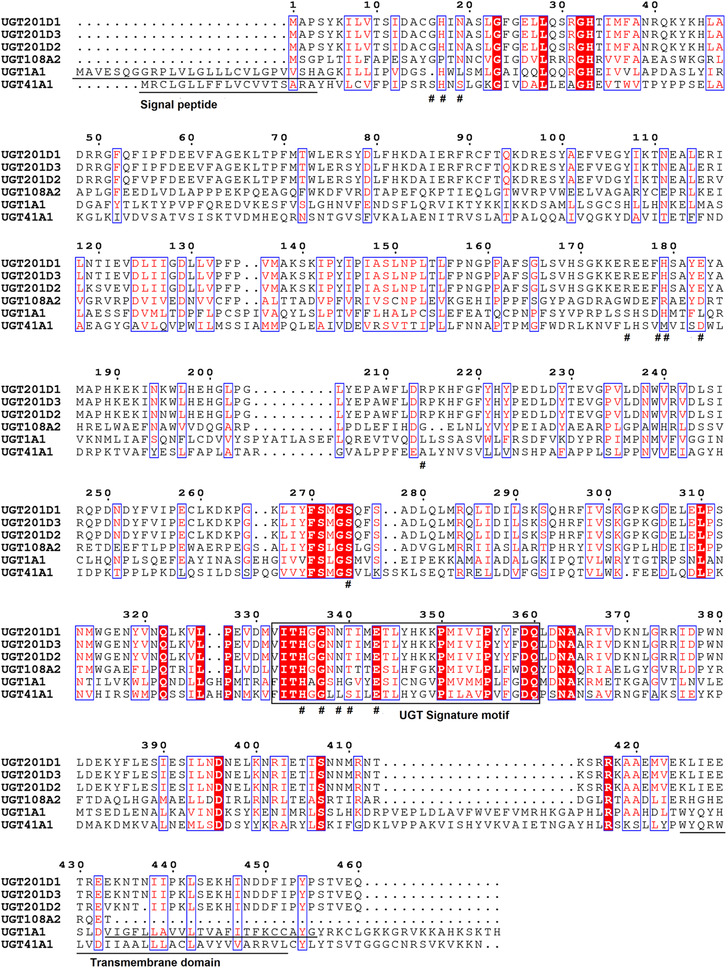
Alignment of amino acid sequence of UGT201D3 in Tetranychus cinnabarinas and uridine diphosphate‐glycosyltransferases (UGTs) in other species. The UGT signature motif is boxed. The active sites predicted by the National Center for Biotechnology Information are be represented by #. (UGT1A1 in human, UGT41A1 in Bombyx mori, UGT108A2 in bacteria, UGT201D1 and UGT201D2 in Tetranychus urticae).
Specific expression of UGT201D3 in different developmental stages, strains and abamectin‐induction treatment
To clarify the characteristics of UGT201D3 gene expression in different developmental stages of T. cinnabarinus, qPCR analysis was performed on the cDNA from eggs, larvae, nymphs and adults of the AbR strain. The relative expression level of UGT201D3 in larvae, nymphs and adults were 7.36‐, 4.16‐ and 4.17‐fold higher than that in eggs (Fig. 3A). Identification of the differences in the levels of expression of UGT201D3 between the SS and AbR strains showed that UGT201D3 was significantly up‐regulated to 4.02‐fold in the AbR strain compared with SS (Fig. 3B). The results of abamectin induction experiment revealed that there were no significant differences between treatments and control in SS, which suggested that the expression of UGT201D3 cannot be induced in SS. By contrast, the transcript of UGT201D3 was significantly increased (2.63‐ and 2.10‐fold, 6 h and 12 h later of abamectin‐exposure, respectively) in the AbR strain (Fig. 3C, D).
Figure 3.
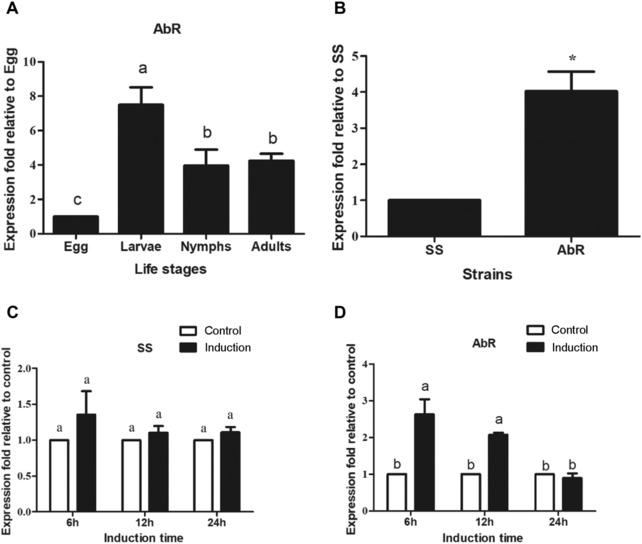
Quantitative polymerase chain reaction analysis of the expression of UGT201D3 in Tetranychus cinnabarinus. (A) The expression of UGT201D3 in different life stages in abamectin‐resistant strain (AbR); (B) the expression of UGT201D3 in different strains (3–5 days old female adult mites); (C, D) the expression of UGT201D3 to abamectin exposure in the susceptible (SS) and AbR strains (3–5 days old female adult mites). The error bars represent the standard deviation of the mean of three independent replicates. Asterisks on the error bars show significant differences between the SS and AbR strains (P < 0.05). Different letters on the error bars show significant differences among different developmental stages or between the abamectin induction and the control (P < 0.05).
Functional analysis of UGT201D3 by RNAi
After RNAi, the relative expression levels of mRNA were detected. The results showed that the transcript levels of UGT201D3 in the SS and AbR strains were significantly decreased by 48.82% and 51.25% compared with the controls, respectively (Fig. 4). Meanwhile, the qPCR data also showed that the expression levels of the other two UGT genes (UGT201B15 and UGT201E2) were not affected by RNAi neither in SS nor in AbR (Fig. 4). These results demonstrated that the transcript of UGT201D3 was successfully knocked down with no off‐target effect.
Figure 4.
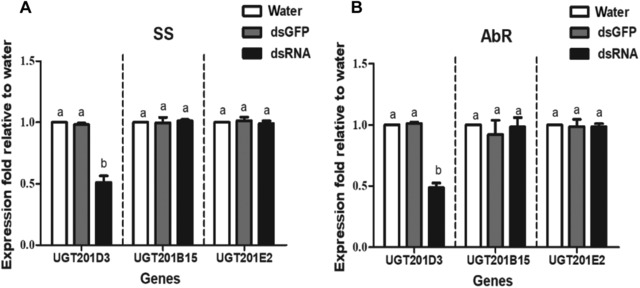
Quantitative polymerase chain reaction analysis of the expression of UGT201D3 after RNA interference (RNAi) in the susceptible (SS) and abamectin‐resistant (AbR) strains of Tetranychus cinnabarinus, relative to expression in the water control. UGT201B15 and UGT201E2 are another two uridine diphosphate‐glycosyltransferase (UGT) genes used to analyze whether there exist off‐target effects in the RNAi. The error bars represent the standard deviation of the mean of three independent replicates. Different letters above bars indicate significant differences among treatments (P < 0.05).
Subsequently, the specific activities of UGT enzymes were detected after decreasing the expression of UGT201D3. The activities of UGTs decreased significantly about 1.68‐fold and 2.72‐fold in SS and AbR strains (Table 3). Furthermore, the mortalities increased significantly by 16.42%, 11.94% and 22.99%, respectively, when treated with three concentrations of abamectin (LC30, LC50 and LC70 of abamectin) in the AbR strain. However, there were no significant differences of the mortalities between the treatment and controls in SS except for the mortality of LC70 of abamectin (Fig. 5). The increased mortalities in AbR implied that down‐regulation of UGT201D3 could decrease the abamectin‐resistance in T. cinnabarinus.
Table 3.
The specific activity of uridine diphosphate‐glycosyltransferases (UGTs) after dsRNA feeding in different strains of T. cinnabarinus
| Specific activity (nmol/mg protein/min)* | ||
|---|---|---|
| Treatments | SS | AbR |
| DEPC‐water | 0.541 ± 0.081 a | 1.727 ± 0.171 a |
| dsGFP | 0.529 ± 0.036 a | 1.716 ± 0.235 a |
| dsRNA | 0.321 ± 0.029 b | 0.634 ± 0.101 b |
*Values within a column followed by different letters are significantly different (P < 0.05).
SS, susceptible strain; AbR, abamectin‐resistant strain; dsRNA, double‐stranded RNA; DEPC, diethylpyrocarbonate; dsGFP, double‐stranded green fluorescent protein.
Figure 5.
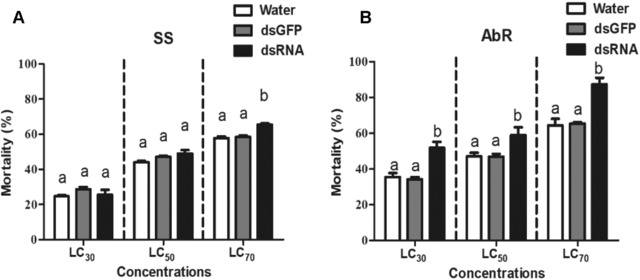
Mortality after RNA interference (RNAi) of UGT201D3 in the susceptible (SS) and abamectin‐resistant (AbR) strains of Tetranychus cinnabarinus. The error bars represent the standard deviation of the mean of three independent replicates. Different letters above bars indicate significant differences among treatments (P < 0.05). LC30: lethal concentration at 30% mortality. LC50: lethal concentration at 50% mortality. LC70: lethal concentration at 70% mortality.
Expression of UGT201D3 in E. coli
To facilitate functionally expressing UGT201D3, the original sequence and N‐terminal modification sequence of UGT201D3 (pET‐28a‐UGT201D3 and pET‐28a‐UGT201D3q) were expressed in E. coli. Induction of the pET‐28a‐UGT201D3 and pET‐28a‐UGT201D3q with 0.1 mmol/L IPTG for 24 h at 28 °C in BL21 (DE3) cells resulted in good levels of protein production. However, only the pET‐28a‐UGT201D3q obtained the majority of soluble protein in the supernatant. After purification using a Ni2+‐NTA agarose gel column, the recombinant UGT201D3 protein was analyzed by SDS‐PAGE and western blotting. These results showed that the recombinant UGT201D3 was well purified. The actual molecular weight was consistent with the predicted molecular mass (51.89 KDa) (Fig. 6).
Figure 6.
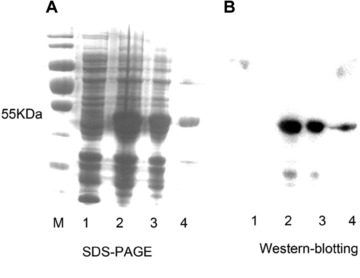
SDS‐PAGE (A) and western blotting (B) analysis of recombinant UGT201D3 expression in Escherichia coli (DE3). Lane M: protein markers; Lane 1: PET‐28a with isopropyl b‐D‐1‐thiogalactopyranoside (IPTG) induction; Lane 2: PET‐28a‐UGT201D3q with IPTG induction; Lane 3: soluble protein; Lane 4: purified by Ni2+‐NTA.
The enzyme activity of purified UGT201D3 was studied using α‐naphthol as substrate and UDP‐glucose as a sugar donor. The specific activity of the recombinant UGT201D3 was 2.81 ± 0.43 nmol/mg/protein/min. The values of K m and V max were 22.73 ± 5.87 μmol/L and 11.08 ± 2.77 nmol/mg/protein/min (Table 4), respectively. The activity of UGT201D3 was inhibited by abamectin and the IC50 was determined at 57.50 ± 3.54 μmol/L (Table 5). Dixon plot analysis showed that the inhibition was competitive for the three linear curves (three different concentrations of α‐naphthol) crossed above the x axis and the inhibition constant (Ki) was determined at 9.9 ± 6.2 μmol/L (Fig. 7).
Table 4.
The specific activity and kinetic variables of recombinant UGT201D3
| Samples | Specific activity (nmol/mg pro/min) | K m (μmol/L) | V max (nmol/mg pro/min) |
|---|---|---|---|
| PET‐28a vector | – | – | – |
| Recombinant UGT201D3 | 2.81 ± 0.43 | 22.73 ± 5.87 | 11.08 ± 2.77 |
K m, Michaelis constant; V max, maximum velocity.
Table 5.
Ability of abamectin to inhibit α‐naphthol glycosylation catalyzed by UGT201D3
| Acaricides | Chemical type | IC50 (μmol/L) |
|---|---|---|
| Abamectin | Macrolide | 57.50 ± 3.54 |
IC50, inhibitory concentration at 50%.
Figure 7.
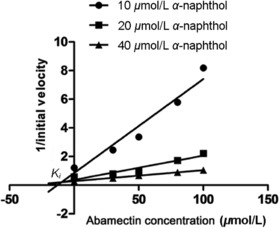
Dixon plot analysis for the inhibition of α‐naphthol conjugating activity of UGT201D3 by different abamectin concentrations. Three concentrations of α‐naphthol (10, 20 and 40 μmol/L) and five concentrations of abamectin (0, 30, 50, 80, 100 μmol/L) were used and data are means of three replicates ± SD. Analysis denoted a competitive type of inhibition and the inhibition constant (Ki) was determined as 9.9 ± 6.2 μmol/L.
Assessing the capability of UGT201D3 to deplete abamectin
To further confirm whether recombinant UGT201D3 protein can metabolize or deplete abamectin directly, catalytic activity was initially assessed by measuring substrate depletion using HPLC. The average recoveries of abamectin at three spiked levels were 93.72%–107.30% with the relative standard deviation ranging from 2.54% to 7.16%, which indicated the developed method was valid and reliable for residual analysis of abamectin in the enzyme metabolism study (Table S3). Compared to the elution buffer, the protein expressed by the empty vector pET‐28a cannot deplete abamectin. Compared with the empty vector, the addition of recombinant UGT201D3 protein detected a significant decrease in the abamectin content, in which the desired new metabolite was not detected. The results showed that the rate of depletion for abamectin was 15.77% ± 3.72% when treated with 150 μg recombinant UGT201D3 for 6 h (Table 6).
Table 6.
In vitro depletion of abamectin by recombinant UGT201D3
| Abamectin content (mg/mL) | ||||
|---|---|---|---|---|
| Protein | Protein content (μg/mL) | Initial | Incubation 6 h | Depleting rate (%) *, † |
| Recombinant UGT201D3 | 150 | 10 | 8.42 ± 0.37 | 15.77% ± 3.72% b |
| PET‐28a vector | – | 10 | 9.93 ± 0.13 | 0.67% ± 1.30% a |
| Elution buffer | – | 10 | 9.92 ± 0.16 | 0.80% ± 1.64% a |
*Values within a column followed by different letters are significantly different (P < 0.05).
†Depleting rate = (initial content of abamectin – abamectin content after incubating 6 h) / initial content of abamectin × 100.
Discussion
Abamectin has been widely used as an insecticide/acaricide for more than 30 years because of its superior bioactivity against insect and mite pest (Xu et al., 2017). So far, many arthropod species, such as P. xylostella, T. urticae and T. cinnabarinus, have evolved various levels of resistance to abamectin worldwide (Leeuwen et al., 2010). The inheritance of abamectin‐resistance was reported in T. cinnabarinus (He et al., 2009a), P. xylostella (Liang et al., 2003) and other insects or mites (Clark et al., 1992; Ferreira et al., 2015), which was controlled by multiple genes and the degree of dominance was incomplete recessive. Polygenic resistance was confirmed by multiple abamectin‐resistant mechanisms documented in insects and mites. Mutations in the target site of glutamate‐gated chloride channels conferred resistance to abamecctin in T. urticae and P. xylostella (Kwon et al., 2010; Dermauw et al., 2012; Wang et al., 2016). Considerable studies showed that CarEs, mixed function oxidases (MFOs) and glutathione‐S‐transferases (GSTs) were the most important enzymes correlated with abamectin‐metabolic resistance in arthropods (Liang et al., 2001; He et al., 2009b; Deokho et al., 2010; Riga et al., 2014). The increased expression of P‐glycoprotein (P‐gp) also contributed to abamectin‐resistance in Drosophila melanogaster (Luo et al., 2013) and T. cinnabarinus (Xu et al., 2016), respectively. Besides the above‐mentioned mechanisms, here we have elucidated another mechanism of abamectin resistance in T. cinnabarinus, which is mediated by the UGTs.
In recent years, the UGTs, which can glycosylate toxins, like plant secondary metabolites, has been identified to be a detoxification enzyme in insects. For example, the larvae of the autumnal moth, Epirrita autumnata, can detoxify toxic flavonoid aglycones present in birch leaves via glycosylation (Salminen et al., 2015). Krempl and colleagues proved that UGTs could metabolize gossypol partially via glycosylation in H. armigera and H. virescens, which might be a crucial step in gossypol detoxification (Krempl et al., 2016). From transcriptome analysis, UGT genes were subsequently found to be over‐transcribed in resistant populations, for instance in DDT‐resistant D. melanogaster (Pedra et al., 2004), carbamate‐resistant Myzus persicae (Silva et al., 2012), neonicotinoid‐resistant Bemisia tabaci (Yang et al., 2013) and permethrin‐induced Anopheles gambiae (Vontas et al., 2005). Recently, some UGT genes were reported have function‐mediating resistance against imidacloprid (Kaplanoglu et al., 2017) and chlorantraniliprole (Li et al., 2017) with the RNAi method. In Caenorhabditis elegans (Laing et al., 2010) and Haemonchus contortus (Vokřál et al., 2012) the covalent linkage of glucose with benzimidazole anthelmintics was catalyzed by UGTs. Moreover, it was also proved that UGTs of H. contortus were able to detoxify naphthalophos, an organophosphate, and they were also involved in anthelmintic‐resistance (Kotze et al., 2014). All these findings implied the potential roles of UGTs in the detoxification of insecticides, and UGTs could mediate metabolic resistance, whereas no further and direct evidence were documented to confirm the interaction between UGT gene products and insecticides until the present study.
It was reported that 5‐nitrouracil (5‐NU), a specific inhibitor of UGTs, could increase the toxicity of insecticides to P. xylostella (Li et al., 2017) and H. contortus (Kotze et al., 2014) by inhibiting UGT activity. In the current study, both enzyme activity assay and enzyme inhibitor bioassay indicated that elevating activity of UGTs was associated with abamectin resistance in T. cinnabarinus. The qPCR analysis in our study showed that the expression of UGT201D3 was higher in AbR strain than that in SS, and the expression of UGT201D3 was increased significantly in AbR after stimulation with abamectin, which implied that UGT201D3 gene could have an important role conferring abamectin resistance in T. cinnabarinus. Similarly, Vontas et al. (2005) found that UGT genes emerged with different transcriptional levels in the permethrin‐resistant strain and showed elevated mRNA levels after permethrin exposure in A. gambiae. It has also been reported that UGT40R3 and UGT46A6 could be induced by deltamethrin in antennae of Spodoptera littoralis (Bozzolan et al., 2014). RNAi has been widely used to investigate gene function in insects and has proved to be an effective technique with high specificity (Zhu, 2013). After silencing the expressions of two UGT genes (UGT1 and UGT2) via RNAi in the imidacloprid‐resistant strain of L. decemlineata, susceptibility of resistant beetles to imidacloprid significantly increased, indicating that the UGT genes were related to imidacloprid resistance (Kaplanoglu et al., 2017). Likewise, similar RNAi results in P. xylostella revealed that UGT2B17 was involved in the detoxification of chlorantraniliprole, and its over‐expression played an important role in chlorantraniliprole‐resistance in P. xylostella (Li et al., 2017). In our study, after knocking down the UGT201D3 via RNAi, the mortalities increased dramatically and the activity of UGTs decreased in the AbR strain, indicating that UGT201D3 could be an important factor involved in abamectin resistance in T. cinnabarinus. It was interesting to note that the mortalities under LC70 of abamectin in the treatment was obviously increased comparing that in the controls in SS. This might be another evidence that UGT201D3 was important in abamectin detoxification and abamectin‐resistance formation in T. cinnabarinus because it worked and resisted high concentrations of abamectin in SS.
Prokaryotic expression is commonly used in the study of gene function in insects or mites. However, due to the inherent characteristics of the expression system of E. coli, there are deficiencies. In practice, the target gene is often modified, including the N‐terminal of the gene. For example, the signal peptide of the P450 gene is removed, and the previous eight amino acids are replaced; or a certain codon of the gene is mutated to facilitate expression (Barnes et al., 1991). It has been found that comparing the N‐terminal modified human P450 expressed in E. coli and the same N‐terminal modified enzyme expressed by insect cells, it was confirmed that the N‐terminal modification did not affect the catalytic properties of P450 (Yamazaki et al., 1999). In addition, the inclusion of hydrophobic groups and β‐sheets in the peptide chain made it easier to form inclusion bodies (Fink, 1998). Mutating immunoglobulin hydrophobic groups into hydrophilic groups could significantly reduce the formation of inclusion bodies in the E. coli expression system (Nieba et al., 1997). Therefore, in order to clarify the function of UGT201D3 gene expressed in T. cinnabarinus in abamectin resistance, this study uses the E. coli expression system to express it in vitro. However, when the original sequence of the gene was expressed, only the inclusion body was obtained. So, we modified the gene via removing the N‐terminal hydrophobic region without affecting the active site and the conserved region. Fortunately, the recombinant soluble protein with UGT activity was obtained. The inhibition test and Dixon plot analysis revealed abamectin could be the substrates of UGT201D3 protein. Subsequently, the HPLC analysis showed that abamectin could be depleted by the recombinant UGT201D3 protein, which provided direct evidence that UGT201D3 played a role in abamectin resistance in T. cinnabarinus. However, we could not say that abamectin was metabolized or degraded by UGT201D3 protein considering the general function of UGTs (glycosylation) and the fact that the novel products from interaction were not detected under the current conditions (Fig. S2). Before the current study about UGTs, we previously documented that the resistance against abamectin in T. cinnabarinus was controlled by polygenes, the detoxifying enzymes, such as CarEs, MFOs, GSTs and P‐gp contributing to abamectin resistance (He et al., 2009a, 2009b; Xu et al., 2016). However, it is hard to state which enzyme is the major factor that mediates abamectin resistance in T. cinnabarinus based on existing results, and systematic and comparative studies should be explored in the future.
In summary, all of the studies we have done demonstrated that UGT201D3 contributed to abamectin‐resistance formation in T. cinnabarinus. This is the first time it has been directly proved that UGT gene up‐regulation can mediate abamectin‐resistance through detoxifying abamectin. These findings will shed light on our understanding of UGT gene function in arthropods and the mechanisms of abamectin‐resistance formation in T. cinnabarinus. It would be reasonable that the similar resistant mechanism may exist in resistant strains of T. cinnabarinus in the filed because the UGT genes mediating resistance have been confirmed in resistant field insects, such as L. decemlineata (Kaplanoglu et al., 2017) and P. xylostella (Li et al., 2017). Furthermore, Pedra et al. (2004) reported that the field and laboratory DDT‐resistant Drosophila genotypes shared much commonalities of over‐transcribed genes and concluded that similar resistance mechanisms may exist between laboratory‐ and field‐selected DDT‐resistant fly lines, which further strengthened the possibility of our inference.
Discloure
The authors declare no conflict of interest.
Supporting information
Table S1. Primers used for cloning, RNAi and qPCR analysis.
Table S2. Sequences used for phylogenetic analysis.
Fig. S1. The analysis of amino acid hydrophilicity/hydrophobicity of UGT201D3. Score > 0 is a hydrophobic region. Inside the red box is 15 amino acids removed.
Acknowledgments
This research is partially funded by Chongqing Research Program of Basic Research and Frontier Technology (No.cstc2017jcyjBX0061) and the National Natural Science Foundation of China (31672085).
*The copyright for this article was changed on June 4, 2020 after original online publication.
References
- Ahn, S.J. , Dermauw, W. , Wybouw, N. , Heckel, D.G. and Van Leeuwen, T. (2014) Bacterial origin of a diverse family of UDP‐glycosyltransferase genes in the Tetranychus urticae genome. Insect Biochemistry and Molecular Biology, 50, 43–57. [DOI] [PubMed] [Google Scholar]
- Ahn, S.J. , Vogel, H. and Heckel, D.G. (2012) Comparative analysis of the UDP‐glycosyltransferase multigene family in insects. Insect Biochemistry and Molecular Biology, 42, 133–147. [DOI] [PubMed] [Google Scholar]
- Auger, P. , Migeon, A. , Ueckermann, E.A. , Tiedt, L. and Navajas, M. (2013) Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): review and new data. Acarologia, 53, 383–415. [Google Scholar]
- Bairoch, A. (1993) The PROSITE dictionary of sites and patterns in proteins, its current status. Nucleic Acids Research, 21, 3097–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, H.J. , Arlotto, M.P. and Waterman, M.R. (1991) Expression and enzymatic activity of recombinant cytochrome P450 17 alpha‐hydroxylase in Escherichia coli . Proceedings of the National Academy of Sciences USA, 88, 5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen, J.D. , Nielsen, H. , Heijne, G.V. and Brunak, S. (2004) Improved Prediction of Signal Peptides: SignalP 3.0. Journal of Molecular Biology, 340, 783–795. [DOI] [PubMed] [Google Scholar]
- Bock, K.W. (2003) Vertebrate UDP‐glucuronosyltransferases: functional and evolutionary aspects. Biochemical Pharmacology, 66, 691–669. [DOI] [PubMed] [Google Scholar]
- Bon, A.M.L. (2010) UDP‐glucuronosyltransferases (UGTs) in neuro‐olfactory tissues: expression, regulation, and function. Drug Metabolism Reviews, 42, 74–97. [DOI] [PubMed] [Google Scholar]
- Bowles, D. , Isayenkova, J. , Lim, E.K. and Poppenberger, B. (2005) Glycosyltransferases: managers of small molecules. Current Opinion in Plant Biology, 8, 254–263. [DOI] [PubMed] [Google Scholar]
- Bozzolan, F. , Siaussat, D. , Maria, A. , Pottier, M.A. , Chertemps, T. , Maibeche‐Coisne, M . et al (2014) Antennal uridine diphosphate (UDP)‐glycosyltransferases in a pest insect: diversity and putative function in odorant and xenobiotics clearance. Insect Molecular Biology, 23, 539–549. [DOI] [PubMed] [Google Scholar]
- Cakmak, I. and Baspinar, H. (2005) Control of the carmine spider mite Tetranychus cinnabarinus Boisduval by the predatory mite Phytoseinlus persimilis (Athias‐Henriot) in protected strawberries in Aydin, Turkey. Turkish Journal of Agriculture and Forestry, 29, 259. [Google Scholar]
- Clark, J.M. , Argentine, J.A. , Lin, H. and Gao, X.Y. (1992) Mechanisms of abamectin resistance in the Colorado potato beetle. Acs Symposium Series, 505,247–263. [Google Scholar]
- de Mendonca, R.S. , Navia, D. , Diniz, I.R. , Auger, P. and Navajas, M. (2011) A critical review on some closely related species of Tetranychus sensu stricto (Acari: Tetranychidae) in the public DNA sequences databases. Experimental and Applied Acarology, 55, 1–23. [DOI] [PubMed] [Google Scholar]
- Deokho, K. , Geonmook, S. , Kang, T.J. and Sihyeock, L. (2010) Multiple resistance mechanisms to abamectin in the two‐spotted spider mite. Journal of Asia‐Pacific Entomology, 13, 229–232. [Google Scholar]
- Dermauw, W. , Ilias, A. , Riga, M. , Tirry, L. , van Leeuwen, T. , Vontas, J . et al (2012) The cys‐loop ligand‐gated ion channel gene family of Tetranychus urticae: implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochemistry and Molecular Biology, 42, 455–465. [DOI] [PubMed] [Google Scholar]
- Devine, G.J. , Barber, M. and Denholm, I. (2001) Incidence and inheritance of resistance to METI‐acaricides in European strains of the two‐spotted spider mite (Tetranychus urticae) (Acari: Tetranychidae). Pest Management Science, 57, 443–448. [DOI] [PubMed] [Google Scholar]
- Feng, Y.N. , Yan, J. , Sun W., Zhao, S. , Lu, W.C. , Li, M. et al (2011) Transcription and induction profiles of two esterase genes in susceptible and acaricide‐resistant Tetranychus cinnabarinus . Pesticide Biochemistry and Physiology, 100, 70–73. [Google Scholar]
- Ferreira, C.B.S. , Andrade, F.H.N. , Rodrigues, A.R.S. , Siqueira, H.A.A. and Gondim, M.G.C. (2015) Resistance in field populations of Tetranychus urticae to acaricides and characterization of the inheritance of abamectin resistance. Crop Protection, 67, 77–83. [Google Scholar]
- Fink A.L. (1998. ) Protein aggregation: folding aggregates, inclusion bodies and amyloid. Folding and Design, 3, R9–R23. [DOI] [PubMed] [Google Scholar]
- Garson, J.A. , Huggett, J.F. , Bustin, S.A. , Pfaffl, M.W. , Vandesompele, J. , Shipley, G.L . et al (2009) Unreliable real‐time PCR analysis of human endogenous retrovirus‐W (HERV‐W) RNA expression and DNA copy number in multiple sclerosis. Aids Research and Human Retroviruses, 25, 379–381. [DOI] [PubMed] [Google Scholar]
- Grancharov, K. , Naydenova, Z. , Lozeva, S. and Golovinsky, E. (2001) Natural and synthetic inhibitors of UDP‐glucuronosyltransferase. Pharmacology & Therapeutics, 89, 171–186. [DOI] [PubMed] [Google Scholar]
- Guo, F.Y. , Zhang, Z.Q. and Zhao, Z.M. (1998) Pesticide resistance of Tetranychus cinnabarinus (Acari: Tetranychidae) in China: a review. Systematic and Applied Acarology, 3, 3–7. [Google Scholar]
- He, L. , Gao, X.W. , Wang, J.J. , Zhao, Z.M. and Liu, N.N. (2009a) Genetic analysis of abamectin resistance in Tetranychus cinnabarinus . Pesticide Biochemistry and Physiology, 95, 147–151. [DOI] [PubMed] [Google Scholar]
- He, L. , Xue, C.H. , Wang, J.J. , Li, M. , Lu, W.C. and Zhao, Z.M. (2009b) Resistance selection and biochemical mechanism of resistance to two acaricides in Tetranychus cinnabarinus (Boiduval). Pesticide Biochemistry and Physiology, 93, 47–52. [DOI] [PubMed] [Google Scholar]
- Huang, F.F. , Chai, C.L. , Zhang, Z. , Dai, F.Y. , Lu, C. , Xiang, Z.H . et al (2008) The UDP‐glucosyltransferase multigene family in Bombyx mori . BMC Genomics, 9, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanoglu, E. , Chapman, P. , Scott, I.M. and Donly, C. (2017) Overexpression of a cytochrome P450 and a UDP‐glycosyltransferase is associated with imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata . Scientific Reports, 7, 1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotze, A.C. , Ruffell, A.P. and Ingham, A.B. (2014) Phenobarbital induction and chemical synergism demonstrate the role of UDP‐glucuronosyltransferases in detoxification of naphthalophos by Haemonchus contortus larvae. Antimicrobial Agents and Chemotherapy, 58, 7475–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempl, C. , Sporer, T. , Reichelt, M. , Ahn, S.J. , Vogel, H. , Joussen, N . et al (2016) Potential detoxification of gossypol by UDP‐glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens . Insect Biochemistry and Molecular Biology, 71, 49–57. [DOI] [PubMed] [Google Scholar]
- Kwon, D.H. , Yoon, K.S. , Clark, J.M. and Lee, S.H. (2010) A point mutation in a glutamate‐gated chloride channel confers abamectin resistance in the two‐spotted spider mite, Tetranychus urticae Koch. Insect Molecular Biology, 19, 583–591. [DOI] [PubMed] [Google Scholar]
- Laing, S.T. , Ivens, A. , Laing, R. , Ravikumar, S. , Woods, D.J. , Gilleard, J.S . et al (2010) Characterization of the xenobiotic response of Caenorhabditis elegans to the anthelmintic drug albendazole and the identification of novel drug glucoside metabolites. Biochemical Journal, 432, 505–514. [DOI] [PubMed] [Google Scholar]
- Leeuwen, T.V. , Vontas, J. , Tsagkarakou, A. , Dermauw, W. and Tirry, L. (2010) Acaricide resistance mechanisms in the two‐spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochemistry and Molecular Biology, 40, 563–572. [DOI] [PubMed] [Google Scholar]
- Li, X.X. , Zhu, B. , Gao, X.W. and Liang, P. (2017) Over‐expression of UDP‐glycosyltransferase gene UGT2B17 is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Pest Management Science, 73, 1402–1409. [DOI] [PubMed] [Google Scholar]
- Liang, P. , Gao, X.W. , Zheng, B.Z. and Dai, H. (2001) Study on resistance mechanism and cross‐resistance of abamectin in diamondback moth Plutella xylostella (L.). Chinese Journal of Pesticide Science, 3, 41–45. [Google Scholar]
- Liang, P. , Gao, X.W. and Zheng, B.Z. (2003) Genetic basis of resistance and studies on cross‐resistance in a population of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Management Science, 59, 1232–1236. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔ C T method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Luo, L. , Sun, Y.J. and Wu, Y.J. (2013) Abamectin resistance in Drosophila is related to increased expression of P‐glycoprotein via the dEGFR and dAkt pathways. Insect Biochemistry and Molecular Biology, 43, 627–634. [DOI] [PubMed] [Google Scholar]
- Mackenzie, P.I. , Bock, K.W. , Burchell, B. , Guillemette, C. , Miners, J.O. , Nebert, D.W . et al (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenetics and Genomics, 15, 677–685. [DOI] [PubMed] [Google Scholar]
- Magdalou, J. , Fournel‐Gigleux, S. and Ouzzine, M. (2010) Insights on membrane topology and structure/function of UDP‐glucuronosyltransferases. Drug Metabolism Reviews, 42, 159–166. [DOI] [PubMed] [Google Scholar]
- McCavera, S. , Walsh, T.K. and Wolstenholme, A.J. (2007) Nematode ligand‐gated chloride channels: an appraisal of their involvement in macrocyclic lactone resistance and prospects for developing molecular markers. Parasitology, 134, 1111–1121. [DOI] [PubMed] [Google Scholar]
- Nieba, L. , Honegger, A. , Krebber, C. and Plückthun A. (1997) Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improved in vivo folding and physical characterization of an engineered scFv fragment. Protein Engineering Design & Selection, 10, 435. [DOI] [PubMed] [Google Scholar]
- Pedra, J.H. , McIntyre, L.M. , Scharf, M.E. and Pittendrigh, B.R. (2004) Genome‐wide transcription profile of field‐ and laboratory‐selected dichlorodiphenyltrichloroethane (DDT)‐resistant Drosophila . Proceedings of the National Academy of Sciences USA, 101, 7034–7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putter, I. , Connell, J.G.M. , Preiser, F.A. , Haidri, A.A. , Ristich, S.S. and Dybas, R.A. (1981) Avermectins: Novel insecticides, acaricides, and nematicides from soil microorganism. Cellular and Molecular Life Sciences, 37, 963–964. [Google Scholar]
- Real, M.D. , Ferré, J. and Chapa, F.J. (1991) UDP‐glucosyltransferase activity toward exogenous substrates in Drosophila melanogaster . Analytical Biochemistry, 194, 349–352. [DOI] [PubMed] [Google Scholar]
- Riga, M. , Tsakireli, D. , Ilias, A. , van Leeuwen, T. , Paine, M. , Vontas, J . et al (2014) Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae . Insect Biochemistry and Molecular Biology, 46, 43–53. [DOI] [PubMed] [Google Scholar]
- Rozen, S. and Skaletsky, H. (2000) Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology, 132, 365–386. [DOI] [PubMed] [Google Scholar]
- Salminen, J.P. , Lahtinen, M. , Lempa, K. , Kapari, L. , Haukioja, E. and Pihlaja, K. (2015) Metabolic modifications of birch leaf phenolics by an herbivorous insect: detoxification of flavonoid aglycones via glycosylation. Zeitschrift Fur Naturforschung C‐A Journal of Biosciences, 59, 437–444. [DOI] [PubMed] [Google Scholar]
- Shi, L. , Zhang, J. , Shen, G. , Zhang, Y.C. , Xu, Q. , He, L . et al (2015) Silencing NADPH‐cytochrome P450 reductase results in reduced acaricide resistance in Tetranychus cinnabarinus (Boisduval). Scientific Reports, 5, 15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel, E. and Baur, R. (1987) Effect of avermectin B1a on chick neuronal gamma‐aminobutyrate receptor channels expressed in Xenopus oocytes. Molecular Pharmacology, 32, 749–752. [PubMed] [Google Scholar]
- Silva, A.X. , Jander, G. , Samaniego, H. , Ramsey, J.S. and Figueroa, C.C. (2012) Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) II: Costs and benefits. PLoS ONE, 7, 880–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf, N. and Nauen, R. (2001) Cross‐resistance, inheritance, and biochemistry of mitochondrial electron transport inhibitor‐acaricide resistance in Tetranychus urticae (Acari: Tetranychidae). Journal of Economic Entomology, 94, 1577–1583. [DOI] [PubMed] [Google Scholar]
- Sun, W. , Jin, Y. , He, L. , Lu, W.C. and Li, M. (2010) Suitable reference gene selection for different strains and developmental stages of the carmine spider mite, Tetranychus cinnabarinus, using quantitative real‐time PCR. Journal of Insect Science, 10, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. , Nei, M. and Kumar, S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen, T. , Vontas, J. , Tsagkarakou, A. , Dermauw, W. and Tirry, L. (2010) Acaricide resistance mechanisms in the two‐spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochemistry and Molecular Biology, 40, 563–572. [DOI] [PubMed] [Google Scholar]
- Vokřál, I. , Bártíková, H. , Prchal, L. , Lamka, J. , Várady, M. , Kubíček, V . et al (2012) The metabolism of flubendazole and the activities of selected biotransformation enzymes in Haemonchus contortus strains susceptible and resistant to anthelmintics. Parasitology, 139, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Vontas, J. , Blass, C. , Koutsos, A.C. , Hemingway, J. , Christophides, G.K. , Ranson, H. et al (2005) Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Molecular Biology, 14, 509–521. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Wang, R. , Yang, Y. , Wu, S. , O'Reilly, A.O. and Wu, Y. (2016) A point mutation in the glutamate‐gated chloride channel of Plutella xylostella is associated with resistance to abamectin. Insect Molecular Biology, 25, 116–125. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Shi, L. , Peng, J. , Shen, G. , Wei, P. , Wu, Q. and He, L. (2016) Analysis of the relationship between P‐glycoprotein and abamectin resistance in Tetranychus cinnabarinus (Boisduval). Pesticide Biochemistry and Physiology, 129, 75–82. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Liu, Y. , Feng, K. , Wang, J. , Smagghe, G.J. , He, L . et al (2017) High gama‐aminobutyric acid contents involved in abamectin resistance and predation, an interesting phenomenon in spider mites. Frontiers in Physiology, 8, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, H. , Nakamura, M. , Shimada, N. , Gillam, E.M. , Guengerich, F.P. , Shimada, T . et al (1999) Enhancement of cytochrome P‐450 3A4 catalytic activities by cytochrome b(5) in bacterial membranes. Drug Metabolism and Disposition, 27, 999. [PubMed] [Google Scholar]
- Yang, N. , Xie, W. , Yang, X. , Wang, S.L. , Wu, Q.J. , Zhang, Y.J . et al (2013) Transcriptomic and proteomic responses of sweetpotato whitefly, Bemisia tabaci, to thiamethoxam. PLoS ONE, 8, 759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Dang, Z. , Zhu, W. , Xu, X. , Liu, D. , Zhou, Z . et al (2012) Enantioselective degradation of hexaconazole in rat hepatic microsomes in vitro . Chirality, 24, 283–288. [DOI] [PubMed] [Google Scholar]
- Zheng, Y. , Hirschberg, B. , Yuan, J. , Wang, A.P. , Schmatz, D.M. , Cully, D.F . et al (2002) Identification of two novel Drosophila melanogaster histamine‐gated chloride channel subunits expressed in the eye. Journal of Biological Chemistry, 277, 2000–2005. [DOI] [PubMed] [Google Scholar]
- Zhu, K.Y. (2013) RNA interference: a powerful tool in entomological research and a novel approach for insect pest management. Insect Science, 20, 1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used for cloning, RNAi and qPCR analysis.
Table S2. Sequences used for phylogenetic analysis.
Fig. S1. The analysis of amino acid hydrophilicity/hydrophobicity of UGT201D3. Score > 0 is a hydrophobic region. Inside the red box is 15 amino acids removed.


