Abstract
Objectives
To evaluate the detection rates of clinically significant prostate cancer classified according to the prostate imaging reporting and data system scoring system using magnetic resonance imaging/ultrasound rigid fusion targeted biopsy.
Methods
A total of 339 patients underwent transperineal magnetic resonance imaging/ultrasound rigid fusion targeted biopsy in our institution between January 2015 and July 2017. Patients with prostate imaging reporting and data system category 1 or 2 and those with a pre‐biopsy prostate‐specific antigen value of >30 ng/mL were excluded from this study. Finally, 310 patients were recruited.
Results
The detection rates of clinically significant prostate cancer with prostate imaging reporting and data system category 3, 4, and 5 were 1.0% (1/98), 35.1% (47/134) and 73.1% (57/78), respectively. The factors affecting the detection of clinically significant prostate cancer with prostate imaging reporting and data system categories 4 and 5 were: (i) prostate imaging reporting and data system category 5; (ii) prostate volume <40 cc; (iii) no previous biopsy; (iv) lesion located in the peripheral zone; and (v) prostate‐specific antigen density >0.35 ng/mL/mL.
Conclusions
The detection rate of clinically significant prostate cancer on magnetic resonance imaging/ultrasound rigid fusion targeted biopsy is very low in patients with prostate imaging reporting and data system category 3; therefore, patients with this classification should not undergo targeted biopsy. Prostate‐specific antigen density, prostate volume, locations of suspected cancer and history of biopsy should be considered to predict the detection rate of clinically significant prostate cancer with prostate imaging reporting and data system categories 4 and 5.
Keywords: clinically significant prostate cancer, multiparametric magnetic resonance imaging, magnetic resonance imaging/ultrasound fusion biopsy, prostate imaging reporting and data system version 2, prostate biopsy
Abbreviations & Acronyms
- AS
active surveillance
- AUC
area under curve
- BPH
benign prostatic hyperplasia
- CI
confidence interval
- CSPC
clinically significant prostate cancer
- DRE
digital rectal examination
- GS
Gleason score
- mpMRI
multiparametric magnetic resonance imaging
- MRI
magnetic resonance imaging
- OR
odds ratio
- PCa
prostate cancer
- PI‐RADS
prostate imaging reporting and data system
- PSA
prostate‐specific antigen
- PV
prostate volume
- PZ
peripheral zone
- SB
systematic biopsy
- TB
targeted biopsy
- TZ
transitional zone
- US
ultrasound
Introduction
A systematic 10‐ to 12‐core biopsy is a standard test for patients with a high PSA level.1 However, concerns regarding overdiagnosis of indolent PCa or unnecessary biopsies have been raised.2 mpMRI and targeted biopsies have a greater diagnostic accuracy for determining the locations of CSPC2, 3 and are thus more useful in the management of patients on AS or undergoing focal therapies for localized low‐grade PCa.4
To improve the diagnostic performance of mpMRI, the European Society of Urogenital Radiology developed the PI‐RADS and released its first version in 2012. The PI‐RADS provides guidelines for evaluating imaging findings of PCa.5 A second version of the PI‐RADS was released in 2014 and was named PI‐RADS v2. The new version achieved good accuracy in detecting CSPC,6 and pathological assessment of 150 prostatectomy specimens revealed PI‐RADS v2 detected 95% of PCa foci ≥0.5 mL.7
In PI‐RADS v2, the probability of CSPC was classified as highly likely, likely and equivocal for lesions with a PI‐RADS category 5, 4 and 3, respectively. PI‐RADS category 3–5 warrant a repeat biopsy for patients with prior negative biopsies according to the American Urological Association and the Society of Abdominal Radiology.8 However, increasing evidence shows that lesions with PI‐RADS category 3 should be monitored without immediate biopsy to avoid unnecessary biopsy.9, 10, 11, 12 In combination with additional clinical parameters, such as previous biopsy history, PV and PI‐RADS score, nomograms have yielded higher CSPC detection rates.13, 14
In the present retrospective study, we analyzed the usefulness of clinical parameters to predict biopsy results based on the CSPC detection rate of real‐time MRI/US rigid fusion guided targeted biopsies at Tokyo Metropolitan Police Hospital, Tokyo, Japan. We also evaluated the necessity of biopsies in patients with PI‐RADS category 3.
Methods
Study design
The present retrospective study was approved by the institutional review board of the Tokyo Metropolitan Police Hospital (reference number 18‐A02), and the need for written informed consent was waived.
Patients
A total of 339 patients underwent transperineal MRI/US‐fusion TB at Tokyo Metropolitan Police Hospital between January 2015 and July 2017. Patients who had undergone MRI in other institutions, those with PI‐RADS category 1 or 2 and those with a pre‐biopsy PSA value >30 ng/mL were excluded (Fig. S1). Finally, 310 patients were included in the present study, and all 454 suspicious lesions categorized as lesions with PI‐RADS category ≥3 underwent transperineal MRI/US rigid fusion TB. All 310 patients underwent pre‐biopsy mpMRI of the prostate. Subsequently, MRI/US rigid fusion TB was recommended for patients with suspicious lesions.
Multiparametric MRI and biopsy method
mpMRI was carried out using a 3T scanner (Achieva 3.0T TX; Philips Medical Systems, Best, the Netherlands) to obtain T2‐weighted fast spin‐echo images in transverse, sagittal and coronal planes as well as diffusion‐weighted and dynamic‐contrast enhanced images. Two experienced radiologists (MO and NK) interpreted the images and scored the suspected lesions according to PI‐RADS v2.15 The highest score of the suspicious lesions was used as the patient's PI‐RADS v2 score.
The MRI parameters have been reported previously.16 T2‐weighted 3‐D/sagittal images (70 slices at 1‐mm thick per slice) were reconstructed for MRI/US rigid fusion TB. A commercially available real‐time virtual sonography system (Hitachi Medical Corporation, Tokyo, Japan) was used for the present study. A linear transrectal probe (HI VISION, Ascendus; Hitachi, Tokyo, Japan) and magnetic position sensors (3D Guidance Trakstar; Ascension, Shelburne, VT, USA) were used to obtain at least two cores from the targeted lesion. Transperineal biopsies were carried out with the patient under general or spinal anesthesia.
Clinically significant prostate cancer and pathology
Clinically insignificant PCa was defined as a GS of ≤3 + 4 and <50% cancer involvement in any one core.17, 18 Meanwhile, CSPC was defined as other cancers. One experienced pathologist (MY) diagnosed Gleason's score. Lymphocytic invasions were defined as >100 lymphocytes in one field of view at ×400 magnification.
Statistical analysis
Clinical parameters for the PV measured by transrectal US, DRE findings, patients’ age and serum PSA were collected from the medical records. The Mann–Whitney U‐test and Pearson's χ2‐test were used to compare continuous and categorical variables, respectively, in the univariate analyses for the predictive factors of CSPC detection rate among patients with PI‐RADS categories 4 and 5. The correlation between continuous values was analyzed using scatter plot and Spearman's coefficient values. Multivariate analyses were carried out using the logistic regression model. All statistical analyses were carried out using jmp 12.2.0 (SAS Institute Inc., Cary, NC, USA), and a P < 0.05 was considered statistically significant.
Scoring system
We assigned points to the independent predictors for CSPC detection in TB for patients with PI‐RADS categories 4 and 5. Additionally, we calculated the CSPC detection rate according to the total points and evaluated prediction accuracy.
Results
The characteristics of the 310 patients are shown in Table 1 according to the standards of reporting for MRI‐targeted biopsy studies criteria.19 The median age was 68.2 years (range 48–89 years), and the median PSA level was 8.6 ng/mL (range 1.65–27.6 ng/mL). The mean number of targeted lesions per patient was 1.5 (range 1–5). A total of 57 patients underwent previous biopsy. Of them, 55 had negative biopsy findings, and two patients had a GS of 3 + 3 and were on AS. CSPC was detected in 105 of the 310 patients (33.9%) from TB, and 136 patients (43.9%) from a combination of TB and SB. The detection rates of CSPC with PI‐RADS category 3, 4 and 5 were 1.0% (1/98), 35.1% (47/134) and 73.1% (57/78) from TB, and 3.1% (3/98), 52.2% (70/134) and 80.8% (63/78) from a combination of TB and SB, respectively. The GSs are listed in Table S1. The agreement of the highest GS detected between TB and SB is shown in Table S2.
Table 1.
Clinical and histological characteristics of patients enrolled in the study
| Patients (n) | 310 |
| Age, years (range) | 68.2 (48–89) |
| Pre‐biopsy PSA, ng/mL (range) | 8.6 (1.65–27.6) |
| Prostate volume, mL (range) | 42.8 (11–113) |
| Positive digital rectal examination result (%) | 64 (20.9) |
| Mean no. targeted lesions per prostate, n (range) | 1.5 (1–5) |
| Targeted cores per prostate, n (range) | 4.0 (2–10) |
| Systematic cores per prostate, n (range) | 15.2 (10–30) |
| Patients without prior biopsy (n) | 253 |
| Patients with negative cancer findings on prior biopsy (n) | 55 |
| Patients under active surveillance (n) | 2 |
| Previous prostate‐related treatment (n) | 0 |
| Gleason score (n) | |
| 6 (3 + 3) | 42 |
| 7 (3 + 4) | 28 |
| 7 (4 + 3) | 11 |
| 8 (4 + 4) | 50 |
| ≥9 (4 + 5, 5 + 4, or 5 + 5) | 16 |
| Clinically significant prostate cancer, n (%) | 105 (33.9) |
Continuous variables were reported as the mean (range).
The characteristics of the patients with PI‐RADS categories 4 and 5 are listed in Table S3. Patients with CSPC showed a statistically higher PSA density, smaller PV and had lesions localized in PZ compared with patients without CSPC. Table 2 and Table S4 show the results of univariate and multivariate analyses of the predictive factors for CSPC with PI‐RADS categories 4 and 5 from TB, and the combination of TB and SB. The scatter plot in Figure S2 presents the existing correlation between PSA level and PV for PI‐RADS categories 4 and 5, with Spearman's P = 0.0138. PSA density and PV were used as parameters to predict CSPC detection. The independent predictive factors for the detection of CSPC were: (i) PI‐RADS category 5; (ii) PV <40 mL; (iii) no history of prostate biopsy; (iv) lesion located in the PZ or both TZ and PZ; and (v) PSA density of >0.35 ng/mL/mL. The positive rates of DRE in PI‐RADS categories 4 and 5 were 37.2% (35/94) and 17.3% (19/110) in PZ and TZ, respectively. Table 3 shows the score for each clinical parameter for patients with PI‐RADS categories 4 and 5. The Spearman's coefficient values between PSA value and PV was 0.029 for patients classified as PI‐RADS categories 4 and 5. The total score ranged from 0 to 6. The AUC was 0.850 and 0.827 between the total score and CSPC detection from TB, and the combination of SB and TB, respectively. Table 4 shows the CSPC detection rates according to the total score. The CSPC detection rates were higher among patients with high scores. Patients were categorized into three risk groups based on the total score. Patients with a total score of 4–6 were assigned to the high CSPC detection risk group, and the CSPC detection rate was 91% (60/66; Fig. 1). Patients with a total score of 2–3 and 0–1 were assigned to the intermediate‐ and low‐risk groups, respectively. The CSPC detection rates among patients with intermediate and low risk were 41.0% (43/105) and 4.9% (2/41), respectively. Histological examinations showed lymphocytic invasion in 15 of 41 (36.6%) intermediate‐risk patients, and in 10 of 29 (34.5%) low‐risk patients.
Table 2.
Univariate and multivariate analysis of predictive factors for detecting clinically significant prostate cancer in patients with PI‐RADS categories 4 and 5 from TB
| Factors | n | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | ||
| PI‐RADS category 5 (yes or no) | 78 | 4.86 | 2.64–8.97 | <0.0001a | 7.22 | 3.11–18.2 | <0.0001a |
| PV <40 mL (yes vs no) | 130 | 4.38 | 2.41–7.98 | <0.0001a | 4.14 | 1.86–9.79 | 0.0004a |
| PV <30 mL (yes vs no) | 71 | 2.80 | 1.55–5.08 | 0.0006a | – | – | – |
| No history of biopsy (yes vs no) | 183 | 6.09 | 1.35–27.5 | 0.0090a | 4.39 | 1.33–17.0 | 0.0139a |
| Lesion in PZ or in both TZ and PZ (yes or no) | 102 | 5.60 | 3.11–10.1 | <0.0001a | 4.68 | 2.27–10.0 | <0.0001a |
| Age >65 years (yes or no) | 143 | 1.56 | 0.88–2.79 | 0.1293 | – | – | – |
| Age >70 years (yes or no) | 97 | 2.06 | 1.19–3.57 | 0.0094a | 1.54 | 0.74–3.19 | 0.2482 |
| Age >75 years (yes or no) | 51 | 1.87 | 0.98–3.55 | 0.0545 | – | – | – |
| PSA density >0.35 ng/mL/mL (yes or no) | 173 | 5.18 | 2.25–11.9 | <0.0001a | 4.08 | 1.58–11.5 | 0.0033a |
| PSA density >0.25 ng/mL/mL (yes or no) | 138 | 2.87 | 1.59–5.17 | 0.0004a | – | – | – |
| PSA density >0.15 ng/mL/mL (yes or no) | 54 | 1.78 | 0.95–3.35 | 0.0701 | – | – | – |
| DRE (yes or no) | 57 | 4.06 | 2.07–7.96 | <0.0001a | 1.38 | 0.56–3.39 | 0.4828 |
| Family history (yes or no) | 12 | 1.49 | 0.46–4.84 | 0.5081 | – | – | – |
Statistically significant.
Table 3.
Scores of each predictive factor for detecting CSPC among the patients with PI‐RADS categories 4 and 5
| Factor | n | Score |
|---|---|---|
| PI‐RADS category | ||
| 5 | 78 | 2 |
| 4 | 134 | 0 |
| PSA density (ng/mL/mL) | ||
| >0.35 | 39 | 1 |
| ≤0.35 | 173 | 0 |
| PV (mL) | ||
| <40 | 130 | 1 |
| ≥40 | 82 | 0 |
| Lesions in PZ or in both PZ and TZ | 102 | 1 |
| Lesions in TZ | 110 | 0 |
| No history of biopsy | 183 | 1 |
| Repeat biopsy | 29 | 0 |
Table 4.
Detection rate of clinically significant prostate cancer through TB according to the total score among patients with PI‐RADS categories 4 and 5
| Total score | CSPC detection risk | CSPC detection rate (%) | 95% CI | Sensitivity (%) | Specificity | PCa detection rate (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| 6 | High | 100 (11/11) | 74.1–100 | 10.6 (11/104) | 100 (108/108) | 100 (11/11) | 74.1–100 |
| 5 | High | 90.9 (20/22) | 72.2–97.5 | 29.8 (31/104) | 98.1 (106/108) | 95.5 (21/22) | 78.2–99.2 |
| 4 | High | 87.9 (29/33) | 72.7–95.2 | 57.7 (60/104) | 94.4 (102/108) | 97.0 (32/33) | 84.7–99.5 |
| 3 | Intermediate | 47.5 (28/61) | 35.5–59.8 | 84.6 (88/104) | 63.9 (69/108) | 60.7 (37/61) | 48.1–71.9 |
| 2 | Intermediate | 31.8 (14/44) | 20.0–46.6 | 98.1 (102/104) | 36.1 (39/108) | 61.4 (27/44) | 46.6–74.3 |
| 1 | Low | 6.4 (2/31) | 1.8–20.7 | 100 (104/104) | 9.3 (10/108) | 29.0 (9/31) | 16.1–46.6 |
| 0 | Low | 0 (0/10) | 0–27.8 | 100 (104/104) | 0.0 (0/108) | 30.0 (3/10) | 10.8–60.3 |
Figure 1.
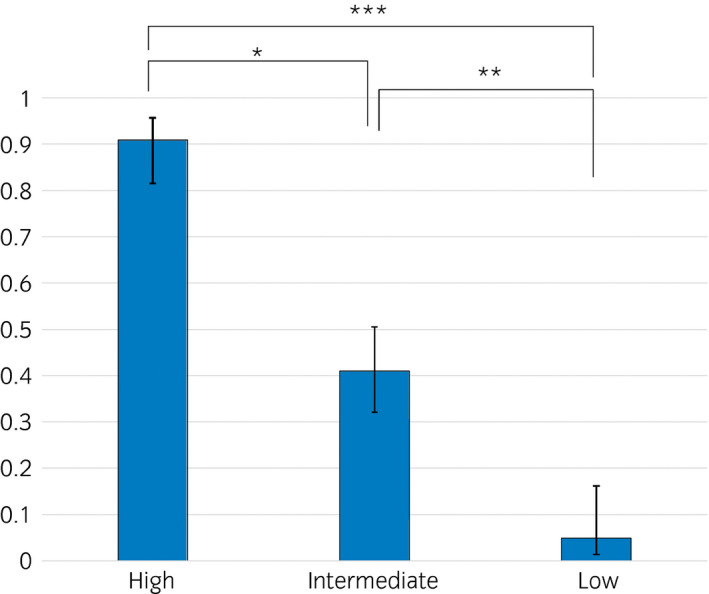
Detection rates of clinically significant prostate cancer according to the CSPC risk stratification for patients with PI‐RADS categories 4 and 5. *Statistically significant with a P < 0.0001. **Statistically significant with a P < 0.0001. ***Statistically significant with a P < 0.0001.
Discussion
The PI‐RADS v2 score and fusion biopsy have improved the diagnostic accuracy for CSPC in prostate biopsy. Recent meta‐analyses showed high sensitivity and moderate specificity for PI‐RADS v2.20, 21 Furthermore, this scoring system achieved good‐to‐moderate interobserver agreement among experienced radiologists.22 In the current study, the CSPC detection rates were greater for lesions with higher PI‐RADS v2 score, indicating that this imaging interpreting system is useful in predicting CSPC.
However, whether biopsies should be carried out for lesions with PI‐RADS category 3 remains unclear. Ullrich et al. reported that a GS of ≥4 + 3 = 7 was detected in three of 118 (2.5%) patients with PI‐RADS category 3, and these patients should undergo follow‐up MRI.9 Liddel et al. reported that two of 86 (2.3%) patients with a GS of 3 + 4 had a PI‐RADS score of 3.10 In our study, the CSPC detection rate for PI‐RADS v2 category 3 was only 1.0% (97/98), and 99% (97/98) of these patients underwent unnecessary TB according to our CSPC definition.
Additionally, we evaluated the CSPC detection rate of PI‐RADS categories 4 and 5. We found that PI‐RADS achieved moderate specificity in detecting CSPC, even though there is still the possibility of detecting no cancer or insignificant PCa in lesions with PI‐RADS categories 4 and 5.20 These patients with no CSPC had previous biopsy, low PSA density and high PV, with the suspicious lesions located in TZs. Multivariate analysis revealed the following four independent predictive factors for CSPC with PI‐RADS categories 4 and 5: (i) PI‐RADS category 5; (ii) PV <40 mL; (iii) no previous history of prostate biopsy; (iv) lesion located in the PZ or both TZ and PZ; and (v) PSA density of >0.35 ng/mL/mL. There was a statistically significant correlation between the location of the suspected malignant lesions and the findings on DRE (P = 0.0015), so the multivariate analysis might not determine the findings of DRE as independent predictors. The detection rate for CSPC in patients with PI‐RADS categories 4 and 5 was categorized according to these predictive factors. The CSPC detection rate of the patients in the low‐risk group was just 4.9% (2/41). In all patients with lesions of PI‐RADS category 4 or 5 lesions on MRI, there was a statistically significant difference between detection rates of CSPC in each risk group.
Several nomograms were reported using PI‐RADS to predict CSPC detection with a high AUC of 0.825–0.897.13, 14 The AUC of our scoring system is acceptable, and we simplified our scoring system to make it more user‐friendly in predicting the risk for CSPC detection compared with these nomograms.
The present results showed that the CSPC detection rate in TZ lesions was lower than that in PZ lesions among patients with PI‐RADS category 4. The detection rate of CSPC in TZ lesions is reportedly lower than that of PZ lesions with PI‐RADS category 3.9, 10 Conversely, Venderink et al. reported that the cancer detection rate with MRI‐guided biopsy is higher in TZ lesions than in PZ lesions23 therefore, the difference in TB approach might be related to the location of the cancer.
As low PV was found to be a predictive factor for CSPC, we hypothesized that false positive findings of BPH might be found on MRI. Unlike nodular BPH, it is often difficult to differentiate PCa from stromal BPH. The stromal BPH might be interpreted as PCa, because imaging enhancement characteristics of stromal BPH overlaps with those of TZ PCa.24 Aside from stromal BPH, prostatitis also often mimics PCa on MRI. Histopathological analysis showed that lymphocytic invasions were found in the low and intermediate CSPC risk group. These findings could result from prostatitis.
In the present study, the cut‐off value of PSA density was relatively higher than that reported elsewhere.11, 23 This study focused on the risk stratification for PI‐RADS categories 4 and 5, whereas previous reports examined risk stratifications for PI‐RADS category 3. We confirmed that the mean PSA density was higher for patients with a higher PI‐RADS v2 score, and it might result in higher PSA density cut‐off.
Furthermore, no previous history of prostate biopsy was a predictive factor of CSPC with PI‐RADS categories 4 and 5 in the present study. Patients who undergo repeat biopsy are reportedly more likely to have negative results compared with biopsy‐naïve patients.25 The present study showed that repeat biopsy carries a risk of false‐positive findings in the patients with PI‐RADS categories 4 and 5.
The present study had several limitations. First, it was a retrospective analysis of patients who underwent biopsies, and selection bias in the process of determining who should receive biopsies could not be excluded. In our institution, biopsies are generally recommended for patients with suspicious lesions detected on MRI. Doctors consider other clinical parameters, such as PSA density or repeat biopsy history, to determine whether a biopsy is necessary. Second, collected data were limited; it is important to collect additional histological data from patients who had undergone radical prostatectomy to analyze the cause of the false positive reading in PI‐RADS categories 4 and 5. Stromal BPH or prostatitis might be among the causes of the false positive findings. This might be confirmed by pathological evaluation for radical prostatectomy. Third, the present study included only TB results, and SB results were not mainly considered. Some prospective studies showed a higher CSPC detection rate for TB than SB.26, 27 In contrast, Baco et al. reported that a 12‐core random biopsy achieved higher detection rates than MRI/US‐guided TB.28 The PI‐RADS v2 score was reported to detect just 24.3% tumors <0.5 mL.7 These small tumors can be detected on SB. Our risk stratification was based on PI‐RADS v2 criteria, and cannot be used as the basis for whether patients should receive SB. Fourth, we applied the rigid fusion method, which compared with elastic fusion biopsy, this biopsy method does not compensate for deformation of prostates and might lead to maltargeting.29 Westhoff et al. reported that patients with large PV might benefit from elastic fusion biopsy, so the elastic fusion technique might detect more CSPC in patients with a PV >40 mL.30
In conclusion, the CSPC detection rate of transperineal MRI/US rigid fusion TB was very low in patients with PI‐RADS category 3. Therefore, immediately carrying out TB is probably unnecessary for these patients. Furthermore, the PI‐RADS categories 4 and 5 included patients with both a high and low risk for CSPC. Immediate target biopsy in low‐risk patients with PI‐RADS category 4 can be postponed. PSA density, PV, locations of the suspected cancer and history of biopsy should be considered when predicting the detection rate of CSPC in patients with lesions of PI‐RADS categories 4 and 5.
Conflict of interest
None declared.
Supporting information
Figure S1. Patient recruitment flow chart.
Figure S2. Scatter plot of the correlation between PSA level and prostate volume for PI‐RADS categories 4 and 5.
Table S1. Pathology and detection rate of prostate cancer and clinically significant prostate cancer.
Table S2. Agreement in the Gleason scores for the targeted and systematic biopsies.
Table S3. Characteristics of patients with PI‐RADS categories 4 and 5.
Table S4. Univariate and multivariate analysis of predictive factors for detecting clinically significant prostate cancer in patients with PI‐RADS categories 4 and 5 from combination of targeted biopsy and systematic biopsy.
References
- 1. Bjurlin MA, Carter HB, Schellhammer P et al Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J. Urol. 2013; 189: 2039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siddiqui MM, Rais‐Bahrami S, Truong H et al Magnetic resonance imaging/ultrasound‐fusion biopsy significantly upgrades prostate cancer versus systematic 12‐core transrectal ultrasound biopsy. Eur. Urol. 2013; 64: 713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur. Urol. 2006; 50: 1163–74. [DOI] [PubMed] [Google Scholar]
- 4. Thomsen FB, Brasso K, Klotz LH, Roder MA, Berg KD, Iversen P. Active surveillance for clinically localized prostate cancer–a systematic review. J. Surg. Oncol. 2014; 109: 830–5. [DOI] [PubMed] [Google Scholar]
- 5. Barentsz JO, Richenberg J, Clements R et al ESUR prostate MR guidelines 2012. Eur. Radiol. 2012; 22: 746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao C, Gao G, Fang D et al The efficiency of multiparametric magnetic resonance imaging (mpMRI) using PI‐RADS Version 2 in the diagnosis of clinically significant prostate cancer. Clin. Imaging 2016; 40: 885–8. [DOI] [PubMed] [Google Scholar]
- 7. Vargas HA, Hotker AM, Goldman DA et al Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole‐mount pathology as standard of reference. Eur. Radiol. 2016; 26: 1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenkrantz AB, Verma S, Choyke P et al Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J. Urol. 2016; 196: 1613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ullrich T, Quentin M, Arsov C et al Risk stratification of equivocal lesions on multiparametric magnetic resonance imaging of the prostate. J. Urol. 2018; 199: 691–8. [DOI] [PubMed] [Google Scholar]
- 10. Liddell H, Jyoti R, Haxhimolla HZ. mp‐MRI prostate characterised PIRADS 3 lesions are associated with a low risk of clinically significant prostate cancer – a retrospective review of 92 biopsied PIRADS 3 lesions. Curr. Urol. 2015; 8: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Washino S, Okochi T, Saito K et al Combination of prostate imaging reporting and data system (PI‐RADS) score and prostate‐specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naive patients. BJU Int. 2017; 119: 225–33. [DOI] [PubMed] [Google Scholar]
- 12. Felker ER, Raman SS, Margolis DJ et al Risk stratification among men with prostate imaging reporting and data system version 2 category 3 transition zone lesions: is biopsy always necessary? AJR Am. J. Roentgenol. 2017; 209: 1272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Leeuwen PJ, Hayen A, Thompson JE et al A multiparametric magnetic resonance imaging‐based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int. 2017; 120: 774–81. [DOI] [PubMed] [Google Scholar]
- 14. Truong M, Wang B, Gordetsky JB et al Multi‐institutional nomogram predicting benign prostate pathology on magnetic resonance/ultrasound fusion biopsy in men with a prior negative 12‐core systematic biopsy. Cancer 2017; 124: 278–85. [DOI] [PubMed] [Google Scholar]
- 15. Barentsz JO, Weinreb JC, Verma S et al Synopsis of the PI‐RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur. Urol. 2016; 69: 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hakozaki Y, Matsushima H, Kumagai J et al A prospective study of magnetic resonance imaging and ultrasonography (MRI/US)‐fusion targeted biopsy and concurrent systematic transperineal biopsy with the average of 18‐cores to detect clinically significant prostate cancer. BMC Urol. 2017; 17: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed HU, El‐Shater Bosaily A, Brown LC et al Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22. [DOI] [PubMed] [Google Scholar]
- 18. van As NJ, Norman AR, Thomas K et al Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur. Urol. 2008; 54: 1297–305. [DOI] [PubMed] [Google Scholar]
- 19. Moore CM, Kasivisvanathan V, Eggener S et al Standards of reporting for MRI‐targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur. Urol. 2013; 64: 544–52. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Tang M, Chen S, Lei X, Zhang X, Huan Y. A meta‐analysis of use of Prostate Imaging Reporting and Data System Version 2 (PI‐RADS V2) with multiparametric MR imaging for the detection of prostate cancer. Eur. Radiol. 2017; 27: 5204–14. [DOI] [PubMed] [Google Scholar]
- 21. Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI‐RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta‐analysis. Eur. Urol. 2015; 67: 1112–21. [DOI] [PubMed] [Google Scholar]
- 22. Schimmoller L, Quentin M, Arsov C et al Inter‐reader agreement of the ESUR score for prostate MRI using in‐bore MRI‐guided biopsies as the reference standard. Eur. Radiol. 2013; 23: 3185–90. [DOI] [PubMed] [Google Scholar]
- 23. Venderink W, van Luijtelaar A, Bomers JG et al Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur. Urol. 2018; 73: 353–60. [DOI] [PubMed] [Google Scholar]
- 24. Quon JS, Moosavi B, Khanna M, Flood TA, Lim CS, Schieda N. False positive and false negative diagnoses of prostate cancer at multi‐parametric prostate MRI in active surveillance. Insights Imaging 2015; 6: 449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Djavan B, Ravery V, Zlotta A et al Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J. Urol. 2001; 166: 1679–83. [PubMed] [Google Scholar]
- 26. Pokorny MR, de Rooij M, Duncan E et al Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound‐guided biopsy versus magnetic resonance (MR) imaging with subsequent MR‐guided biopsy in men without previous prostate biopsies. Eur. Urol. 2014; 66: 22–9. [DOI] [PubMed] [Google Scholar]
- 27. Panebianco V, Barchetti F, Sciarra A et al Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol. Oncol. 2015; 33: 17.e1–7. [DOI] [PubMed] [Google Scholar]
- 28. Baco E, Rud E, Eri LM et al A Randomized controlled trial to assess and compare the outcomes of two‐core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12‐core systematic biopsy. Eur. Urol. 2016; 69: 149–56. [DOI] [PubMed] [Google Scholar]
- 29. Ukimura O, Desai MM, Palmer S et al 3‐Dimensional elastic registration system of prostate biopsy location by real‐time 3‐dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J. Urol. 2012; 187: 1080–6. [DOI] [PubMed] [Google Scholar]
- 30. Westhoff N, Siegel FP, Hausmann D et al Precision of MRI/ultrasound‐fusion biopsy in prostate cancer diagnosis: an ex vivo comparison of alternative biopsy techniques on prostate phantoms. World J. Urol. 2017; 35: 1015–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient recruitment flow chart.
Figure S2. Scatter plot of the correlation between PSA level and prostate volume for PI‐RADS categories 4 and 5.
Table S1. Pathology and detection rate of prostate cancer and clinically significant prostate cancer.
Table S2. Agreement in the Gleason scores for the targeted and systematic biopsies.
Table S3. Characteristics of patients with PI‐RADS categories 4 and 5.
Table S4. Univariate and multivariate analysis of predictive factors for detecting clinically significant prostate cancer in patients with PI‐RADS categories 4 and 5 from combination of targeted biopsy and systematic biopsy.


