Abstract
Parenteral iron is used to restore the body's iron pool before and during erythropoiesis‐stimulating agent (ESA) therapy; together these agents form the backbone of anemia management in end‐stage renal disease (ESRD) patients undergoing hemodialysis. ESRD patients receiving chronic intravenous iron products, which exceed their blood loss are exposed to an increased risk of positive iron balance. Measurement of the liver iron concentration (LIC) reflects total body iron stores in patients with secondary hemosiderosis and genetic hemochromatosis. Recent studies of LIC in hemodialysis patients, measured by quantitative MRI and magnetic susceptometry, have demonstrated a high risk of iron overload in dialysis patients treated with IV iron products at doses advocated by current anemia management guidelines for dialysis patients. Liver iron overload causes increased production of hepcidin and elevated plasma levels, which can activate macrophages of atherosclerotic plaques. This mechanism may explain the results of 3 long‐term epidemiological studies which showed the association of excessive IV iron doses with increased risk of cardiovascular morbidity and mortality among hemodialysis patients. A more physiological approach of iron therapy in ESRD is needed. Peritoneal dialysis patients, hemodialysis patients infected with hepatitis C virus, and hemodialysis patients with ferritin above 1000 μg/L without a concomitant inflammatory state, all require specific and cautious iron management. Two recent studies have shown that most hemodialysis patients will benefit from lower maintenance IV iron dosages; their results are applicable to American hemodialysis patients. Novel pharmacometric and economic approaches to iron therapy and anemia management are emerging which are designed to lessen the potential side effects of excessive IV iron while maintaining hemoglobin stability without an increase in ESA dosing.
1. INTRODUCTION AND GENERAL REMARKS
Partial correction of anemia with routine use of recombinant erythropoiesis‐stimulating agents (ESA) over the past three decades has deeply improved quality of life in most end‐stage renal disease (ESRD) patients, reducing the need for blood transfusions and anemia‐related morbidities.1, 2, 3 ESA use frequently leads to functional iron deficiency (termed iron‐restricted anemia), due to massive transfer of stored iron to erythroid progenitor cells and inadequate iron mobilization from repleted stores (related to high levels of hepcidin), together with true iron deficiency due to blood loss related to the hemodialysis procedure, uremic enteropathy and blood sampling.1, 2, 3 True iron deficiency is thus a major clinical concern, giving rise to ESA resistance, impaired cellular function and aggravated cardiac insufficiency which is frequently encountered in this setting.4, 5
Intravenous (IV) iron is typically administered during dialysis sessions,6, 7 with almost all ESA‐treated hemodialysis patients receiving parenteral iron to restore the body iron pool before and during ESA therapy. Iron therapy is considered, with ESA, as the mainstay of anemia treatment in ESRD.2, 3 Iron metabolism is a closed system, critically regulated by hepcidin without any excretory mechanisms for excess iron. As a result, iron levels in ESRD patients chronically treated with IV iron products may exceed their ongoing blood losses, exposing them to an increased risk of positive iron balance.3, 8, 9 These twin risks of iron deficiency and iron overload must be closely monitored.3 Iron overload was considered exceptional in the ESA era, but is now recognized as a potential clinical issue.3 In the last two decades, the use of IV iron products in ESRD patients has risen dramatically in the US and Europe, with a parallel increase in ferritin levels.10, 11
The liver is the main iron storage site, and liver iron concentration (LIC) gives an accurate picture of total body iron stores in patients with secondary hemosiderosis (thalassemia major, sickle cell disease) and genetic hemochromatosis.3, 9 In the last decade, major progress has been made in noninvasive LIC measurements using radiological techniques to replace liver biopsies for diagnosing and monitoring iron overload disorders.12, 13 Methods include application of superconducting quantum interference devices (SQUID), quantitative computed tomography and magnetic resonance imaging (MRI).14 MRI is now the preferred technique, due to its reproducibility, sensitivity, availability and the ability to scan multiple organs (liver, spleen, heart, pancreas) in the same session.13 There are 3 MRI modalities for liver iron assays: the signal‐intensity ratio with the Rennes algorithm, R2 relaxometry, and R2* relaxometry, published in 2004 to 2005 and rigorously validated in cohorts of patients with secondary hemosiderosis, genetic hemochromatosis and hepatic disorders requiring liver biopsy for biochemical iron assay.15, 16, 17 A recent pilot study in hemodialysis patients showed, using liver histology (Perls staining), that determination of liver iron based on signal‐intensity ratio MRI with the Rennes algorithm, accurately identifies iron load.18
Recent LIC studies using quantitative MRI in hemodialysis patients, as well as the overlooked SQUID study performed 13 years ago, have demonstrated a high risk of iron overload in dialysis patients treated with IV iron products at doses advocated by current anemia management guidelines for dialysis patients.19, 20, 21, 22 Moreover, liver iron overload resulted in increased production and elevation of plasma hepcidin levels.21, 22 By activating macrophages, elevated hepcidin can cause atherosclerotic plaque instability and increase the risk of ischemic cardiovascular complications.23 High hepcidin‐25 levels were recently linked to cardiovascular events in dialysis patients,24 pointing to its role as a mediator of cardiovascular morbidity encountered in patients with iatrogenic iron overload.3, 23, 24 This detrimental effect induced by liver iron overload may explain the results of the 3 long‐term epidemiological studies (including the DOPPS study of 32 435 hemodialysis patients followed for a median of 1.7 years in 12 industrialized countries) demonstrating the association of excessive IV iron doses with increased risk of cardiovascular morbidity and mortality among hemodialysis patients.25, 26, 27
Taking into account the lack of availability of MRI in many countries and the costs for ESRD patients in others (eg, USA, Israel), data from published studies with these new research tools were extrapolated in this article into current practices (eg, mg of IV iron infused per month and levels of iron biomarkers for follow‐up such as ferritin and transferrin saturation); my goal is to help physicians to manage anemia and protect patients from potential harm while minimizing the cost of anemia treatment. A number of specific scenarios are addressed.
2. IV IRON PRODUCTS AS SECOND‐LINE TREATMENT IN PERITONEAL DIALYSIS PATIENTS
Iron metabolism differs markedly between patients undergoing peritoneal dialysis (PD) and hemodialysis (HD). Peritoneal dialysis has fewer sources of iron deficiency, including blood loss directly related to the hemodialysis technique and occult gastrointestinal tract bleeding aggravated by anticoagulation of hemodialysis circuits.3, 6 This lower need for iron store replenishment explains the more conservative strategy advocated for PD patients in current guidelines, with a ferritin target of >100 μg/L and oral iron for first‐line therapy.28, 29, 30, 31 Moreover, IV iron is not recommended by the KDIGO guideline and ERBP statement as an ESA‐sparing agent for peritoneal dialysis patients, contrary to HD patients, but is reserved for peritoneal dialysis patients who do not tolerate or who respond poorly to oral iron, or who have high iron requirements.30, 31, 32
Two recent French publications shed light on this. One presents data from the French Registry of Peritoneal Dialysis (RDPLF) on anemia management,33 reporting 568 PD patients between 2010 and 2017. Mean patient age was 71 years and mean peritoneal dialysis duration was 13 months; 42% of patients were women and 40% had diabetes mellitus. Overall, 72% of patients were treated with ESA, 23% received oral iron products and only 11% were treated with IV iron. Mean hemoglobin was close to 12 g/dL, and median CRP was ~5 mg/L. Ferritin levels increased to 270 μg/L in 2013 and stabilized at 200 μg/L in 2017. Transferrin saturation was between 23% and 25% from 2010 to 2017. In line with these epidemiological data, a recent prospective observational study measured LIC using signal‐intensity ratio MRI (with Rennes algorithm), in a cohort of 32 peritoneal dialysis patients in the greater Paris area. In contrast to hemodialysis patients, LIC was normal in most peritoneal dialysis patients, with few patients having liver iron overload, which was mostly mild.34
Taking into consideration the efficacy and safety of the strategy of anemia management advocated by current guidelines, IV iron products should remain as second‐line treatment in peritoneal dialysis.
3. IRON PRODUCTS SHOULD BE USED WITH CAUTION IN DIALYSIS HEPATITIS C PATIENTS
John Daugirdas recently stated “Another potential risk of loading the liver with iron has to do with the course of chronic hepatitis. Both iron‐loading and chronic viral infection increase the risk of liver fibrosis”.35 Overall, 80% of hepatitis C (HCV) viremic patients progress to chronic hepatitis, 20% of whom will develop cirrhosis; HCV infection is currently one of the most common causes of liver transplantation in developed countries.36 According to the DOPPS study, the prevalence of HCV infection among dialysis patients between 2012 and 2015 in North America, Europe and Japan was 8.7%, and nosocomial HCV spread in hemodialysis facilities was still occurring.37 HCV‐infected dialysis patients have an increased risk of mortality from liver disease (HR 4.4; 95% CI: 3.14‐6.15).37
Increased hepatic tissue iron plays a deleterious role in the course of HCV evolution, favoring development of fibrosis, cirrhosis and cancer.38 Mild or moderate liver iron deposits are common in liver biopsies of patients with chronic HCV and are associated with the severity of liver disease (higher histological inflammatory activity score). These iron deposits were shown to decrease significantly with interferon.39 The reduction of excess liver and body iron stores by phlebotomy also potentiated the effects of interferon (amelioration of histologic lesions) and improved the course of HCV in patients who could not receive interferon.40, 41, 42 Similarly, elevated liver tissue iron (mild or moderate) is encountered in about 40%‐50% of patients with chronic hepatitis B infection (HBV) and with nonalcoholic steatohepatitis (NASH) and has also been linked to the severity of these 2 latter liver diseases (higher activity scores and fibrosis).43, 44 In gerbils, iron induced liver fibrosis by increasing TGF‐β expression and collagen,45 and when applied (as FeSO4) to cultured human hepatocytes (non‐neoplastic PH5CH8 cells) it enhanced HCV replication about ten‐fold.46
Both HCV and HBV have been recognized as powerful liver carcinogens, causing about 75% of all cases of hepatocellular carcinoma (HCC).47 In HCV infections, HCC develops almost exclusively in livers with cirrhosis or established fibrosis. Dual HBV and HCV infections in cirrhotic patients dramatically increase the risk of HCC with an odds ratio of 165 vs 17 and 23 for HCV and HBV infection alone respectively.47 The relative risk of HCC in patients with genetic hemochromatosis and cirrhosis is thought to be up to a 20‐fold increase.48 Patients with thalassemia and those with African iron overload are also at a 10‐fold greater risk of HCC.48 Besides iron overload disorders, other nonviral factors have been linked to HCC, namely alcohol, tobacco, aflatoxin, schistosomiasis, diabetes and obesity, and some liver diseases.48 Transgenic C57BL/6 mice expressing HCV polyprotein and fed an iron‐rich diet had an increased liver iron content and hepatocyte proliferation and 50% developed hepatic tumors at 12 months, including HCC.49 At the molecular level, iron can induce liver carcinogenesis by promoting cyclin D1 gene expression in hepatocytes (cyclin D1 is involved in the G1 phase of the cell cycle).50 The pathophysiological role of iron in liver carcinogenesis is shown by the beneficial impact of phlebotomy on the risk of HCC in HCV infection; in 2 Japanese studies, the incidence of liver cancer in HCV‐infected patients not responsive to interferon‐ribavirin therapy was reduced four‐fold by phlebotomy after 8‐10 years of observation.51, 52
Importantly, there is a residual risk of liver cancer in HCV‐infected patients even after being cured with antiviral drugs. A recent meta‐analysis of 30 retrospective observational studies (31 528 patients followed for 2.5 to 14.4 years) showed a significant reduction in the risk of HCC in HCV‐infected patients with a sustained response to antiviral therapy (HR 0.24, 95% CI: 0.18‐0.31) but not its disappearance.53 Therefore, routine life‐long screening for HCC with twice‐yearly ultrasonography is warranted even in virologically healed HCV patients.47 There is a scarcity of data on the use of IV iron in HCV hemodialysis patients. Two studies (including 89 and 66 patients defined by positive serology associated with liver enzyme abnormalities) have suggested that parenteral iron therapy given per current guidelines can contribute to hepatocellular injury in these patients as evidenced by the increase in transaminase levels after 3 months of treatment.54, 55
Most hepatologists avoid iron therapy (oral and parenteral) in HCV‐infected patients without kidney disease with the goal of avoiding a rise in viral replication, aggravation of histological lesions, and HCC development.56 However, in ESRD patients it is impossible to withhold iron supplementation due to recurrent blood loss which invariably results in depletion of iron stores and severe iron deficiency.6, 38 Thus, iron replacement should be used cautiously with careful monitoring in HCV‐infected ESRD patients.38 It was recently proposed to use in this setting the minimalist Japanese strategy applying to iron therapy.38, 57, 58
The Japanese Society for Dialysis, out of concern for iron overload, proposed as early as 2011 that minimal IV iron (up to 650 mg in the induction phase) be given to hemodialysis patients and only in cases of true iron deficiency (ferritin < 100 μg/L), and also warned against maintenance IV iron therapy.57 This careful strategy of iron repletion allowed adequate hemoglobin levels with low ferritin, moderate transferrin saturation without any detrimental effect on survival and was again advocated this year for Japanese dialysis populations.58, 59, 60
Taking into account the lower body surface area of Japanese patients, the proposed dose of iron for repletion (600 mg) should be adapted to around 800 mg in European patients and 1000 mg in US patients.38 A recent French study designed to determine the accuracy of biomarkers of iron metabolism in dialysis patients on parenteral iron therapy may offer physicians further insight.61 It combined quantitative MRI (Rennes signal‐intensity ratio) with data‐mining and classical statistical analysis, in 212 hemodialysis patients free of overt inflammation and malnutrition (and treated for anemia per current guidelines) to identify the accurate target values for biological markers of iron metabolism. Among the biological markers, only serum ferritin showed a strong correlation with LIC and logistical analyses correctly classified patients as having normal liver iron stores (LIC ≤ 50 μmol/g) or elevated stores (LIC > 50 μmol/g).60 Serum ferritin was the iron biomarker with the best discriminatory capacity in ROC curve analysis (AUC: 0.77); the optimal serum ferritin cutoff was 162 μg/L for LIC > 50 μmol/g (mild iron overload; sensitivity 67%; specificity 77%; diagnostic accuracy 69%).61 Thus, an upper target of 150 μg/L ferritin in HCV‐ESRD patients would greatly minimize the risk of increased liver iron load with potential harmful consequences. This careful iron strategy may also apply to HBV and NASH patients who share the same risks of liver iron overload.
Conversely, this minimalist strategy is not advocated for patients with advanced liver disease where ferritin is intrinsically high and where portal hypertension leads to large blood losses related to esophageal varices and portal gastritis.
4. STOPPING IV IRON IN HEAVILY OVERLOADED HEMODIALYSIS PATIENTS (FERRITIN > 1000 μG/L AND NO INFLAMMATORY SYNDROME)
The increased use of IV iron products in ESRD in the last decade translates into an increase in ferritin levels, reaching a mean value close to 850 μg/L in American patients (DOPPS monitor April 2018) and from 400 to 600 μg/L in Europe.2, 10, 11, 62 Thus, heavily overloaded hemodialysis patients (ferritin chronically above 1000 μg/L without concomitant inflammatory syndrome) can be encountered, especially in the USA. A recent Israeli study focused on iron overload in this subset of hemodialysis patients with serum ferritin levels above 1000 μg/L.21 Relaxometry R2*‐MRI was used to measure liver and spleen iron content and pancreatic and cardiac iron deposits were evaluated in 21 hemodialysis patients, giving a median ferritin level of 2220 μg/L (range: 1220‐6820). Hepatic siderosis was present in 90% of patients and spleen involvement in 95%.
Pancreatic involvement was evaluated in the 8 most motivated patients and was present in 3 cases (37%). The presence of iron deposits in the pancreas (in a third of investigated patients) mean these patients fulfill the criteria recently proposed as radiological surrogates of iron toxicity in dialysis patients (eg, visceral iron deposits external to the liver, in the heart and/or pancreas) by a panel of specialists at an international advisory board meeting, sponsored by Vifor Fresenius Medical Care Renal Pharma, a pharmaceutical firm which manufactures several iron replacement therapies.63 Iron deposits in the pancreas predict iron cardiomyopathy in thalassemia and act as an early warning system for cardiac iron‐loading in thalassemic patients;64 moreover, pancreatic iron deposits are also predictive for diabetes mellitus in various iron overload disorders.64, 65, 66 None of the 21 dialysis patients had an abnormal cardiac R2*/T2* but the small number of patients studied makes it difficult to draw definitive conclusions about the risk of cardiac iron deposits in this high‐ferritin subset of dialysis patients.
Of note, iron‐overloaded dialysis patients have exhibited ESA hyporesponsiveness, as have patients with iron deficiency.67 Similarly patients with low‐risk myelodysplastic syndrome with iron overload have a defect in erythropoiesis related to iron adverse effects on erythroid precursors (inhibition of burst‐forming unit colony formation and erythroblast differentiation) which benefit from iron chelation.68 Finally, some of these heavily iron‐overloaded dialysis patients can paradoxically exhibit functional iron deficiency (iron‐restricted anemia) which could classically be overcome by IV iron as demonstrated by the DRIVE‐trial performed in patients with ferritin levels between 500 and 1200 μg/L.69
In light of recent knowledge, it seems wise in this latter setting to use ascorbic acid which mobilizes sequestered iron for erythropoiesis, and to stop IV iron products in all cases of ferritin above 1000 μg/L without concomitant inflammatory syndrome.67, 70 It has been shown that iron therapy withdrawal for a year with ESA continuation strongly improved iron metabolism biomarkers without any detrimental effect on erythropoiesis in this subset of iron‐overloaded hemodialysis patients.21
5. BENEFITS OF MAINTENANCE IV IRON THERAPY < 250 MG/MONTH IN HEMODIALYSIS PATIENTS, WITHOUT LOSS OF EFFICACY ON HEMOGLOBIN OR INCREASED ESA REQUIREMENT
Two recent publications highlight the possibility of drastically reducing the amount of iron infused for maintenance therapy without a detrimental effect on hemoglobin levels or ESA cost.71, 72 In light of recent literature, a team at the Nancy academic hospital in France performed a proof of concept study, changing their iron maintenance strategy over a period of 8 months, and greatly reducing infused iron in 45 stable hemodialysis patients.71 Their former strategy targeted a transferrin saturation between 30% and 50% irrespective of serum ferritin. With the new strategy, IV iron was only administered if transferrin saturation was below 20% and ferritin below 200 μg/L. Routine ESA practices were unchanged with a hemoglobin target between 10 and 12 g/dL; ESA (methoxy‐polyethylene glycol‐epoetin‐beta) was administered monthly,71 and the dose was corrected by 25% as necessary and discontinued temporarily if hemoglobin was over 13 g/dL.71
Over the 6 months of follow‐up, the mean monthly iron dose declined from 310 mg to 97 mg (P = .0003)(Figure 1), mean serum ferritin decreased from 948 ± 1056 to 571 ± 424 μg/L (P = .0001) and transferrin saturation decreased from 42% ± 22 to 33% ± 14 (P = .01) (Figure 2). Most importantly, hemoglobin levels remained stable throughout the study (11.1 g/dL ± 1.05 vs 11.0 g/dL ± 1.2, P = .54) as did the ESA dosage (126 μg/28 days ± 92 vs 108 μg/28 days ± 113, P = .07) (Figure 2).
Figure 1.
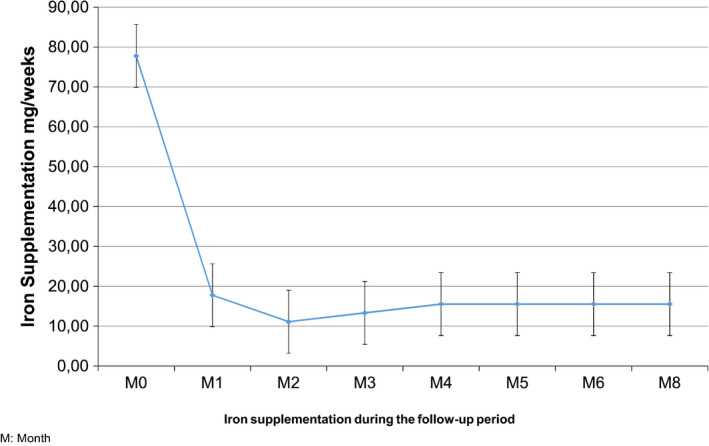
Evolution of iron supplementation during the follow‐up of 45 patients in the Nancy study, Reproduced from Peters N.O., Jay N., Cridlig J., Rostoker G., Frimat L. Targets for adapting intravenous iron dose in hemodialysis: a proof of concept study. BMC Nephrology 2017;18:97 [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
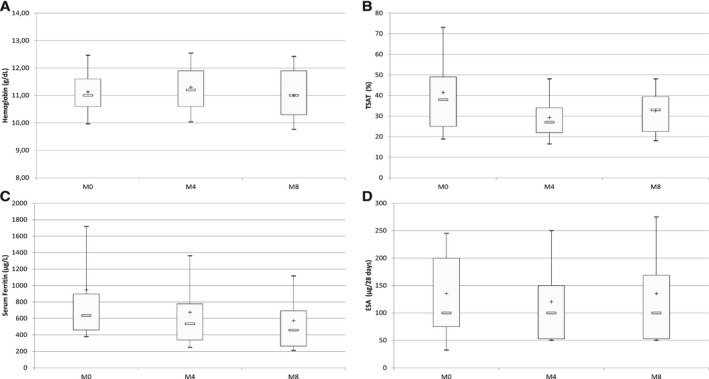
Box and whisker plots showing the change in hemoglobin levels and biochemical indicators of iron status and ESA doses during the follow‐up of 45 hemodialysis patients in the Nancy study. A, Hemoglobin levels during the follow‐up period (p = .54). B, TSAT during the follow‐up (p = .01). C, Serum ferritin during the follow‐up (p = .0001). D, Change in prescribed ESA doses during the follow‐up (p = .07). Limits of boxes are 1st quartile (lower limit) and 3rd quartile (upper limit), with + and − being the mean and median values, respectively. Ends of whiskers indicate 1st decile (lower end) and 9th decile (upper end). M: Month. Reproduced from Peters N.O., Jay N., Cridlig J., Rostoker G., Frimat L. Targets for adapting intravenous iron dose in hemodialysis: a proof of concept study. BMC Nephrology 2017;18:97
The authors concluded that a stable hemoglobin level can be maintained using usual ESA doses combined with IV iron doses adapted to transferrin saturation and serum ferritin thresholds lower than those used in routine clinical dialysis practice, reducing the risk of iron overload. It is very likely that most of these patients were liver iron‐overloaded as evidenced by the study of Castillo et al, analyzing 47 hemodialysis patients with ferritin above 500 μg/L which found increased liver content at MRI in 91% of the patients.72 Taking into account current levels of ferritin in US patients (~850 μg/L) and in the French study (947 μg/L), the Nancy strategy minimizing maintenance iron warrants consideration in the USA.
These results are in line with the recent DOPPS study devoted to the effectiveness of iron dosing for anemia management.73 This prospective study analyzed 9 471 hemodialysis patients from 11 countries between 2009 and 2011, for associations of IV iron dose (3‐month average, categorized as 0, <300, ≥300 mg/month) with 3‐month changes in hemoglobin, transferrin saturation, ferritin, and ESA dose. Thirty percent of patients received no IV iron over 3 months, 40% received <300 mg/month, and 30% received ≥300 mg/month. Interestingly, IV iron dose distributions in North America (58% of the sample) were identical to those in Europe, Australia and New Zealand (42% of the sample). The most common doses were 100 mg/month (in patients with IV iron < 300 mg/month) and 400 mg/month (in patients with IV iron ≥300 mg/month). Most patients (73%) remained in the same IV iron dose category during the 3 months of the study, and 66% remained in the same IV iron dose category for the following 9 months.73 Age, sex and comorbidities were similar in the different IV iron categories. Absolute ESA dose changes, ferritin and transferrin saturation changes were all minimal (near zero) with IV iron < 300 mg/month, compared to no iron (0 mg/month) or larger doses of IV iron (≥300 mg/month).
This study also provided estimates of the magnitude of different IV iron dosing on hemoglobin in current dialysis practices: 0.18 g/dL for iron doses < 300 mg/month compared to no iron and 0.13 g/dL for iron doses > 300 mg/month compared to iron doses < 300 mg/month. The authors concluded that IV iron dosing <300 mg/month, commonly seen with a maintenance dosing of 100‐200 mg/month, may be a more effective therapeutic approach to support hemoglobin stability than the higher IV iron doses (300‐400 mg/month) often given in the USA and many European countries.
This DOPPS‐advocated dose of maintenance IV iron of 100‐200 mg/month will also promote safety since it is lower than the toxic iron doses associated in recent epidemiological studies with increased mortality in hemodialysis patients eg, >200 mg/month in Japan (albeit where body surface area is lower than in Europe and USA)26 and 300 mg/month in the DOPPS study published in 2015.27 The recommended dosage is also lower than the 250 mg/month associated with iron overload in hepatic MRIs shown in a French study published in 2014 in 199 hemodialysis patients analyzed with tree‐learning analysis.74
6. CONCLUSIONS
Two years ago, Steven Fishbane, in an elegant and premonitory review on the balance of risks and benefit of iron therapy for anemia management, warned of the difficulty of justifying ferritin values above 500 μg/L in most dialysis patients.2
It appears today that in addition to specific populations which require cautious iron management (peritoneal dialysis, HCV‐infected, and ferritin above 1000 μg/L without concomitant inflammation patients), most hemodialysis patients in the Western world may benefit from lower maintenance IV iron dosage (after adequate replenishment of iron stores as advocated by the labels) as recently shown by the Nancy proof of concept study71 and the DOPPS researchers in an international epidemiological analysis.73
Most importantly, these 2 latter studies can be applied to USA hemodialysis patients. Thus, new pharmacometric and economic approaches to iron therapy and anemia management are clearly emerging with the aim of lessening the potential side effects of excessive IV iron with the ability to maintain hemoglobin stability without the concerns and costs of higher ESA doses.
CONFLICTS OF INTEREST
Received grants from AMGEN‐France, BAXTER‐France, GAMBRO‐France, HOSPAL‐FRANCE and NIPRO France. Is a member of the following boards: Amgen Aranesp European Board 2012‐2014. Roche‐France scientific board of the study MIRIADE 2012‐2016. Astellas‐France scientific board of anemia treatment in ESRD 2018. Received payment for lectures for: Fresenius Renal Pharma‐France (2013 and 2015), Amgen‐France (2013), Novartis‐France (2015), Sanofi‐Israël (2016), Baxter‐France (2016, 2017 and 2018), Fresenius Renal Pharma‐Africa (2017), Astellas‐France (2017), Aguettant‐France (2017), Baxter‐Benelux (2018).
REFERENCES
- 1. Hörl WH. Clinical aspects of iron use in the anemia of kidney disease. J Am Soc Nephrol. 2007;18:382‐393. [DOI] [PubMed] [Google Scholar]
- 2. Fishbane S. Balance of benefit and risk in intravenous iron treatment in chronic kidney disease. Semin Nephrol. 2016;36:119‐123. [DOI] [PubMed] [Google Scholar]
- 3. Rostoker G, Vaziri ND. Iatrogenic iron overload and its potential consequences in patients on hemodialysis. Presse Med. 2017;46(12 Pt 2):e312‐e328. [DOI] [PubMed] [Google Scholar]
- 4. Macdougall IC, Bircher AJ, Eckardt KU, et al. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;89:28‐39. [DOI] [PubMed] [Google Scholar]
- 5. Malyszko J, Anker S. Iron therapy in heart failure patients without anemia: possible implications for chronic kidney disease patients. Clin Kidney J. 2017;10(Suppl 1):i25‐i31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rottembourg J, Rostoker G. Use of intravenous iron supplementation in chronic kidney disease: interests, limits, and recommendations for a better practice. Neprol Therap. 2015;11:531‐542. [DOI] [PubMed] [Google Scholar]
- 7. Macdougall IC, Geisser P. Use of intravenous iron supplementation in chronic kidney disease: an update. Iran J Kidney Disease. 2013;7:9‐22. [PubMed] [Google Scholar]
- 8. Daher R, Manceau H, Karim Z. Iron metabolism and the role of the iron‐regulating hormone hepcidin in health and disease. Presse Med. 2017;46(12 Pt 2):e272‐e278. [DOI] [PubMed] [Google Scholar]
- 9. Kohgo Y, Ikuta K, Ohtake T, et al. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freburger JK, Ng LJ, Bradbury BD, et al. Changing patterns of anemia management in US hemodialysis patients. Am J Med. 2012;125:906‐914. E9 [DOI] [PubMed] [Google Scholar]
- 11. Bailie GR, Larkina M, Goodkin DA, et al. Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2013;28:2570‐2579. [DOI] [PubMed] [Google Scholar]
- 12. Rostoker G. The changing landscape of iron overload disorders at the beginning of the 21st century. Presse Med. 2017;46(12 Pt 2):e269‐e271. [DOI] [PubMed] [Google Scholar]
- 13. Paisant A, d'Assignies G, Bannier E, Bardou‐Jacquet E, Gandon Y. MRI for the measurement of liver iron content, and for the diagnosis and follow‐up of iron overload disorders. Presse Med. 2017;46(12 Pt 2):e279‐e287. [DOI] [PubMed] [Google Scholar]
- 14. Barton JC, Edwards CQ, Phatak PD, Britton RS, Bacon BR. Handbook of Iron Overload Disorders. UK: Cambridge University Press; 2010. [Google Scholar]
- 15. Gandon Y, Olivié D, Guyader D, et al. Non‐invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357‐362. [DOI] [PubMed] [Google Scholar]
- 16. St Pierre TG, Clark PR, Chua‐Anusom W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855‐861. [DOI] [PubMed] [Google Scholar]
- 17. Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion‐dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460‐1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rostoker G, Laroudie M, Blanc R, et al. Signal‐intensity‐ratio MRI accurately estimates hepatic iron load in hemodialysis patients. Heliyon. 2017;3:e00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Canavese C, Bergamo D, Ciccone G, et al. Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int. 2004;65:1091‐1098. [DOI] [PubMed] [Google Scholar]
- 20. Ferrari P, Kulkarni H, Dheda S, et al. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:77‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghoti H, Rachmilewitz EA, Simon‐Lopez R, et al. Evidence for tissue iron overload in long‐term hemodialysis patients and the impact of withdrawing parenteral iron. Eur J Haematol. 2012;89:87‐93. [DOI] [PubMed] [Google Scholar]
- 22. Rostoker G, Griuncelli M, Loridon C, et al. Hemodialysis‐associated hemosiderosis in the era of erythropoiesis‐stimulating agents: a MRI study. Am J Med. 2012;125:991‐999. [DOI] [PubMed] [Google Scholar]
- 23. Van der Weerd NC, Grooteman MP, Nubé MJ, et al. Hepcidin in chronic kidney disease: not an anaemia management tool, but promising as a cardiovascular biomarker. The Netherlands J Med. 2015;73:108‐118. [PubMed] [Google Scholar]
- 24. Van der Weerd NC, Grooteman MPC, Bots ML, et al. Hepcidin‐25 is related to cardiovascular events in chronic haemodialysis patients. Nephrol Dial Transplant. 2013;28:3062‐3071. [DOI] [PubMed] [Google Scholar]
- 25. Kuo KL, Hung SC, Lin YP, et al. Intravenous ferric chloride hexahydrate supplementation induced endothelial dysfunction and increased cardiovascular risk among hemodialysis patients. PLoS ONE. 2012;7:e50295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuragano T, Matsumura O, Matsuda A, et al. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 2014;86:845‐854. [DOI] [PubMed] [Google Scholar]
- 27. Bailie GR, Larkina M, Goodkin DA, et al. Data from the dialysis outcomes and practice patterns study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015;87:162‐168. [DOI] [PubMed] [Google Scholar]
- 28. KDOQI National Kidney Foundation . Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47(5 Suppl. 3):Sl6‐S85. [DOI] [PubMed] [Google Scholar]
- 29. Locatelli F, Covic A, Eckardt KU, Wiecek A, Vanholder R and on behalf of the ERA‐EDTA ERBP advisory board . Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2009;24:348‐354. [DOI] [PubMed] [Google Scholar]
- 30. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group . KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney inter Suppl. 2012;2:279‐335. [Google Scholar]
- 31. Locatelli F, Bárány P, Covic A, et al. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European renal best practice position statement. Nephrol Dial Transplant. 2013;28:1346‐1359. [DOI] [PubMed] [Google Scholar]
- 32. Wish JB. Intravenous iron: not just for hemodialysis patients anymore. Peritoneal Dialysis Int. 2008;28:126‐129. [PubMed] [Google Scholar]
- 33. Issad B, Cohen Y, Griuncelli M, et al. Results of anemia module (RDPLF) and iron stock assessment in patients with PD using hepatic MRI. Fourth Symposium of Home Dialysis. Brussels 6 and 7 June 2018.
- 34. Issad B, Ghali N, Beaudreuil S, et al. Hepatic iron load at magnetic resonance imaging is normal in most patients receiving peritoneal dialysis. Kidney Int Rep. 2017;2:1219‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Daugirdas JT. Iron and anemia in chronic kidney disease: new treatments changing old paradigms. Hemodialysis Int. 2017;21(Suppl 1):S3‐S5. [DOI] [PubMed] [Google Scholar]
- 36. Petruzziello A, Marigliano S, Loquercio G, et al. Global epidemiology of hepatitis C virus infection: an update of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824‐7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodkin DA, Bieber B, Jadoul M, et al. Mortality, hospitalization and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol. 2017;12:287‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rostoker G, Vaziri ND. Impact of iatrogenic iron overload on the course of hepatitis C in dialysis population: a plea for caution. Hemodialysis Int. 2017;21(Suppl 1):S68‐S77. [DOI] [PubMed] [Google Scholar]
- 39. Boucher E, Bourienne A, Adams P, Turlin B, Brissot P, Deugnier Y. Liver iron concentration and distribution in chronic hepatitis C before and after interferon treatment. Gut. 1997;41:115‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sartori M, Andorno S, Rossini A, et al. Phlebotomy improves histology in chronic hepatitis C males with mild iron overload. World J Gastroenterol. 2010;16:596‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franchini M, Targher G, Capra F, Montagnana M, Lippi G. The effect of iron depletion on chronic hepatitis C virus infection. Hepatol Int. 2008;2:335‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Desai TK, Jamil LH, Balasubramaniam M, Koff R, Bonkovsky HL. Phlebotomy improves therapeutic response to interferon in patients with chronic hepatitis C: a meta‐analysis of six prospective randomized controlled trials. Dig Dis Sci. 2008;53:815‐822. [DOI] [PubMed] [Google Scholar]
- 43. Martinelli AL, Filho AB, Franco RF, et al. Liver iron deposits in hepatitis B patients: association with severity of liver disease but not with hemochromatosis gene mutations. J Gastrenterol and Hepatol. 2004;19:1036‐1041. [DOI] [PubMed] [Google Scholar]
- 44. George DK, Goldwurm S, MacDonald GA, et al. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311‐318. [DOI] [PubMed] [Google Scholar]
- 45. Pietrangelo A, Gualdi R, Casalgrandi G, Montosi G, Ventura E. Molecular and cellular aspects of iron‐induced hepatic cirrhosis in rodents. J Clin Invest. 1995;95:1824‐1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kakizaki S, Takagi H, Horiguchi N, et al. Iron enhances hepatitis C virus replication in cultured human hepatocytes. Liver. 2000;20:125‐128. [DOI] [PubMed] [Google Scholar]
- 47. Balogh J, Victor D 3rd, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocellular carcinoma. 2016;3:41‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hamed MA, Ali SA. Non‐viral factors contributing to hepatocelular carcinoma. World J Hepatol. 2013;5:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Furutani T, Hino K, Okuda M, et al. Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2006;130:2087‐2098. [DOI] [PubMed] [Google Scholar]
- 50. Traodec MB, Courselaud B, Détivaud L, et al. Iron overload promotes cyclin D1 expression and alters cell cycle in mouse hepatocytes. J Hepatol. 2006;44:391‐399. [DOI] [PubMed] [Google Scholar]
- 51. Nirei K, Matsuoka S, Nakamura H, Matsumura H, Moriyama M. Incidence of hepatocellular carcinoma reduced by phlebotomy treatment in patients with chronic hepatitis C. Intern Med. 2015;54:107‐117. [DOI] [PubMed] [Google Scholar]
- 52. Kato J, Miyanishi K, Kobune M, et al. Long‐term phlebotomy with low‐iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830‐836. [DOI] [PubMed] [Google Scholar]
- 53. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck‐Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta‐analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329‐337. [DOI] [PubMed] [Google Scholar]
- 54. Ozdemir A, Yalinbas B, Selamet U, et al. Relationship between iron replacement and hepatic functions in hepatitis C virus‐positive chronic haemodialysis patients. Nephrology. 2005;10:433‐437. [DOI] [PubMed] [Google Scholar]
- 55. Kahraman S, Yilmaz R, Genctoy G, et al. Efficacy and safety of intravenous iron therapy for HCV‐positive haemodialysis patients. Nephron Clin Pract. 2005;100:c78‐c85. [DOI] [PubMed] [Google Scholar]
- 56. Milic S, Mikolasevic I, Orlic L, et al. The role of iron and iron overload in chronic liver disease. Med Sci Monit. 2016;22:2144‐2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamamoto H, Tsubakihara Y. Limiting iron supplementation for anemia in dialysis patients–the Basis for Japan's conservative guidelines. Semin Dial. 2011;24:269‐271. [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto H, Shinichi N, Tadashi T, et al. 2015 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3:36. [Google Scholar]
- 59. Ogawa C, Tsuchiya K, Kanda F, Maeda T. Low levels of serum ferritin lead to adequate hemoglobin levels and good survival in hemodialysis patients. Am J Nephrol. 2014;40:561‐570. [DOI] [PubMed] [Google Scholar]
- 60. Ogawa C, Tsuchiya K, Tomosugi N, et al. Low levels of serum ferritin and moderate transferrin saturation lead to adequate hemoglobin levels in hemodialysis patients, retrospective observational study. PLoS ONE. 2017;12:e0179608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rostoker G, Griuncelli M, Loridon C, et al. Reassessment of iron biomarkers for prediction of dialysis iron overload: an MRI study. PLoS ONE. 2015;10:e0132006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. The DOPPS Practice Monitor. Slide browser. Available from: http://www.dopps.org/dpm/. Accessed June 18, 2018.
- 63. Wish JB, Aronoff GR, Bacon BR, et al. Positive iron balance in chronic kidney disease: how much is too much and how to tell? Am J Nephrol. 2018;47:72‐83. [DOI] [PubMed] [Google Scholar]
- 64. Noetzli LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. 2009;114:4021‐4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Restaino G, Meloni A, Positano V, et al. Regional and global pancreatic T*2 MRI for iron overload assessment in a large cohort of healthy subjects: normal values and correlation with age and gender. Magn Reson Med. 2011;65:764‐769. [DOI] [PubMed] [Google Scholar]
- 66. Henninger B, Rauch S, Zoller H, et al. R2*‐relaxometry of the pancreas in patients with human hemochromatosis protein associated hereditary hemochromatosis. Eur J Radiol. 2017;89:149‐155. [DOI] [PubMed] [Google Scholar]
- 67. Tarng DC, Huang TP, Chen TW, Yang WC. Erythropoietin hyporesponsiveness: from iron deficiency to iron overload. Kidney Int Suppl. 1999;69:S107‐S118. [PubMed] [Google Scholar]
- 68. Shenoy N, Vallumsetla N, Rachmilewitz E, Verma A, Ginzburg Y. Impact of iron overload and potential benefit from iron chelation in low‐risk myelodysplasic syndrome. Blood. 2014;124:873‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coyne DW, KapoianT T, Suki W, et al. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18:975‐984. [DOI] [PubMed] [Google Scholar]
- 70. Tarng DC, Huang TP, Wei YH. Erythropoietin and iron: the role of ascorbic acid. Nephrol Dial Transplant. 2001;16(Suppl 5):35‐39. [DOI] [PubMed] [Google Scholar]
- 71. Peters NO, Jay N, Cridlig J, Rostoker G, Frimat L. Targets for adapting intravenous iron dose in hemodialysis: a proof of concept study. BMC Nephrol. 2017;18:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Castillo NL, Boixadera H, Romeu M, et al. Factors associated with the magnetic resonance imaging estimated liver concentration in long‐term hemodialysis patients receiving intravenous iron supplementation. Nephrol Dial Transplant. 2016;31(Suppl 1):i276. [Google Scholar]
- 73. Robinson BM, Larkina M, Bieber B, et al. Evaluating the effectiveness of IV iron dosing for anemia management in common clinical practice: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). BMC Nephrol. 2017;18:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rostoker G, Griuncelli M, Loridon C, et al. Maximal standard dose of parenteral iron for hemodialysis patients: an MRI‐Based decision tree learning analysis. PLoS ONE. 2014;9:e115096. [DOI] [PMC free article] [PubMed] [Google Scholar]


