Summary
Background
Although existing psoriasis treatments are effective and well tolerated in many patients, there is still a need for new effective targeted treatment options. Tofacitinib is an oral Janus kinase inhibitor that has been investigated in patients with moderate‐to‐severe chronic plaque psoriasis.
Objectives
To consider the benefits and risks of tofacitinib in patients with moderate‐to‐severe psoriasis.
Methods
Data were pooled from one phase II, four phase III and one long‐term extension study comprising 5204 patient‐years of tofacitinib treatment. Efficacy end points included patients achieving Physician's Global Assessments of ‘clear’ or ‘almost clear’, ≥ 75% and ≥ 90% reduction in Psoriasis Area and Severity Index (coprimary end points) and improvements in Dermatology Life Quality Index score, Hospital Anxiety and Depression Scale depression score and Itch Severity Item score, at weeks 16 and 52. Safety data were summarized for 3 years of tofacitinib exposure.
Results
Tofacitinib 5 and 10 mg twice daily (BID) showed superiority over placebo for all efficacy end points at week 16, with response maintained for 52 weeks of continued treatment. Tofacitinib improved patients’ quality of life and was well tolerated. Rates of safety events of interest (except herpes zoster) were similar to those in the published literature and healthcare databases for other systemic psoriasis therapies. Tofacitinib 10 mg BID demonstrated greater efficacy than 5 mg BID.
Conclusions
Tofacitinib has a benefit–risk profile in moderate‐to‐severe psoriasis consistent with that of other systemic treatments.
Short abstract
What's already known about this topic?
Psoriasis is a chronic, systemic inflammatory disease, which has a significant impact on patients’ health‐related quality of life.
Although several existing psoriasis treatments are efficacious and well tolerated in many patients, some patients require treatment switching, and a proportion of patients remain untreated or undertreated.
Potential challenges to the use of existing therapies include safety issues and limited efficacy in some patients with conventional oral psoriasis treatments, inconvenience of topical treatments and the requirement for parenteral administration of biologics.
What does this study add?
Consistent efficacy and a safety profile consistent with that seen in rheumatoid arthritis, psoriatic arthritis and ulcerative colitis were demonstrated for oral tofacitinib in patients with moderate‐to‐severe psoriasis.
Tofacitinib has a benefit–risk profile in patients with moderate‐to‐severe psoriasis that is consistent with that of other systemic psoriasis treatments.
Linked Comment: Fleming. Br J Dermatol 2019; 180:13–14.
Plain language summary available online
Psoriasis is a chronic, systemic inflammatory disease with a global prevalence in the range of 1–3%.1, 2, 3 Several treatments are available for psoriasis that are effective and well tolerated in many patients.4, 5 However, conventional systemic therapies are associated with limited efficacy in some patients,4 clinical response to biologics can decrease over time,6 and both types of drugs have recognized safety issues.7, 8 Consequently, some patients with psoriasis switch between multiple treatments over their lifetime, and a significant proportion of patients remain untreated or undertreated.9
The treatment satisfaction of patients with psoriasis has been reported to be poor, but it is generally higher with biological agents than with traditional therapies,9, 10, 11 due to the better efficacy of biologics.10, 11 However, patients generally prefer oral to parenteral treatments.12
Tofacitinib is a Janus kinase inhibitor. In contrast to current biological therapies, tofacitinib is administered orally. This analysis aimed to assess the benefit–risk profile of tofacitinib in patients with moderate‐to‐severe chronic plaque psoriasis by pooling the data from six clinical trials.13, 14, 15, 16, 17, 18
Patients and methods
Study design
These analyses were based on pooled data from one phase II double‐blind randomized controlled trial (ClinicalTrials.gov identifier NCT00678210),18 four phase III double‐blind randomized controlled trials (collectively referred to as the index studies) (OPT Pivotal 1, NCT01276639;15 OPT Pivotal 2, NCT01309737;15 OPT Compare, NCT01241591;14 OPT Retreatment, NCT01186744)13 and one open‐label long‐term extension study (NCT01163253).19 Further details of the study designs and treatments are provided in Table S1 (see Supporting Information).
Efficacy end points
The main efficacy end points for the current analyses were the proportion of patients achieving Physician's Global Assessment (PGA) ratings of ‘clear’ or ‘almost clear’ and the proportion of patients achieving ≥ 75% reduction in Psoriasis Area and Severity Index (PASI 75) at weeks 16 and 52 of treatment (these were the coprimary end points at week 16 in the OPT Pivotal 1 and 2 studies). Other efficacy end points included the proportions of patients achieving PASI 90; a score of ≤ 1 on the Dermatology Life Quality Index (DLQI), indicating no effect of skin disease on the patient's life;20 a score of ≤ 1 on the Itch Severity Item (ISI), equating to ‘little or no itch’;21 a depression score of < 8 on the Hospital Anxiety and Depression Scale (HADS), indicating no symptoms of depression;22 ≥ 75% reduction in the Nail Psoriasis Severity Index (NAPSI 75) in patients with nail psoriasis at baseline; and change from baseline in body surface area affected by psoriasis.
Safety end points
The incidence rates (IRs; numbers of patients with events per 100 patient‐years) of serious infections, opportunistic infections, herpes zoster, major adverse cardiovascular events (MACE), malignancy excluding nonmelanoma skin cancer (NMSC), and NMSC were assessed. Treated infections that required parenteral antimicrobial therapy, required hospitalization or met other criteria that required the infection to be reported as a serious adverse event (AE) were considered a serious infection. Opportunistic infections, defined as infections that occur more frequently in immunocompromised individuals, also included disseminated herpes zoster and herpes zoster affecting nonadjacent or more than two adjacent dermatomes. MACE was defined as the occurrence of a myocardial infarction, cerebrovascular event or cardiovascular death. Independent adjudication committees, blinded to treatment assignment, reviewed all potential opportunistic infections and all cardiovascular events.
Statistical analysis
Efficacy data
Data from OPT Pivotal 1 and OPT Pivotal 2, up to week 52, were pooled for the efficacy analyses. The full analysis set included all patients who were randomized and received at least one dose of study drug in either Pivotal study.
For dichotomized data, missing observations were set to nonresponders unless otherwise stated. The Cochran–Mantel–Haenszel approach to adjust the study effect was used to estimate the treatment difference among treatment groups at week 16 (before placebo‐treated patients advanced to tofacitinib). Statistical testing was based on normal approximation to the binomial proportions. No statistical analyses were applied to data beyond week 16.
Safety data
Data from patients who received at least one dose of tofacitinib, including all events through to 4 April 2014 (interim data for the long‐term extension study), were considered for the safety analyses.
Safety data from the phase II and III studies were summarized for tofacitinib 5 mg and 10 mg twice daily (BID) over 16 weeks (vs. placebo) and over 1 year (no placebo arm; for patients initially receiving placebo then advanced to tofacitinib at week 16, the data after week 16 were included). Safety data were also summarized for combined tofacitinib exposure for up to 3 years in the index and long‐term extension studies. Total tofacitinib exposure data are presented for patients who received tofacitinib 10 mg BID for ≥ 80% of their study duration (10‐mg BID group) and for pooled tofacitinib doses (tofacitinib all). For patients who switched from placebo or etanercept to tofacitinib, a new baseline was established, with the first day of tofacitinib treatment considered as day 1.
To contextualize IRs for safety events, a systematic review of published randomized clinical trials and observational studies reporting AEs with other psoriasis treatments was undertaken (Appendix S1 and Table S2; see Supporting Information). Additionally, data from the six tofacitinib clinical trials were benchmarked against two Pfizer‐commissioned comparison cohorts of patients with moderate‐to‐severe psoriasis, derived from U.S. Medicare and Kaiser Permanente Northern California (KPNC) databases (Appendix S1).
All clinical studies were conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and with the International Council for Harmonisation Good Clinical Practice Guidelines. All documentation was reviewed by the institutional review board and/or independent ethics committee at each of the investigational centres. All patients provided written informed consent.
Results
Patients
For efficacy analyses, 745, 741 and 373 patients were included who received tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively, in OPT Pivotal 1 and 2. The patient demographics and baseline disease characteristics have previously been reported for these patients and were balanced between the treatment groups.15
For safety analyses during the 16‐week placebo‐controlled period, the numbers of patients receiving at least one dose of tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo were 1123 (299 patient‐years of exposure), 1120 (302 patient‐years) and 530 (136 patient‐years), respectively. For safety analyses over 1 year, 1217 (801 patient‐years) and 1219 (869 patient‐years) patients received at least one dose of tofacitinib 5 mg and 10 mg BID, respectively. For analyses of safety across all tofacitinib‐treated patients up to 3 years, 3623 patients were included (5204 patient‐years of exposure), and 2915, 2362, 995 and 55 patients had received treatment with tofacitinib for at least 6 months, 1 year, 2 years and 3 years, respectively.
The demographic and other baseline characteristics for all tofacitinib‐treated patients are shown in Table S3 (see Supporting Information). The median DLQI score was 11, indicating a very large effect of psoriasis on the patients’ quality of life.23
Efficacy
Based on pooled data from the OPT Pivotal 1 and 2 studies, significantly greater proportions of patients receiving tofacitinib 5 mg or 10 mg BID vs. placebo achieved a PGA of ‘clear’ or ‘almost clear’ (44·0%, 59·1% and 10·0%, respectively; P < 0·001) and PASI 75 (43·1%, 59·4% and 8·9%, respectively; P < 0·001) at week 16 (Fig. 1a, b). Further improvements were seen with both tofacitinib doses up to week 28. At week 52, ≥ 57% of patients who achieved a PGA response at week 16 and ≥ 66% of patients who achieved PASI 75 at week 16 maintained that response.
Figure 1.
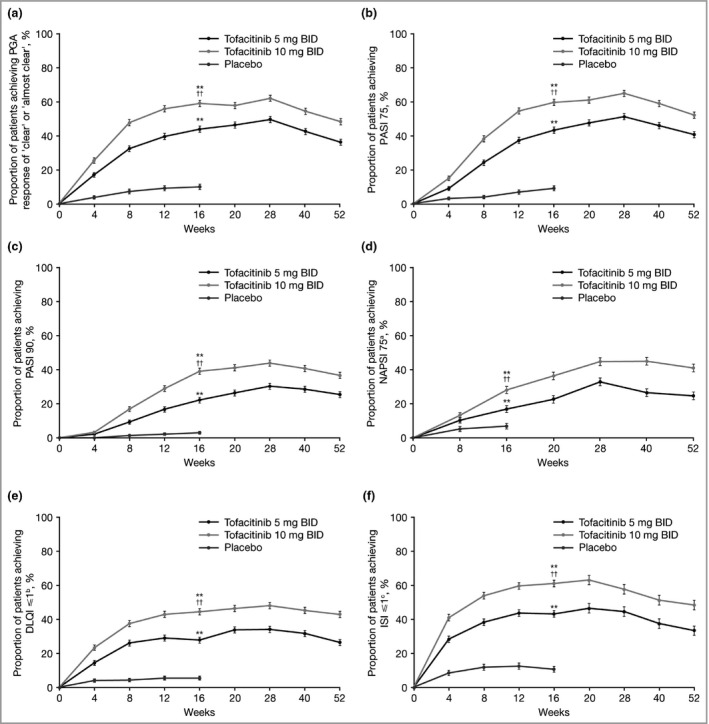
Proportions of patients (mean ± SE) achieving (a) Physician's Global Assessment (PGA) response of ‘clear’ or ‘almost clear’, (b) ≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75), (c) PASI 90, (d) ≥ 75% improvement in Nail Psoriasis Severity Index (NAPSI 75), (e) Dermatology Life Quality Index (DLQI) response and (f) Itch Severity Item (ISI) response (full analysis set, nonresponder imputation). aOf those patients with nail psoriasis at baseline, bof those patients with baseline DLQI > 1, cof those patients with baseline ISI > 1. **P < 0·0001 tofacitinib vs. placebo; †† P < 0·0001 tofacitinib 10 mg twice daily (BID) vs. tofacitinib 5 mg BID. P‐values are shown at week 16 only (the time at which the main efficacy end points were collected across the various studies).
The more stringent efficacy thresholds of PASI 90 and NAPSI 75 were also achieved in more patients receiving tofacitinib 5 mg or 10 mg BID than placebo at week 16 (PASI 90: 22·2%, 39·1% and 3·0%, respectively; P < 0·001; NAPSI 75: 16·9%, 28·1% and 6·8%, respectively; P < 0·001) (Fig. 1c, d). At week 52, ≥ 65% of those who achieved PASI 90 at week 16 maintained that response.
At week 16, a DLQI total score ≤ 1 and an ISI score ≤ 1 were achieved in significantly more patients receiving tofacitinib 5 mg or 10 mg BID (DLQI: 27·7% and 44·3%, respectively; ISI: 43·0% and 60·9%, respectively) than placebo (DLQI: 5·3%; ISI: 10·5%; P < 0·001) (Fig. 1e, f). The proportions of patients achieving a DLQI total score ≤ 1, and an ISI score ≤ 1 continued to increase to week 28. Of those patients who had a HADS depression score indicative of depression (≥ 8) at baseline, a higher proportion receiving tofacitinib 5 mg BID (52·4%) or 10 mg BID (61·6%) compared with placebo (35·8%) had a HADS depression score indicating they were not depressed (< 8) at week 16 (Fig. S1; see Supporting Information).
Tofacitinib was efficacious in subgroups of patients with moderate or severe disease, who had previous experience of systemic treatment or were systemic treatment naive, and who had lower or higher bodyweight. Tofacitinib 10 mg BID showed greater efficacy than 5 mg BID in all subgroups, and the overall efficacy was lower in patients with baseline PASI < 20, prior experience of systemic treatment and bodyweight ≥ 90 kg at baseline (Table 1).
Table 1.
Proportions of patients achieving ≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75) with tofacitinib 10 mg twice daily (BID) vs. tofacitinib 5 mg BID by subgroup (full analysis set, nonresponder imputation)
| Week 16 | Week 52 | |||
|---|---|---|---|---|
| Tofacitinib 5 mg BID (n = 739) | Tofacitinib 10 mg BID (n = 734) | Tofacitinib 5 mg BID (n = 739) | Tofacitinib 10 mg BID (n = 734) | |
| Baseline PASI | ||||
| < 20 | 38·6 (33·7–43·6) | 57·1 (52·0–62·2) | 36·5 (31·6–41·4) | 49·7 (44·6–54·9) |
| ≥ 20 | 47·5 (42·5–52·6) | 61·7 (56·8–66·7) | 44·4 (39·3–49·4) | 54·1 (50·0–59·2) |
| Previous systemic treatment | ||||
| No | 57·0 (50·6–63·3) | 70·0 (64·5–75·6) | 54·2 (47·9–60·6) | 64·2 (58·4–70·1) |
| Yes | 36·4 (32·2–40·6) | 53·6 (49·1–58·1) | 34·0 (29·9–38·1) | 45·2 (40·7–49·6) |
| Baseline weight | ||||
| < 90 kg | 46·0 (41·3–50·8) | 62·7 (58·0–67·3) | 45·0 (40·2–49·7) | 56·4 (51·7–61·2) |
| ≥ 90 kg | 39·1 (33·7–44·5) | 55·1 (49·6–60·6) | 34·3 (29·0–39·6) | 45·9 (40·4–51·4) |
Values are the percentage (95% confidence interval).
Safety
Safety data from patients with psoriasis receiving 16 weeks of treatment with tofacitinib 5 mg and 10 mg BID vs. placebo have been previously reported.15 During the placebo‐controlled period, AEs were reported in 606 (54·0%) patients who received tofacitinib 5 mg BID, 660 (58·9%) who received tofacitinib 10 mg BID and 267 (50·4%) who received placebo; 29 (2·6%), 35 (3·1%) and 26 (4·9%), respectively, discontinued treatment due to AEs.
During 1 year of treatment with tofacitinib 5 mg and 10 mg BID, there were few dose‐related differences in the IRs for events of special interest. Although IRs for serious infections, herpes zoster and NMSC were numerically greater with tofacitinib 10 mg BID than with 5 mg BID, the 95% confidence intervals (CIs) overlapped (Table S4; see Supporting Information).
Across all tofacitinib‐treated patients in the six clinical trials included in this analysis, the most frequently observed serious infections were pneumonia (n = 16, 0·4%), herpes zoster (n = 8, 0·2%) and appendicitis (n = 7, 0·2%). There was no association between the occurrence of serious infections and cases of confirmed neutropenia or lymphopenia. Opportunistic infections were infrequent, with 12 infections adjudicated to be opportunistic, and no dose dependency was observed. Nine herpes zoster events, reported in eight of the 130 patients with herpes zoster (6%), were adjudicated to be opportunistic infections. Other opportunistic infections were listeria encephalitis, fungal pneumonia, herpes simplex meningitis and cryptococcal pneumonia, each reported in one patient, and all of which resolved on anti‐infective treatment.
Of the 130 cases of herpes zoster, > 90% were classified as mild or moderate in severity. Most cases of herpes zoster resolved with antiviral therapy without complication; eight patients discontinued due to herpes zoster. Postherpetic neuralgia (PHN) was reported by 10 (8%) of the tofacitinib‐treated patients with herpes zoster, equating to 0·3% of all the tofacitinib‐treated patients and an IR of 0·19 per 100 patient‐years. A higher IR for herpes zoster was observed in Asian (particularly Japanese) patients with psoriasis receiving tofacitinib: the IR (95% CI) over the total tofacitinib exposure for Asian patients was 5·75 (3·65–8·63), compared with 2·55 (2·13–3·03) for the global population.
The IR of malignancies excluding NMSC was similar between patients receiving tofacitinib 5 mg and 10 mg BID. The IRs (95% CIs) for total exposure were 0·97 (0·64–1·40) and 1·00 (0·75–1·31) for tofacitinib 10 mg BID and all tofacitinib doses, respectively. No increase in the IR of malignancies excluding NMSC was observed over time in patients treated with tofacitinib for up to 3 years. The IR of NMSC was higher in patients receiving tofacitinib 10 mg BID over 1 year compared with 5 mg BID (Table S4). The IRs (95% CIs) for total exposure were 0·90 (0·58–1·33) and 0·74 (0·52–1·01) for tofacitinib 10 mg BID and all tofacitinib doses, respectively. Of the adjudicated cardiovascular events, 19 occurring in patients receiving tofacitinib were considered to be MACE during the entire tofacitinib exposure, with no dose dependency observed over 1 year (Table S4). The IRs (95% CIs) for MACE over the total tofacitinib exposure were 0·32 (0·15–0·61) and 0·37 (0·22–0·57) for tofacitinib 10 mg BID and all tofacitinib doses, respectively.
During the placebo‐controlled phases of the psoriasis clinical trials analysed in this study, the IR of death for patients receiving tofacitinib was low and comparable with that observed in the placebo treatment groups. Across all studies, there was a total of 10 deaths up to 30 days after discontinuation of tofacitinib, with an IR of 0·19 (95% CI 0·09–0·35). By comparison, there was one death in a patient receiving placebo (IR 0·73, 95% CI 0·02–4·09) and no deaths reported in patients receiving etanercept. Of note, only 335 patients received etanercept for up to 12 weeks (74·7 patient‐years of exposure) and only 530 patients received placebo for 12–16 weeks (136 patient‐years of exposure). Causes of death in patients receiving tofacitinib included myocardial infarction (n = 4), cardiac arrest (n = 2), acute respiratory distress syndrome (n = 1), lung cancer (n = 1), hepatic failure (n = 1) and road traffic accident (n = 1).
Changes were seen in some laboratory parameters in patients receiving tofacitinib.13, 14, 15 Small, dose‐dependent decreases in haemoglobin, stable after 3 months of treatment and reversible on treatment withdrawal, were observed. Across all tofacitinib‐treated patients in the six studies, the mean ± SE change from baseline to the last observation in haemoglobin levels was −0·34 ± 0·02 g dL−1. Small mean decreases in neutrophil counts were observed at week 4, gradually reverting back towards baseline values after week 8, and generally stabilizing after month 4. Increases in lymphocyte counts were seen at week 4, persisting through week 8, followed by slight decreases through month 24. Across all tofacitinib‐treated patients, the mean change from baseline to the last observation in neutrophil count was −0·04 × 109 cells L−1, and in lymphocyte count it was 0·18 × 109 cells L−1.
Small, nonprogressive increases occurred in mean total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol and triglycerides, but total : HDL cholesterol or LDL : HDL cholesterol ratios were unaffected. Across all tofacitinib‐treated patients, the mean ± SE percentage changes from baseline to the last observation were 9·9 ± 0·35% for total cholesterol, 12·0 ± 0·57% for LDL cholesterol, 12·5 ± 0·40% for HDL cholesterol and 14·6 ± 1·13% for triglycerides. Small, dose‐dependent median (interquartile range) increases in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were observed over 1 year of treatment: 2·5 IU L−1 (−1·0 to 7·0) for AST and 2·0 IU L−1 (−2·0 to 9·0) for ALT in the tofacitinib 5 mg BID group, and 3·0 IU L−1 (−1·0 to 9·0) for AST and 3·0 IU L−1 (−3·0 to 11·0) for ALT in the tofacitinib 10 mg BID group; they did not increase further on longer exposure. There were no confirmed cases of Hy's law or drug‐induced liver injury.
IRs of safety events reported in published randomized controlled trials and observational studies, and those reported in the Pfizer‐commissioned comparison cohorts (Medicare and KPNC) for other systemic psoriasis treatments, are summarized in Table 2, alongside data from the tofacitinib clinical programme. Detailed results are given in Figure S2 (see Supporting Information).
Table 2.
Incidence rate ranges (events per 100 patient‐years) for safety events of interest reported in randomized controlled trials, observational studies, Pfizer‐commissioned comparison cohorts (Medicare, KPNC) and the tofacitinib clinical trials
| Published RCTsa | Published observational studies | Medicare comparison cohort | KPNC comparison cohort | Tofacitinib allb | |
|---|---|---|---|---|---|
| Serious infections | 0–4·55 | 0·83–2·62 | NA | 1·51–2·17 | 1·68 |
| Herpes zoster | 4·75 | 0·00–3·23 | 1·11–1·43 | 0·86–1·03 | 2·55 |
| Malignancies (excluding NMSC) | 0–7·69 | 0·54–2·9 | 0·50–1·29 | 0·75–1·15 | 1·00 |
| NMSC | 0–2·08 | 0·07–1·89 | 2·24–5·36 | 1·14–1·24 | 0·74 |
| MACE | 0–1·16 | 0·36–4·45c | NA | NA | 0·37 |
KPNC, Kaiser Permanente Northern California; MACE, major adverse cardiovascular events; NA, not available; NMSC, nonmelanoma skin cancer. aPublished randomized controlled trial (RCT) data refer to data from the full duration (typically 1 year) of clinical trials of agents other than tofacitinib (secukinumab, ustekinumab, adalimumab, etanercept, infliximab, apremilast and oral methotrexate); they do not include data from long‐term extension phases. bIncludes data for combined tofacitinib exposure for up to 3 years in the index and long‐term extension studies. cRange cited is for rates where definition of MACE included any death. When cardiovascular death only was included, the upper bound was 2·22 rather than 4·45 events per 100 patient‐years.
Discussion
Despite a range of biological treatment options being available for plaque psoriasis, orally administered agents with clinically meaningful efficacy and an acceptable safety profile with long‐term use are needed. The efficacy of tofacitinib in patients with moderate‐to‐severe psoriasis has been demonstrated in the extensive clinical development programme, with improvements observed for all clinical end points and quality of life. Together with safety data from 5204 patient‐years of exposure to tofacitinib, these findings suggest a benefit–risk profile for tofacitinib in psoriasis that is consistent with those of other systemic treatments.
Significant differences for tofacitinib vs. placebo were seen for all efficacy end points at week 16. A loss of efficacy was seen in some patients after 28 weeks; the mechanism of action for this is unknown, and a clinical response is maintained in many patients for up to 2 years.19 Improvements in nail psoriasis (reported to be nonresponsive to traditional psoriasis treatments),24, 25 relief of itch within 2 days of treatment,14 sustained improvements in quality of life, and improvements in the HADS depression score were seen with both tofacitinib doses. Tofacitinib 5 mg BID has consistently demonstrated superior efficacy to placebo, and tofacitinib 10 mg BID has shown additional efficacy compared with 5 mg BID.13, 14, 15
Based on the results of OPT Compare (NCT01241591), tofacitinib 10 mg BID had similar efficacy to etanercept 50 mg twice weekly (PASI 75: 63·6% and 58·5%, respectively; PGA response: 68·2% and 66·3%, respectively), whereas 5 mg BID did not meet the noninferiority criteria (PASI 75: 39·5%; PGA response: 47·1%).14 A meta‐analysis conducted by Pfizer has shown the efficacy of tofacitinib 5 mg BID to be similar to those of oral methotrexate and etanercept 25 mg twice weekly, and numerically higher than with the oral psoriasis therapies acitretin and apremilast.26 The meta‐analysis also showed the efficacy of tofacitinib 10 mg BID to be in the range of those of injectable tumour necrosis factor antagonists (currently the most widely prescribed biological drugs for psoriasis), similar to that of ciclosporin, and greater than those of other oral treatments (apremilast, acitretin, oral methotrexate and fumarates).26, 27
Tofacitinib demonstrated efficacy in subgroups defined by baseline PASI score, prior systemic treatment and bodyweight. Lower efficacy was seen in patients with PASI baseline score < 20, higher bodyweight and prior systemic treatment, as has been observed with biological therapy.28, 29
Tofacitinib 5 mg BID and 10 mg BID were generally well tolerated, and safety events were similar to those reported with tofacitinib in rheumatoid arthritis, ulcerative colitis and psoriatic arthritis.30, 31, 32, 33, 34 Only one study is available comparing tofacitinib head to head with another psoriasis treatment,14 and comparisons between treatments must therefore be interpreted cautiously. However, comparisons with data from published studies and Pfizer‐commissioned comparison cohorts demonstrate that, with the exception of herpes zoster, rates of key safety events in the tofacitinib clinical trials analysed fall within the range of rates for the same events across a range of systemic psoriasis therapies. IRs for herpes zoster were generally higher in the tofacitinib clinical trials in the comparison cohorts, although there was some overlap in the CIs around estimates for tofacitinib and ustekinumab in the Medicare comparison cohort (Fig. S2d; see Supporting Information).
The rates were also higher than the majority of those in published observational studies, but were lower than the estimate for ustekinumab in one publication, and the 95% CIs overlapped those around estimates for ciclosporin, infliximab, oral methotrexate with biologics, acitretin with biologics and psoralen plus ultraviolet A (Fig. S2c; see Supporting Information). The majority of cases of herpes zoster were nonserious, were of mild or moderate severity and resolved on treatment. PHN was reported by 8% of patients receiving tofacitinib who experienced herpes zoster (IR for PHN: 0·2 per 100 patient‐years); no published data on the rates of PHN in patients with psoriasis were found in the literature.
IRs of serious infections and herpes zoster were numerically higher with tofacitinib 10 mg BID than with 5 mg BID; however, the IR of serious infections with tofacitinib 10 mg BID was below that reported in some observational studies for tumour necrosis factor antagonists (Table S2), and the dose‐dependent difference in the IR for herpes zoster was fairly small (1·3%). Given the greater observed efficacy of the higher dose of tofacitinib, the overall benefit–risk profile of tofacitinib 10 mg BID is maintained and consistent with those of other systemic treatments for moderate‐to‐severe psoriasis. In the global clinical studies in psoriasis, patients in Japan were noted to show an increased incidence of herpes zoster compared with other patients, reflecting findings reported elsewhere.35
A number of changes in laboratory parameters were seen in patients receiving tofacitinib in the psoriasis clinical trials analysed in this study, but they were reversible upon withdrawal of tofacitinib. There were no indications that tofacitinib was associated with the safety issues that are seen with oral methotrexate treatment, such as hepatic toxicity36, 37, 38 or bone marrow suppression,37, 39 or with the renal toxicity known to be associated with ciclosporin use;39 these findings are supported by data from 4967 patients with rheumatoid arthritis and a total tofacitinib exposure of 17 738 patient‐years that also showed no indication of hepatic or renal toxicity with tofacitinib.31
The authors acknowledge some limitations of this study. The study included data from clinical trials, and the selection criteria for the trials were such that the study populations may not fully reflect the characteristics of patients seen in everyday clinical practice. The clinical trials included limited numbers of patients and involved relatively short follow‐up periods compared with the time course of a chronic disease like psoriasis, and therefore estimates of rare safety events, such as malignancies, may be less reliable than when long‐term data are available.
As psoriasis is a chronic disease, often occurring from an early age, over their lifetimes most patients will receive multiple therapies, with some not responding well to any; there is therefore an unmet need for new and effective treatment options with acceptable safety profiles. While the efficacy of tofacitinib is somewhat lower than that of some biological psoriasis treatments, the efficacy of tofacitinib 10 mg BID is comparable with that of etanercept 50 mg twice weekly.26
The safety profile of tofacitinib is generally comparable with that of biologics, with the exception of the higher rates of herpes zoster seen with tofacitinib. However, the parenteral administration of biologics might be an issue for some patients. The oral route of administration of tofacitinib is more convenient and may be preferred by patients over subcutaneous injection or intravenous infusion;12, 40, 41 it also bypasses the risk of injection‐ or infusion‐site‐related AEs, one of the most frequently reported AEs for biological therapies.42, 43
Overall, tofacitinib has a benefit–risk profile in patients with moderate‐to‐severe plaque psoriasis consistent with those of other systemic psoriasis treatments, with significant improvement in clinical signs and symptoms and an acceptable safety profile.
Supporting information
Appendix S1 Contextualization of the findings.
Table S1 Details of the included studies.
Table S2 Incidence rate (events per 100 patient‐years) ranges reported in published randomized controlled trials.
Table S3 Demographic and baseline characteristics of the study patients (all studies).
Table S4 Incidence rates (patients with events per 100 patient‐years) for safety events of interest in patients with psoriasis treated with tofacitinib over 1 year in the phase III studies.
Fig S1. Proportions of patients with Hospital Anxiety and Depression Scale (HADS) depression score < 8 at week 16 among those with HADS depression score ≥ 8 at baseline.
Fig S2. Incidence rates (events per 100 patient‐years) in observational studies and Pfizer‐commissioned comparison cohorts.
Acknowledgments
The authors would like to thank Maryam Asgari and Charlie Quesenberry, principal investigators of the KPNC database cohort study, and Kevin Winthrop and Jeffrey Curtis, principal investigators of the Medicare database cohort. This study was supported by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Sandrine M. Dupré, PhD, and Carole Evans, PhD, at and on behalf of Complete Medical Communications, Manchester, U.K., and was funded by Pfizer Inc., New York, NY, U.S.A., in accordance with the Good Publication Practice (GPP3) guidelines.44
Conflicts of interest: B.E.S. has been a consultant and advisory board member for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene, Cutanea‐Maruho, Dermira, Janssen, LEO Pharma, Lilly, Medac, Novartis, Pfizer Inc., Sun Pharma, UCB and Valeant (honoraria for all); an investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, GlaxoSmithKline, Novartis, Janssen, Lilly, Merck, Pfizer Inc. and Sun Pharma (payments to the University of Connecticut, not to B.E.S.) and scientific director for the CORRONA Psoriasis Registry (consulting fee); and received grant support to the University of Connecticut for a fellowship programme from AbbVie and Janssen (payments to the University of Connecticut, not to B.E.S.). A.B.G. has been a consultant or advisory board member for AbbVie, Aclaris, Actelion, Akros, Allergan, Amgen, Amicus, Astellas, Baxalta, Beiersdorf, Bristol‐Myers Squibb, Canfite, Catabasis, Celgene, Centocor (Janssen), Coronado, Crescendo Bioscience, CSL Behring Biotherapies for Life, Dermipsor, Dermira, Genentech, GlaxoSmithKline, Incyte, Karyopharm, Kineta One, KPI Therapeutics, Lilly, Meiji Seika Pharma Co., Mitsubishi Tanabe Pharma Development America, Novartis, Novo Nordisk, Pfizer Inc., Reddy Labs, Takeda, TEVA, UCB, Valeant, Vertex and Xenoport; and received research or educational grants from Janssen Incyte. P.C.M.vdK. has been a consultant for Abbott, Almirall, Amgen, Celgene, Centocor, Galderma, Janssen‐Cilag, LEO Pharma, Lilly, Mitsubishi, Novartis, Pfizer Inc., Philips and Sandoz; and an investigator for AbbVie, Amgen, Basilea, Janssen‐Cilag, LEO Pharma, Lilly, Pfizer Inc. and Philips. L.P. has been an advisory board member, speaker, consultant or investigator for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer Inc., Regeneron, Roche, Sandoz, Sanofi and Sun Pharma. H.B. has been an advisory board member, speaker, consultant or investigator for AbbVie, Amgen, Anaptysbio, Baxalta, Bayer, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer Inc., Pierre Fabre, Sun Pharma and Takeda. E.C. has been a speaker, consultant or investigator for AbbVie, Galderma, Lilly, Merck, Novartis, Pfizer Inc. and Roche. S.I. has been a speaker or consultant for AbbVie, Celgene, Janssen, Lilly, Maruho, Novartis, Pfizer Inc. and Torii Yakuhin. D.T. has received research support from AbbVie, Almirall, Amgen, Astellas, Biogen Idec, Boehringer Ingelheim, Celgene, Dignity, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Lilly, MSD, Novartis, Pfizer Inc., Regeneron and Sandoz; has received honoraria from AbbVie, Biogen Idec, Celgene, Janssen, LEO Pharma, Novartis and Pfizer Inc.; has been a consultant for AbbVie, Celgene, Dignity, Galapagos, Novartis, Pfizer Inc. and Xenoport; and has been on scientific advisory boards for AbbVie, Celgene, GlaxoSmithKline, Janssen, LEO Pharma, Lilly, Novartis, Pfizer Inc., Sandoz and Sanofi. H.T., H.V., P.G., V.F. and R.W. are employees and shareholders of Pfizer Inc. M.K. was an employee of Pfizer Inc. at the time the studies were conducted.
Funding sources This study was funded by Pfizer Inc.
Conflicts of interest Conflicts of interest statements can be found in the Appendix.
Plain language summary available online
References
- 1. Gelfand JM, Weinstein R, Porter SB et al Prevalence and treatment of psoriasis in the United Kingdom: a population‐based study. Arch Dermatol 2005; 141:1537–41. [DOI] [PubMed] [Google Scholar]
- 2. Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol 2009; 60:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Augustin M, Reich K, Glaeske G et al Co‐morbidity and age‐related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venereol 2010; 90:147–51. [DOI] [PubMed] [Google Scholar]
- 4. Pathirana D, Ormerod AD, Saiag P et al European S3‐guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009; 23(Suppl. 2):1–70. [DOI] [PubMed] [Google Scholar]
- 5. Menter A, Korman NJ, Elmets CA et al Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case‐based presentations and evidence‐based conclusions. J Am Acad Dermatol 2011; 65:137–74. [DOI] [PubMed] [Google Scholar]
- 6. Wadhwa M, Knezevic I, Kang HN, Thorpe R. Immunogenicity assessment of biotherapeutic products: an overview of assays and their utility. Biologicals 2015; 43:298–306. [DOI] [PubMed] [Google Scholar]
- 7. Weidmann A, Foulkes AC, Kirkham N, Reynolds NJ. Methotrexate toxicity during treatment of chronic plaque psoriasis: a case report and review of the literature. Dermatol Ther (Heidelb) 2014; 4:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mansouri Y, Goldenberg G. Biologic safety in psoriasis: review of long‐term safety data. J Clin Aesthet Dermatol 2015; 8:30–42. [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong AW, Robertson AD, Wu J et al Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol 2013; 149:1180–5. [DOI] [PubMed] [Google Scholar]
- 10. Callis Duffin K, Yeung H, Takeshita J et al Patient satisfaction with treatments for moderate‐to‐severe plaque psoriasis in clinical practice. Br J Dermatol 2014; 170:672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Cranenburgh OD, de Korte J, Sprangers MA et al Satisfaction with treatment among patients with psoriasis: a web‐based survey study. Br J Dermatol 2013; 169:398–405. [DOI] [PubMed] [Google Scholar]
- 12. Wilson LS, Loucks A, Gipson G et al Patient preferences for attributes of multiple sclerosis disease‐modifying therapies: development and results of a ratings‐based conjoint analysis. Int J MS Care 2015; 17:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bissonnette R, Iversen L, Sofen H et al Tofacitinib withdrawal and retreatment in moderate‐to‐severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015; 172:1395–406. [DOI] [PubMed] [Google Scholar]
- 14. Bachelez H, van de Kerkhof PC, Strohal R et al Tofacitinib versus etanercept or placebo in moderate‐to‐severe chronic plaque psoriasis: a phase 3 randomised non‐inferiority trial. Lancet 2015; 386:552–61. [DOI] [PubMed] [Google Scholar]
- 15. Papp KA, Menter MA, Abe M et al Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo‐controlled, phase III trials. Br J Dermatol 2015; 173:949–61. [DOI] [PubMed] [Google Scholar]
- 16. Asahina A, Etoh T, Igarashi A et al Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double‐blind, phase 3 study. J Dermatol 2016; 43:869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menter A, Papp KA, Tan H et al Efficacy of tofacitinib, an oral Janus kinase inhibitor, on clinical signs of moderate‐to‐severe plaque psoriasis in different body regions. J Drugs Dermatol 2014; 13:252–6. [PubMed] [Google Scholar]
- 18. Papp KA, Menter A, Strober B et al Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo‐controlled dose‐ranging study. Br J Dermatol 2012; 167:668–77. [DOI] [PubMed] [Google Scholar]
- 19. Papp KA, Krueger JG, Feldman SR et al Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: long‐term efficacy and safety results from 2 randomized phase‐III studies and 1 open‐label long‐term extension study. J Am Acad Dermatol 2016; 74:841–50. [DOI] [PubMed] [Google Scholar]
- 20. Hongbo Y, Thomas CL, Harrison MA et al Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? J Invest Dermatol 2005; 125:659–64. [DOI] [PubMed] [Google Scholar]
- 21. Valenzuela F, Paul C, Mallbris L et al Tofacitinib versus etanercept or placebo in patients with moderate to severe chronic plaque psoriasis: patient‐reported outcomes from a phase 3 study. J Eur Acad Dermatol Venereol 2016; 30:1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52:69–77. [DOI] [PubMed] [Google Scholar]
- 23. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19:210–16. [DOI] [PubMed] [Google Scholar]
- 24. Demirsoy EO, Kiran R, Salman S et al Effectiveness of systemic treatment agents on psoriatic nails: a comparative study. J Drugs Dermatol 2013; 12:1039–43. [PubMed] [Google Scholar]
- 25. Jemec GB, Ibler KS. Treatment of nail psoriasis with TNF‐α or IL12/23 inhibitors. J Drugs Dermatol 2012; 11:939–42. [PubMed] [Google Scholar]
- 26. Checchio T, Ahadieh S, Gupta P et al Quantitative evaluations of time‐course and treatment effects of systemic agents for psoriasis: a model‐based meta‐analysis. Clin Pharmacol Ther 2017; 102:1006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sticherling M, Mrowietz U, Augustin M et al Secukinumab is superior to fumaric acid esters in treating subjects with moderate to severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol 2017; 177:1024–32. [DOI] [PubMed] [Google Scholar]
- 28. Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol 2011; 25:1007–11. [DOI] [PubMed] [Google Scholar]
- 29. Mazzotta A, Esposito M, Costanzo A, Chimenti S. Efficacy and safety of etanercept in psoriasis after switching from other treatments: an observational study. Am J Clin Dermatol 2009; 10:319–24. [DOI] [PubMed] [Google Scholar]
- 30. Cohen SB, Tanaka Y, Mariette X et al Long‐term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8·5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017; 76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wollenhaupt J, Silverfield J, Lee EB et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open‐label, long‐term extension studies over 9 years. Presented at the American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, San Diego, CA, U.S.A., 3–8 November 2017; Abstr. 522
- 32. Sandborn WJ, Su C, Sands BE et al Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376:1723–36. [DOI] [PubMed] [Google Scholar]
- 33. Mease P, Hall S, Fitzgerald O et al Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017; 377:1537–50. [DOI] [PubMed] [Google Scholar]
- 34. Gladman D, Rigby W, Azevedo VF et al Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017; 377:1525–36. [DOI] [PubMed] [Google Scholar]
- 35. Asahina A, Torii H, Ohtsuki M et al Safety and efficacy of adalimumab treatment in Japanese patients with psoriasis: results of SALSA study. J Dermatol 2016; 43:1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amital H, Arnson Y, Chodick G, Shalev V. Hepatotoxicity rates do not differ in patients with rheumatoid arthritis and psoriasis treated with methotrexate. Rheumatology (Oxford) 2009; 48:1107–10. [DOI] [PubMed] [Google Scholar]
- 37. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low‐dose long‐term treatment. J Eur Acad Dermatol Venereol 2000; 14:382–8. [DOI] [PubMed] [Google Scholar]
- 38. National Institutes of Health . LiverTox. Clinical and research information on drug‐induced liver injury. Available at: https://livertox.nih.gov (last accessed 28 September 2018).
- 39. Lebwohl M, Feldman SR, Walther R et al Clinical management of psoriasis: principles and practice. Cutis 2001; 67:1–15. [PubMed] [Google Scholar]
- 40. Fraenkel L, Gulanski B, Wittink D. Patient willingness to take teriparatide. Patient Educ Couns 2007; 65:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Louder AM, Singh A, Saverno K et al Patient preferences regarding rheumatoid arthritis therapies: a conjoint analysis. Am Health Drug Benefits 2016; 9:84–93. [PMC free article] [PubMed] [Google Scholar]
- 42. Farahnik B, Beroukhim K, Nakamura M et al Anti‐IL‐17 agents for psoriasis: a review of phase III data. J Drugs Dermatol 2016; 15:311–16. [PubMed] [Google Scholar]
- 43. Sorenson E, Koo J. Evidence‐based adverse effects of biologic agents in the treatment of moderate‐to‐severe psoriasis: providing clarity to an opaque topic. J Dermatolog Treat 2015; 26:493–501. [DOI] [PubMed] [Google Scholar]
- 44. Battisti WP, Wager E, Baltzer L et al Good Publication Practice for communicating company‐sponsored medical research: GPP3. Ann Intern Med 2015; 163:461–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Contextualization of the findings.
Table S1 Details of the included studies.
Table S2 Incidence rate (events per 100 patient‐years) ranges reported in published randomized controlled trials.
Table S3 Demographic and baseline characteristics of the study patients (all studies).
Table S4 Incidence rates (patients with events per 100 patient‐years) for safety events of interest in patients with psoriasis treated with tofacitinib over 1 year in the phase III studies.
Fig S1. Proportions of patients with Hospital Anxiety and Depression Scale (HADS) depression score < 8 at week 16 among those with HADS depression score ≥ 8 at baseline.
Fig S2. Incidence rates (events per 100 patient‐years) in observational studies and Pfizer‐commissioned comparison cohorts.


