Summary
Background
Hidradenitis suppurativa (HS), a chronic cutaneous disease, can negatively affect work life.
Objectives
This retrospective cohort study evaluates the indirect burden among employed patients with HS in the U.S.A.
Methods
Newly diagnosed and general patients with HS, who were employees (age 18–64 years) from a large claims database (Q1 1999 to Q1 2015), were matched 1 : 5 to controls. Income growth and risk of leaving the workforce were assessed among the newly diagnosed HS and control cohorts in the 5‐year study period. Income, work loss days and indirect costs (absenteeism and disability) were assessed among the general HS and control cohorts in the 1‐year study period.
Results
Newly diagnosed (n = 1003, mean age 39·5 years, 66·3% female) and general patients with HS (n = 1204, mean age 39·9 years, 69·1% female) were matched to 5015 and 6020 controls, respectively. Newly diagnosed patients with HS had significantly slower income growth ($324 per year) and higher risk of leaving the workforce (adjusted hazard ratio 1·65, 95% confidence interval 1·45–1·88) compared with controls (all P < 0·05). General patients with HS had more total days of work loss (18·4 vs. 7·7), higher annual total indirect costs ($2925 vs. $1483) and lower annual income ($54 925 vs. $62 357) than controls (all P < 0·001).
Conclusions
Patients with newly diagnosed HS and general patients with HS experienced a greater indirect burden than matched controls.
Short abstract
What's already known about this topic?
Hidradenitis suppurativa (HS), a chronic inflammatory disease, can negatively affect work life.
What does this study add?
This retrospective claims analysis found that HS is associated with high indirect burden, in terms of slower income growth, higher risk of leaving the workforce and higher indirect costs.
These results indicate that patients with HS have unmet disease management needs.
Linked Editorial: Zouboulis. Br J Dermatol 2019; 181:7–8.
Plain language summary available online
Hidradenitis suppurativa (HS) is a chronic inflammatory cutaneous disease that affects apocrine‐gland‐bearing regions of the skin, and can be potentially debilitating.1, 2 Estimates of the overall prevalence of HS in the U.S.A. population have varied widely, from 0·1% to 4%,3, 4 but it affects women approximately two to three times more frequently than men.5, 6 HS typically develops between puberty and the age of 40 years, and thus impacts individuals at working age.7
The overarching goals of pharmacological therapy in HS are to clear or reduce the number and extent of current lesions, prevent new lesions from forming and minimize pain and scarring.8 The most commonly used treatments for HS include topical or systemic antibiotics and intralesional or systemic corticosteroids.9 However, no single pharmacological agent is universally effective in all patients with HS,10, 11 and to date the anti‐tumour necrosis factor biologic adalimumab, approved in 2015, is the first and only U.S.A. Food and Drug Administration‐approved treatment for HS.12
The painful lesions and chronic nature of HS affect many facets of patients’ lives, including having a detrimental impact on quality of life.1, 13, 14 Patients affected by HS often experience distress, depression and other psychological comorbidities, and the physical symptoms can lead to feelings of embarrassment, shame and social isolation.15, 16 In addition, patients with HS experience a substantial economic burden, with the total direct healthcare costs of patients with HS estimated to be $6783 higher than those of controls over a 3‐year period.17 HS has also been demonstrated to have a significant impact on patients’ work productivity and negatively affect opportunities for career advancement. In a survey of employed patients with HS, 58% reported that the disease caused a work absence, with a mean absence of around 34 days annually.18 However, little is known regarding the indirect burden of HS such as lost income, the risk of leaving the workforce, days of work lost and indirect costs.
To address this gap in the literature, this study assessed the impact of HS on income growth and risk of leaving the workforce comparing patients with newly diagnosed (i.e. incident) HS and matched controls. In addition, this study evaluated the impact of HS on the annual income, days of work lost and annual indirect costs comparing general patients with HS (i.e. incident or prevalent) and matched controls.
Patients and methods
Data source
The data for this study were derived from OptumHealth Care Solutions, Inc. (Q1 1999 to Q1 2015). This database includes the administrative claims for over 18 million privately insured individuals covered by 84 self‐insured Fortune 500 companies across the U.S.A. Medical and drug claims and eligibility data are available for all beneficiaries. Measures of work loss were available for primary policyholders (i.e. employees) in 43 companies, including patient records on disability (short term and long term, leave of absence and early retirement). Employer‐reported income data were available for a subgroup of employees. The data were deidentified and complied with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. Thus, no institutional board review was required.
Study population and study cohorts
Two patient populations were selected: a newly diagnosed HS cohort and a general HS cohort. Newly diagnosed patients with HS were selected to evaluate the impact of HS diagnosis on income and the risk of leaving the workforce over time. A general HS cohort (incident or prevalent HS) was selected to evaluate annual income, days of work lost and annual indirect costs to illustrate the indirect burden among the overall population of patients with HS.
The HS cohorts included patients with at least two diagnoses for HS [International Classification of Diseases, 9th Revision (ICD‐9) code 705·83] and who had continuous enrolment in a healthcare plan, were aged 18–64 years, were employed or actively employed and had income data available. The risk of leaving the workforce was assessed among newly diagnosed patients with HS with work loss data available. All patients in the general HS cohort were required to have work loss data. The index date was defined as the initial date of HS diagnosis for the newly diagnosed HS cohort and as a randomly selected HS diagnosis date for the general HS cohort. It was required that the newly diagnosed patients with HS had no claims with HS during the 12 months prior to the index date.
The control cohorts included patients without any HS diagnosis through their entire claims history, and they were otherwise required to meet the same selection criteria as the corresponding HS cohorts. Controls were matched 5 : 1 to the HS cohorts exactly on sex, birth year, index year and region. Health insurance plan type was also matched to the newly diagnosed HS cohort.
For the newly diagnosed HS and control cohorts, the study period ran from the index date to the earliest event among the end of continuous eligibility, end of employment, patients turning 65 years old and 5 years after the index date; for the general HS and control cohorts, the study period was the 12‐month period after the index date. Further details are provided in Appendix S1 (see Supporting Information).
Study measures
Baseline characteristics
For the newly diagnosed HS and control cohorts, patient characteristics summarized during the baseline period included demographics (age, sex, region and type of health insurance), comorbidities and annual income. HS‐related comorbidities and modified Charlson Comorbidity Index (CCI) (excluding HS‐related comorbidities) were identified using ICD‐9 diagnostic codes (Tables S1 and S2; see Supporting Information). Annual income was measured by employer‐reported annualized income (wage and salary).
In the general HS and control cohorts, in addition to the patient characteristics listed above, total work loss days (disability days and medically related absenteeism days) and annual indirect costs (disability costs and medically related absenteeism costs) were also estimated during the baseline period. Disability days were calculated based on patient records in the disability claims. Medically related absenteeism days were calculated based on the use of medical services during business days (e.g. an office visit or a hospital inpatient visit during Monday to Friday), as well as the waiting period in advance of the start of disability benefit (e.g. five missed days of work due to illness).
The algorithm assumed that each hospitalization day and emergency room visit accounted for a full day of work loss, while each outpatient or other medical visit accounted for half a day of work loss. Disability costs were based on actual employer disability payments reported in the data. Medically related absenteeism costs were calculated as days of medically related absenteeism multiplied by the employee's daily income. Income and costs were inflated to 2015 U.S.A. dollars using the all‐item component of the Consumer Price Index.
Study outcomes
In the newly diagnosed HS and control cohorts, income growth over time and risk of leaving the workforce were assessed during the study period of up to 5 years. Annual income during the study period was estimated using the same methods as described in the baseline period. Patients were observed during the study period as to whether they left the workforce due to the following events (based on patient records from the data): short‐term disability, long‐term disability, leave of absence and early retirement.
In the general HS and control cohorts, days of work loss, annual indirect costs and annual income were assessed during the 1‐year study period. Total days of work loss, annual total indirect costs and annual income during the study period were estimated using the same methods as described in the baseline period.
Statistical methods
All statistical analyses were performed using SAS software version 9·4 (IBM, Armonk, NY, U.S.A.), and a P‐value of 0·05 was used to determine statistical significance.
Baseline characteristics (except for the matched variables) were compared between study cohorts using generalized estimating equations, which accounted for the correlation between the patients with HS and their matched controls. In the newly diagnosed HS and control cohorts, income growth over time was assessed using a multivariable generalized linear mixed model, which accounted for matching and the correlation within patients due to the repeated measures of income. The multivariable model adjusted for modified CCI score and baseline annual income. Kaplan–Meier analyses with log‐rank tests were used to assess the risk of leaving the workforce. The time to each workforce leave event was defined as the time from the index date to the earliest record that indicated the event of interest in the disability claims. Patients without a workforce leave event during the study period were censored. Cox proportional hazards models were used with adjustment for modified CCI.
In the general HS and control cohorts, incidence rates of work loss days per patient‐year, annual indirect costs and annual income were described and compared between study cohorts using multivariable generalized estimating equation models adjusted for modified CCI and health insurance plan at baseline.
Results
Sample selection
In total, 1003 patients with HS were included in the newly diagnosed HS cohort, and were matched with 5015 controls for the analysis of income growth (Fig. 1). In the newly diagnosed HS and control cohorts, 798 patients with HS and 3990 matched controls had work loss data available and were included in the analysis for risk of leaving the workforce.
Figure 1.
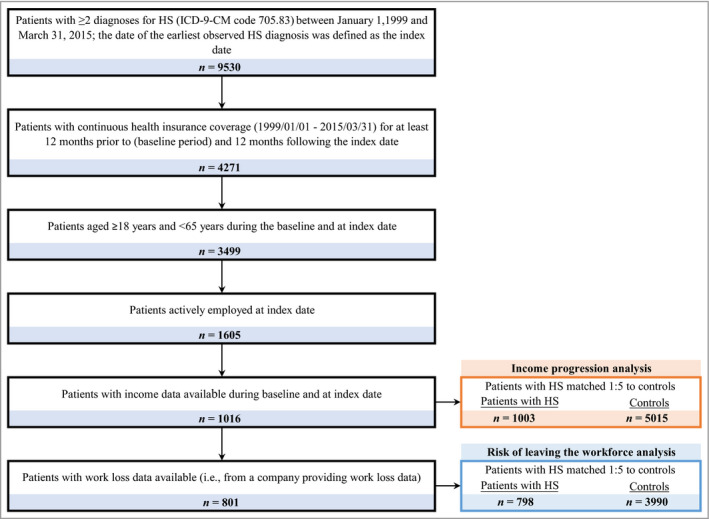
Sample selection of newly diagnosed patients with hidradenitis suppurativa (HS). ICD‐9‐CM, International Classification of Diseases, 9th Revision, Clinical Modification.
The general HS cohort was comprised of 1204 patients with HS, who were matched with 6020 controls for work loss and indirect cost analyses (Fig. 2). In the general HS cohort, 1086 patients with HS and 5430 matched controls were actively employed at the index date and were included in the annual income analysis.
Figure 2.
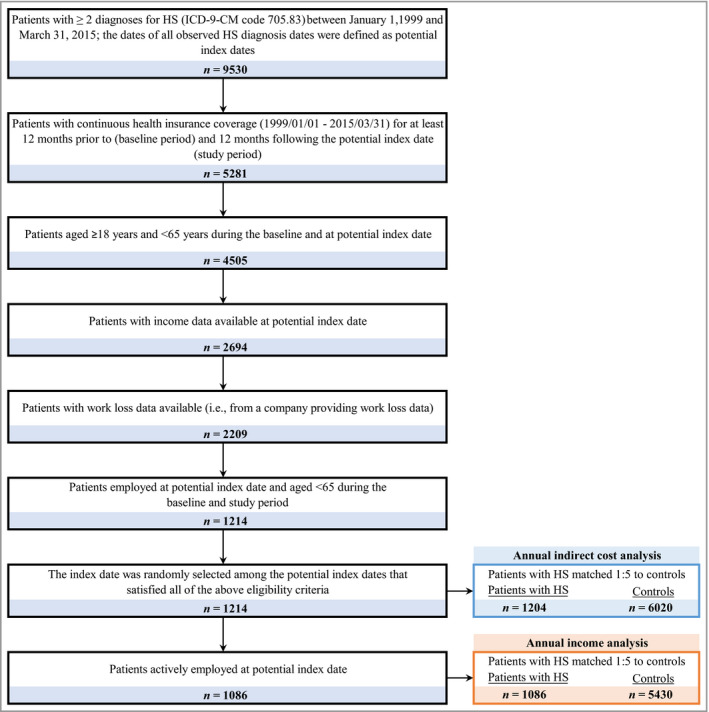
Sample selection of patients with general hidradenitis suppurativa (HS). ICD‐9‐CM, International Classification of Diseases, 9th Revision, Clinical Modification.
Baseline characteristics
The mean ages of patients in the newly diagnosed and general HS cohorts were 39·5 and 39·9 years, respectively; 66·3% vs. 69·1% were female, and 64·0% vs. 62·2% had preferred provider organization insurance. In both cohorts, patients with HS had significantly higher percentages of HS‐related comorbidities (P < 0·001 for all except for anxiety), higher rates of smoking (P < 0·001) and a higher mean modified CCI than matched controls. Patients with HS had significantly lower annual income during the baseline period (both cohorts P < 0·001) (Tables S3 and S4; see Supporting Information).
Income growth and risk of leaving the workforce in the newly diagnosed hidradenitis suppurativa and control cohorts
Among the cohort of newly diagnosed patients with HS and matched controls, the adjusted results of the analysis of income growth over time showed that income growth in the first year after HS diagnosis was $547 less for patients with HS than for the control cohort (Fig. 3). After the first year, income growth for the HS cohort was on average $324 less per year than for the control cohort (P = 0·002). By the fifth year after the index date, the cumulative income growth for the HS cohort was $1843 less than that of matched controls ($4299 vs. $6142).
Figure 3.
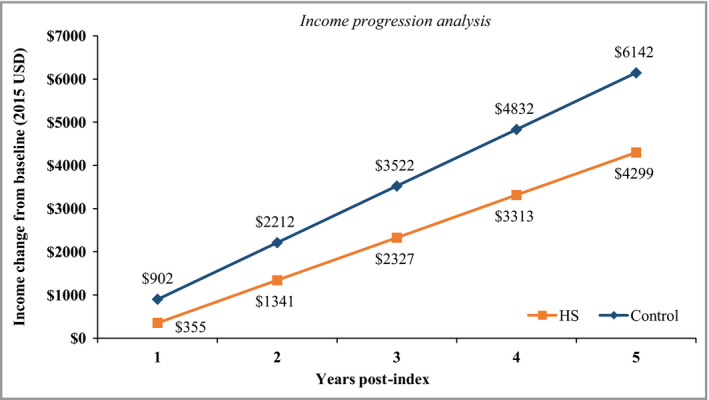
Regression‐adjusted income growth from baseline for patients with hidradenitis suppurativa (HS) vs. control patients. Modified Charlson Comorbidity Index and baseline income were adjusted for in the model. USD, U.S.A. dollars.
The median follow‐up times for the analysis of the risk of leaving the workforce were 3·4 years for the HS cohort and 2·2 years for matched controls. Over the 5 years following HS diagnosis, patients with HS were significantly more likely than matched controls to have a leave of absence, short‐term disability or long‐term disability, or to leave the workforce for any reason (all log‐rank test P < 0·05) (Fig. 4). Moreover, patients with HS were less likely than controls to have early retirement (P < 0·001).
Figure 4.
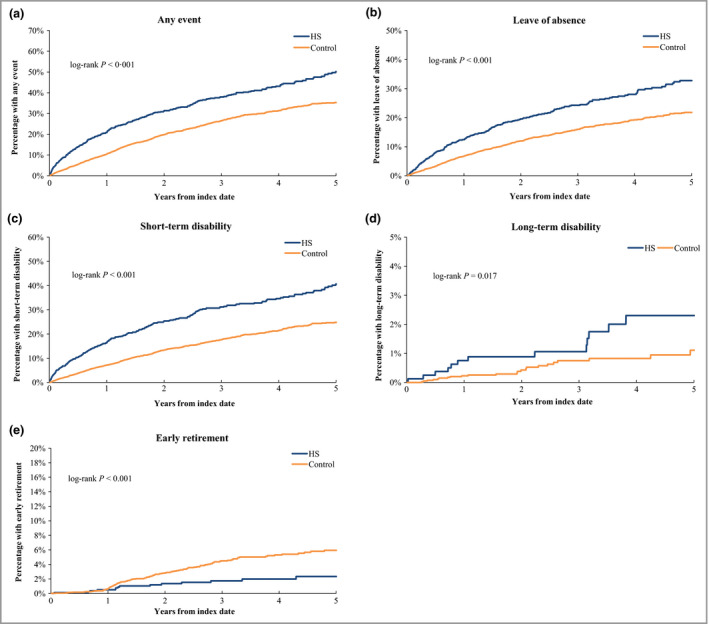
Kaplan–Meier analysis for risk of leaving the workforce in the hidradenitis suppurativa (HS) and control cohorts. The median was not reached in all of the analyses.
The results of the multivariate analyses were consistent with the unadjusted analyses (Table 1). Compared with control patients, patients with HS were more likely to experience a work leave event [adjusted hazard ratio (HR) 1·7, 95% confidence interval (CI) 1·5–1·9; P < 0·001], including a leave of absence (HR 1·6, 95% CI 1·4–1·9; P < 0·001), short‐term disability (HR 1·9, 95% CI 1·7–2·2; P < 0·001) and long‐term disability (HR 2·1, 95% CI 1·0–4·4; P = 0·044). Additionally, control patients were more likely to leave the workforce due to early retirement than patients with HS (adjusted HR 0·4, 95% CI 0·2–0·7; P = 0·001).
Table 1.
Cox proportional hazard models for risk of leaving the workforce associated with hidradenitis suppurativa (HS)
| Events | Adjusted HR (95% CI)a | P‐value |
|---|---|---|
| Any event | 1·65 (1·45–1·88) | < 0·001 |
| Leave of absence | 1·64 (1·40–1·93) | < 0·001 |
| Short‐term disability | 1·94 (1·67–2·24) | < 0·001 |
| Long‐term disability | 2·12 (1·02–4·42) | 0·044 |
| Early retirement | 0·40 (0·23–0·70) | 0·001 |
HR, hazard ratio; CI, confidence interval. aModified Charlson Comorbidity Index was adjusted for in the models. P‐values < 0·05 are significant.
Work loss, indirect costs and annual income in the general hidradenitis suppurativa and control cohorts
Among the general HS cohort and matched controls, on average, patients with HS had significantly more total days of work loss than controls [18·4 vs. 7·7; adjusted incidence rate ratio (IRR) 2·3, 95% CI 2·0–2·6], more medically related absenteeism days (7·8 vs. 4·1; adjusted IRR 1·8, 95% CI 1·7–2·0) and more disability days (10·6 vs. 3·6; adjusted IRR 2·8, 95% CI 2·2–3·5) (all P < 0·001; Table 2).
Table 2.
Medically related absenteeism and disability days comparing HS and control cohorts
| Observed incidence rate | Unadjusted IRR | Adjusted IRRa | ||||
|---|---|---|---|---|---|---|
| HS patients | Control patients | IRR (95% CI) | P‐value | IRR (95% CI) | P‐value | |
| N = 1204 | N = 6020 | |||||
| Total days of lost work | 18·37 | 7·72 | 2·38 (2·09, 2·71) | <0·001* | 2·29 (2·03, 2·59) | <0·001* |
| Medically‐related absenteeism days | 7·75 | 4·14 | 1·87 (1·76, 2·00) | <0·001* | 1·83 (1·71, 1·95) | <0·001* |
| Disability days | 10·62 | 3·58 | 2·97 (2·36, 3·73) | <0·001* | 2·79 (2·23, 3·49) | <0·001* |
CI, confidence interval; HS, hidradenitis suppurativa; IRR, incidence rate ratio; N, number. *P < 0·05. aModified CCI and health insurance plan were adjusted for in the models.
In addition, patients with HS were observed to have higher total annual indirect costs ($2925 vs. $1483, respectively), medically related absenteeism costs ($1598 vs. $1021) and disability costs ($1328 vs. $461) compared with matched controls (all P < 0·001). In the adjusted analysis, the cost difference was $1317 for total indirect costs, $538 for medically related absenteeism costs and $765 for disability costs between patients with HS and matched controls (all P < 0·001; Table 3). Patients with HS also had significantly lower mean annual income than matched controls ($54 925 vs. $62 357, respectively), with an adjusted difference of −$7291 (P < 0·001; Table 3).
Table 3.
Annual indirect costs and income comparing HS and control cohorts
| Observed value | Unadjusted difference (2015 USD) (HS vs. Control) | P‐value | Adjusted differencea (2015 USD) (HS vs. Control) | P‐value | ||
|---|---|---|---|---|---|---|
| HS patients | Control patients | |||||
| N = 1204 | N = 6020 | |||||
| Total indirect healthcare costs | 2925 ± 6160 | 1483 ± 3572 | 1442 | <0·001* | 1317 | <0·001* |
| Medically‐related absenteeism costs | 1598 ± 2119 | 1021 ± 1955 | 577 | <0·001* | 538 | <0·001* |
| Disability costs | 1328 ± 5841 | 461 ± 2834 | 867 | <0·001* | 765 | <0·001* |
| Annual income | 54 925 ± 36 189 | 62 357 ± 52 792 | −7432 | <0·001* | −7291 | <0·001* |
HS, hidradenitis suppurativa; N, number; USD, US dollars. *P < 0·05. aModified CCI and health insurance plan were adjusted for the models.
Discussion
Because HS predominantly affects individuals during working age and can negatively impact patients both physically and mentally, it is expected that HS would have a substantial indirect burden. However, to date there is limited information in the published literature on the indirect burden of HS. The current study addressed this knowledge gap by comparing the annual income and income trajectory, risk of leaving the workforce, work loss days and indirect cost between working‐age patients with HS and matched controls.
Few studies have comprehensively examined the indirect burden of HS, and these have been limited by small sample sizes and a focus on non‐U.S.A. patients. Matusiak et al. studied the impact of HS on quality of life and professional activity among 54 Polish patients with HS aged 16–65 years.18 They reported that HS caused work absence in 58% of 30 employed and professionally active patients with HS; on average, there were three incidents of missed work annually, totalling 34 days.18 The results of the current study are largely consistent with the findings from the previous literature; the differences in results are likely due to the methodological differences and small sample sizes of the previous studies. For example, the difference in annual work loss among patients with HS observed in this study (18 days) vs. Matusiak et al. (34 days) was likely introduced by the difference in the reporting algorithm (employer reported in claims analysis vs. self‐reported in survey, respectively).
To the best of our knowledge, this is the first study to assess the indirect burden of privately insured patients with HS in the U.S.A., and the current approach has several strengths. Firstly, various components of the indirect burden associated with HS were assessed in this study, including income, risk of leaving the workforce, days of work loss and indirect costs. Secondly, we assessed the indirect burden of HS from the perspectives of both newly diagnosed and general (incident and prevalent) patients with HS. The newly diagnosed cohort provided evidence on how income growth and risk of leaving the workforce evolved over time compared with those of patients without HS, and the general HS and control cohorts provided a random snapshot of the indirect burden associated with incident and prevalent HS, including days of work loss, indirect costs and annual income. Thirdly, matching and multivariable regression analyses mitigated the differences between the patients with HS and the controls. The consistent results across multiple outcomes strengthened the findings and comprehensively depicted the overall indirect burden of HS on the work life of patients. Compared with matched controls, patients with HS had a higher risk of taking leaves of absence and short‐ and long‐term disability, and were less likely to retire early. The early‐retirement results might be due to the financial constraints experienced by patients with HS due to the increased medical costs and reduced income compared with matched controls.
The results of this study should be considered in light of several limitations. Some of the limitations are common across retrospective observational studies based on healthcare claims data, including potential data omissions, coding errors and unmeasured differences between the patients with HS and the matched control patients. The database did not contain information regarding the severity of HS; thus, subgroup analyses based on disease severity could not be performed. Similarly, the reasons for leaving the workforce were not available, and HS‐specific events leading to leaving the workforce could not be evaluated. In addition, all patients were required to have work loss and income data available, which might not be generalizable to the broader HS population. However, the availability of work loss data from each company is likely random and not associated with the work loss outcomes; it should therefore have minimal impact on the generalizability of the results. Lastly, as the cost of presenteeism was not captured in this study, the indirect costs may have been underestimated.
In conclusion, this study provides real‐world evidence that HS is associated with a considerable indirect burden including lower annual income and income growth, higher risk of leaving the workforce, more days of work loss and higher indirect costs. Effective treatments for HS may reduce this incremental indirect burden.
Supporting information
Appendix S1 Diagnostic criteria in the newly diagnosed and general hidradenitis suppurativa cohorts.
Table S1 International Classification of Diseases, 9th Revision codes used to identify hidradenitis suppurativa‐related comorbidities.
Table S2 International Classification of Diseases, 9th Revision codes used to identify Charlson Comorbidity Index comorbidities, excluding hidradenitis suppurativa‐related comorbidities.
Table S3 Baseline characteristics of newly diagnosed patients with hidradenitis suppurativa and controls.
Table S4 Baseline characteristics of general patients with hidradenitis suppurativa and controls.
Powerpoint S1. Journal Club Slide Set.
Acknowledgments
The authors would like to thank Jing Zhao, PhD from Analysis Group for contributions towards medical writing and analytical support. This support was funded by AbbVie.
Funding sources
Design, study conduct and financial support for the study were provided by AbbVie, Inc.; AbbVie participated in the interpretation of the data and review and approval of the manuscript. All authors contributed to the development of the publication and maintained control over the final content.
Conflicts of interest
H.Y., F.M. and J.S. are employees of Analysis Group, Inc., which received research funding from AbbVie, Inc. for this study. B.C. is an employee and stockholder of AbbVie, Inc. T.T. participates in advisory boards for UCB and AbbVie.
Plain language summary available online
References
- 1. Wolkenstein P, Loundou A, Barrau K et al Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol 2007; 56:621–3. [DOI] [PubMed] [Google Scholar]
- 2. Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2009; 23:985–98. [DOI] [PubMed] [Google Scholar]
- 3. Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009; 60:539–61. [DOI] [PubMed] [Google Scholar]
- 4. Cosmatos I, Matcho A, Weinstein R et al Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol 2013; 69:819. [DOI] [PubMed] [Google Scholar]
- 5. Garg A, Kirby JS, Lavian J et al Sex‐ and age‐adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol 2017a; 153:760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg A, Lavian J, Lin G et al Incidence of hidradenitis suppurativa in the United States: a sex‐ and age‐adjusted population analysis. J Am Acad Dermatol 2017b; 77:118–22. [DOI] [PubMed] [Google Scholar]
- 7. von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2000; 14:389–92. [DOI] [PubMed] [Google Scholar]
- 8. Jemec GBE. Hidradenitis suppurativa. N Engl J Med 2012; 366:158–64. [DOI] [PubMed] [Google Scholar]
- 9. Nazary M, van der Zee HH, Prens EP et al Pathogenesis and pharmacotherapy of hidradenitis suppurativa. Eur J Pharmacol 2011; 672:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Micheletti RG. An update on the diagnosis and treatment of hidradenitis suppurativa. Cutis 2015; 96(6 Suppl.):7–12. [PubMed] [Google Scholar]
- 11. Kerdel FA. Current and emerging nonsurgical treatment options for hidradenitis suppurativa. Semin Cutan Med Surg 2014; 33(3 Suppl.):S57–9. [DOI] [PubMed] [Google Scholar]
- 12. Kimball AB, Okun MM, Williams DA et al Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016; 375:422–34. [DOI] [PubMed] [Google Scholar]
- 13. Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol 2011; 91:328–32. [DOI] [PubMed] [Google Scholar]
- 14. Delany E, Gormley G, Hughes R et al A cross‐sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol 2018; 32:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010a; 90:264–8. [DOI] [PubMed] [Google Scholar]
- 16. Miller IM, McAndrew RJ, Hamzavi I. Prevalence, risk factors, and comorbidities of hidradenitis suppurativa. Dermatol Clin 2016; 34:7–16. [DOI] [PubMed] [Google Scholar]
- 17. Kirby JS, Miller JJ, Adams DR, Leslie D. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol 2014; 150:937–44. [DOI] [PubMed] [Google Scholar]
- 18. Matusiak Ł, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol 2010b; 62:706–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Diagnostic criteria in the newly diagnosed and general hidradenitis suppurativa cohorts.
Table S1 International Classification of Diseases, 9th Revision codes used to identify hidradenitis suppurativa‐related comorbidities.
Table S2 International Classification of Diseases, 9th Revision codes used to identify Charlson Comorbidity Index comorbidities, excluding hidradenitis suppurativa‐related comorbidities.
Table S3 Baseline characteristics of newly diagnosed patients with hidradenitis suppurativa and controls.
Table S4 Baseline characteristics of general patients with hidradenitis suppurativa and controls.
Powerpoint S1. Journal Club Slide Set.


