Abstract
Devil's claw is used for the treatment of inflammatory symptoms and degenerative disorders in horses since many years, but without the substantive pharmacokinetic data. The pharmacokinetic parameters of harpagoside, the main active constituent of Harpagophytum procumbens DC ex Meisn., were evaluated in equine plasma after administration of Harpagophytum extract FB 8858 in an open, single‐dose, two‐treatment, two‐period, randomized cross‐over design. Six horses received a single dose of Harpagophytum extract, corresponding to 5 mg/kg BM harpagoside, and after 7 days washout period, 10 mg/kg BM harpagoside via nasogastric tube. Plasma samples at certain time points (before and 0–24 hr after administration) were collected, cleaned up by solid‐phase extraction, and harpagoside concentrations were determined by LC‐MS/MS using apigenin‐7‐glucoside as internal standard. Plasma concentration‐time data and relevant parameters were described by noncompartmental model through PKSolver software. Harpagoside could be detected up to 9 hr after administration. C max was found at 25.59 and 55.46 ng/ml, t 1/2 at 2.53 and 2.32 hr, respectively, and t max at 1 hr in both trials. AUC 0–inf was 70.46 and 117.85 ng hr ml−1, respectively. A proportional relationship between dose, C max and AUC was observed. Distribution (V z/F) was 259.04 and 283.83 L/kg and clearance (CL/F) 70.96 and 84.86 L hr−1 kg−1, respectively. Treatment of horses with Harpagophytum extract did not cause any clinically detectable side effects.
Keywords: Devil's Claw, Harpagophytum procumbens, harpagoside, horse, pharmacokinetics
1. INTRODUCTION
Harpagophytum procumbens DC ex Meisn., also called grapple plant, wood spider, and most commonly devil's claw, is an important traditional medicinal plant of the sesame family (Pedaliaceae), growing in the Kalahari region of southern Africa (Van Wyk & Gericke, 2000).
In the middle of the last century, several systematic studies revealed that extracts of the plant's secondary tuberous roots are effective in the treatment of degenerative rheumatoid arthritis, osteoarthritis and tendonitis (Stewart & Cole, 2005).
The plant and its healing effects, since, gained an international reputation as a potent anti‐inflammatory and analgesic agent. The current monograph of the European Scientific Cooperative on Phytotherapy (ESCOP) recommends Harpagophytum preparations for painful osteoarthritis, rheumatism and arthritic ailments (Mncwangi, Chen, Vermaak, Viljoen, & Gericke, 2012; Stewart & Cole, 2005).
In cases of chronic pain wherein the equine patient requires ongoing, daily pharmaceutical support to maintain functionality and quality of life, the usefulness of NSAIDs is offset by the potential of their toxicological implications like the development of gastric and intestinal ulcer (Reed, Messer, Tessman, & Keegan, 2006). In addition, long‐term treatment with NSAIDs has been associated with the suppression of normal proteoglycan synthesis in horses (Beluche, Bertone, Anderson, & Rohde, 2001). The well‐known adverse effects in long‐term use of NSAIDs are likely the contributors to the growing use of “alternative” treatments for chronic inflammatory disorders in horses (Trumble, 2005).
As a result of a number of recent studies, Harpagophytum is proposed as a complementary treatment for chronic rheumatism, tendonitis, osteoarthritis, and arthritis (Colas, Garcia, & Popot, 2006).
In vitro and in vivo studies showed that the active principle of Harpagophytum extract seems to be a sum of co‐active constituents (Chrubasik et al., 2006). Recent investigations in humans documented the iridoid glycoside harpagoside to be the pharmacologically most active (Van Haelen et al., 1983) and most effective antiphlogistic component of Harpagophytum in animal models of inflammation (Baghdikian, Lanhers, & Fleurentin, 1997; Lanhers, Fleurentin, Mortier, Vinche, & Younos, 1992; Loew, Möllerfeld, Schrödter, Puttkammer, & Kaszkin, 2001; Soulimani, Younos, Mortier, & Derrieu, 1994). Harpagoside is also referred to be the main characteristic marker of both species H. procumbens and Harpagophytum zeyheri, other iridoid glycosides such as harpagide and 8‐para‐coumaroyl harpagide are not always present in Harpagophytum extracts (Colas et al., 2006).
Various chemical and pharmacological studies on H. procumbens have been carried out to demonstrate its bioactive compounds and pharmacological effects. Recent data provide evidence that devil′s claw extracts have a broader mechanism of action than NSAIDs (Chrubasik, Model, Black, & Pollak, 2003; Chrubasik & Pollak, 2002). H. procumbens extracts were shown to exert anti‐inflammatory and analgesic activity (Georgiev et al., 2010; Mncwangi et al., 2012) by the inhibition of inflammatory mediators such as COX‐2 mediated prostaglandin (Fiebich, Heinrich, Hiller, & Kammerer, 2001; Fiebich, Munoz, Rose, Weiss, & McGregor, 2012; Huang et al., 2005; Jang et al., 2003) and suppression of leukotriene biosynthesis (Loew et al., 2001). The inhibition of COX‐1 and COX‐2 activity and nitric oxide (NO) production by an extract of H. procumbens was shown in humans (Anaute, Torres, & De Mello, 2010). In‐vitro‐data also demonstrated Harpagophytum extracts preventing the release of mediators of cartilage destruction, such as TNF‐α, IL‐1β, IL‐6, matrix metalloproteinases and, nitric oxide (Fiebich et al., 2001, 2012; Huang et al., 2005; Kazunori, Kazuya, Shunsuke, & Hideaki, 2010; Schopohl, Grüneberg, & Melzig, 2016; Schulze‐Tanzil, Hansen, & Shakibaei, 2004) and elastase (Boje, Lechtenberg, & Nahrstedt, 2003). Immune modulatory function by facilitating cell migration into the inflamed tissue, whereby in consequence the anti‐inflammatory activity of the resident macrophages was also found to be promoted by harpagoside (Schopohl et al., 2016). The reported anti‐inflammatory effect of H. procumbens could also be attributed to its antioxidant activity (Schaffer et al., 2013). The chondroprotective effect of H. procumbens in which the tissue inhibitor metalloproteinase‐2 (TIMP‐2) is involved was also proven in a study using an animal model of osteoarthritis (Chrubasik et al., 2006). Concerning the analgetic properties of H. procumbens, it was determined that the opioid system seems to be involved in the antinociceptive effect (Uchida et al., 2008).
Pharmacokinetic data of H. procumbens have previously been documented in human plasma (Loew et al., 2001). Transferring the pharmacokinetic profile of a drug from one species to another might cause problems in clinical application concerning the design of dosage schedules (Lees, 2003). Although a liquid chromatography/electrospray ionization mass spectrometric method was published to detect iridoids in equine urine and plasma by the Association of Official Racing Chemists (Colas et al., 2006), there is no report to our knowledge on the pharmacokinetic data of harpagoside in equine plasma after administration of a therapeutic dose of Harpagophytum extract.
The objective of the present study was to evaluate the main pharmacokinetic parameters of harpagoside after single intragastric administration of two different doses of the H. procumbens extract FB 8858 in horses. As biopharmaceutical data are primarily important to link results from in vitro assays and clinical studies, the study of the pharmacokinetic parameters of harpagoside (the main active constituent of H. procumbens) will also provide basic data in order to design a rational dosage regimen for extracts of devil's claw and obtain useful information for clinical treatment.
2. MATERIALS AND METHODS
2.1. Experimental trials: Horses and equine samples
Six adult clinically healthy warm‐blooded horses (1 gelding, 5 mares), with no history of joint disease, weighing 495–560 kg, were included in the study. They received three meals of a commercial feed per day. During the experimental period, each horse was kept in an individual straw‐bedded stable and given water ad libitum. The horses were kept on paddocks between the experiments.
The horses were randomly assigned to two treatment groups, using an open, single‐dose, two‐treatment, two‐period, and randomized cross‐over design. A 7 days washout period was arranged between administrations. The horses were weighed the day before each drug administration to exactly calculate the required dose of the Harpagophytum extract. The dosage was calculated based on the recommended daily dosage in humans (Chrubasik, 2004; ESCOP Monographs, 2009) considering metabolic body weight and equine body mass, respectively, and is reflecting the empirical applied dosages in horses based on the results of a clinical trial with the same H. procumbens extract conducted at the University of Veterinary Medicine Vienna, data not published. Each horse received a single harpagoside‐dose of 5 mg/kg BM (trial 1) and 10 mg/kg BM (trial 2) via nasogastric tube. A catheter was placed into the left jugular vein to facilitate blood sampling. Blood samples (8 ml) were collected into heparinized tubes before and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12 and 24 hr after intragastric administration. The blood samples were immediately centrifuged at 3900 g for 10 min. The plasma collected was stored at −20°C until analysis.
The study was discussed and approved by the institutional ethics committee (BMWF‐68.205/0109‐II/10b/2009) according to international guidelines.
2.2. Chemicals and instrumentation
Finzelberg‐Harpagophytum‐Extract FB 8858 (Ch.‐Nr. 05120116) of H. procumbens (Ch.‐Nr. 05110037) with a content of 25.3% harpagoside was provided by Finzelberg GmbH & Co. KG (Andernach, Germany).
Harpagoside, ROTICHROM®, apigenin‐7‐glucoside, ROTICHROM®, both HPLC grade, methanol, ROTISOLV®, and formic acid (98%) p.a., ROTIPURAN®, were purchased from Carl Roth GmbH & Co KG (Karlsruhe, Germany), and Vacutainer LH from Greiner Bio‐One GmbH (Kremsmünster, Austria).
Deionized water was produced using a Milli‐Q Millipore water system (Merck Millipore, Billerica, MA, USA). Supel™‐Select HLB 54182 U 60 mg/3 ml SPE Tubes for a Supelco Visiprep™ 12‐Port Model SPE workstation were obtained from Supelco, Sigma‐Aldrich GmbH (St. Louis, MO, USA).
Instrumentation: Vacuum pump: Vacobox B‐17 (Büchi Labortechnik AG, Flawil, Switzerland); SpeedVac Concentrator Savant SC 100 with Refrigerated Condensation Trap Savant RT 100A (Savant Instruments, Holbruck, NY, USA);
LC‐MS/MS: Agilent 1100 series consisting of an inline‐degasser (G1379A), binary pump (G1312A), autosampler (G1367A, WP) and UV‐detector (G1314A, VWD) (Agilent Technologies, Santa Clara, CA, USA) coupled to Esquire HCT mass spectrometer with an electrospray ionization source ESI (Bruker Daltonics, Bremen, Germany).
2.3. Sample preparation and plasma analysis
Plasma samples were cleaned up by solid‐phase extraction (SPE), according to the method described by Colas et al. (2006) with small modifications. SPE was performed with hydrophilic styrene‐based polymer Supel™‐Select HLB 60 mg/3 ml SPE cartridges using a Supelco Visiprep™ 12‐Port Model SPE workstation and a vacuum pump. The column was conditioned with 3 ml MeOH and 3 ml H2O. A 2 ml of equine plasma was loaded. The column was washed with 3 ml H2O. Harpagoside was eluted with 2 ml MeOH/CH3CN (ratio 50/50 vol/vol). The eluted samples were evaporated to dryness at 43°C with a SpeedVac concentrator. The residual extract was reconstituted in 200 μl MeOH. A 20 μl apigenin‐7‐glucoside (1 μg/ml) was added as internal standard (IS), prior to LC‐MS/MS‐analysis.
LC‐MS/MS analysis was performed on an Agilent 1100 series LC system with a Synergy Hydro RP column (2.5 μm, 150 × 2 mm), Phenomenex (Torrance, CA, USA).
The flow rate was 0.2 ml/min and an 11 min gradient from 30% to 95% methanol in 0.1% formic acid was applied within a total runtime of 25 min. A 10 μl of sample was injected.
After electrospray ionisation (ESI) harpagoside was detected using an Esquire HCT mass spectrometer in MRM mode (multiple reaction monitoring). Following, MS/MS transitions were found for harpagoside in scan mode: precursor m/z 517.2 Da fragmented to m/z 369.3, 351.3, 203.0 and 185.0 Da as product ions. For the IS apigenin‐7‐glucoside, the precursor ion m/z 433.2 Da fragmented to m/z 270.9 and 272.0 Da. Quantitation was performed adding the areas of following MS2 masses: Harpagoside [351.2, 352.2, 369.2, 370.0, 203.0 Da] and apigenin‐7‐glucoside [270.9, 272.0 Da]. The retention time of harpagoside was 12.9 min, apigenin‐7‐glucoside eluted after 11.2 min.
2.4. Validation of the LC‐MS/MS method
To determine the selectivity of the method, the chromatograms of blank, spiked and drug administered equine plasma were compared to determine the level of interference by endogenous coeluent components.
Quantification of harpagoside was performed using apigenin‐7‐glucoside as internal standard. Stock standard solutions of harpagoside (100 ng/ml) and apigenin‐7‐glucoside (IS, 1 μg/ml) were prepared separately in methanol. These solutions were stored at 4°C away from light and were found to be stable for at least 6 months. For the calibration curves, a series of 1 ml blank equine plasma was spiked with harpagoside and IS from the stock standard solutions to give harpagoside concentrations of 2.5, 5, 10, 15, 20 and 30 ng/ml. Sample preparation and LC‐MS/MS analysis of the calibration samples were performed as described above. Quantification was based on the ratio of the harpagoside peak area to internal standard peak area.
The limit of detection (LOD) was defined as the lowest concentration of spiked plasma samples resulting in a signal‐to‐noise ratio (S/N) of 3:1, and for the limit of quantification (LOQ) signal‐to‐noise ratio of 10:1. Both were determined using plasma samples spiked with harpagoside standards in concentrations of 2.5–30 ng/ml (n = 6 each). For each spiked standard the peak intensity of harpagoside was compared to the noise in the near neighborhood of the harpagoside peak. The resulting linear regression of S/N versus concentrations was used for determination of LOD and LOQ.
The accuracy of the method was assessed by adding known amounts (10 ng/ml) of the active pharmaceutical ingredient (harpagoside) to the equine blank plasma before SPE enrichment. Recovery (%) was calculated analysing four replicate QC samples. These were prepared in the same way as the calibration samples.
In order to evaluate precision, the repeatability (intra‐day and inter‐day) was analysed using the same QC samples spiked with 10 ng/ml harpagoside. They were injected into the LC‐MS/MS system six times on two different days. This allowed the percentage relative standard deviations (RSD, %) of the data to be calculated.
The stability of harpagoside in the plasma was assessed using spiked QC samples (2.5, 5, 10 ng/ml) under three conditions (room temperature, 12 hr; −4°C, 30 days; −20°C, 30 days).
2.5. Pharmacokinetic analysis
For determination of pharmacokinetic parameters, the software PKSolver (Zhang, Huo, Zhou, & Xie, 2010), an add‐in programme for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel, was used. The utilized model was a noncompartmental analysis of plasma data after extravascular input. Linear trapezoidal calculation method was used for AUC.
2.6. Safety assessment
Participant horses were monitored for adverse reactions, if any, with special attention to gastrointestinal disturbances as reported in humans. Safety monitoring included clinical investigation and evaluation of clinical signs as appetite and feed intake.
3. RESULTS
3.1. Optimization of sample preparation and LC‐MS/MS conditions
Selectivity of the extraction process was verified for plasma samples by comparison of a blank with the same sample spiked at a known concentration of harpagoside. No interfering peaks were observed at the expected retention time.
Linearity was obtained in the harpagoside concentration range 2.5–30 ng/ml. Samples were analysed in two batches. For each batch, six sets of standard calibration curves were measured.
The LOD (S/N = 3:1) for harpagoside was 2 ng/ml and the LOQ (S/N = 10:1) was 8 ng/ml.
The determination of precision using plasma samples spiked with 10 ng harpagoside per ml showed 6.3 % RSD intra‐day and 12.8 % inter‐day reproducibility. Accuracy of the sample preparation method including SPE showed a mean recovery of 96.8 %.
To determine the stability of harpagoside equine spiked plasma samples (2.5, 5, 10 ng/ml) were assessed under three conditions (room temperature, 12 hr; −4°C, 30 days; −20°C, 30 days). Harpagoside was stable under these conditions, storage did not affect concentrations.
3.2. Pharmacokinetics
Plasma samples of six horses following single intragastric administration of H. procumbens extract FB 8858 were analysed by the above described method. The active pharmaceutical ingredient harpagoside was detected in equine plasma at 30 min after administration via nasogastric tube and reached the maximum plasma concentration (C max) 25.59 ng/ml in trial 1 and 55.46 ng/ml in trial 2 at time to peak concentration (t max) of 1 hr in both trials. Harpagoside could be detected up to 9 hr after administration by the established method. The pharmacokinetic parameters of harpagoside after single intragastric administration of the H. procumbens extract FB 8858 corresponding to 5 and 10 mg/kg body mass (BM) harpagoside, respectively, in six horses are presented in Table 1. There was a proportional relationship between dose and C max or dose and AUC.
Table 1.
Mean pharmacokinetic parameters calculated from equine drug plasma concentrations of harpagoside in six horses after single intragastric administration of Harpagophytum procumbens extract FB 8858 corresponding to 5 mg/kg BM versus 10 mg/kg BM harpagoside by PKSolver using a noncompartmental analysis of plasma data after extravascular administration. Linear trapezoidal calculation method was used for AUC
| Parameter | Unit | 5 mg/kg BM harpagoside (trial 1) | 10 mg/kg BM harpagoside (trial 2) |
|---|---|---|---|
| λz | 1/hr | 0.27 | 0.30 |
| t 1/2 | hr | 2.53 | 2.32 |
| t max | hr | 1 | 1 |
| C max | ng/ml | 25.59 | 55.46 |
| C last_obs/C max | 0.03 | 0.03 | |
| AUC0–t | ng hr ml−1 | 67.24 | 113.12 |
| AUC0–inf_obs | ng hr ml−1 | 70.46 | 117.85 |
| AUC0–t/0–inf_obs | 0.95 | 0.96 | |
| AUMC0–inf_obs | ng h2 ml−1 | 186.89 | 258.35 |
| MRT0–inf_obs | hr | 2.65 | 2.19 |
| V z/F_obs | L/kg | 259.04 | 283.83 |
| CL/F_obs | L hr−1 kg−1 | 70.96 | 84.86 |
AUC0–inf, Area under the plasma concentration‐time curve from time zero to infinity; AUC0–t, Area under the plasma concentration‐time curve from time zero to time t; AUMC0–inf, Area under the first moment of the plasma concentration‐time curve from time zero to infinity; CL/F, apparent total clearance of the drug from plasma after extravascular administration; C max, maximum plasma drug concentration; MRT, mean residence time from time zero to infinity; t max, time to maximum plasma concentration; V z/F, apparent volume of distribution during terminal phase after extravascular administration; λz, terminal rate constant; t 1/2, half‐life.
The mean harpagoside plasma concentration‐time profiles are depicted in Figure 1.
Figure 1.
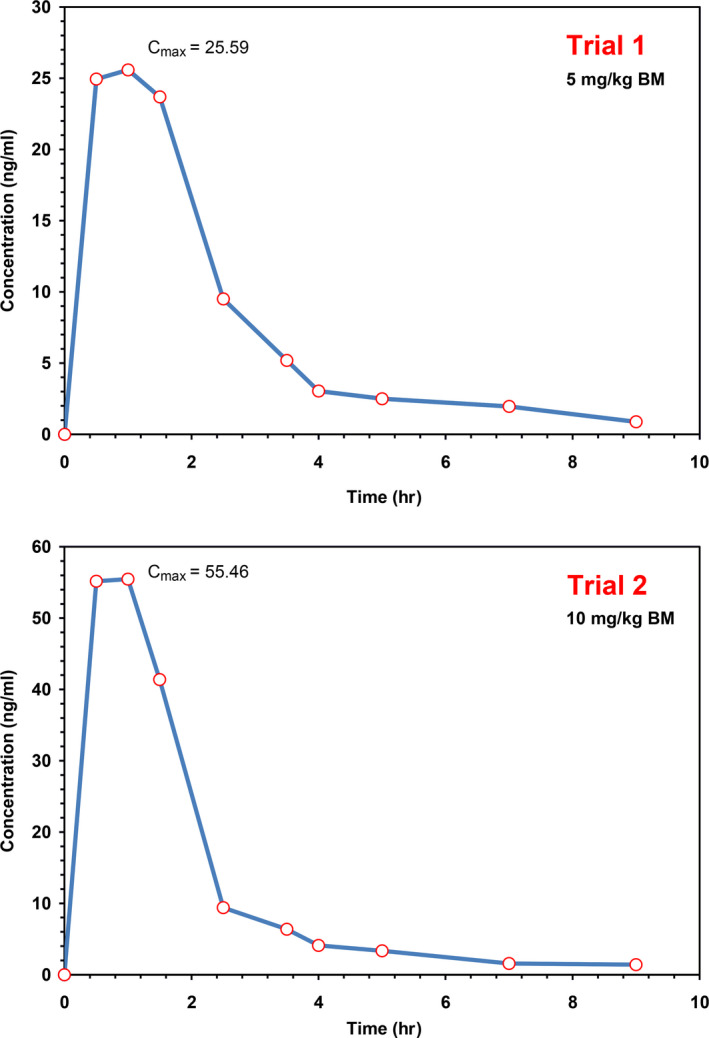
Mean plasma concentration‐time profile of harpagoside after single intragastric dosing of Harpagophytum procumbens extract FB 8858 corresponding to 5 mg/kg BM harpagoside (trial 1) and 10 mg/kg BM harpagoside (trial 2) to six horses [Colour figure can be viewed at wileyonlinelibrary.com]
No clinical signs of systemic drug intolerance or adverse reactions, such as gastrointestinal disturbances, were seen during the period of observation after the administration in any of the treated horses.
4. DISCUSSION
Extracts of devil's claw show analgetic and anti‐inflammatory effects and improve significantly the symptomatology of chronic‐inflammatory diseases like e.g., arthrosis of the hips and knees in human (Chrubasik, Pollak, & Black, 2002; Mahomed & Ojewole, 2004; Wegener & Lüpke, 2003). In addition, an increasing amount of in vitro studies confirm the anti‐inflammatory, anti‐oxidative and analgetic properties of H. procumbens making it a promising medicinal plant (Mncwangi et al., 2012). In traditional medicine, H. procumbens preparations are mainly used against chronic inflammation of the joints (Schopohl et al., 2016). In Veterinary Medicine, they are used for the prevention of inflammatory symptoms in competition horses and in the treatment of animals with lameness (Colas, Garcia, & Popot, 2008).
The Food and Drug Administration of the United States of America claimed that botanical drug products should be investigated with regard to the blood levels of known active constituents, representative markers and major chemical compounds.
Some pharmacokinetic data of devil's claw have been published in human (Loew et al., 2001) and analytical methods for analysis of harpagoside in equine urine and plasma have been established by Colas et al. (2006, 2008), but to the best of our knowledge this is the first study presenting substantive pharmacokinetic data of harpagoside, the main active constituent of H. procumbens and H. zeyheri, in horses.
Prior to pharmacokinetic data acquisition, a sample preparation workflow was established using solid phase extraction for cleanup and enrichment of harpagoside in equine plasma followed by analysis with LC‐ion‐trap‐MS/MS. Calibration curves established with harpagoside spiked blank plasma in order to minimize matrix suppression and enhancement effects in the ion source of the MS as well as the application of apigenin‐7‐glucoside as internal standard provided a good basis for quantitation of harpagoside in the low nanogram range (LOD 2 ng/ml).
Desired clinical properties of an anti‐inflammatory drug appropriate to long‐term treatment in case of osteoarthritis are fast onset of action, sufficient oral bioavailability, long duration of action, adequate therapeutic effect, and no or few side effects, as well as low affinity to participate in drug interactions. These properties are partly related to the pharmacokinetic parameters. A rapid absorption is often correlated with a fast onset of action.
In the present study, C max of harpagoside in equine plasma was found at 25.59 ng/ml following administration of the extract FB 8858 corresponding to a dosage of 5 mg/kg BM harpagoside. Administration of the same extract in the dosage of 10 mg/kg BM harpagoside after a washout period of 7 days led to a twice as high concentration (C max = 55.46 ng/ml). Time to reach maximal plasma concentration (t max) was 1 hr in both trials. Loew et al. (2001) published pharmacokinetic data of humans after administration of varying doses of different Harpagophytum extracts (i.e. HF 8858 and WS 1531). The authors suppose that although the letters of the names of the extract HF 8858 in the study of Loew et al. (2001) and FB 8858 in our study differ, these extracts are identical coming both from Andernach with the same content of 25% harpagoside. Therefore, human and equine pharmacokinetic data of these extracts can be directly compared. Assuming a body weight of 70 kg for a man, in the study of Loew et al. (2001) a dose corresponding to 2.14 mg/kg BM harpagoside was administered. This led to a C max of 32.2 ng/ml at a t max of 1.3 hr. These values are very similar to our findings, although the dosage was more than twice as high. There was a proportional relationship between the intragastric administered dose and C max of harpagoside concentrations in postadministration equine plasma samples, which has also been reported for humans (Loew et al., 2001).
In contrast to humans, for whom in time course of plasma levels a second increase in harpagoside 8 hours after oral administration of the extract WS 1531 was found (Loew et al., 2001), potentially indicating the enterohepatic circulation of harpagoside, there was no second harpagoside peak in equine plasma in the present study. Conjugates (glucuronides) are commonly measurable in plasma. As samples from the treated horses have not been subjected to de‐glucuronidation by adding glucuronidase prior to sample clean up, only the free harpagoside was measured in plasma in the present study. There are no signs that harpagoside is subjected to Phase II conjugation reaction in horses based on the current results. Further investigations are necessary to prove whether harpagoside undergoes an enterohepatic circulation in humans as well as in horses.
Colas et al. (2006) tested their developed analytical method in equine plasma and urine after administration of commercially available Harpagophytum preparations containing different amounts of powdered roots of devil's claw corresponding to 0.2–0.4 mg/kg BM harpagoside per day, when an average BM of 550 kg of the horses is assumed. The authors detected harpagoside up to 4 hr in plasma and up to 24 hr in urine. Harpagoside seemed not to be proportional to the administered dose in the postadministration urine samples (Colas et al., 2006). In the present study, it was possible to determine the concentration of harpagoside in equine plasma up to 9 hr after single intragastric administration of a Harpagophytum extract. The fact that harpagoside could be detected up to 24 hr in urine in the study of Colas et al. (2006) might be important for analysis of postcompetition samples for controlling drug exposure in the horse. However, controlling the drug effect, rather than the drug exposure, makes urine a less than ideal matrix (Toutain & Lassourd, 2002).
The present data showed low inter‐individual variability in harpagoside plasma concentrations in the horse. In consideration that this is a characteristic for drugs which have limited biotransformation, harpagoside seems to be metabolized to a relatively low extent, minimizing the risk of metabolic drug‐drug interactions. There are no reports on Harpagophytum regarding other drug‐interactions in various species (Harman, 2002); moreover, no evidence of interactions has been reported in horses (Williams & Lamprecht, 2008).
The half‐life of harpagoside 2.53 and 2.32 hr, respectively, in the present study, is also indicative that multiple doses of Harpagophytum extract are required throughout the day. This also brings up the importance of the galenic formulation. By the way, detection time is a variable influenced by many factors unrelated to the drug properties, such as dose, route of administration, pharmaceutical formulation or LOD (Toutain & Lassourd, 2002).
There are no published data about the distribution of harpagoside in human plasma to be compared. A low volume of distribution of a substance indicates that the drug does not have the attempt to go specifically to “deep” tissues in the peripheral compartments or is stored or pooled somewhere in the peripheral compartment, such as fat or is bound to specific biological material (Ritschel, Banarer, Diaz, Margary, & Otero, 1980). It is suggested that harpagoside could not easily be accumulated in equine plasma.
As arthrosis and related orthopedic disorders are chronic, common practical therapeutic use encompassing repeated daily application requires gastro‐intestinal disturbances in long‐term use to be excluded. A few negative impacts of devil's claw have been shown by the potential to cause gastrointestinal upset linked to gastric ulcers in human (Izzo, Di Carlo, Borelli, & Ernst, 2005), so there was no restriction of the food‐supply during this experiment to minimize risk, if any, of gastrointestinal irritation (except 3 hr after the administration of Harpagophytum extract via nasogastric tube to avoid food aspiration). However, Devil's claw root preparations are usually well‐tolerated with a low incidence of minor adverse events reported in 20 human studies involving 4,274 patients: mainly mild gastro‐intestinal disturbances (diarrhoea, nausea, dyspepsia, flatulence) and even three cases of allergic skin reactions (pruritus and skin etching) have been attributed to treatment with devil's claw root extracts. In none of the double‐blind studies, the incidence of adverse events during treatment with devil's claw preparations was higher than during placebo treatment (ESCOP Monographs, 2009). No side effects associated with Harpagophytum have been reported in horses. To our knowledge, there is only one clinical study investigating the efficacy of a H. procumbens preparation in horses with tarsal osteoarthritis (Montavon, 1994). Even in the present study, no clinical signs of gastrointestinal adverse reactions could be seen in any of the treated horses. However, further investigations should be done to confirm long‐term safety of Harpagophytum preparations in horses.
5. CONCLUSION
The main pharmacokinetic data of harpagoside in equine plasma are essential to understand the time course of this efficacious substance after administration of an extract of Harpagophytum procumbens (Devil's Claw) in horses. Devil's claw is a safe drug and well‐tolerated on oral administration route. Treatment of horses with Harpagophytum extract did not cause any clinically detectable side effects such as gastrointestinal irritation in our study. The knowledge of basic pharmacokinetics of devil's claw in the horse, based on the results of this study, will help to link results from in vitro assays and clinical studies and optimize therapeutic efficacy.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTION
S. Axmann, K. Hummel, K. Nöbauer, E. Razzazi‐Fazeli and K. Zitterl‐Eglseer state that they have read and approved the final manuscript. S. Axmann: Performance of the project, including sample collection, clinical supervision of the horses, clean‐up of plasma samples, data analyses, evaluation and interpretation of the results, contributed to drafting the manuscript. K. Hummel: Performance of LC‐MS/MS analysis. K. Nöbauer: Performance of LC‐MS/MS analysis. E. Razzazi‐Fazeli: Supervisor and advisor for LC‐MS/MS analysis. K. Zitterl‐Eglseer: Project manager, supervisor, made important suggestions for revision of the draft manuscript, responsible for study design and final version of the manuscript, correspondence author.
ACKNOWLEDGMENTS
Finzelberg‐Harpagophytum‐Extract FB 8858 (Ch.‐Nr. 05120116) was kindly provided by Finzelberg GmbH & Co. KG (Andernach, Germany).
Axmann S, Hummel K, Nöbauer K, Razzazi‐Fazeli E, Zitterl‐Eglseer K. Pharmacokinetics of harpagoside in horses after intragastric administration of a Devil's claw (Harpagophytum procumbens) extract. J vet Pharmacol Therap. 2019;42:37–44. 10.1111/jvp.12716
REFERENCES
- Anaute, M. , Torres, L. , & De Mello, S. (2010). Effect of isolated fractions of Harpagophytum procumbens D.C. (devil's claw) on COX‐1, COX‐2 activity and nitric oxide production on whole‐blood assay. Phytotherapy Research, 24, 1365–1369. 10.1002/ptr.3124 [DOI] [PubMed] [Google Scholar]
- Baghdikian, B. , Lanhers, M. , & Fleurentin, J. (1997). An analytical study, anti‐inflammatory and analgesic effects of Harpagophytum procumbens and Harpagophytum zeyheri . Planta Medica, 63, 171–176. 10.1055/s-2006-957638 [DOI] [PubMed] [Google Scholar]
- Beluche, L. , Bertone, A. , Anderson, D. , & Rohde, C. (2001). Effects of oral administration of phenylbutazone to horses on in vitro articular cartilage metabolism. American Journal of Veterinary Research, 62, 1916–1921. 10.2460/ajvr.2001.62.1916 [DOI] [PubMed] [Google Scholar]
- Boje, K. , Lechtenberg, M. , & Nahrstedt, A. (2003). New and known iridoid‐ and phenylethanoid glycosides from Harpagophytum procumbens and their inhibition of human leukocyte elastase. Planta Medica, 69, 820–825. [DOI] [PubMed] [Google Scholar]
- Chrubasik, S. (2004). Addendum to the ESCOP monograph on Harpagophytum procumbens . Phytomedicine, 11, 691–695. 10.1016/j.phymed.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Chrubasik, J. , Faller‐Marquart, M. , Chrubasik, S. , Lindhorst, E. , Gerlach, U. , & Torda, T. (2006). Potential molecular basis of the chondroprotective effect of Harpagophytum procumbens . Phytomedicine, 13, 589–600. [DOI] [PubMed] [Google Scholar]
- Chrubasik, S. , Model, A. , Black, A. , & Pollak, S. (2003). A randomized double‐blind pilot study comparing Doloteffin and Vioxx in the treatment of low back pain. Rheumatology, 42, 141–148. 10.1093/rheumatology/keg053 [DOI] [PubMed] [Google Scholar]
- Chrubasik, S. , & Pollak, S. (2002). Herbal antirheumatics in treatment of pain. Wiener medizinische Wochenschrift, 7, 198–203. 10.1046/j.1563-258X.2002.11115.x [DOI] [PubMed] [Google Scholar]
- Chrubasik, S. , Pollak, S. , & Black, A. (2002). Effectiveness of devil's claw for osteoarthritis. Rheumatology, 41, 1332–1333. 10.1093/rheumatology/41.11.1332 [DOI] [PubMed] [Google Scholar]
- Colas, C. , Garcia, P. , & Popot, M. (2006). Liquid chromatography/electrospray ionization mass spectrometric characterization of Harpagophytum in equine urine and plasma. Rapid Communications in Mass Spectrometry, 20, 3257–3266. 10.1002/(ISSN)1097-0231 [DOI] [PubMed] [Google Scholar]
- Colas, C. , Garcia, P. , & Popot, M. (2008). Optimization of solid‐phase extraction for the liquid chromatography‐mass spectrometry analysis of harpagoside, 8‐para‐coumaroyl harpagide, and harpagide in equine plasma and urine. Journal of Chromatographic Science, 46, 174–183. 10.1093/chromsci/46.2.174 [DOI] [PubMed] [Google Scholar]
- ESCOP Monographs (2009). The scientific foundation for herbal medicinal products, 2nd ed. (pp. 135–146). Stuttgart: Thieme. [Google Scholar]
- Fiebich, B. , Heinrich, L. , Hiller, K. , & Kammerer, N. (2001). Inhibition of TNF‐alpha synthesis in LPS‐stimulated primary human monocytes by Harpagophytum extract SteiHap 69. Phytomedicine, 8, 28–30. 10.1078/0944-7113-00002 [DOI] [PubMed] [Google Scholar]
- Fiebich, B. , Munoz, E. , Rose, T. , Weiss, G. , & McGregor, G. (2012). Molecular targets of the antiinflammatory Harpagophytum procumbens (Devil′s claw): inhibition of TNFα and COX‐2 gene expression by preventing activation of AP‐1. Phytotherapy Research, 26, 806–811. 10.1002/ptr.3636 [DOI] [PubMed] [Google Scholar]
- Georgiev, M. , Alipieva, K. , Pashova, S. , Denev, P. , Angelova, M. , Kerns, G. , & Bley, T. (2010). Antioxidant activity of Devil's Claw cell biomass and its active constituents. Food Chemistry, 121, 967–972. 10.1016/j.foodchem.2010.01.028 [DOI] [Google Scholar]
- Harman, J. (2002). The toxicology of herbs in equine practice. Clinical Techniques in Equine Practice, 8, 74–80. 10.1053/ctep.2002.34475 [DOI] [Google Scholar]
- Huang, T. , Tran, V. , Duke, R. , Tan, S. , Chrubasik, S. , Roufogalis, B. , & Duke, C. (2005). Harpagoside suppresses lipopolysaccharid‐induced iNOS and COX‐2 expression through inhibition of NF‐kB activation. Journal of Ethnopharmacology, 13, 11–15. [DOI] [PubMed] [Google Scholar]
- Izzo, A. , Di Carlo, G. , Borelli, F. , & Ernst, E. (2005). Cardiovascular pharmacotherapy and herbal medicines: The risk of drug interaction. International Journal of Cardiology, 98, 1–14. 10.1016/j.ijcard.2003.06.039 [DOI] [PubMed] [Google Scholar]
- Jang, M. , Lim, S. , Han, S. , Park, H. , Shin, I. , Kim, J. , … Kim, C. (2003). Harpagophytum procumbens suppresses lipopolysaccharid‐stimulated expressions of cyclooxygenase‐2 and inducible nitric oxide synthase in fibroblast cell line L929. Journal of Pharmacological Sciences, 93, 367–371. 10.1254/jphs.93.367 [DOI] [PubMed] [Google Scholar]
- Kazunori, I. , Kazuya, M. , Shunsuke, N. , & Hideaki, M. (2010). Inhibitory effects of devil's claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. Journal of Natural Medicines, 64, 219–222. [DOI] [PubMed] [Google Scholar]
- Lanhers, M. , Fleurentin, J. , Mortier, F. , Vinche, A. , & Younos, C. (1992). Antiinflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens . Planta Medica, 58, 117–123. 10.1055/s-2006-961411 [DOI] [PubMed] [Google Scholar]
- Lees, P. (2003). Veterinary pharmacology: Where to in the 21st century? The Veterinary Journal, 165, 95–97. 10.1016/S1090-0233(02)00177-6 [DOI] [PubMed] [Google Scholar]
- Loew, D. , Möllerfeld, J. , Schrödter, A. , Puttkammer, S. , & Kaszkin, M. (2001). Investigations on the pharmacokinetic properties of Harpagophytum extracts and their effects on eicosanoid biosynthesis in vitro and ex vivo. Clinical pharmacology and therapeutics, 69, 356–364. 10.1067/mcp.2001.115445 [DOI] [PubMed] [Google Scholar]
- Mahomed, I. , & Ojewole, J. (2004). Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytotherapy Research, 18, 982–989. 10.1002/(ISSN)1099-1573 [DOI] [PubMed] [Google Scholar]
- Mncwangi, N. , Chen, W. , Vermaak, I. , Viljoen, A. , & Gericke, N. (2012). Devil's Claw ‐ A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens . Journal of Ethnopharmacology, 143, 755–771. 10.1016/j.jep.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Montavon, S. (1994). Efficacy of a phytotherapeutic preparation based on Harpagophytum procumbens in case of bone spavin of adult horses. Pratique Vétérinaire Équine, 26, 49–53. [Google Scholar]
- Reed, S. , Messer, N. , Tessman, R. , & Keegan, K. (2006). Effects of phenylbutazone alone or in combination with flunixin meglumine on blood protein concentrations in horses. American Journal of Veterinary Research, 67, 398–402. 10.2460/ajvr.67.3.398 [DOI] [PubMed] [Google Scholar]
- Ritschel, W. , Banarer, M. , Diaz, D. , Margary, J. , & Otero, J. (1980). Prediction of the volume of distribution from in vitro data and use for estimating the absolute extent of absorption. International Journal of Clinical Pharmacology, Therapeutics, and Toxicology, 18, 298–316. [PubMed] [Google Scholar]
- Schaffer, L. , Peroza, L. , Boligon, A. , Athayde, M. , Alves, S. , Fachinetto, R. , & Wagner, C. (2013). Harpagophytum procumbens prevents oxidative stress and loss of cell viability in vitro. Neurochemical Research, 38, 2256–2267. 10.1007/s11064-013-1133-x [DOI] [PubMed] [Google Scholar]
- Schopohl, P. , Grüneberg, P. , & Melzig, M. (2016). The influence of harpagoside and harpagide on TNFα‐secretion and cell adhesion molecule mRNA‐expression in IFNγ/LPS‐stimulated THP‐1 cells. Fitoterapia, 110, 157–165. 10.1016/j.fitote.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Schulze‐Tanzil, G. , Hansen, S. , & Shakibaei, M. (2004). Effect of a Harpagophytum procumbens DC extract on matrix metalloproteinases in human chondrocytes in vitro. Arzneimittel‐Forschung, 54, 213–220. [DOI] [PubMed] [Google Scholar]
- Soulimani, R. , Younos, C. , Mortier, F. , & Derrieu, C. (1994). The role of stomachal digestion on the pharmacological activity of plant extracts, using as an example extract of Harpagophytum procumbens . Canadian Journal of Physiology and Pharmacology, 72, 1532–1536. 10.1139/y94-220 [DOI] [PubMed] [Google Scholar]
- Stewart, K. , & Cole, D. (2005). The commercial harvest of Devil's Claw (Harpagophytum spp.) in Southern Africa: The devil's in the details. Journal of Ethnopharmacology, 100, 225–236. 10.1016/j.jep.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Toutain, P. , & Lassourd, V. (2002). Pharmacokinetic/pharmacodynamic approach to assess irrelevant plasma or urine drug concentrations in postcompetition samples for drug control in the horse. The Veterinary Journal, 34, 242–249. [DOI] [PubMed] [Google Scholar]
- Trumble, T. (2005). The use of nutraceuticals for osteoarthritis in horses. The Veterinary Clinics of North America: Equine Practice, 21, 575–597. [DOI] [PubMed] [Google Scholar]
- Uchida, S. , Hirai, K. , Hatanak, J. , Hanato, J. , Umegaki, K. , & Yamada, S. (2008). Antinociceptive effects of St. John's wort, Harpagophytum procumbens extract and grape seed Proanthocyanidins extract in mice. Biological and Pharmaceutical Bulletin, 31, 240–245. 10.1248/bpb.31.240 [DOI] [PubMed] [Google Scholar]
- Van Haelen, M. , Van Haelen Fastré, R. , Samaey‐Fontaine, J. , Elchamid, A. , Niebes, P. , & Matagne, D. (1983). Aspects botaniques, constitution chimique et activité pharmacologique d′Harpagophytum procumbens . Phytotherapy, 5, 7–13. [Google Scholar]
- Van Wyk, B. , & Gericke, N. (2000). Peoples Plants. A Guide to Useful Plants of Southern Africa. Pretoria: Briza Publications. [Google Scholar]
- Wegener, T. , & Lüpke, N. (2003). Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil's claw (Harpagophytum procumbens DC.). Phytotherapy Research, 17, 1165–1172. 10.1002/ptr.1322 [DOI] [PubMed] [Google Scholar]
- Williams, C. , & Lamprecht, E. (2008). Some commonly fed herbs and other functional foods in equine nutrition: A review. The Veterinary Journal, 178, 21–31. 10.1016/j.tvjl.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Huo, M. , Zhou, J. , & Xie, S. (2010). PKSolver: An add‐in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Computer Methods and Programs in Biomedicine, 99, 306–314. 10.1016/j.cmpb.2010.01.007 [DOI] [PubMed] [Google Scholar]


