Summary
Microbial populations exist to great depths on Earth, but with apparently insufficient energy supply. Earthquake rock fracturing produces H2 from mechanochemical water splitting, however, microbial utilization of this widespread potential energy source has not been directly demonstrated. Here, we show experimentally that mechanochemically generated H2 from granite can be directly, long‐term, utilized by a CH4 producing microbial community. This is consistent with CH4 formation in subsurface rock fracturing in the environment. Our results not only support water splitting H2 generation as a potential deep biosphere energy source, but as an oxidant must also be produced, they suggest that there is also a respiratory oxidant supply in the subsurface which is independent of photosynthesis. This may explain the widespread distribution of facultative aerobes in subsurface environments. A range of common rocks were shown to produce mechanochemical H2, and hence, this process should be widespread in the subsurface, with the potential for considerable mineral fuelled CH4 production.
Introduction
The majority of prokaryotes on Earth live in the subsurface and are present to depths in excess of 3 km (Parkes et al., 2014). These prokaryotes are far away from photosynthetically derived organic matter and oxygen and are under severe energy limitation (Hoehler and Jorgensen, 2013). Therefore, subsurface microorganisms maybe be more reliant on the geosphere for energy supply (Pedersen, 2000), including H2 which has a range of geosphere sources. For example: (i) oxidation of ferrous iron containing minerals, predominantly at elevated temperatures – serpentinization (Holm et al., 2015); (ii) radiolysis of water (Lin et al., 2005); (iii) pyrite formation from FeS and H2S (Drobner et al., 1990); and (iv) high temperature conversion of water in minerals into H2 and peroxy linkages (Freund, 1985). Low temperature (~20 °C) basalt weathering/oxidation had been suggested to fuel a H2‐based microbial ecosystem in the Columbia River Basalt Aquifer (Stevens and McKinley, 1995). However, this community subsequently was considered to be heterotrophic instead, as little H2 formation occurred under simulated in situ conditions and also because ferrous iron concentrations would have been limiting (Anderson et al., 1998). Despite this, total H2 flux in continental rocks has been suggested to be highly significant at 0.36–2.27 x 1011 mol per year (Lollar et al., 2014), and comparable to the seafloor hydrothermal H2 fluxes that support spectacular marine ecosystems. This flux would help explain the large terrestrial subsurface biosphere, but H2 from water radiolysis and serpentinization would be restricted to rocks with radioactive compounds or ferrous iron minerals respectively.
Another source of geologically‐generated H2 is from mechanochemical splitting of water due to free radical reactions on fractured rock surfaces (Kita et al., 1982; Freund et al., 2002) or rocks under tension (Balk et al., 2009). However, mechanochemical H2 formation is rarely considered as a deep biosphere energy source despite this process being widespread and not limited to a few specific rock types (Kita et al., 1982; Freund et al., 2002). Although fracturing is concentrated around earthquake zones (Wakita et al., 1980; Brauer et al., 2005), rock comminution during erosion (Telling et al., 2015) and seismic events (Sleep and Zoback, 2007), are also sources of mechanochemical H2 and together these should be widespread in the subsurface. Estimates of mechanochemically produced H2 at 3.4 × 1016 mol per year (Hirose et al., 2011; 2012) show that it is a larger global H2 source than serpentinization and water radiolysis combined. In addition, the presence of CH4 in earthquake zones (Brauer et al., 2005) suggests that some of this mechanically produced H2 is being used directly by subsurface methanogens.
However, it is unknown if the production rates and concentrations of mineral‐H2, the conditions for its production (e.g. temperature and pressures) and/or the by‐products of the reactions (e.g. highly reactive oxygen species), would actually enable utilization by anaerobic microbial communities. Investigating whether mechanically‐produced H2 can be directly utilized by prokaryotic communities is not only important for understanding deep biosphere energy sources, if a significant amount of this H2 is utilized to form CH4, this would also be important for accurate quantification of greenhouse gas formation and global warming. Furthermore, mechanochemical‐H2 formation may have been important for early life on Earth and could potentially maintain subsurface biospheres on other planets (McMahon et al., 2016). We, therefore, conducted laboratory rock‐crushing experiments under optimal conditions for H2‐utilizing methanogens to test whether mechanochemical‐H2 formation could directly fuel microbial activity, and hence, potentially microbial ecosystems.
Results and discussion
Mineral‐H2 formation on crushing
To determine the mineral H2 formation conditions for subsequent microbial utilization, pure silica (2 g) in vials with aluminium balls under anaerobic conditions were heated at 25, 38, 67, 84 and 100 °C for 30 min and then contents ground using a ball mill (60 min, Supporting Information Fig. S1A; see Supporting Information for Experimental procedures). The vials were then heated for a further 30 min before headspace gas was analysed. Above ~40 °C H2 concentrations increased with temperature (P < 0.05), reaching 178 nmol H2 L−1 headspace at 100 °C for silica only with milling. All controls, including silica plus water, were not significantly different from an empty vial (Fig. 1). These results show that milling and silica were essential for producing significant H2, and that other potential sources of H2 on heating, such as thermal breakdown of organic matter contaminants and rubber stoppers, were negligible sources of H2 under the prevailing conditions. Furthermore, milling of water with silica produced considerably less H2 compared with silica without water (Fig. 1), suggesting that the added water reduced milling efficiency. This further emphasizes the importance of milling for H2 formation as does the experiment with silica plus water without milling which produced even less H2 (~20 nmol L−1). Dry grinding of minerals produces H2 with the water coming from between mineral grains or from reaction of hydroxyl groups (Kameda et al., 2004). Although H2 formation from silica, and granite, has been shown to increase with temperature, up to a maximum at ~200–220 °C (Kita et al., 1982), lower temperatures are required for direct coupling with microbial H2 utilization, as the upper temperature for prokaryotes and methanogenesis is around 120 °C (Takai et al., 2008). Hence, there is a compromise between the temperature required for maximum mechanochemical mineral‐H2 formation, and the temperature range enabling its direct microbial utilization. From the temperature range tested (Fig. 1) 67 °C was selected for further experiments to enable subsequent coupling with the deep‐sea, thermophilic methanogen Methanothermococcus okinawensis (growth optimum 60–65 °C, range 40–75 °C, Takai et al., 2002). Prokaryotes at similar thermophilic temperatures have been detected in deep, subsurface sediments (Roussel et al., 2008) and in water from deep rock fracture zones (Takai et al., 2003, Moser et al., 2005).
Figure 1.
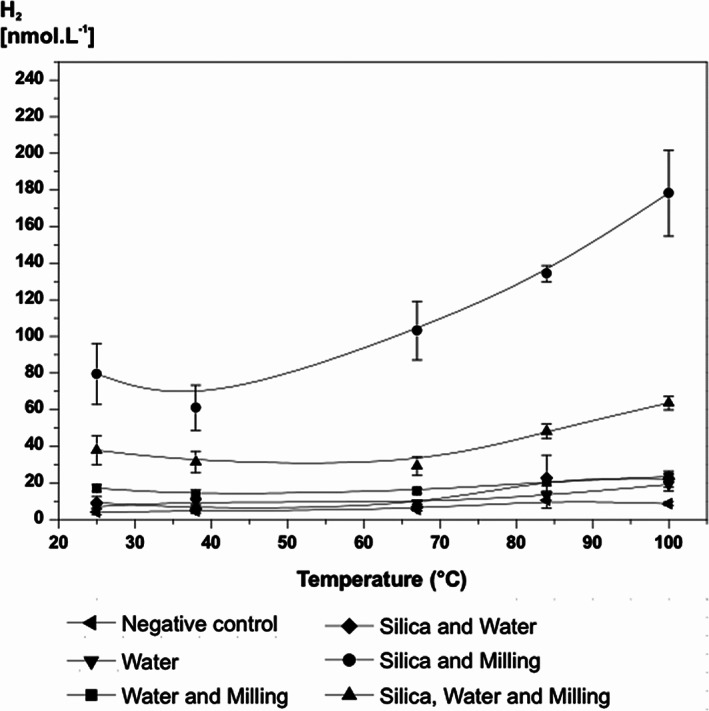
Effect of milling on H2 formation from silica between 25 and 100 °C (mean of triplicates and standard error bars shown).
To enhance mineral‐H2 formation at 67 °C, silica milling was conducted in an oil bath (Supporting Information Fig. S1B) to provide extended periods of heated milling and this was combined with headspace flushing (Fig. 2). Initially with milling, there was rapid H2 formation decreasing slightly after ~30 h. However, after headspace flushing H2 rapidly returned to its original concentration, ~490 nmol L−1. Flushing was repeated another three times up to ~140 h, with the same result, even though milling had stopped after ~55 h. After a further three flushes up to 216 h, the amount of H2 produced reduced considerably (lowest ~90 nmol L−1), indicating that most reactive surfaces had been utilized. However, flushing had resulted in a ~2.5 times increase in the amount of H2 formed. Another period of milling increased H2 to above the initial concentration (~760 nmol L−1), although subsequent flushing resulted in only low H2 concentrations (Fig. 2). This sequence was repeated in another two milling periods, followed by an extra period without flushing which yielded the maximum H2 concentrations of 1213 nmol L−1, after a total of ~530 h. Free radical concentrations also increased with crushing time (Supporting Information Fig. S2) corresponding with increasing H2 formation. These results show that continuous H2 formation can be obtained by a mixture of (i) additional crushing, and (ii) H2 removal by headspace flushing. The latter is consistent with feedback inhibition and suggests that microbial H2 consumption might sustain or even enhance H2 formation. Cumulative H2 formation totalled 7186 nmol L−1.
Figure 2.
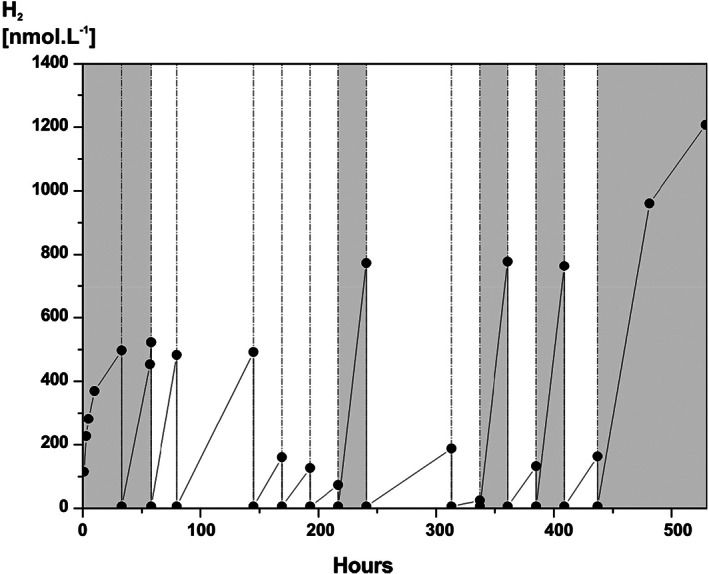
Effect of milling on H2 from silica at 67 °C.
Dotted lines denote headspace flushing (x3 with oxygen free nitrogen). Shading shows milling intervals.
Similar results were obtained with crushing basalt with total H2 production after ~120 h of 350 nmol L−1, with one initial milling period (~75 h). These results are similar to the initial H2 formation in low temperature basalt weathering experiments conducted previously by Anderson and colleagues (1998), who also suggested that initial H2 formation was possibly due to reactive mineral surfaces, however, our milling was probably more effective, resulting in significant H2 formation after headspace flushing, which did not occur in the Anderson et al. experiments. Milling at 67 °C for ~30 h also produced H2 from other minerals in the order of highest concentrations: granite > quartz > silica and borosilicate glass > basalt, ranging in maximum concentration from 1133 to 142 nmol L−1 (Supporting Information Fig. S3). Mineralogical changes with crushing granite, including reduction of quartz and formation of new minerals (Supporting Information Fig. S4, overall decrease in peaks labelled Q for quartz, including some also with other minerals and appearance of additional peaks respectively), confirmed that H2 generation occurred together with breakage of Si—O bonds in phyllosilicates, which together with free radical formation (Supporting Information Fig. S2) is consistent with mechanochemical reactions.
Coupling mineral derived H2 with methanogenesis
For further experiments, the high H2 producing granite was used in increasing amounts (15–40 g) with 30 g giving maximum H2 production and then this amount was subsequently used as standard. Under these conditions ~500 μmol L−1 H2 was produced, but there was no H2 consumption or CH4 production when the system was inoculated with a M. okinawensis culture (Supporting Information Fig. S1D), despite repeated attempts (Supporting Information Fig. S5). Under our culture conditions the H2 threshold for significant CH4 production by M. okinawensis was between ~200 and 1500 μmol L−1, so sufficient H2 was present in our mineral experiments for the methanogen to use. However, mechanochemical splitting of water also produces highly reactive oxygen species (Balk et al., 2009), which could have inhibited this strictly, anaerobic methanogen. Subsurface environments are generally reducing (e.g. H2S and reduced metal species), so reactive oxygen species would be reduced, and/or be used directly or indirectly (oxidized products of reduced species ‐ thiosulfate and metal oxides) by facultative aerobes/anaerobes. This would not substantially occur in our pure culture methanogen experiments. Therefore, we specifically enriched a methanogenic community (see Supporting Information Fig. S6 and Experimental Procedures) under low oxygen concentrations (and low H2, ~400 μmol L−1) to inoculate further experiments, which could both cope with oxidized species and produce CH4 from H2 (Fig. 4, Supporting Information Fig. S1). The same enrichment subculture was then used to inoculate all subsequent experiments (Supporting Information Fig. S6b), to ensure that the community composition was identical for each.
Three experiments were conducted each with a different grinding mechanism to ensure that H2 formation was not restricted to a specific grinding process. Experiment 1 ‐ rotation with granite balls; Experiment 2 ‐ grinding with a magnetic stirring bar and Experiment 3 ‐ grinding with an abrasive resistant bar to prevent the iron magnet being exposed and contributing to H2 formation. All experiments resulted in H2 consumption and CH4 production after inoculation with the methanogenic community (Fig. 3). In Experiments 1 and 2, CH4 production was almost twice the amount expected from measured H2 consumption (4H2 + CO2 ➔ CH4 + 2H2O), which presumably reflects simultaneous mineral‐H2 production and H2 consumption by methanogens. In addition, enhanced H2 formation similar to the effect of headspace flushing previously documented, and of a similar magnitude (Fig. 2), may be occurring due to the methanogenic H2 consumption. In Experiment 3 (Fig. 3C), water (4 ml) was added after grinding for 682 h, to further increase H2 formation (~500 μmol L−1) and to demonstrate that the H2 increase previously observed on addition of the inoculum in Experiments 1 and 2 (Fig. 3A and B) was due to increased water availability after grinding. After ~1266 h the experiment was inoculated and almost immediately H2 was consumed and CH4 produced. By ~250 h incubation most of the H2 was removed (to ~20 μmol L−1) and CH4 then stabilized around 140 μmol L−1. Shortly after this, grinding was restarted (after 1587 h) and CH4 production restarted immediately, but for H2 there was a delay of ~140 h before concentrations increased, presumably due to its initial consumption for methanogenesis. During this second phase of grinding, CH4 and H2 concentrations plateaued at ~160 and 115 μmol L−1 respectively). Grinding was then stopped (2161 h) and H2 and CH4 (small decrease) production ceased. After ~60 h grinding was restarted and immediately H2 was produced, CH4 concentrations, however, decreased until the system was re‐inoculated (inoculum presumably dried out), after which H2 again was removed along with CH4 production, some 2800 h/120 days after the beginning of the experiment. The initial period of methanogenesis was much more rapid in this experiment compared with Experiments 1 and 2 (2–4 times, Fig. 3), and the H2:CH4 ratio was as expected for hydrogenotrophic methanogenesis. The second period of CH4 formation, however, was much slower, presumably reflecting the much lower H2 concentrations and the H2:CH4 ratio was similar to the Experiments 1 and 2. Probably the large and very rapid initial phase of H2 consumption in Experiment 3 masked the effect of continuing mineral H2 formation. In controls, including an inoculated empty crushing bottle, and an autoclaved inoculum, no coupled H2 removal and CH4 production occurred (Supporting Information Fig. S7). Some CH4 was released into the inoculated empty bottle control (max 26 μmol L−1), but this represented only a fraction of the CH4 produced in the inoculated mineral H2 experiments. The controls demonstrate that CH4 production was not a result of thermal breakdown of cells or organic matter in the inoculum. The rapid response to renewed mineral grinding (Fig. 3C) also demonstrates how tight and effective this mineral H2 methanogenesis system is.
Figure 3.
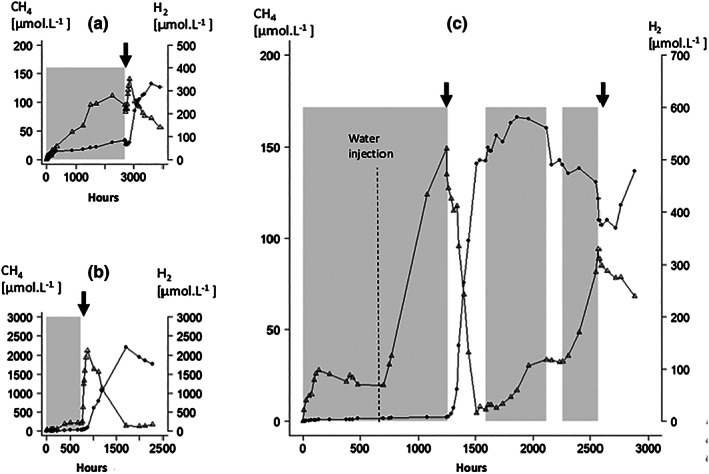
- Experiment 1: rotating with granite balls.
- Experiment 2 grinding with magnetic stirrer.
- Experiment 3: grinding with an abrasive resistant stirring bar. Triangle = H2, solid circle = CH4. Shading shows milling periods and arrow shows inoculation with methanogenic community.
The methanogenic community
The composition of the methanogenic community was screened by methanogen functional mcrA gene and 16S rRNA gene sequence analysis (Supporting Information Fig. S8 and Fig. 4). Only one methanogenic archaeon was detected in the inoculum and this had 96% (mcrA gene) and 99% (16S rRNA gene) nucleotide sequence similarity to Methanothermobacter crinale, a methanogen often isolated from subsurface oil and gas reservoirs and thought to develop co‐operative relationships with Bacteria (Cheng et al., 2011). In addition to the methanogen, the methanogenic community also contained several bacterial 16S rRNA gene sequences (Fig. 4), predominantly thermophilic Firmicutes, belonging to the orders Thermonanaerobacterales, and Clostridiales, including Desulfotomaculum species, within the Clostridia class. Both of the above bacterial families commonly occur in the deep hot biosphere or deep subsurface environments (Aullo et al., 2013; Parkes et al., 2014, O'Sullivan et al., 2015, Purkamo et al., 2016). An association of methanogens with Clostridiales species have been shown to dominate in deep hot subterranean environments such as deep gold mines (Moser et al., 2003). Many bacterial sequences were related to cultured species (45%), including those from hot springs (Perevalova et al., 2013, Brockia lithotrophica, H2‐utilizing, obligate anaerobic, spore‐former), where a H2 driven methanogenic community has been documented (Chapelle et al., 2002); hot salty environments (Cayol et al., 1994, Halothermothrix orenii, an anaerobic, chemoorganotroph); and oil reservoirs (Nilsen et al., 1996), and some have known syntrophic interactions with methanogens (Nilsen et al., 1996, Desulfotomaculum thermocisternum, a thermophilic, H2‐utilizing, spore‐forming sulfate‐reducer). In addition, some Desulfotomaculum species have genes encoding for enzymes that can protect against reactive oxygen compounds (Spring et al., 2009). In our system, the presence of Desulfotomaculum species with the methanogen could help methanogenesis to occur despite the presence of oxidized compounds. One of the most common sequences (15%) was related to uncultured Bacteria colonizing young ocean crust (Fig. 4), which probably supports significant autotrophic microbial biomass (Bach and Edwards, 2003). Comparison of the bacteria in our methanogenic community (see Supporting Information Fig. S9) with those detected in H2‐utilizing SLIME environments (Stevens and McKinley, 1995) such as aging and young ocean crust (Cowen et al., 2003; Orcutt et al., 2011) or thermophilic methanogenic community fed from cathode‐derived hydrogen (Fu et al., 2013) shows that bacteria in our methanogenic community were representative of populations fuelled by H2.
Figure 4.
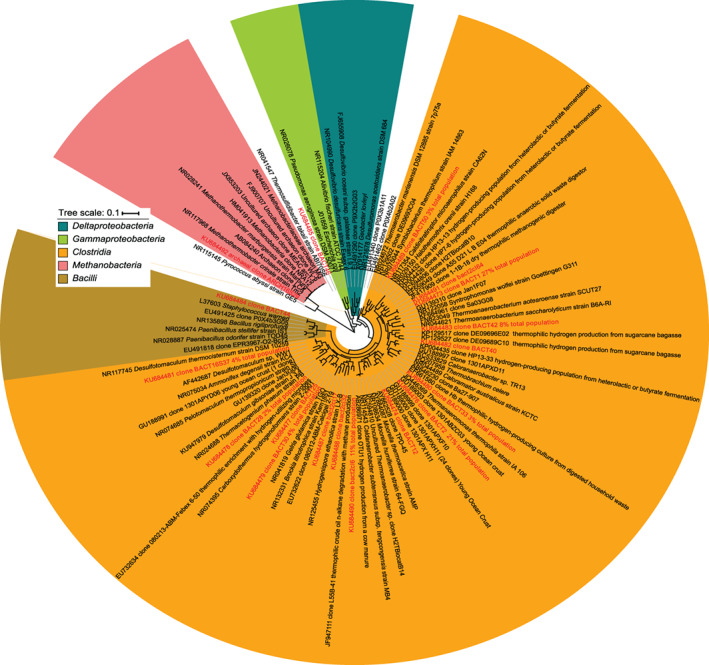
Phylogenetic tree of bacterial and archaeal 16S rRNA gene diversity in the methanogenic community inoculum.
Neighbour‐joining tree prepared with MEGA 5.2.2 software (method: Jukes‐Cantor model, bootstrap test: 500 replicates) and edited with the Interactive Tree of Life (ITOL) using sequences aligned with the ClustalW2 program. Sequences detected in this study are highlighted in red and bold.
Summary
These results demonstrate considerable H2 formation from a range of common rocks and minerals by crushing induced mechanochemistry. This H2 formation can be sustained by repeated crushing under anaerobic conditions and at temperatures (25–100 °C) well within the range of prokaryotes. Higher temperatures produce even greater amounts of H2 (up to a maximum at ~200–220 °C, Kita et al., 1982) and this could diffuse upwards into the temperature limited base of the subsurface biosphere. Granite derived H2 was directly utilized by a CH4‐producing community in which the methanogen could effectively compete for H2 (over the 120 days of the experiment), and which was resilient to the oxidized species also mechanochemically produced, but not by a pure methanogen culture on its own. However, this production of oxidized species is also environmentally important as this could help sustain respiratory prokaryotes, independent of oxygen from surface photosynthesis, in the deep subsurface, including those using H2 or H2‐derived products, such as CH4. Subsurface oxidants may also help explain the considerable number of facultative aerobic prokaryotes in the deep subsurface (Pedersen, 1993; Miettinen et al., 2015).
Estimated earthquake‐derived H2 flux is five orders of magnitude higher (Hirose et al., 2012) than from radiolysis and serpentinization (Lollar et al., 2014) and, therefore, should be a much more important energy source for the deep biosphere, and provide a continuing fuel for deep CH4 formation and flux to the atmosphere, which is second only to wetland emissions (Etiope, 2012). If all of the estimated flux of mechanochemically derived H2 (Freund et al., 2002; Hirose et al., 2012) was converted to CH4 this would be ~1000 times higher than current estimates of geological CH4 greenhouse gas production (Kirschke et al., 2013). As tectonics on Earth probably occurred by ~3 Ga (Hirose et al., 2011), mechanochemical reaction products would also have been available to early life. Mechanochemistry from landslides, glacial bedrock comminution (Telling et al., 2015) and meteorite impacts add further to this tectonically driven water splitting, rock energy source, which could occur on many other planets (Hurowitz et al., 2007; Yin, 2012).
Author contributions
RJP designed the project and wrote the paper. SB, EGR, GW and HB conducted the practical work, acquired and analysed data, and contributed to paper writing. All co‐authors contributed to the final version of the manuscript. Data supporting the paper and Experimental Procedures are presented in the Supplementary Information. GenBank Sequence accession numbers KU684473 to KU684492.
Supporting information
Fig. S1. Apparatus used for milling experiments: A. Ball mill (150 r.p.m.). B. Rotary milling in a 67 °C oil‐bath with 25 ml Wheaton® vials (150 r.p.m.). C. Rotary milling in a 67 °C oil‐bath with 100 ml Duran® bottles. D. Grinding with a magnetic stirring bar in a beaker water‐bath on heated‐stirrer at 67 °C, with or without a separate methanogenic community inoculum.
Fig. S2. Free radical production from milled silica at 67 °C based on consumption of a radical scavenger (DPPH: Damm & Peukert (2009)). Black squares are milled silica; red circles are the non‐milled negative control.
Fig. S3. H2 and CO formation during milling a range of minerals at 67 °C. Circles = granite, triangles = quartz, squares = silica, star = borosilicate glass, diamonds = basalt.
Fig. S4. XRD profiles of fresh powdered granite initially used in the experiment (top) and the granite after crushing at 67 °C with a magnetic stirrer (bottom).
Fig. S5. Inoculation of granite derived H2 experiments with Methanothermococcus okinawensis at 67 °C and changes in CH4 (filled circle) and H2 concentrations (triangles). Shaded area represents the grinding period; dotted line denotes injection of sterile medium to enhance H2 production and arrows are injection of the methanogen pure culture. Replicate experiments a and b.
Fig. S6. Enrichment of air tolerant methanogenic community at 67 °C using sediments from the Tamar Estuary, UK in mineral medium: a) initial enrichment slurry with successive air additions, b) enrichment after successive subculture at low H2 concentrations in a vial mimicking experimental conditions. CH4 (filled circle) and H2 (triangles).
Fig. S7. Control experiments at 67 °C with a) autoclaved (x3) enrichment inoculated into an experiment with 30 g of crushed granite (shaded area is the grinding period) and H2 adjusted to ~300 μmol L−1. b) Active methanogenic enrichment inoculated into an empty device. Experiment 1 shown by solid lines and Experiment 2 by dashed lines. CH4 (filled circle) and H2 (triangles).
Fig. S8. Phylogenetic tree of methanogen functional mcrA gene clones from the methanogenic community inoculum. All clones were closely related (96%) to Methanothermobacter crinale.
Fig. S9. Comparison at the class‐level of bacterial 16S rRNA genes detected in this study with other studies of subsurface environments. 1: Parkes et al., (this study) crushing experiments, hot condition; 2: Fu et al. (2013) cathode hydrogen production sustaining methanogenic community, hot condition; 3: Cowen et al., (2003) aging ocean crust, hot condition; 4: Orcutt et al., (2011) young ocean crust, hot condition; 5: Diksma et al., (2016) dark C fixation in coastal marine sediments, cold condition; 6: Le Campion et al., unpublished, continental subsurface aquifer; 7–9: Dong et al., (2014) 1.8 km deep subsurface Cambrian sandstone reservoir, thermophilic; 10–12: Edlund et al., (2008) Baltic sea sediments, cold conditions (10 = redox depth ‐337 mV; 11 = redox depth − 169 mV; 12 = redox depth − 64 mV [b1]).
Acknowledgements
This research was supported by the Natural Environment Research Council UK, grant number NE/H02042X/1 and for GW NE/J011177/1. Professor Tom Blenkinsop, Cardiff University, read and commented on a draft version of the paper.
References
- Anderson, R.T. , Chapelle, F.H. , and Lovley, D.R. (1998) Evidence against hydrogen‐based microbial ecosystems in basalt aquifers. Science 281: 976–977. [DOI] [PubMed] [Google Scholar]
- Aullo, T. , Ranchou‐Peyruse, A. , Ollivier, B. , and Magot, M. (2013) Desulfotomaculum spp. and related gram‐positive sulfate‐reducing bacteria in deep subsurface environments. Front Microbiol 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, W. , and Edwards, K.J. (2003) Iron and sulfide oxidation within the basaltic ocean crust: implications for chemolithoautotrophic microbial biomass production. Geochim Cosmochim Acta 67: 3871–3887. [Google Scholar]
- Balk, M. , Bose, M. , Ertem, G. , Rogoff, D.A. , Rothschild, L.J. , and Freund, F.T. (2009) Oxidation of water to hydrogen peroxide at the rock‐water interface due to stress‐activated electric currents in rocks. Earth Planet Sci Lett 283: 87–92. [Google Scholar]
- Brauer, K. , Kampf, H. , Faber, E. , Koch, U. , Nitzsche, H.M. , and Strauch, G. (2005) Seismically triggered microbial methane production relating to the Vogtland ‐ NW Bohemia earthquake swarm period 2000, Central Europe. Geochem J 39: 441–450. [Google Scholar]
- Cayol, J.L. , Ollivier, B. , Patel, B.K. , Prensier, G. , Guezennec, J. , and Garcia, J.L. (1994) Isolation and characterization of Halothermothrix orenii gen. nov., sp. nov., a halophilic, thermophilic, fermentative, strictly anaerobic bacterium. Int J Syst Bacteriol 44: 534–540. [DOI] [PubMed] [Google Scholar]
- Chapelle, F.H. , O'Neill, K. , Bradley, P.M. , Methe, B.A. , Ciufo, S.A. , Knobel, L.L. , and Lovley, D.R. (2002) A hydrogen‐based subsurface microbial community dominated by methanogens. Nature 415: 312–315. [DOI] [PubMed] [Google Scholar]
- Cheng, L. , Dai, L. , Li, X. , Zhang, H. , and Lu, Y. (2011) Isolation and characterization of Methanothermobacter crinale sp nov., a novel Hydrogenotrophic methanogen from the Shengli oil field. Appl Environ Microbiol 77: 5212–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen, J.P. , Giovannoni, S.J. , Kenig, F. , Johnson, H.P. , Butterfield, D. , Rappe, M.S. , et al (2003) Fluids from aging ocean crust that support microbial life. Science 299: 120–123. [DOI] [PubMed] [Google Scholar]
- Damm, C. , and Peukert, W. (2009) Kinetics of radical formation during the mechanical activation of quartz. Langmuir 25: 2264–2270. [DOI] [PubMed] [Google Scholar]
- Drobner, E. , Huber, H. , Wachtershauser, G. , Rose, D. , and Stetter, K.O. (1990) Pyrite formation linked with hydrogen evolution under anaerobic conditions. Nature 346: 742–744. [Google Scholar]
- Etiope, G. (2012) Climate science: methane uncovered. Nat Geosci 5: 373–374. [Google Scholar]
- Freund, F. (1985) Conversion of dissolved "water" into molecular hydrogen and peroxy linkages. J Non Cryst Solids 71: 195–202. [Google Scholar]
- Freund, F. , Dickinson, J.T. , and Cash, M. (2002) Hydrogen in rocks: an energy source for deep microbial communities. Astrobiology 2: 83–92. [DOI] [PubMed] [Google Scholar]
- Fu, Q. , Kobayashi, H. , Kuramochi, Y. , Xu, J. , Wakayama, T. , Maeda, H. , and Sato, K. (2013) Bioelectrochemical analyses of a thermophilic biocathode catalyzing sustainable hydrogen production. Int J Hydrogen Energy 38: 15638–15645. [Google Scholar]
- Hirose, T. , Kawagucci, S. , and Suzuki, K. (2011) Mechanoradical H2 generation during simulated faulting: implications for an earthquake‐driven subsurface biosphere. Geophys Res Lett 38: 3. [Google Scholar]
- Hirose, T. , Kawagucci, S. , and Suzuki, K. (2012) Correction to “Mechanoradical H2 generation during simulated faulting: implications for an earthquake‐driven subsurface biosphere”. Geophys Res Lett 39: L23304. [Google Scholar]
- Hoehler, T.M. , and Jorgensen, B.B. (2013) Microbial life under extreme energy limitation. Nat Rev Microbiol 11: 83–94. [DOI] [PubMed] [Google Scholar]
- Holm, N.G. , Oze, C. , Mousis, O. , Waite, J.H. , and Guilbert‐Lepoutre, A. (2015) Serpentinization and the formation of H2 and CH4 on celestial bodies (planets, moons, comets). Astrobiology 15: 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurowitz, J.A. , Tosca, N.J. , McLennan, S.M. , and Schoonen, M.A.A. (2007) Production of hydrogen peroxide in Martian and lunar soils. Earth Planet Sci Lett 255: 41–52. [Google Scholar]
- Kameda, J. , Saruwatari, K. , and Tanaka, H. (2004) H2 generation during dry grinding of kaolinite. J Colloid Interface Sci 275: 225–228. [DOI] [PubMed] [Google Scholar]
- Kirschke, S. , Bousquet, P. , Ciais, P. , Saunois, M. , Canadell, J.G. , Dlugokencky, E.J. , et al (2013) Three decades of global methane sources and sinks. Nat Geosci 6: 813–823. [Google Scholar]
- Kita, I. , Matsuo, S. , and Wakita, H. (1982) H2 generation by reaction between H2O and crushed rock ‐ an experimental‐study on H2 degassing from the active fault zone. J Geophys Res 87: 789–795. [Google Scholar]
- Lin, L.H. , Hall, J. , Lippmann‐Pipke, J. , Ward, J.A. , Lollar, M.D. , Rothmel, R. , et al (2005) Radiolytic H2 in continental crust: nuclear power for deep subsurface microbial communities. Geochem Geophys Geosyst 6: Q07003. [Google Scholar]
- Lollar, B.S. , Onstott, T.C. , Lacrampe‐Couloume, G. , and Ballentine, C.J. (2014) The contribution of the Precambrian continental lithosphere to global H2 production. Nature 516: 379–382. [DOI] [PubMed] [Google Scholar]
- McMahon, S. , Parnell, J. , and Blamey, N.J. (2016) Evidence for Seismogenic hydrogen gas, a potential microbial energy source on earth and Mars. Astrobiology 16: 690–702. [DOI] [PubMed] [Google Scholar]
- Miettinen, H. , Kietäväinen, R. , Sohlberg, E. , Numminen, M. , Ahonen, L. , and Itävaara, M. (2015) Microbiome composition and geochemical characteristics of deep subsurface high‐pressure environment, Pyhäsalmi mine Finland. Front Microbiol 6: 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, D.P. , Gihring, T.M. , Brockman, F.J. , Fredrickson, J.K. , Balkwill, D.L. , Dollhopf, M.E. , et al (2005) Desulfotomaculum and Methanobacterium spp. dominate a 4‐ to 5‐kilometer‐deep fault. Appl Environ Microbiol 71: 8773–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, D.P. , Onstott, T.C. , Fredrickson, J.K. , Brockman, F.J. , Balkwill, D.L. , Drake, G.R. , et al (2003) Temporal shifts in the geochemistry and microbial community structure of an ultradeep mine borehole following isolation. Geomicrobiol J 20: 517–548. [Google Scholar]
- Nilsen, R.K. , Torsvik, T. , and Lien, T. (1996) Desulfotomaculum thermocisternum sp nov, a sulfate reducer isolated from a hot North Sea oil reservoir. Int J Syst Bact 6: 397–402. [Google Scholar]
- O'Sullivan, L.A. , Roussel, E.G. , Weightman, A.J. , Webster, G. , Hubert, C. , Bell, E. , et al (2015) Survival of Desulfotomaculum spores from estuarine sediments after serial autoclaving and high‐temperature exposure. ISME J 9: 922–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcutt, B.N. , Bach, W. , Becker, K. , Fisher, A.T. , Hentscher, M. , Toner, B.M. , et al (2011) Colonization of subsurface microbial observatories deployed in young ocean crust. ISME J 5: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes, R.J. , Cragg, B. , Roussel, E. , Webster, G. , Weightman, A. , and Sass, H. (2014) A review of prokaryotic populations and processes in sub‐seafloor sediments, including biosphere:geosphere interactions. Mar Geol 352: 409–425. [Google Scholar]
- Pedersen, K. (1993) The deep subterranean biosphere. Earth Sci Rev 34: 243–260. [Google Scholar]
- Pedersen, K. (2000) Exploration of deep intraterrestrial microbial life: current perspectives. FEMS Microbiol Lett 185: 9–16. [DOI] [PubMed] [Google Scholar]
- Perevalova, A.A. , Kublanov, I.V. , Baslerov, R.V. , Zhang, G. , and Bonch‐Osmolovskaya, E.A. (2013) Brockia lithotrophica gen. nov., sp. nov., an anaerobic thermophilic bacterium from a terrestrial hot spring. Int J Syst Evol Microbiol 63: 479–483. [DOI] [PubMed] [Google Scholar]
- Purkamo, L. , Bomberg, M. , Kietäväinen, R. , Salavirta, H. , Nyyssönen, M. , Nuppunen‐Puputti, M. , et al (2016) Microbial co‐occurrence patterns in deep Precambrian bedrock fracture fluids. Biogeosciences 13: 3091–3108. [Google Scholar]
- Roussel, E.G. , Cambon‐Bonavita, M.A. , Querellou, J. , Cragg, B.A. , Webster, G. , Prieur, D. , and Parkes, R.J. (2008) Extending the sub‐sea‐floor biosphere. Science 320: 1046–1046. [DOI] [PubMed] [Google Scholar]
- Sleep, N.H. , and Zoback, M.D. (2007) Did earthquakes keep the early crust habitable? Astrobiology 7: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Spring, S. , Lapidus, A. , Schröder, M. , Gleim, D. , Sims, D. , Meincke, L. , et al (2009) Complete genome sequence of Desulfotomaculum acetoxidans type strain (5575(T)). Stand Genomic Sci 1: 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, T.O. , and McKinley, J.P. (1995) Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 270: 450–454. [Google Scholar]
- Takai, K. , Inoue, A. , and Horikoshi, K. (2002) Methanothermococcus okinawensis sp nov., a thermophilic, methane‐producing archaeon isolated from a Western Pacific deep‐sea hydrothermal vent system. Int J Syst Evol Microbiol 52: 1089–1095. [DOI] [PubMed] [Google Scholar]
- Takai, K. , Kobayashi, H. , Nealson, K.H. , and Horikoshi, K. (2003) Sulfurihydrogenibium subterraneum gen. nov., sp nov., from a subsurface hot aquifer. Int J Syst Evol Microbiol 53: 823–827. [DOI] [PubMed] [Google Scholar]
- Takai, K. , Nakamura, K. , Toki, T. , Tsunogai, U. , Miyazaki, M. , Miyazaki, J. , et al (2008) Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high‐pressure cultivation. Proc Natl Acad Sci U S A 105: 10949–10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling, J. , Boyd, E.S. , Bone, N. , Jones, E.L. , Tranter, M. , MacFarlane, J.W. , et al (2015) Rock comminution as a source of hydrogen for subglacial ecosystems. Nat Geosci 8: 851–855. [Google Scholar]
- Wakita, H. , Nakamura, Y. , Kita, I. , Fujii, N. , and Notsu, K. (1980) Hydrogen release: new indicator of fault activity. Science 210: 188–190. [DOI] [PubMed] [Google Scholar]
- Yin, A. (2012) Structural analysis of the Valles Marineris fault zone: possible evidence for large‐scale strike‐slip faulting on Mars. Lithosphere 4: 286–330. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Apparatus used for milling experiments: A. Ball mill (150 r.p.m.). B. Rotary milling in a 67 °C oil‐bath with 25 ml Wheaton® vials (150 r.p.m.). C. Rotary milling in a 67 °C oil‐bath with 100 ml Duran® bottles. D. Grinding with a magnetic stirring bar in a beaker water‐bath on heated‐stirrer at 67 °C, with or without a separate methanogenic community inoculum.
Fig. S2. Free radical production from milled silica at 67 °C based on consumption of a radical scavenger (DPPH: Damm & Peukert (2009)). Black squares are milled silica; red circles are the non‐milled negative control.
Fig. S3. H2 and CO formation during milling a range of minerals at 67 °C. Circles = granite, triangles = quartz, squares = silica, star = borosilicate glass, diamonds = basalt.
Fig. S4. XRD profiles of fresh powdered granite initially used in the experiment (top) and the granite after crushing at 67 °C with a magnetic stirrer (bottom).
Fig. S5. Inoculation of granite derived H2 experiments with Methanothermococcus okinawensis at 67 °C and changes in CH4 (filled circle) and H2 concentrations (triangles). Shaded area represents the grinding period; dotted line denotes injection of sterile medium to enhance H2 production and arrows are injection of the methanogen pure culture. Replicate experiments a and b.
Fig. S6. Enrichment of air tolerant methanogenic community at 67 °C using sediments from the Tamar Estuary, UK in mineral medium: a) initial enrichment slurry with successive air additions, b) enrichment after successive subculture at low H2 concentrations in a vial mimicking experimental conditions. CH4 (filled circle) and H2 (triangles).
Fig. S7. Control experiments at 67 °C with a) autoclaved (x3) enrichment inoculated into an experiment with 30 g of crushed granite (shaded area is the grinding period) and H2 adjusted to ~300 μmol L−1. b) Active methanogenic enrichment inoculated into an empty device. Experiment 1 shown by solid lines and Experiment 2 by dashed lines. CH4 (filled circle) and H2 (triangles).
Fig. S8. Phylogenetic tree of methanogen functional mcrA gene clones from the methanogenic community inoculum. All clones were closely related (96%) to Methanothermobacter crinale.
Fig. S9. Comparison at the class‐level of bacterial 16S rRNA genes detected in this study with other studies of subsurface environments. 1: Parkes et al., (this study) crushing experiments, hot condition; 2: Fu et al. (2013) cathode hydrogen production sustaining methanogenic community, hot condition; 3: Cowen et al., (2003) aging ocean crust, hot condition; 4: Orcutt et al., (2011) young ocean crust, hot condition; 5: Diksma et al., (2016) dark C fixation in coastal marine sediments, cold condition; 6: Le Campion et al., unpublished, continental subsurface aquifer; 7–9: Dong et al., (2014) 1.8 km deep subsurface Cambrian sandstone reservoir, thermophilic; 10–12: Edlund et al., (2008) Baltic sea sediments, cold conditions (10 = redox depth ‐337 mV; 11 = redox depth − 169 mV; 12 = redox depth − 64 mV [b1]).


